A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.1.1. Environmental Microplastics
2.1.2. Pre-Production Microplastics
- virgin colourless polyethene (PE) pre-production pellets (size 5000 µm);
- virgin green polyethene (PE) pre-production microparticles (size <500 µm);
- virgin colorless polypropylene (PP) pre-production pellets (size 5000 µm).
2.1.3. Water Samples
2.2. Chemical Identification
2.2.1. Pollutants Extraction from Microplastics Samples
2.2.2. Pollutant Extraction from River Water Samples
2.3. Target Compound Analysis by GC–MS/MS
2.4. Non-Target Compounds Screening by GC–HRMS
3. Results and Discussion
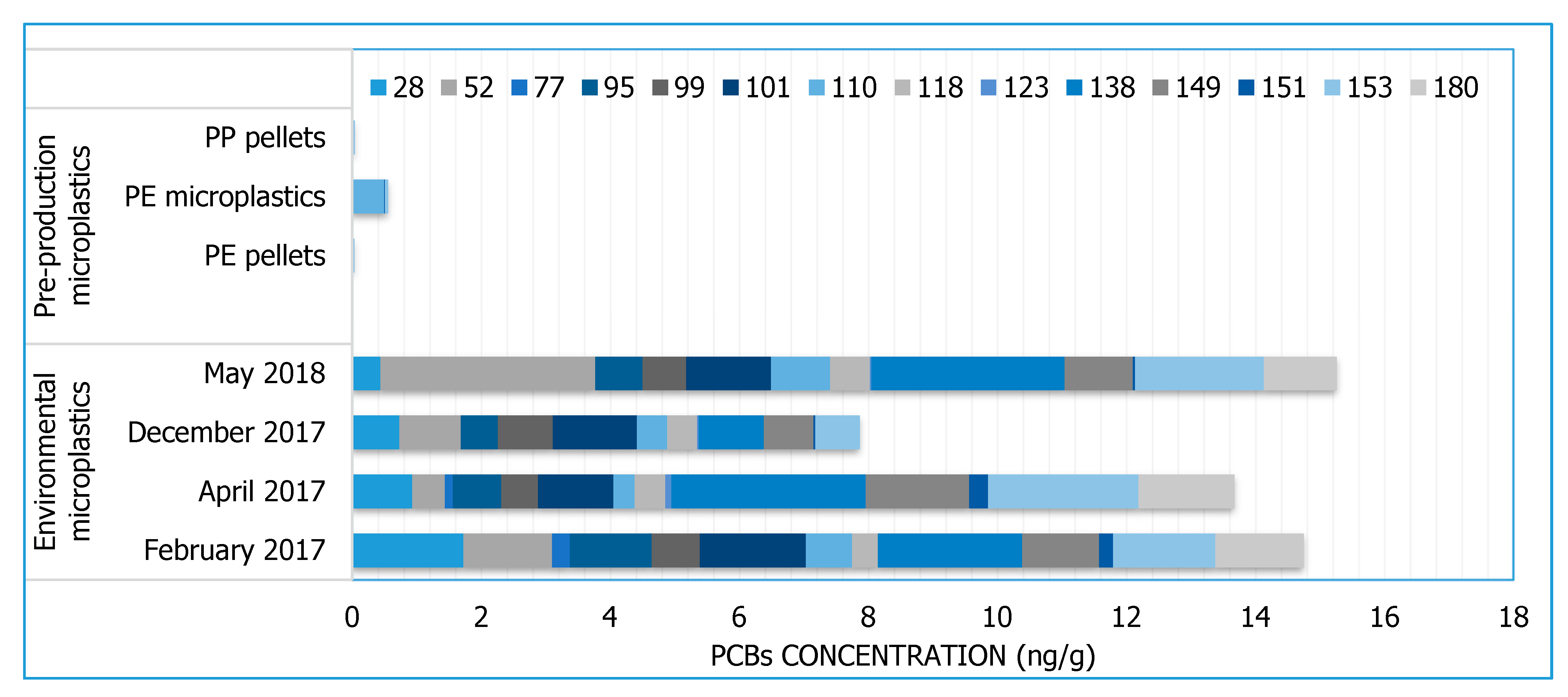
3.1. PCBs
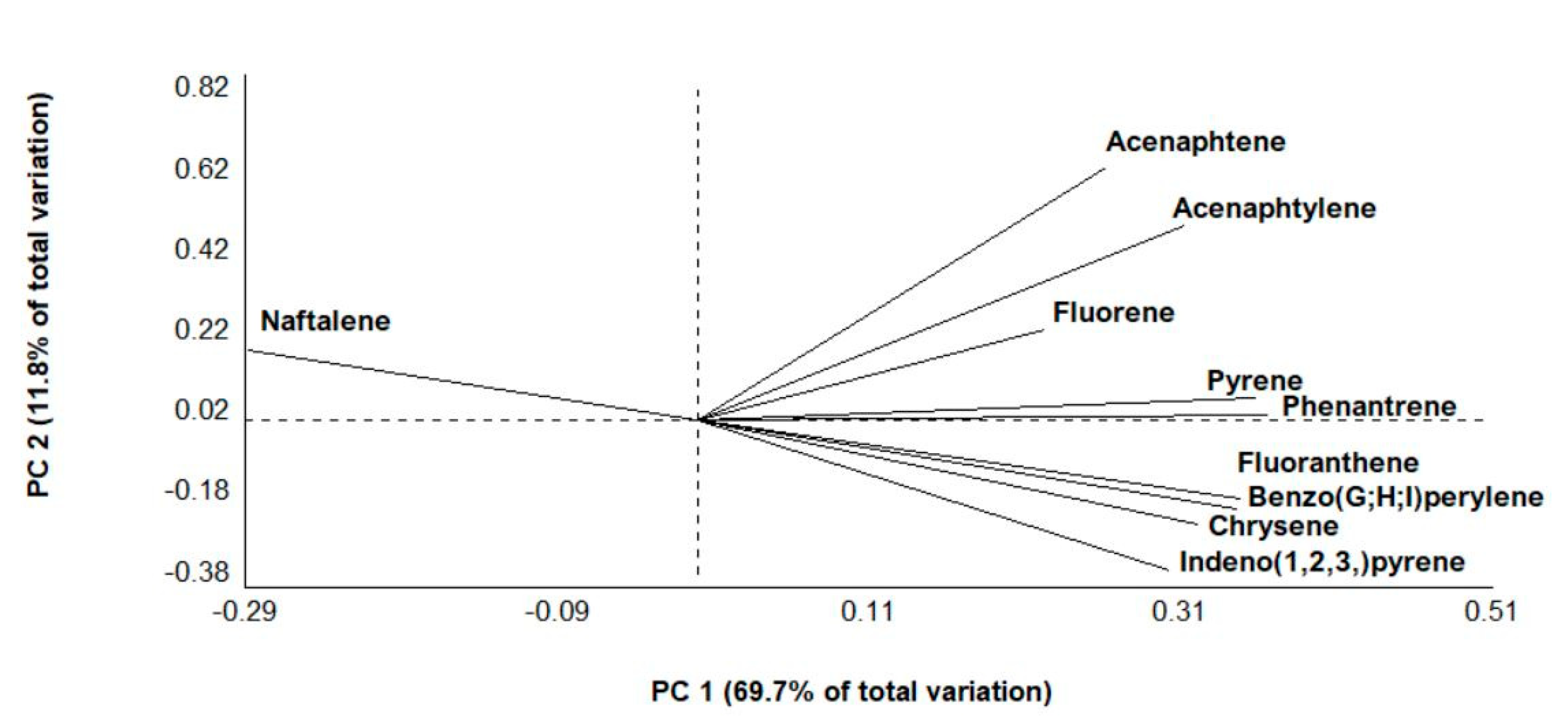
3.2. PAHs
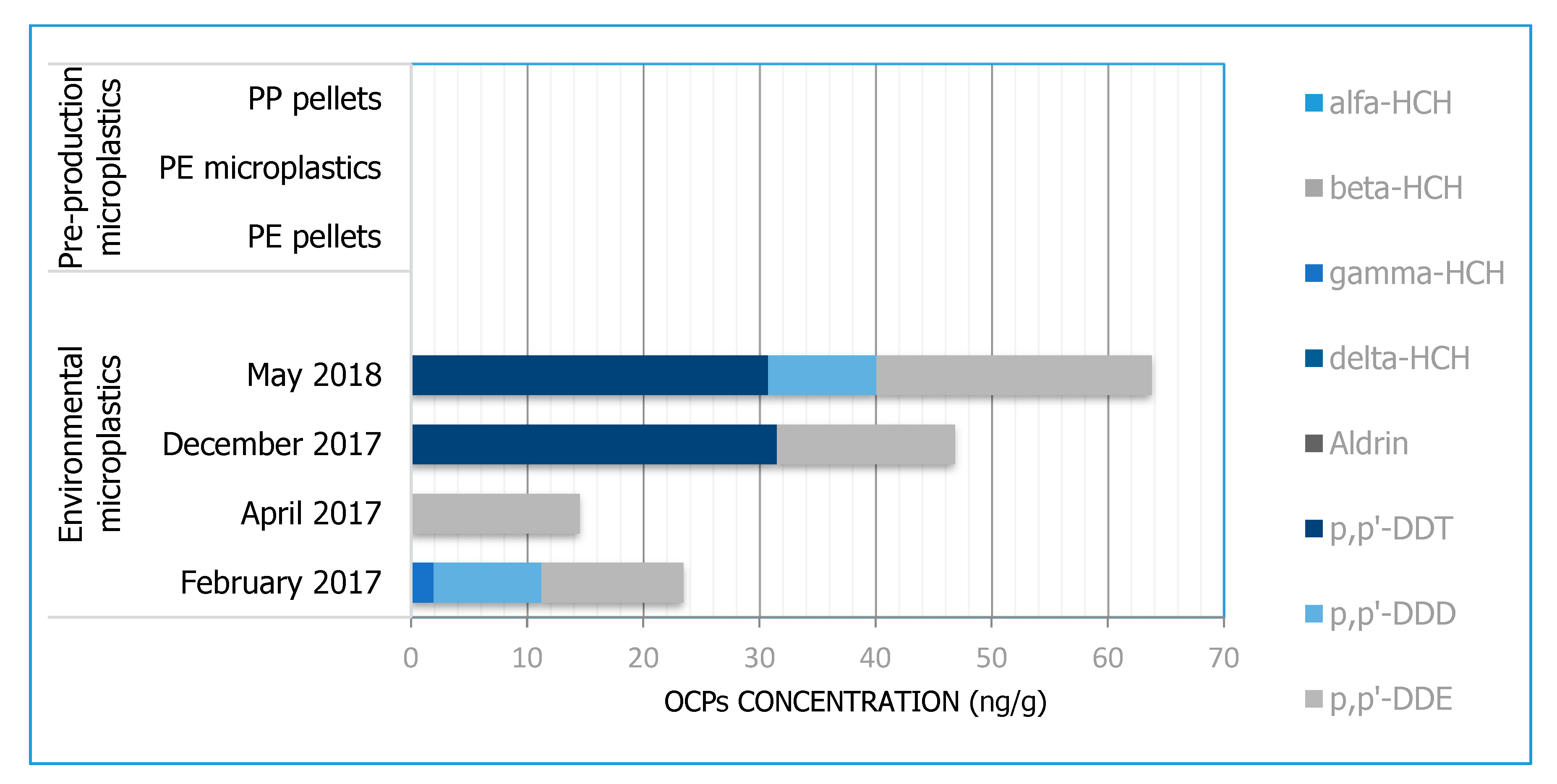
3.3. OCPs
3.4. Non-Target Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Rios, L.M.; Jones, P.R.; Moore, C.J.; Narayan, U.V. Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s “eastern garbage patch”. J. Environ. Monit. 2010, 12, 2226–2236. [Google Scholar] [CrossRef]
- Ryan, P.G.; Bouwman, H.; Moloney, C.L.; Yuyama, M.; Takada, H. Long-term decreases in persistent organic pollutants in South African coastal waters detected from beached polyethylene pellets. Mar. Pollut. Bull. 2012, 64, 2756–2760. [Google Scholar] [CrossRef]
- Van, A.; Rochman, C.M.; Flores, E.M.; Hill, K.L.; Vargas, E.; Vargas, S.A.; Hoh, E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 2012, 86, 258–263. [Google Scholar] [CrossRef]
- Eriksson, C.; Burton, H.; Fitch, S.; Schulz, M.; van den Hoff, J. Daily accumulation rates of marine debris on sub-Antarctic island beaches. Mar. Pollut. Bull. 2013, 66, 199–208. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.S.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater microplastics. Emerging environmental contaminants? In The Handbook of Environmental Chemistry; Barceló, D., Kostianoy, A.G., Eds.; Springer Open: Cham, Switzerland, 2018; Volume 58, pp. 1–16. [Google Scholar]
- León, V.M.; García, I.; González, E.; Samper, R.; Fernández-González, V.; Muniategui-Lorenzo, S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ. Pollut. 2018, 236, 442–453. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- León, V.M.; García-Agüera, I.; Moltó, V.; Fernández-González, V.; Llorca-Pérez, L.; Andrade, J.M.; Muniategui-Lorenzo, S.; Campillo, J.A. PAHs, pesticides, personal care products and plastic additives in plastic debris from Spanish Mediterranean beaches. Sci. Total. Environ. 2019, 670, 672–684. [Google Scholar] [CrossRef]
- Gorman, D.; Moreira, F.T.; Turra, A.; Fontenelle, F.R.; Combi, T.; Bícego, M.C.; Martins, C.D.C. Organic contamination of beached plastic pellets in the South Atlantic: Risk assessments can benefit by considering spatial gradients. Chemosphere 2019, 223, 608–615. [Google Scholar] [CrossRef]
- Graca, B.; Bełdowska, M.; Wrzesień, P.; Zgrundo, A. Styrofoam debris as a potential carrier of mercury within ecosystems. Environ. Sci. Pollut. Res. 2014, 21, 2263–2271. [Google Scholar] [CrossRef]
- Holmes, L.A. Interactions of Trace Metals with Plastic Production Pellets in the Marine Environment. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2013. [Google Scholar]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Hong, S.H.; Shim, W.J.; Hong, L. Methods of analysing chemicals associated with microplastics: A review. Anal. Methods 2017, 9, 1361–1368. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, A.V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total. Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Zhao, Y.; Xiang, Y.; He, D.; Pan, X. Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environ. Pollut. 2020, 257, 113475. [Google Scholar] [CrossRef]
- Murphy, J. Additives for Plastics Handbook, 2nd ed.; Elsevier Science Ltd.: Oxford, UK; New York, NY, USA; Tokyo, Japan, 2001; pp. 30–60. [Google Scholar]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Gauquie, J.; Devriese, L.I.; Robbens, J.; De Witte, B. A qualitative screening and quantitative measurement of organic contaminants on different types of marine plastic debris. Chemosphere 2015, 138, 348–356. [Google Scholar] [CrossRef]
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Mendoza, L.M.R.; Balcer, M. Association of hazardous compounds with microplastics in freshwater ecosystems. In Microplastics in Water and Wastewater; IWA Publishing: London, UK, 2019; pp. 15–25. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Agilent SureMass, Agilent Technologies Technical Overview, Publication Number 5991-8048EN (2017); Agilent Technologies: Wood Dale, IL, USA, 2017.
- Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope, Carbon Neutral Printing-Natureoffice: Wiesbaden, Germany, 2019. [Google Scholar]
- Council Directive 76/769/EEC of 27 July 1976 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations; Official Journal of the European Communities: Bruxelles, Belgium, 1976.
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Engler, R.E. The Complex Interaction between Marine Debris and Toxic Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuarine Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gomez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total. Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; Van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Frias, J.; Sobral, P.; Ferreira, A. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Jayasiri, H.B.; Purushothaman, C.S.; Vennila, A. Bimonthly variability of persistent organochlorines in plastic pellets from four beaches in Mumbai coast, India. Environ. Monit. Assess. 2015, 187, 469. [Google Scholar] [CrossRef]
- Antunes, J.C.; Frias, J.G.L.; Micaelo, A.C.; Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuarine Coast. Shelf Sci. 2013, 130, 62–69. [Google Scholar] [CrossRef]
- Mizukawa, K.; Takada, H.; Ito, M.; Geok, Y.B.; Hosoda, J.; Yamashita, R.; Saha, M.; Suzuki, S.; Miguez, C.; Frias, J.; et al. Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets. Mar. Pollut. Bull. 2013, 70, 296–302. [Google Scholar] [CrossRef]
- Taniguchi, S.; Colabuono, F.I.; Dias, P.S.; Oliveira, R.; Fisner, M.; Turra, A.; Izar, G.M.; Abessa, D.M.; Saha, M.; Hosoda, J.; et al. Spatial variability in persistent organic pollutants and polycyclic aromatic hydrocarbons found in beach-stranded pellets along the coast of the state of São Paulo, southeastern Brazil. Mar. Pollut. Bull. 2016, 106, 87–94. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- International Pellet Watch. Available online: http://www.pelletwatch.org (accessed on 10 July 2020).
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.C.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef]
- Fisner, M.; Taniguchi, S.; Moreira, F.; Bícego, M.C.; Turra, A. Polycyclic aromatic hydrocarbons (PAHs) in plastic pellets: Variability in the concentration and composition at different sediment depths in a sandy beach. Mar. Pollut. Bull. 2013, 70, 219–226. [Google Scholar] [CrossRef]
- Simoneau, C.; Van Den Eede, L.; Valzacchi, S. Identification and quantification of the migration of chemicals from plastic baby bottles used as substitutes for polycarbonate. Food Addit. Contam. Part A 2011, 29, 469–480. [Google Scholar] [CrossRef]
- Pfaender, F.K.; Alexander, M. Extensive microbial degradation of DDT in vitro and DDT metabolism by natural communities. J. Agric. Food Chem. 1972, 20, 842–846. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W.Y.; Ma, K.C. Illustrated handbook of physical—Chemical properties and environmental fate for organic compounds. Lewis Publishers, Chelsea magna. Environ. Monit. Assess. 1992, 187, 1–14. [Google Scholar]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- ARPA (Regional Agency for Environmental Prevention and Protection) 2016. Monitoraggio Qualitativo dei Corpi Idrici Superficiali per il Triennio 2016–2018. Available online: www.arpa.puglia.it›document_library›get_file (accessed on 8 September 2020).
- Costantini, E.A.C.; Dazzi, C. The Soils of Italy; Springer Science & Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- ISPRA. Audizione dell’Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA) presso la Commissione Agricoltura, congiuntamente con la Commissione Ambiente, della Camera sul consumo di suolo, Audizione; ISPRA Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2014. [Google Scholar]
- Thiombane, M.; Petrik, A.; Di Bonito, M.; Albanese, S.; Zuzolo, D.; Cicchella, D.; Lima, A.; Qu, C.; Qi, S.-H.; De Vivo, B. Status, sources and contamination levels of organochlorine pesticide residues in urban and agricultural areas: A preliminary review in central–southern Italian soils. Environ. Sci. Pollut. Res. 2018, 25, 26361–26382. [Google Scholar] [CrossRef]
- Bacci, E. Ecotoxicology of Organic Contaminants; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Zhang, A.; Chen, Z.; Ahrens, L.; Liu, W.; Li, Y.-F. Concentrations of DDTs and Enantiomeric Fractions of Chiral DDTs in Agricultural Soils from Zhejiang Province, China, and Correlations with Total Organic Carbon and pH. J. Agric. Food Chem. 2012, 60, 8294–8301. [Google Scholar] [CrossRef]
- Montuori, P.; Cirillo, T.; Fasano, E.; Nardone, A.; Esposito, F.; Triassi, M. Spatial distribution and partitioning of polychlorinated biphenyl and organochlorine pesticide in water and sediment from Sarno River and Estuary, Southern Italy. Environ. Sci. Pollut. Res. 2014, 21, 5023–5035. [Google Scholar] [CrossRef]
- Qu, C.; Albanese, S.; Chen, W.; Lima, A.; Doherty, A.L.; Piccolo, A.; Arienzo, M.; Qi, S.; De Vivo, B. The status of organochlorine pesticide contamination in the soils of the Campanian Plain, southern Italy, and correlations with soil properties and cancer risk. Environ. Pollut. 2016, 216, 500–511. [Google Scholar] [CrossRef]
- Rashid, M.M.; Sarker, M. Waste polyethylene terephthalate (PETE) and polystyrene (PS) into fuel. Int. J. Sci. Technol. Res. 2013, 2, 176–189. [Google Scholar]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative Analysis of Additives in Plastic Marine Debris and Its New Products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Screening of Plastic Toys for Chemical Composition and Hazards—Market Surveillance in the Netherlands; Report ND05o610/01; Food and Safety Authority (VWA): Zutphen, The Netherlands, 2005.
- Rogan, W.J.; Gladen, B.C. Neurotoxicology of PCBs and related compounds. Neurotoxicology 1992, 13, 27–36. [Google Scholar] [PubMed]
- De Duffard, A.M.E.; Duffard, R. Behavioral toxicology, risk assessment, and chlorinated hydrocarbons. Environ. Heal. Perspect. 1996, 104, 353–360. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance; Official Journal of the European Communities: Bruxelles, Belgium, 2011.
- Detoni, C.B.; Paese, K.; Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Nanosized and Nanoencapsulated Sunscreens. In Nanocosmetisc and Nanomedicines; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 333–362. [Google Scholar] [CrossRef]
- Council Directive 67/548/EEC of 27 June 1967 on the Approximation of Laws, Regulations and Administrative Provisions Relating to the Classification, Packaging and Labelling of Dangerous Substances; Official Journal of the European Communities: Bruxelles, Belgium, 1967.
- Phthalate Strategy; Environmental Project No. 1488; The Danish EPA: Copenhagen, Denmark, 2013; ISBN no.978-87-93026-22-3.
- Lau, O.-W.; Wong, S.-K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characteriszation of plastic packaging additives: Food contact, stability and toxicity. Arabian J. Chem. 2017, 10, S1938–S1954. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Invasions by marine life on plastic debris. Nature 2002, 416, 808–809. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Carson, H.S.; Eriksen, M. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar. Biol. 2014, 161, 1441–1453. [Google Scholar] [CrossRef]
- Food and Consumer Product Safety Authority. Migration of Bisphenol A and Plasticizers from Plastic Feeding Utensils for Babies; Report No ND05o410; Food and Consumer Product Safety Authority: Utrecht, The Netherland, 2005; p. 11. [Google Scholar]
- Food and Consumer Product Safety Authority. Screening of Plastic Toys for Chemical Composition And hazards. Market Surveillance in the Netherlands; Report ND05o610/01; Food and Consumer Product Safety Authority: Utrecht, The Netherlans, 2005; p. 17. [Google Scholar]
- Bradley, E.; Coulier, L. An Investigation into the Reaction and Breakdown Products from Starting Substances Used to Produce Food Contact Plastics; Report FD 07/01; CSL Sand Hutton: York, UK, 2007; p. 628. [Google Scholar]
- Hansen, E.; Nilsson, N.; Vium, K.S.R. Hazardous Substances in Plastics. Survey of Chemical Substances in Consumer Products No. 132; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2014; ISBN 978-87-93283-31-2. [Google Scholar]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanermen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef]
- Jobling, S.; Reynolds, T.; White, R.; Parker, M.G.; Sumpter, J.P. A variety of environmentally persistent chemicals, including some phthalate plasticiszers, are weakly estrogenic. Environ. Health Perspect. 1995, 103, 582–587. [Google Scholar] [CrossRef]
- Duty, S.M.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Chen, Z.; Herrick, R.F.; Christiani, D.C.; Hauser, R. Phthalate Exposure and Human Semen Parameters. Epidemiology 2003, 14, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Calafat, A. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticiszers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.; Struijs, J. Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol. Environ. Saf. 2006, 63, 204–215. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Bonet, J.; Velasco, G.; Lacorte, S. Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci. Total. Environ. 2009, 407, 4157–4167. [Google Scholar] [CrossRef]
- Bergé, A. Identification of Sources of Alkylphenols and Phthalates in Urban Area. Comparison of Domestic Discharges to Pure Industrial Wastewater. Master’s Thesis, University Paris-Est, Créteil, France, 2012. [Google Scholar]
- Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-analysis of environmental contamination by phthalates. Environ. Sci. Pollut. Res. 2013, 20, 8057–8076. [Google Scholar] [CrossRef]
- Stanley, M.K.; Robillard, K.A.; Staples, C.A. Phthalate esters (The Handbook of Environmental Chemistry); Staples, C.A., Ed.; Springer: Berlin, Germany, 2003; Volume 3, p. 1. [Google Scholar]
- Llompart, M.; Garcia-Jares, C.; Landin, P. Phthalates Esters. In Chromatographic Analysis of the Environment; Nollet, L.M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 1103. [Google Scholar]
- Bergé, A.; Gasperi, J.; Rocher, V.; Gras, L.; Coursimault, A.; Moilleron, R. Phthalates and alkylphenols in industrial and domestic effluents: Case of Paris conurbation (France). Sci. Total. Environ. 2014, 489, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Kühn, S.; Van Oyen, A.; Booth, A.M.; Meijboom, A.; Van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar] [CrossRef]
- Cambridge Polymer Group. Rubber Duck Deformulation; Cambridge Polymer Group Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Liu, F.-F.; Liu, G.-Z.; Zhu, Z.-L.; Wang, S.-C.; Zhao, F.-F. Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 2019, 214, 688–694. [Google Scholar] [CrossRef]
- Gray, J.L.E.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.N.R.; Parks, L. Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicol. Sci. 2000, 58, 350–365. [Google Scholar] [CrossRef]
- Ema, M.; Miyawaki, E. Adverse effects on development of the reproductive system in male offspring of rats given monobutyl phthalate, a metabolite of dibutyl phthalate, during late pregnancy. Reprod. Toxicol. 2001, 15, 189–194. [Google Scholar] [CrossRef]
- Fisher, J.S.; MacPherson, S.; Marchetti, N.; Sharpe, R.M. Human ’testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 2003, 18, 1383–1394. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, L.; Yuan, L.; Wang, X.; Zhang, W. Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butylphthalate (DBP). Toxicology 2007, 232, 286–293. [Google Scholar] [CrossRef]
- Shrivastava, A. Introduction to Plastics Engineering—3 Plastic Properties and Testing; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780323396196. [Google Scholar]
- Subramanian, M.N. Plastics Additives and Testing; Scrivener Publishing LLC: Salem, MA, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-11890-0. [Google Scholar]
- Bolgar, M.; Hubball, J.; Groeger, J.; Meromeck, S. Plasticizers. In Handbook for the Chemical Analysis of Plastic and Polymer Additives, 1st ed.; AccuStandard: New Haven, CT, USA; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; Available online: https://www.taylorfrancis.com/books/9780429140204 (accessed on 6 October 2020).
- Wang, H.; Wang, C.; Wu, W.; Mo, Z.; Wang, Z. Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere 2003, 50, 557–562. [Google Scholar] [CrossRef]
- Lu, Z.; De Silva, A.O.; Peart, T.E.; Cook, C.J.; Tetreault, G.R.; Servos, M.R.; Muir, D.C.G. Distribution, partitioning and bioaccumulation of substituted diphenylamine antioxidants and benzotriazole UV stabilisers in an urban creek in Canada. Environ. Sci. Technol. 2016, 50, 9089–9097. [Google Scholar] [CrossRef]
- Wick, A.; Jacobs, B.; Kunkel, U.; Heininger, P.; Ternes, T.A. Benzotriazole UV stabiliszers in sediments, suspended particulate matter and fish of German rivers: New insights into occurrence, time trends and persistency. Environ. Pollut. 2016, 212, 401–412. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Chang, S.; Hong, S.H.; Shim, W.J. Microplastics as a vector of hydrophobic contaminants: Importance of hydrophobic additives: Hydrophobic organic contaminants from microplastics. Integr. Environ. Assess. Manag. 2017, 13, 494–499. [Google Scholar] [CrossRef]
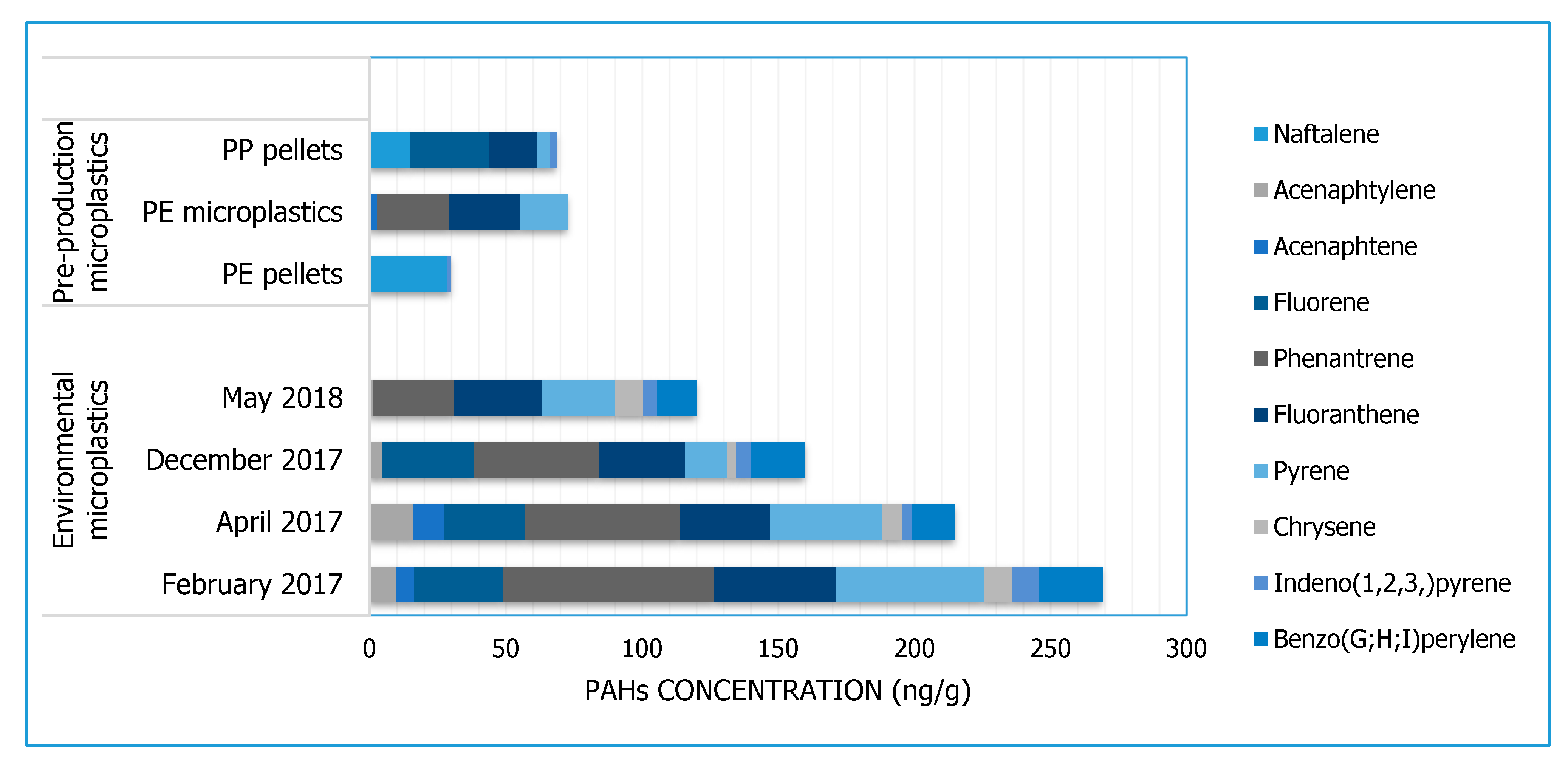
| Pollutants Analysed | PAHs | Naphthalene, acenaphtylene, acenaphtene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(123)pyrene, dibenzo(ah)anthracene, benzo(ghi)perylene | |
| OCPs | Alfa-HCH, beta-HCH, gamma-HCH, delta-HCH, aldrin, p,p’-DDE, p,p’-DDD, p,p’-DDT | ||
| PCBs | 18, 28, 52, 44, 95, 101, 99, 81, 77, 110, 151, 123, 149, 118, 114, 146, 153, 105, 138, 126, 187, 183, 128,167, 177, 156, 157, 180, 169, 170, 189 | ||
| Microplastics Analysed | Pre-production microplastics | PE transparent pellets (size 5000 µm) PP transparent pellets (size 5000 µm) PE green microplastics (size 300 µm) | |
| Environmental microplastics | February 2017 | Polymer: PE 77%, PS 13%, PP 10% Morphology: fragments 50%, pellets 30%, lines 10% Color: transparent 50%, black 40%, colored 10% Size: 5000–1000 µm | |
| April 2017 | |||
| December 2017 | |||
| May 2018 | |||
| Environmental Microplastics | ||||||
|---|---|---|---|---|---|---|
| Category | CAS# | Compound Name | Match Factor | Formula | Library Molecular Weight | Common Name and Comments |
| Hydrocarbons/ alkanes and substituted hydrocarbons | 544-76-3 | Hexadecane | 96.33 | C16H34 | 226.266 | n/a Originate from the paraffin wax used as an external lubricant in PVC and other polymers. Alkanes are also used as a solvent and are oligomers originating from poly olefines (PP, PE, and PS) during recycling [62,63]. |
| 593-49-7 | Heptacosane | 94.68 | C27H56 | 380.438 | ||
| 630-04-6 | Hentriacontane | 93.29 | C31H64 | 436.501 | ||
| 630-02-4 | Octacosane | 91.34 | C28H58 | 394.454 | ||
| 1186-53-4 | Pentane, 2,2,3,4-tetramethyl- | 92.25 | C9H20 | 128.157 | ||
| 62108-23-0 | Decane, 2,5,6-trimethyl- | 89.72 | C13H28 | 184.219 | ||
| 1002-43-3 | Undecane, 3-methyl- | 89.41 | C12H26 | 170.203 | ||
| 53366-38-4 | Cyclopentane, (2-methylbutyl)- | 88.11 | C10H20 | 140.157 | ||
| 17301-32-5 | Undecane, 4,7-dimethyl- | 85.37 | C13H28 | 184.219 | ||
| 563-16-6 | Hexane, 3,3-dimethyl- | 82.51 | C8H18 | 114.141 | ||
| Hydrocarbons/ alkenes | 629-89-0 | 1-Octadecyne | 88.42 | C18H34 | 250.266 | n/a Starter compounds for several additives and polymers or are formed as a by-product in the olefin polymerisation [64]. |
| 765-13-9 | 1-Pentadecyne | 87.09 | C15H28 | 208.219 | ||
| 74685-30-6 | 5-Eicosene | 82.09 | C20H40 | 280.313 | ||
| Halogenated hydrocarbons | 4292-19-7 | Dodecane, 1-iodo- | 90.5 | C12H25I | 296.1 | n/a Toxicity of chlorinated hydrocarbons is documented, and these compounds have been considered as persistent organic pollutants [65,66]. |
| 1000406-32-0 | Tetracosane, 1-iodo- | 85.6 | C24H49I | 464.288 | ||
| Cyclic hydrocarbons | 294-62-2 | Cyclododecane | 90.1 | C12H24 | 168.188 | n/a Probably reaction products or decomposition products [66]. |
| Plastic additives/UV stabilisers | 1843-05-6 | Octabenzone | 94.19 | C21H26O3 | 326.188 | Uvinul 3008 UV stabiliser used to protect polymers against damage by UV [67]. |
| 5466-77-3 | 2-Propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester | 92.45 | C18H26O3 | 290.188 | n/a UV-B filter, a common ingredient in sunscreen and other skincare products to minimise DNA photodamage [68]. | |
| 97-76-4 | 2,4-Di-tert-butylphenol | 98.0 | C14H22O | 206.167 | n/a | |
| 764-42-1 | Fumaronitrile | 95.1 | C4H2N2 | 78.022 | n/a | |
| 2082-79-3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | 88.13 | C35H62O3 | 530.47 | Irganox 1076 | |
| Plastic additives/ phtalathes | 84-69-5 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 91.99 | C16H22O4 | 278.152 | DiBP |
| 117-81-7 | Bis(2-ethylhexyl) phthalate | 94.6 | C24H38O4 | 390.277 | DEHP reprotoxic category 2, T (toxic) [69]. | |
| 84-66-2 | Diethyl phthalate | 88.20 | C12H14O4 | 222.089 | DEP most broadly used in medicinal products [70]. | |
| 84-74-2 | Dibutyl phthalate | 87.10 | C16H22O4 | 278.152 | DBP reprotoxic category 3, T (toxic), N (dangerous for the environment) [71,72]; most widely used in medicinal products. | |
| 28029-89-2 | Didecan-2-yl phthalate | 87.3 | C28H46O4 | 446.34 | 1,2-Benzenedicarboxylic acid | |
| Plastic additives/ antioxidants | 3896-11-5 | 2-tert-Butyl-6-(5-chloro-2H-benzotriazol- 2-yl)-4-methylphenol | 89.61 | C17H18ClN3O | 315.114 | Bumetrizole. Used to slow the oxidation process of the polymers exposed to UV light [71,72]. |
| 101-72-4 | 1,4-Benzenediamine, N-(1-methylethyl)-N’-phenyl- | 86.59 | C15H18N2 | 226.147 | Santoflex IPPD | |
| 95906-11-9 | Tris(2,4-di-tert-butylphenyl) phosphate | 86.12 | C42H63O4P | 662.446 | n/a | |
| 15721-78-5 | Benzenamine, 4-(1,1,3,3-tetramethylbutyl)-N-[4-(1,1,3,3-tetramethylbutyl)phenyl]- | 84.4 | C28H43N | 393.34 | n/a | |
| Intermediate | 4337-65-9 | Hexanedioic acid, mono(2-ethylhexyl)ester | 91.05 | C14H26O4 | 258.183 | n/a |
| Alcohols | 10042-59-8 | 1-Heptanol, 2-propyl- | 95.90 | C10H22O | 158.167 | Chemical intermediate or internal lubricant. Impurities or degradation products [73,74,75]. Screening of plastic toys and feeding utensils for babies showed the presence of alcohols [76,77]. Possible degradation products of plastic [26,63,78]. |
| 3913-02-8 | 1-Octanol, 2-butyl- | 86.88 | C12H26O | 186.198 | ||
| 54004-41-0 | 1-Pentanol, 4-methyl-2-propyl- | 84.85 | C9H20O | 144.151 | ||
| Biofilm and algae compounds | 83-47-6 | Gamma-sitosterol | 82.02 | C29H50O | 414.386 | n/a |
| 201358-24-9 | 24-Noroleana-3,12-diene | 88.59 | C29H46 | 394.36 | n/a | |
| 502-69-2 | 2-Pentadecanone, 6,10,14-trimethyl- | 88.43 | C18H36O | 268.277 | n/a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanale, C.; Dierkes, G.; Massarelli, C.; Bagnuolo, G.; Uricchio, V.F. A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics. Toxics 2020, 8, 100. https://doi.org/10.3390/toxics8040100
Campanale C, Dierkes G, Massarelli C, Bagnuolo G, Uricchio VF. A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics. Toxics. 2020; 8(4):100. https://doi.org/10.3390/toxics8040100
Chicago/Turabian StyleCampanale, Claudia, Georg Dierkes, Carmine Massarelli, Giuseppe Bagnuolo, and Vito Felice Uricchio. 2020. "A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics" Toxics 8, no. 4: 100. https://doi.org/10.3390/toxics8040100
APA StyleCampanale, C., Dierkes, G., Massarelli, C., Bagnuolo, G., & Uricchio, V. F. (2020). A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics. Toxics, 8(4), 100. https://doi.org/10.3390/toxics8040100







