Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications
Abstract
1. Introduction
2. Chemical and Physical Methods
2.1. Chemical Precipitation
2.1.1. Hydroxide Precipitation
2.1.2. Sulfide Precipitation
2.1.3. Other Techniques Using Chemical Precipitation
- Ferrous sulfate is usually used together with lime for water softening. The aggregates consist of calcium sulfate and ferric hydroxide. The condition for a successful removal process is that the wastewater must contain dissolved oxygen that is required by the chemical reaction to form the precipitate [17].
- Aluminum for the removal of phosphate and water softening. The reaction takes place with phosphate or various alkaline compounds (i.e., bicarbonate, hydroxide or carbonate) that form insoluble aluminum slats [17].
- Polymers, which can be anionic, cationic or nonionic. They can be used for neutralization or as links coagulants after they are added to wastewaters [19].
2.2. Ion Exchange
2.3. Adsorption
2.3.1. Carbon-Based Adsorbents
2.3.2. Carbon Nanotube (CNT) Absorbents
2.3.3. Low-Cost Bioadsorbents
2.4. Membrane Filtration
2.4.1. Ultrafiltration (UF)
2.4.2. Reverse Osmosis (RO)
2.4.3. Nanofiltration (NF)
2.5. Coagulation and Flocculation
2.6. Flotation
3. Electrochemical Depolluting Treatments
3.1. Electrodialysis (ED)
3.2. Electroflotation (EF)
3.3. Electrocoagulation (EC)
- Formation of coagulants by electrolytic oxidation of the sacrificial anode electrodes.
- Destabilization of the contaminants, particulate suspension and breaking of emulsions.
- Aggregation of the destabilized phases to form flocs.
- -
- Monopolar electrodes in parallel connection, where current is divided between all electrodes;
- -
- Monopolar electrodes in a serial connections system, where every couple of sacrificial electrodes are connected with each other;
- -
- Bipolar electrode in serial connections where there is not any electrical connection between the inner electrodes because the outer electrodes are linked to power.
3.4. Electrochemical Technologies in Wastewater Treatment
4. Electrochemical Detection and Removal of Heavy Metals
4.1. Methods to Obtain Nanostructured Electrodes
4.1.1. Electroless Deposition
4.1.2. Electrochemical Template Synthesis of Nanostructures
4.1.3. Electrochemical Template Synthesis for Nanostructure Arrays
4.2. Electrochemical Detection of Heavy Metals
- Structure-directed agents, i.e., tubular assembly of a surfactant that encapsulate oxides; similar to multiwall CNTs, oxide nanotubes obtained by this method have a multiwall structure composed of a mixture of oxide and organic components [221]. Unlike CNTs, oxide nanotubes can be obtained in gram quantities by chemistry synthesis at low temperature.
- Template directed growth: nanoporous alumina (AAO) or carbon nanotubes as templates. Polycrystalline nanotubes of ZrO2 [222], V2O5 [223], etc. For certain applications, the synthesis of single-crystalline oxide nanotube is required. Li et al. obtained single-crystalline In2O3 NTs [224] and single-crystalline MgO NTs [225].
- Fill in nanotubes Hollow cavities with high aspect ratio such as nanotubes can be filled in to create nanocable structures. Ajayan and Iijima were first to insert by capillarity low-melting-point metals in the hollow cavities of carbon nanotubes [226,227]. This method also works well with oxides due to their high-melting points. Li et al. [224] obtained single-crystalline In2O3 NTs loaded with metallic In by evaporating a mixture of indium and indium oxide in vacuum and single-crystalline MgO NTs filled with Ga [225]. The removal of the template without destroying the nanotubes remains an unresolved problem.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- USEPA. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; Epa 822-f-18-001; Office of Water, U.S. Environmental Protection Agency (usepa): Washington, DC, USA, 2018. [Google Scholar]
- Organisation, W.H. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- UN. United Nations Guide to the Globally Harmonized System of Classification and Labeling of Chemicals (ghs); United Nations: Geneva, Switzerland, 2015; p. 90. [Google Scholar]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.H.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Scheierling, S.M.; Bartone, C.R.; Mara, D.D.; Drechsel, P. Toward an agenda for improving wastewater use in agriculture. Water Int. 2011, 36, 20. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Favier, L.; Harja, M.; Simion, A.I.; Rusu, L.; Kadmi, Y.; Pacala, M.L.; Bouzaza, A. Advanced oxidation process for the removal of chlorinated phenols in aqueous suspensions. J. Environ. Prot. Ecol. 2016, 17, 10. [Google Scholar]
- Ardeleanu, M.N.; Popescu, I.N.; Udroiu, I.N.; Diaconu, E.M.; Mihai, S.; Lungu, E.; Alhalaili, B.; Vidu, R. Novel pdms-based sensor system for mpwm measurements of picoliter volumes in microfluidic devices. Sensors 2019, 19, 4886. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Dragan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211-212, 317–331. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2016, 7, 387–419. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef]
- Tünay, O.; Kabdaşli, N.I. Hydroxide precipitation of complexed metals. Water Res. 1994, 28, 2117–2124. [Google Scholar] [CrossRef]
- Gorny, J.; Billon, G.; Noiriel, C.; Dumoulin, D.; Lesven, L.; Made, B. Chromium behavior in aquatic environments: A review. Environ. Rev. 2016, 24, 503–516. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Jumawan, A.B.; Grieves, R.B. Separation of toxic heavy metals by sulfide precipitation. Sep. Sci. Technol. 1979, 14, 441–452. [Google Scholar] [CrossRef]
- Monea, M.C.; Löhr, D.K.; Meyer, C.; Preyl, V.; Xiao, J.; Steinmetz, H.; Schönberger, H.; Drenkova-Tuhtan, A. Comparing the leaching behavior of phosphorus, aluminum and iron from post-precipitated tertiary sludge and anaerobically digested sewage sludge aiming at phosphorus recovery. J. Clean. Prod. 2020, 247, 119129. [Google Scholar] [CrossRef]
- Monea, M.C.; Meyer, C.; Steinmetz, H.; Schönberger, H.; Drenkova-Tuhtan, A. Phosphorus recovery from sewage sludge – phosphorus leaching behavior from aluminum-containing tertiary and anaerobically digested sludge. Water Sci. Technol. 2020. [Google Scholar] [CrossRef]
- EPA. Innovative and Alternative Technology Assessment Manual; EPA 430/9-78-009; Agency, E.P.: Washington, DC, USA, 1980. [Google Scholar]
- Gonzalez-Munoz, M.J.; Rodriguez, M.A.; Luque, S.; Alvarez, J.R. Recovery of heavy metals from metal industry waste waters by chemical precipitation and nanofiltration. Desalination 2006, 200, 742–744. [Google Scholar] [CrossRef]
- Kumar, P.; Pournara, A.; Kim, K.-H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-organic frameworks: Challenges and opportunities for ion-exchange/sorption applications. Prog. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Hoch, L.B.; Mack, E.J.; Hydutsky, B.W.; Hershman, J.M.; Skluzacek, I.M.; Mallouk, T.E. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environ. Sci. Technol. 2008, 42, 2600–2605. [Google Scholar] [CrossRef]
- Naja, G.; Volesky, B. Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. In Heavy Metals in the Environment; Springer Nature: Cham, Switzerland, 2009; pp. 13–61. [Google Scholar]
- Narayani, M.; Shetty, K.V. Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 955–1009. [Google Scholar] [CrossRef]
- Sarkar, B. Heavy Metals in the Environment; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Tonini, D.R.; Gauvin, D.A.; Soffel, R.W.; Freeman, W.P. Achieving low mercury concentrations in chlor-alkah wastewaters. Environ. Prog. 2003, 22, 167–173. [Google Scholar] [CrossRef]
- Zagorodni, A. Ion exchange Materials: Properties and Applications: Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Fan, Q.H.; Li, Z.; Zhao, H.G.; Jia, Z.H.; Xu, J.Z.; Wu, W.S. Adsorption of pb(ii) on palygorskite from aqueous solution: Effects of ph, ionic strength and temperature. Appl. Clay Sci. 2009, 45, 111–116. [Google Scholar] [CrossRef]
- A.B. Application of the Ion Exchange Process for the Treatment of Radioactive Waste and Management of Spent Ion Exchangers; International Atomic Energy Agency: Vienna, Austria, 2002. [Google Scholar]
- Bortun, A.I.; Bortun, L.N.; Clearfield, A. Evaluation of synthetic inorganic ion exchangers for cesium and strontium removal from contaminated groundwater and wastewater. Solvent Extr. Ion Exch. 1997, 15, 909–929. [Google Scholar] [CrossRef]
- Chitpong, N.; Husson, S.M. High-capacity, nanofiber-based ion-exchange membranes for the selective recovery of heavy metals from impaired waters. Sep. Purif. Technol. 2017, 179, 94–103. [Google Scholar] [CrossRef]
- Ma, H.Y.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membrane for heavy metal ion adsorption. Curr. Org. Chem. 2013, 17, 1361–1370. [Google Scholar] [CrossRef]
- Geay, M.; Marchetti, V.; Clement, A.; Lonbinoux, B.; Gerardin, P. Decontamination of synthetic solutions containing heavy metals using chemically modified sawdusts bearing polyacrylic acid chains. J. Wood Sci. 2000, 46, 331–333. [Google Scholar] [CrossRef]
- Guclu, G.; Gurdag, G.; Ozgumus, S. Competitive removal of heavy metal ions by cellulose graft copolymers. J. Appl. Polym. Sci. 2003, 90, 2034–2039. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for pb(ii) and cd(ii) uptake. Langmuir 2014, 30, 120–131. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Sogbaike, C.E.; Ebhoaye, J.E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep. Purif. Technol. 2005, 44, 85–89. [Google Scholar] [CrossRef]
- Stephen, M.; Catherine, N.; Brenda, M.; Andrew, K.; Leslie, P.; Corrine, G. Oxolane-2,5-dione modified electrospun cellulose nanofibers for heavy metals adsorption. J. Hazard. Mater. 2011, 192, 922–927. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.W. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhou, J.P.; Zhou, D.C.; Tang, Y.R. Adsorption of cadmium and strontium on cellulose/alginic acid ion-exchange membrane. J. Membr. Sci. 1999, 162, 103–109. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Zhao, Y.H.; Bai, R.B. Development of a multifunctional membrane for chromatic warning and enhanced adsorptive removal of heavy metal ions: Application to cadmium. J. Membr. Sci. 2011, 379, 69–79. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Devi, T.K.S.; Raajenthiren, M. Effect of silica particles on cellulose acetate blend ultrafiltration membranes: Part i. Sep. Purif. Technol. 2008, 64, 38–47. [Google Scholar] [CrossRef]
- Han, R.L.; Zhang, S.H.; Liu, C.; Wang, Y.T.; Jian, X.G. Effect of naa zeolite particle addition on poly(phthalazinone ether sulfone ketone) composite ultrafiltration (uf) membrane performance. J. Membr. Sci. 2009, 345, 5–12. [Google Scholar] [CrossRef]
- Liu, F.; Ma, B.R.; Zhou, D.; Xiang, Y.H.; Xue, L.X. Breaking through tradeoff of polysulfone ultrafiltration membranes by zeolite 4a. Microporous Mesoporous Mater. 2014, 186, 113–120. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J. Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation. J. Membr. Sci. 2011, 368, 100–109. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.Y.; Hupp, J.T.; Farha, O.K. Metal-organic frameworks for applications in remediation of oxyanion/cation-contaminated water. Crystengcomm 2015, 17, 7245–7253. [Google Scholar] [CrossRef]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 2016, 7, 2438. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, S.G.; Wei, J.Q.; Zhang, X.F.; Xu, C.L.; Luan, Z.K.; Wu, D.H.; Wei, B.Q. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metal pb(ii) in aqueous solution. Mater. Sci. Eng. A-Structural Mater. Prop. Microstruct. Process. 2007, 466, 201–206. [Google Scholar] [CrossRef]
- Lu, C.Y.; Liu, C.T.; Su, F.S. Sorption kinetics, thermodynamics and competition of ni2+ from aqueous solutions onto surface oxidized carbon nanotubes. Desalination 2009, 249, 18–23. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Ebrahimi, M. Thermodynamics and kinetics of adsorption of cu(ii) from aqueous solutions onto multi-walled carbon nanotubes. J. Saudi Chem. Soc. 2014, 18, 792–801. [Google Scholar] [CrossRef]
- Li, Y.H.; Ding, J.; Luan, Z.K.; Di, Z.C.; Zhu, Y.F.; Xu, C.L.; Wu, D.H.; Wei, B.Q. Competitive adsorption of pb2+, cu2+ and cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Naghizadeh, A. Comparison between activated carbon and multiwall carbon nanotubes in the removal of cadmium(ii) and chromium(vi) from water solutions. J. Water Supply Res. Technol. -Aqua 2015, 64, 64–73. [Google Scholar] [CrossRef]
- Yang, S.T.; Li, J.X.; Shao, D.D.; Hu, J.; Wang, X.K. Adsorption of ni(ii) on oxidized multi-walled carbon nanotubes: Effect of contact time, ph, foreign ions and paa. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef]
- Lu, C.Y.; Liu, C.; Rao, G.P. Comparisons of sorbent cost for the removal of ni2+ from aqueous solution by carbon nanotubes and granular activated carbon. J. Hazard. Mater. 2008, 151, 239–246. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Chiu, H.S. Adsorption of zinc(ii) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Ho, J.H.; Yeh, Y.N.; Wang, H.W.; Khoo, S.K.; Chen, Y.H.; Chow, C.F. Removal of nickel and silver ions using eggshells with membrane, eggshell membrane, and eggshells. Food Sci. Technol. Res. 2014, 20, 337–343. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, S.W.; Yang, J.K.; Kim, B.G.; Lee, S.M. Removal of heavy metals using waste eggshell. J. Environ. Sci. 2007, 19, 1436–1441. [Google Scholar] [CrossRef]
- Chavan, M.; Mane, S. Removal of copper and zinc from aqueous solutions by using low cost adsorbents. Int. J. Sci. Res. (IJSR) 2015, 4, 3076–3080. [Google Scholar]
- Renge, V.C.; Khedkar, S.V.; Pande, S.V. Removal of heavy metals from wastewater using low cost adsorbents: A review. Sci. Rev. Chem. Commun. 2012, 2, 5. [Google Scholar]
- Yeddou, N.; Bensmaili, A. Equilibrium and kinetic modelling of iron adsorption by eggshells in a batch system: Effect of temperature. Desalination 2007, 206, 127–134. [Google Scholar] [CrossRef]
- Rohaizar, N.A.; Hadi, N.; Sien, W.C. Removal of cu (ii) from water by adsorption on chicken eggshell. Int. J. Eng. Technol. IJET-IJENS 2013, 13, 6. [Google Scholar]
- Arunlertaree, C.; Kaewsomboon, W.; Kumsopa, A.; Pokethitiyook, P.; Panyawathanakit, P. Removal of lead from battery manufacturing wastewater by egg shell. Songklanakarin J. Sci. Technol. 2007, 29, 12. [Google Scholar]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Abdulrasaq, O.O.; Osinfade, G.B. Removal of copper (ii), iron (iii) and lead (ii) ions from mono-component simulated waste effluent by adsorption on coconut husk. Afr. J. Environ. Sci. Technol. 2010, 4, 6. [Google Scholar]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Kutty, S.R.M.; Yusop, S.H. Copper and cadmium adsorption by activated carbon prepared from coconut coir. Nat. Environ. Pollut. Technol. 2010, 9, 4. [Google Scholar]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of crystal violet from aqueous solution onto naoh-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Kanwal, F.; Rehman, R.; Anwar, J.; Mahmud, T. Adsorption studies of cadmium (ii) using novel composites of polyaniline with rice husk and saw dust of eucalyptus camaldulensis. Electron. J. Environ. Agric. Food Chem. 2011, 10, 14. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Utilization of rice husks as a feedstock for preparation of activated carbon by microwave induced koh and k2co3 activation. Bioresour. Technol. 2011, 102, 9814–9817. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Abhinaya, R.V.; Kirupha, S.D.; Murugesan, A.; Sivanesan, S. Adsorption of metal ions onto the chemically modified agricultural waste. Clean-Soil Air Water 2012, 40, 188–197. [Google Scholar] [CrossRef]
- Ounnar, A.; Favier, L.; Bouzaza, A.; Bentahar, F.; Trari, M. Kinetic study of spiramycin removal from aqueous solution using heterogeneous photocatalysis. Kinet. Catal. 2016, 57, 200–206. [Google Scholar] [CrossRef]
- Vázquez, G.; Mosquera, O.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Alkaline pre-treatment of waste chestnut shell from a food industry to enhance cadmium, copper, lead and zinc ions removal. Chem. Eng. J. 2012, 184, 147–155. [Google Scholar] [CrossRef]
- Vázquez, G.; Sonia Freire, M.; González-Alvarez, J.; Antorrena, G. Equilibrium and kinetic modelling of the adsorption of cd2+ ions onto chestnut shell. Desalination 2009, 249, 855–860. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Huang, J.-H.; Zeng, G.-M.; Zhou, C.-F.; Li, X.; Shi, L.-J.; He, S.-B. Adsorption of surfactant micelles and cd 2+/zn 2+ in micellar-enhanced ultrafiltration. J. Hazard. Mater. 2010, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Danis, U.; Aydiner, C. Investigation of process performance and fouling mechanisms in micellar-enhanced ultrafiltration of nickel-contaminated waters. J. Hazard. Mater. 2009, 162, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Landaburu-Aguirre, J.; Pongrácz, E.; Perämäki, P.; Keiski, R.L. Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: Use of response surface methodology to improve understanding of process performance and optimisation. J. Hazard. Mater. 2010, 180, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Ferella, F.; Prisciandaro, M.; De Michelis, I. Removal of heavy metals by surfactant-enhanced ultrafiltration from wastewaters. Desalination 2007, 207, 125–133. [Google Scholar] [CrossRef]
- Ennigrou, D.J.; Gzara, L.; Romdhane, M.R.B.; Dhahbi, M. Cadmium removal from aqueous solutions by polyelectrolyte enhanced ultrafiltration. Desalination 2009, 246, 363–369. [Google Scholar] [CrossRef]
- Camarillo, R.; Llanos, J.; García-Fernández, L.; Pérez, Á.; Canizares, P. Treatment of copper (ii)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition. Sep. Purif. Technol. 2010, 70, 320–328. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper (ii) and nickel (ii) from aqueous media using the complexation–ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of cu2+ and ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Ipek, U. Removal of ni (ii) and zn (ii) from an aqueous solutionby reverse osmosis. Desalination 2005, 174, 161–169. [Google Scholar] [CrossRef]
- Chan, B.; Dudeney, A. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates. Miner. Eng. 2008, 21, 272–278. [Google Scholar] [CrossRef]
- Tanninen, J.; Mänttäri, M.; Nyström, M. Nanofiltration of concentrated acidic copper sulphate solutions. Desalination 2006, 189, 92–96. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Guha, B. Effect of ph on rejection of hexavalent chromium by nanofiltration. Desalination 2008, 219, 171–178. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Pauer, V.; Mizsey, P. Recovery of copper from process waters by nanofiltration and reverse osmosis. Desalination 2009, 240, 132–142. [Google Scholar] [CrossRef]
- El Samrani, A.G.; Lartiges, B.S.; Villieras, F. Chemical coagulation of combined sewer overflow: Heavy metal removal and treatment optimization. Water Res. 2008, 42, 951–960. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, G. Study on the macromolecular coagulant pex which traps heavy metals. Chem. Eng. Sci. 2007, 62, 4636–4643. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, M.; Wang, J.X. Removal of cu2+ and turbidity from wastewater by mercaptoacetyl chitosan. J. Hazard. Mater. 2009, 169, 621–625. [Google Scholar] [CrossRef]
- Lundh, M.; Jonsson, L.; Dahlquist, J. Experimental studies of the fluid dynamics in the separation zone in dissolved air flotation. Water Res. 2000, 34, 21–30. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Meng, Y.T.; Zeng, G.M.; Fang, Y.Y.; Shi, J.G. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf. a-Physicochem. Eng. Asp. 2008, 317, 256–261. [Google Scholar] [CrossRef]
- Capponi, F.; Sartori, M.; Souza, M.L.; Rubio, J. Modified column flotation of adsorbing iron hydroxide colloidal precipitates. Int. J. Miner. Process. 2006, 79, 167–173. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Taama, W.; Scott, K.; Jachuck, R.; Slade, R.; Varcoe, J. Comparative performance of ion exchange membranes for electrodialysis of nickel and cobalt. Sep. Purif. Technol. 2003, 30, 113–127. [Google Scholar] [CrossRef]
- Jakobsen, M.R.; Fritt-Rasmussen, J.; Nielsen, S.; Ottosen, L.M. Electrodialytic removal of cadmium from wastewater sludge. J. Hazard. Mater. 2004, 106, 127–132. [Google Scholar] [CrossRef]
- Belkacem, M.; Khodir, M.; Abdelkrim, S. Treatment characteristics of textile wastewater and removal of heavy metals using the electroflotation technique. Desalination 2008, 228, 245–254. [Google Scholar] [CrossRef]
- Khosla, N.K.; Venkatachalam, S.; Somasundaran, P. Pulsed electrogeneration of bubbles for electroflotation. J. Appl. Electrochem. 1991, 21, 986–990. [Google Scholar] [CrossRef]
- Mansour, L.B.; Chalbi, S.; Kesentini, I. Experimental study of hydrodynamic and bubble size distributions in electroflotation process. Indian J. Chem. Technol. 2007, 14, 253–257. [Google Scholar]
- Burns, S.E.; Yiacoumi, S.; Tsouris, C. Microbubble generation for environmental and industrial separations. Sep. Purif. Technol. 1997, 11, 221–232. [Google Scholar] [CrossRef]
- Ketkar, D.R.; Mallikarjunan, R.; Venkatachalam, S. Electroflotation of quartz fines. Int. J. Miner. Process. 1991, 31, 127–138. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (ec)—science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process- a systematic review. J. Environ. Health Sci. Eng. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Khandegar, V.; Saroha, A.K. Removal of chromium from electroplating industry effluent using electrocoagulation. J. Hazard. Toxic Radioact. Waste 2013, 17. [Google Scholar] [CrossRef]
- Akbal, F.; Camci, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214–222. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. J. Hazard. Mater. 2011, 192, 26–34. [Google Scholar] [CrossRef]
- Malakootian, M.; Yousefi, N.; Fatehizadeh, A. Survey efficiency of electrocoagulation on nitrate removal from aqueous solution. Int. J. Environ. Sci. Technol. 2011, 8, 107–114. [Google Scholar] [CrossRef]
- Pociecha, M.; Lestan, D. Using electrocoagulation for metal and chelant separation from washing solution after edta leaching of pb, zn and cd contaminated soil. J. Hazard. Mater. 2010, 174, 670–678. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and fe-al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Kabdasli, I.; Vardar, B.; Arslan-Alaton, I.; Tunay, O. Effect of dye auxiliaries on color and cod removal from simulated reactive dyebath effluent by electrocoagulation. Chem. Eng. J. 2009, 148, 89–96. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Moein, H.; Mostafapour, F.K.; Nakhaie, S. Application of electrocoagulation process for dairy wastewater treatment. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 1–28. [Google Scholar] [CrossRef]
- Apaydin, K.U.; Gonullu, M.T. An investigation on the treatment of tannery wastewater by electrocoagulation. Global Nest J. 2009, 11. [Google Scholar]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Kumar, M.S. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, R.; Vasudevan, S. Evaluation of electrocoagulation process for the removal of strontium and cesium from aqueous solution. Chem. Eng. Res. Des. 2015, 93, 522–530. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Ebrahimi, S.J.A.-D.; Mesdaghinia, A.; Gharibi, H.; Sowlat, M.H. Performance evaluation of a continuous bipolar electrocoagulation/electrooxidation–electroflotation (eceo–ef) reactor designed for simultaneous removal of ammonia and phosphate from wastewater effluent. J. Hazard. Mater. 2011, 192, 1267–1274. [Google Scholar] [CrossRef]
- Isa, M.H.; Ezechi, E.H.; Ahmed, Z.; Magram, S.F.; Kutty, S.R.M. Boron removal by electrocoagulation and recovery. Water Res. 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Ownagh, K.A.; Mahvi, A.H. Application of electrocoagulation process using iron and aluminum electrodes for fluoride removal from aqueous environment. E-J. Chem. 2012, 9, 102629. [Google Scholar] [CrossRef]
- Edris Bazrafshan, E.; Mahvi, A.H.; Naseri, S.; Mesdaghinia, A.R. Performance evaluation of electrocoagulation process for removal of chromium6+ from synthetic chromium solutions using iron and aluminum electrodes. Turkish J. Eng. Env. Sci. 2008, 32, 8. [Google Scholar]
- Mansoorian, H.J.; Rajabizadeh, A.; Bazrafshan, E.; Mahvi, A.H. Practical assessment of electrocoagulation process in removing nickel metal from aqueous solutions using iron-rod electrodes. Desalin. Water Treat. 2012, 44, 29–35. [Google Scholar] [CrossRef]
- Chaturvedi, S.I. Mercury removal using al-al electrodes by electrocoagulation. Int. J. Mod. Eng. Res. (IJMER) 2013, 3, 7. [Google Scholar]
- Shafaei, A.; Pajootan, E.; Nikazar, M.; Arami, M. Removal of co (ii) from aqueous solution by electrocoagulation process using aluminum electrodes. Desalination 2011, 279, 121–126. [Google Scholar] [CrossRef]
- Pouet, M.F.; Grasmick, A. Urban wastewater treatment by electrocoagulation and flotation. Water Sci. Technol. 1995, 31, 275–283. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Park, D.-S.; Ahn, K.-H. Combining fluidized metal-impregnated granular activated carbon in three-dimensional electrocoagulation system: Feasibility and optimization test of color and cod removal from real cotton textile wastewater. Sep. Purif. Technol. 2015, 146, 154–167. [Google Scholar] [CrossRef]
- Adjeroud, N.; Dahmoune, F.; Merzouk, B.; Leclerc, J.-P.; Madani, K. Improvement of electrocoagulation–electroflotation treatment of effluent by addition of opuntia ficus indica pad juice. Sep. Purif. Technol. 2015, 144, 168–176. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Azizabadi, H.; Mahvi, A. Performance evaluation of electrocoagulation process for phenol removal from aqueous solutions. Fresenius Environ. Bull. 2012, 21, 364–371. [Google Scholar]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an ultra-low-pressure reverse osmosis membrane (ulprom) for separating heavy metal: Effects of interference parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Kryvoruchko, A.; Yurlova, L.; Kornilovich, B. Purification of water containing heavy metals by chelating-enhanced ultrafiltration. Desalination 2002, 144, 243–248. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Kabdasli, I.; Arslan, T.; Olmez-Hanci, T.; Arslan-Alaton, I.; Tunay, O. Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J. Hazard. Mater. 2009, 165, 838–845. [Google Scholar] [CrossRef]
- Alvarez, M.T.; Crespo, C.; Mattiasson, B. Precipitation of zn2+, cu2+ and pb2+ at bench scale using biogenic hydrogen sulphide produced from the utilization of volatile fatty acids by sulphate reducing bacteria. J. Biotechnol. 2005, 118, S181. [Google Scholar]
- Martins, A.S.; Nunez, L.; Lanza, M.R.D. Enhanced photoelectrocatalytic performance of tio2 nanotube array modified with wo3 applied to the degradation of the endocrine disruptor propyl paraben. J. Electroanal. Chem. 2017, 802, 33–39. [Google Scholar] [CrossRef]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.; Paulose, M.; Seabold, J.A.; Choi, K.-S.; Grimes, C.A. Recent advances in the use of tio2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Wu, W.Y.; Huang, Z.H.; Lim, T.T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. a-Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Tan, C.; Xiang, B.; Li, Y.; Fang, J.; Huang, M. Preparation and characteristics of a nano-pbo2 anode for organic wastewater treatment. Chem. Eng. J. 2011, 166, 15–21. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, L.; Xue, H.; Han, W.; Wang, L.; Sun, X.; Li, J. Preparation and characterization of tio2-nts/sno2-sb electrodes by electrodeposition. J. Electroanal. Chem. 2010, 648, 119–127. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Sun, Z.R. Preparation and characterization of cerium-doped multiwalled carbon nanotubes electrode for the electrochemical degradation of low-concentration ceftazidime in aqueous solutions. Electrochim. Acta 2016, 199, 80–91. [Google Scholar] [CrossRef]
- Sudhagar, P.; Juarez-Perez, E.J.; Kang, Y.S.; Mora-Sero, I. Quantum dot-sensitized solar cells. In Low-Cost Nanomaterials: Toward Greener and More Efficient Energy Applications; Lin, Z., Wang, J., Eds.; Springer Nature: Cham, Switzerland, 2014; pp. 89–136. [Google Scholar]
- De Leo, M.; Pereira, F.C.; Moretto, L.M.; Scopece, P.; Polizzi, S.; Ugo, P. Towards a better understanding of gold electroless deposition in track-etched templates. Chem. Mater. 2007, 19, 5955–5964. [Google Scholar] [CrossRef]
- Menon, V.P.; Martin, C.R. Fabrication and evaluation of nanoelectrode ensembles. Anal. Chem. 1995, 67, 1920–1928. [Google Scholar] [CrossRef]
- Wirtz, M.; Martin, C.R. Template-fabricated gold nanowires and nanotubes. Adv. Mater. 2003, 15, 455–458. [Google Scholar] [CrossRef]
- Vidu, R.; Perez-Page, M.; Quach, D.V.; Chen, X.Y.; Stroeve, P. Electrodeposition of ni and te-doped cobalt triantimonide in citrate solutions. Electroanalysis 2015, 27, 2845–2856. [Google Scholar] [CrossRef]
- Azizi, A.; Mohammadi, M.; Sadrnezhaad, S.K. End-closed nicofe-b nanotube arrays by electroless method. Mater. Lett. 2011, 65, 289–292. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Li, X.T.; Geng, W.C.; Qiu, S.L. Synthesis of metallic nanotube arrays in porous anodic aluminum oxide template through electroless deposition. Mater. Res. Bull. 2006, 41, 1417–1423. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Wu, G.S.; Xie, T.; Lin, Y.; Zhang, L.D. Self-assembly synthesis and magnetic studies of co-p alloy nanowire arrays. Nanotechnology 2004, 15, 59–61. [Google Scholar] [CrossRef]
- Mbindyo, J.K.N.; Reiss, B.D.; Martin, B.R.; Keating, C.D.; Natan, M.J.; Mallouk, T.E. DNA-directed assembly of gold nanowires on complementary surfaces. Adv. Mater. 2001, 13, 249. [Google Scholar] [CrossRef]
- Moon, J.M.; Wei, A. Uniform gold nanorod arrays from polyethylenimine-coated alumina templates. J. Phys. Chem. B 2005, 109, 23336–23341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Tian, M.L.; Mallouk, T.E.; Chan, M.H.W. Microstructure and interdiffusion of template-synthesized au/sn/au junction nanowires. Nano Lett. 2004, 4, 1313–1318. [Google Scholar] [CrossRef]
- Budden, M.G.; Wilkinson, D.S. Skin and nail lesions from gold potassium cyanide. Contact Dermat. 1978, 4, 172–173. [Google Scholar] [CrossRef]
- Wright, I.H.; Vesey, C.J. Acute poisoning with gold cyanide. Anaesthesia 1986, 41, 936–939. [Google Scholar] [CrossRef]
- Wu, M.L.; Tsai, W.J.; Ger, A.; Deng, J.F.; Tsay, S.H.; Yang, M.H. Cholestatic hepatitis caused by acute gold potassium cyanide poisoning. J. Toxicol. -Clin. Toxicol. 2001, 39, 739–743. [Google Scholar] [CrossRef]
- Baltrunas, G.; Valiuniene, A.; Vienozinskis, J.; Gaidamauskas, E.; Jankauskas, T.; Margarian, Z. Electrochemical gold deposition from sulfite solution: Application for subsequent polyaniline layer formation. J. Appl. Electrochem. 2008, 38, 1519–1526. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, X.L.; Chen, S.T.; Fang, Y.; Li, N.; Zhai, X.M.; Liu, Y. Size-controlled synthesis of au nanoparticles and nanowires and their application as sers substrates. Colloids Surfaces a-Physicochem. Eng. Asp. 2011, 384, 345–351. [Google Scholar] [CrossRef]
- Kan, C.X.; Cai, W.P.; Li, Z.S.; Fu, G.H.; Zhang, L.D. Reduction effect of pore wall and formation of au nanowires inside monolithic mesoporous silica. Chem. Phys. Lett. 2003, 382, 318–324. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.H.; Sun, L.L.; Guo, C.L.; Sun, Y.J.; Li, Z. Controllable synthesis of gold nanowires. Mater. Lett. 2008, 62, 4124–4126. [Google Scholar] [CrossRef]
- Kim, F.; Sohn, K.; Wu, J.S.; Huang, J.X. Chemical synthesis of gold nanowires in acidic solutions. J. Am. Chem. Soc. 2008, 130, 14442. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.J.; Hong, F.C.N. Electroless nanowelding of silver nanowires at room temperature. Rsc. Adv. 2014, 4, 40330–40338. [Google Scholar] [CrossRef]
- Muench, F.; Kunz, U.; Wardenga, H.F.; Kleebe, H.J.; Ensinger, W. Metal nanotubes and nanowires with rhombohedral cross-section electrolessly deposited in mica templates. Langmuir 2014, 30, 10878–10885. [Google Scholar] [CrossRef]
- Kawamori, M.; Yagi, S.; Matsubara, E. Formation of nickel nanowires by electroless deposition. In Nanotechnology; Leonte, O.M., Chen, F., Mustain, W., Eds.; ECS Transactions, The Electrochemical Society: Pennington, NJ, USA, 2012; Volume 30, pp. 1–7. [Google Scholar]
- Balela, M.D.L.; Yagi, S.; Matsubara, E. Fabrication of cobalt nanowires by electroless deposition under external magnetic field. J. Electrochem. Soc. 2011, 158, D210–D216. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.G.; Noh, Y.; Kim, W.B. An overview of one-dimensional metal nanostructures for electrocatalysis. Catal. Surv. Asia 2015, 19, 88–121. [Google Scholar] [CrossRef]
- Perez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-based syntheses for shape controlled nanostructures. Adv. Colloid Interface Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Xu, W.W.; Cui, X.D.; Wang, Y. Recent progress in metal-organic frameworks and their derived nanostructures for energy and environmental applications. Chemsuschem 2017, 10, 1645–1663. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-based synthesis of nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Martin, C.R. A general template-based method for the preparation of nanomaterials. J. Mater. Chem. 1997, 7, 1075–1087. [Google Scholar] [CrossRef]
- Kautek, W.; Reetz, S.; Pentzien, S. Template electrodeposition of nanowire arrays on gold foils fabricated by pulsed-laser deposition. Electrochim. Acta 1995, 40, 1461–1468. [Google Scholar] [CrossRef]
- Dryden, D.M.; Vidu, R.; Stroeve, P. Nanowire formation is preceded by nanotube growth in templated electrodeposition of cobalt hybrid nanostructures. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.C.; Xu, Z. Template synthesis of ordered nano-system and their applications. Chin. J. Inorg. Chem. 2002, 18, 965–975. [Google Scholar]
- Ku, J.R.; Vidu, R.; Talroze, R.; Stroeve, P. Fabrication of nanocables by electrochemical deposition inside metal nanotubes. J. Am. Chem. Soc. 2004, 126, 15022–15023. [Google Scholar] [CrossRef]
- Benfield, R.E.; Grandjean, D.; Dore, J.C.; Esfahanian, H.; Wu, Z.H.; Kroll, M.; Geerkens, M.; Schmid, G. Structure of assemblies of metal nanowires in mesoporous alumina membranes studied by exafs, xanes, X-ray diffraction and saxs. Faraday Discuss. 2004, 125, 327–342. [Google Scholar] [CrossRef]
- Cao, H.Q.; Xu, Z.; Sheng, D.; Hong, J.M.; Sang, H.; Du, Y.W. An array of iron nanowires encapsulated in polyaniline nanotubules and its magnetic behavior. J. Mater. Chem. 2001, 11, 958–960. [Google Scholar] [CrossRef]
- Dryden, D.M.; Sun, T.; McCormick, R.; Hickey, R.; Vidu, R.; Stroeve, P. Anomalous deposition of co-ni alloys in film and nanowire morphologies from citrate baths. Electrochim. Acta 2016, 220, 595–600. [Google Scholar] [CrossRef]
- Daub, M.; Enculescu, I.; Neumann, R.; Spohr, R. Ni nanowires electrodeposited in single ion track templates. J. Optoelectron. Adv. Mater. 2005, 7, 865–870. [Google Scholar]
- Vidu, R.; Predescu, A.M.; Matei, E.; Berbecaru, A.; Pantilimon, C.; Dragan, C.; Predescu, C. Template-assisted co-ni nanowire arrays. Nanomaterials 2019, 9, 1446. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.; Delvaux, M. Preparation of polymeric and metallic nanostructures using a template-based deposition method. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2001, 15, 269–271. [Google Scholar] [CrossRef]
- Wirtz, M.; Parker, M.; Kobayashi, Y.; Martin, C.R. Molecular sieving and sensing with gold nanotube membranes. Chem. Rec. 2002, 2, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Parker, M.; Kobayashi, Y.; Martin, C.R. Template-synthesized nanotubes for chemical separations and analysis. Chem. -A Eur. J. 2002, 8, 3573–3578. [Google Scholar] [CrossRef]
- Wirtz, M.; Yu, S.F.; Martin, C.R. Template synthesized gold nanotube membranes for chemical separations and sensing. Analyst 2002, 127, 871–879. [Google Scholar] [CrossRef]
- Delvaux, M.; Demoustier-Champagne, S.; Walcarius, A. Flow injection amperometric detection at enzyme-modified gold nanoelectrodes. Electroanalysis 2004, 16, 190–198. [Google Scholar] [CrossRef]
- Delvaux, M.; Walcarius, A.; Demoustier-Champagne, S. Electrocatalytic h2o2 amperometric detection using gold nanotube electrode ensembles. Anal. Chim. Acta 2004, 525, 221–230. [Google Scholar] [CrossRef]
- Delvaux, M.; Demoustier-Champagne, S. Immobilisation of glucose oxidase within metallic nanotubes arrays for application to enzyme biosensors. Biosens. Bioelectron. 2003, 18, 943–951. [Google Scholar] [CrossRef]
- Zhang, G.M.; Emmanuel, R.; Liu, H.W.; Liu, W.M.; Hou, S.M.; Kui, Y.Z.; Xue, Z.Q. Field emission from an array of free-standing metallic nanowires. Chin. Phys. Lett. 2002, 19, 1016–1018. [Google Scholar]
- Tian, M.L.; Wang, J.G.; Snyder, J.; Kurtz, J.; Liu, Y.; Schiffer, P.; Mallouk, T.E.; Chan, M.H.W. Synthesis and characterization of superconducting single-crystal sn nanowires. Appl. Phys. Lett. 2003, 83, 1620–1622. [Google Scholar] [CrossRef]
- Tian, M.L.; Wang, J.U.; Kurtz, J.; Mallouk, T.E.; Chan, M.H.W. Electrochemical growth of single-crystal metal nanowires via a two-dimensional nucleation and growth mechanism. Nano Lett. 2003, 3, 919–923. [Google Scholar] [CrossRef]
- Schonenberger, C.; van der Zande, B.M.I.; Fokkink, L.G.J.; Henny, M.; Schmid, C.; Kruger, M.; Bachtold, A.; Huber, R.; Birk, H.; Staufer, U. Template synthesis of nanowires in porous polycarbonate membranes: Electrochemistry and morphology. J. Phys. Chem. B 1997, 101, 5497–5505. [Google Scholar] [CrossRef]
- Liu, H.W.; Hou, S.M.; Zhang, G.M.; Shen, Z.Y.; Liu, W.M.; Wu, J.L.; Xue, Z.Q.; Roy, E.; Zhang, K.Y. Structure and electrical properties of gold nanowires grown with electrochemical deposition. Acta Phys. -Chim. Sin. 2002, 18, 359–363. [Google Scholar]
- Wang, H.; Wang, J.; Tian, M.; Bell, L.; Hutchinson, E.; Rosario, M.M.; Liu, Y.; Amma, A.; Mallouk, T. Metallic contacts with individual ru nanowires prepared by electrochemical deposition and the suppression of superconductivity in ultrasmall ru grains. Appl. Phys. Lett. 2004, 84, 5171–5173. [Google Scholar] [CrossRef]
- Wang, J.G.; Tian, M.L.; Mallouk, T.E.; Chan, M.H.W. Microtwinning in template-synthesized single-crystal metal nanowires. J. Phys. Chem. B 2004, 108, 841–845. [Google Scholar] [CrossRef]
- Wang, X.J.; Bohn, P.W. Anisotropic in-plane gradients of poly(acrylic acid) formed by electropolymerization with spatiotemporal control of the electrochemical potential. J. Am. Chem. Soc. 2004, 126, 6825–6832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Fan, S.C.; Lee, K.R.; Li, C.L.; Huang, S.H.; Tsai, H.A.; Lai, J.Y. Polyamide/sds-clay hybrid nanocomposite membrane application to water-ethanol mixture pervaporation separation. J. Membr. Sci. 2004, 239, 219–226. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, J.J.; Ye, C.H.; Fang, X.S.; Zhang, L.D. Thermal expansion of cu nanowire arrays. Nanotechnology 2004, 15, 1437–1440. [Google Scholar] [CrossRef]
- Barbic, M.; Mock, J.J.; Smith, D.R.; Schultz, S. Single crystal silver nanowires prepared by the metal amplification method. Journal Of Applied Physics 2002, 91, 9341–9345. [Google Scholar] [CrossRef]
- Valizadeh, S.; George, J.M.; Leisner, P.; Hultman, L. Electrochemical deposition of co nanowire arrays; quantitative consideration of concentration profiles. Electrochim. Acta 2001, 47, 865–874. [Google Scholar] [CrossRef]
- Pignard, S.; Goglio, G.; Huynen, I.; Radulescu, A.; Piraux, L. Ferromagnetic resonance in submicron metallic wires. IEEE Trans. Magn. 2000, 36, 3482–3484. [Google Scholar] [CrossRef]
- Pignard, S.; Goglio, G.; Radulescu, A.; Piraux, L.; Dubois, S.; Declemy, A.; Duvail, J.L. Study of the magnetization reversal in individual nickel nanowires. J. Appl. Phys. 2000, 87, 824–829. [Google Scholar] [CrossRef]
- Ge, S.H.; Li, C.; Ma, X.; Li, W.; Li, C.X. The influence of magnetic field on the crystal growth of electrodeposited co nanowires. Acta Phys. Sin. 2001, 50, 149–152. [Google Scholar]
- Ge, S.H.; Li, C.; Ma, X.; Li, W.; Xi, L.; Li, C.X. Approach to fabricating co nanowire arrays with perpendicular anisotropy: Application of a magnetic field during deposition. J. Appl. Phys. 2001, 90, 509–511. [Google Scholar] [CrossRef]
- Ge, S.H.; Ma, X.; Li, C.; Li, W. Fabrication of electro deposited co nanowire arrays with perpendicular anisotropy. J. Magn. Magn. Mater. 2001, 226, 1867–1869. [Google Scholar] [CrossRef]
- Tai, Y.L.; Teng, H.S. Template synthesis and electrochemical characterization of nickel-based tubule electrode arrays. Chem. Mater. 2004, 16, 338–342. [Google Scholar] [CrossRef]
- Ohgai, T.; Gravier, L.; Hoffer, X.; Lindeberg, M.; Hjort, K.; Spohr, R.; Ansermet, J.P. Template synthesis and magnetoresistance property of ni and co single nanowires electrodeposited into nanopores with a wide range of aspect ratios. J. Phys. D-Appl. Phys. 2003, 36, 3109–3114. [Google Scholar] [CrossRef]
- Pra, L.D.D.; Ferain, E.; Legras, R.; Demoustier-Champagne, S. Fabrication of a new generation of track-etched templates and their use for the synthesis of metallic and organic nanostructures. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2002, 196, 81–88. [Google Scholar]
- Schuchert, I.U.; Molares, M.E.T.; Dobrev, D.; Vetter, J.; Neumann, R.; Martin, M. Electrochemical copper deposition in etched ion track membranes—Experimental results and a qualitative kinetic model. J. Electrochem. Soc. 2003, 150, C189–C194. [Google Scholar] [CrossRef]
- Molares, M.E.T.; Brotz, J.; Buschmann, V.; Dobrev, D.; Neumann, R.; Scholz, R.; Schuchert, I.U.; Trautmann, C.; Vetter, J. Etched heavy ion tracks in polycarbonate as template for copper nanowires. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2001, 185, 192–197. [Google Scholar] [CrossRef]
- Molares, M.E.T.; Buschmann, V.; Dobrev, D.; Neumann, R.; Scholz, R.; Schuchert, I.U.; Vetter, J. Single-crystalline copper nanowires produced by electrochemical deposition in polymeric ion track membranes. Adv. Mater. 2001, 13, 62. [Google Scholar] [CrossRef]
- Enculescu, I.; Siwy, Z.; Dobrev, D.; Trautmann, C.; Toimil Molares, M.E.; Neumann, R.; Hjort, K.; Westerberg, I.; Spohr, R. Copper nanowires electrodeposited in etched single-ion track templates. Appl. Phys. A 2003, 77, 751–755. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.J.; Zhang, L.D.; Zhang, X.Y.; Zhang, J.; Li, G.H. Fabrication and optical absorption of ordered indium oxide nanowire arrays embedded in anodic alumina membranes. Chem. Phys. Lett. 2001, 334, 298–302. [Google Scholar] [CrossRef]
- Cheng, B.; Samulski, E.T. Fabrication and characterization of nanotubular semiconductor oxides in2o3 and ga2o3. Journal of Materials Chemistry 2001, 11, 2901–2902. [Google Scholar] [CrossRef]
- Ding, G.Q.; Shen, W.Z.; Zheng, M.J.; Zhou, Z.B. Indium oxide “rods in dots” nanostructures. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Jeong, J.S.; Lee, J.Y.; Lee, C.J.; An, S.J.; Yi, G.C. Synthesis and characterization of high-quality in2o3 nanobelts via catalyst-free growth using a simple physical vapor deposition at low temperature. Chem. Phys. Lett. 2004, 384, 246–250. [Google Scholar] [CrossRef]
- O’Dwyer, C.; Szachowicz, M.; Visimberga, G.; Lavayen, V.; Newcomb, S.B.; Torres, C.M.S. Bottom-up growth of fully transparent contact layers of indium tin oxide nanowires for light-emitting devices. Nat. Nanotechnol. 2009, 4, 239–244. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.H.; Han, S.; Liu, X.L.; Tang, T.; Zhou, C.W. Diameter-controlled growth of single-crystalline in2o3 nanowires and their electronic properties. Adv. Mater. 2003, 15, 143. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, N.H.; Lee, C. An mocvd route to in2o3 one-dimensional materials with novel morphologies. Appl. Phys. a-Mater. Sci. Process. 2005, 81, 1135–1138. [Google Scholar] [CrossRef]
- Liu, Q.; Zou, R.J.; Bando, Y.; Golberg, D.; Hu, J.Q. Nanowires sheathed inside nanotubes: Manipulation, properties and applications. Prog. Mater. Sci. 2015, 70, 1–49. [Google Scholar] [CrossRef]
- Muhr, H.J.; Krumeich, F.; Schonholzer, U.P.; Bieri, F.; Niederberger, M.; Gauckler, L.J.; Nesper, R. Vanadium oxide nanotubes—A new flexible vanadate nanophase. Adv. Mater. 2000, 12, 231. [Google Scholar] [CrossRef]
- Bao, J.C.; Xu, D.P.; Zhou, Q.F.; Xu, Z. An array of concentric composite nanostructure of metal nanowires encapsulated in zirconia nanotubes: Preparation, characterization, and magnetic properties. Chem. Mater. 2002, 14, 4709–4713. [Google Scholar] [CrossRef]
- Huang, X.; Rui, X.H.; Hng, H.H.; Yan, Q.Y. Vanadium pentoxide-based cathode materials for lithium-ion batteries: Morphology control, carbon hybridization, and cation doping. Part. Part. Syst. Charact. 2015, 32, 276–294. [Google Scholar] [CrossRef]
- Li, Y.B.; Bando, Y.; Golberg, D. Single-crystalline in2o3 nanotubes filled with in. Adv. Mater. 2003, 15, 581–585. [Google Scholar] [CrossRef]
- Li, Y.B.; Bando, Y.; Golberg, D.; Liu, Z.W. Ga-filled single-crystalline mgo nanotube: Wide-temperature range nanothermometer. Appl. Phys. Lett. 2003, 83, 999–1001. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Iijima, S. Smallest carbon nanotube. Nature 1992, 358, 23. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Iijima, S. Capillarity-induced filling of carbon nanotubes. Nature 1993, 361, 333–334. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, J.; Zhang, Q.L.; Wei, J.; Xu, G.Y. Bismuth nano-flower modified cpe for anodic stripping voltammetry detection of cd(ii). Int. J. Electrochem. Sci. 2019, 14, 4483–4495. [Google Scholar] [CrossRef]
- Zeng, Z.T.; Fang, S.Y.; Tang, D.; Xiao, R.; Tang, L.; Peng, B.; Gong, J.L.; Long, B.Q.; Ouyang, X.L.; Zeng, G.M. Ultrasensitive sensor based on novel bismuth carbon nanomaterial for lead and cadmium determination in natural water, contaminated soil and human plasma. Microporous Mesoporous Mater. 2019, 284, 177–185. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, C.; Su, Y.C.; Mu, W.; Han, X.J. Simultaneous detection of trace cd(ii) and pb(ii) by differential pulse anodic stripping voltammetry using a bismuth oxycarbide/nafion electrode. Inorg. Chem. Commun. 2020, 111. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Wei, Y.M. A simple strategy for the detection of cu(ii), cd(ii) and pb(ii) in water by a voltammetric sensor on a tc4a modified electrode. New J. Chem. 2019, 43, 1544–1550. [Google Scholar] [CrossRef]
- Gao, F.; Gao, N.N.; Nishitani, A.; Tanaka, H. Rod-like hydroxyapatite and nafion nanocomposite as an electrochemical matrix for simultaneous and sensitive detection of hg2+, cu2+, pb2+ and cd2+. J. Electroanal. Chem. 2016, 775, 212–218. [Google Scholar] [CrossRef]
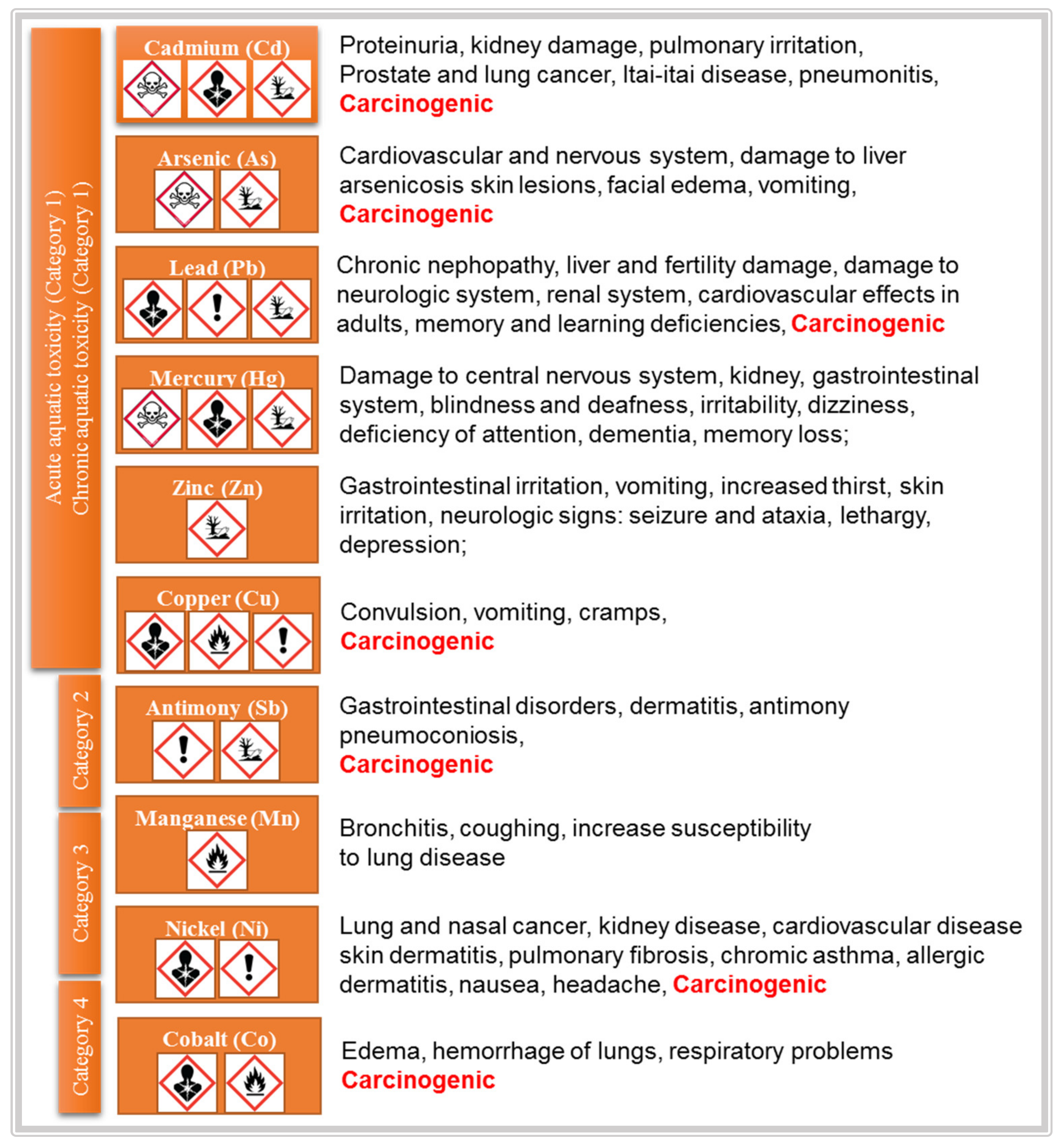
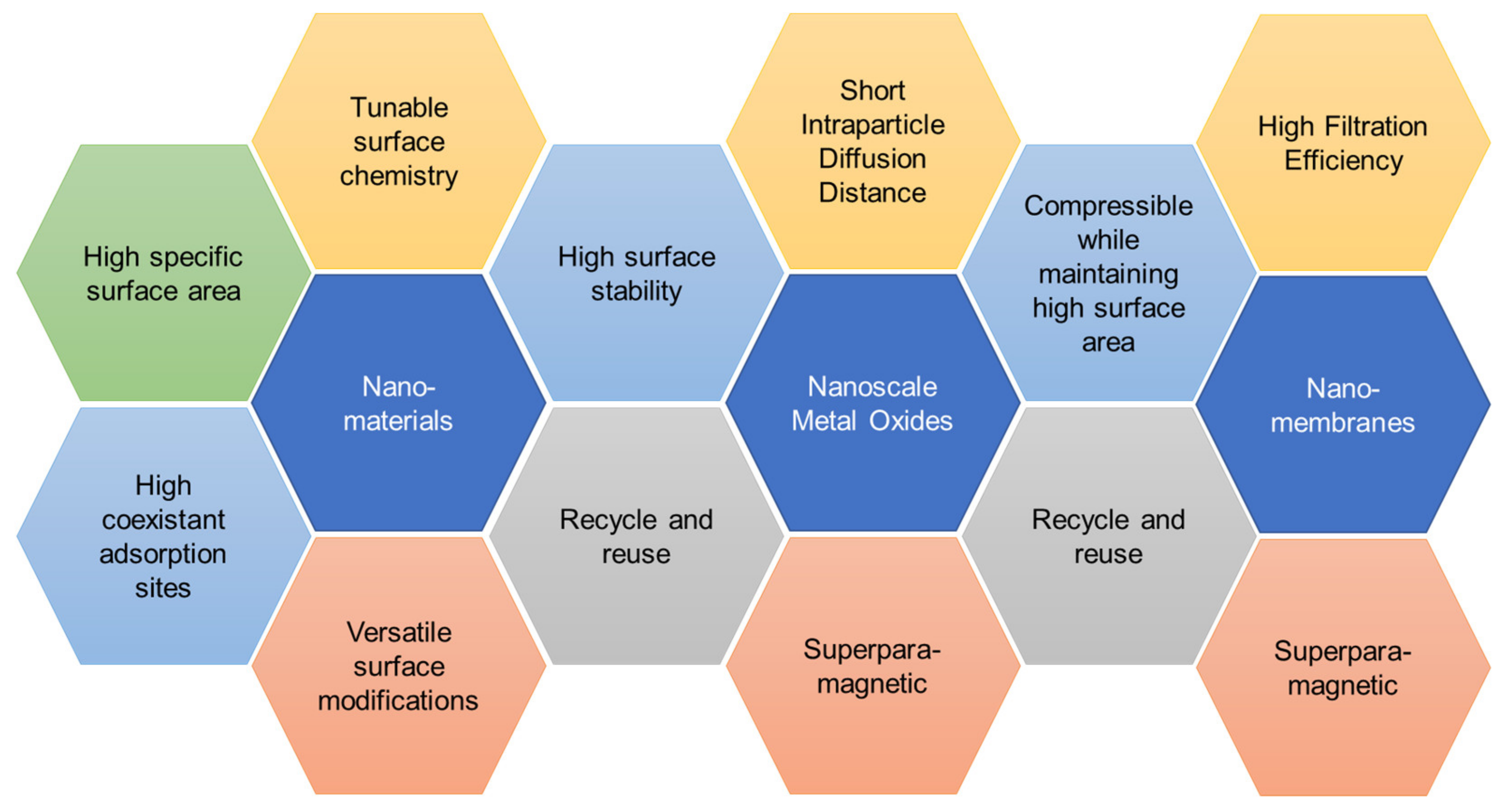

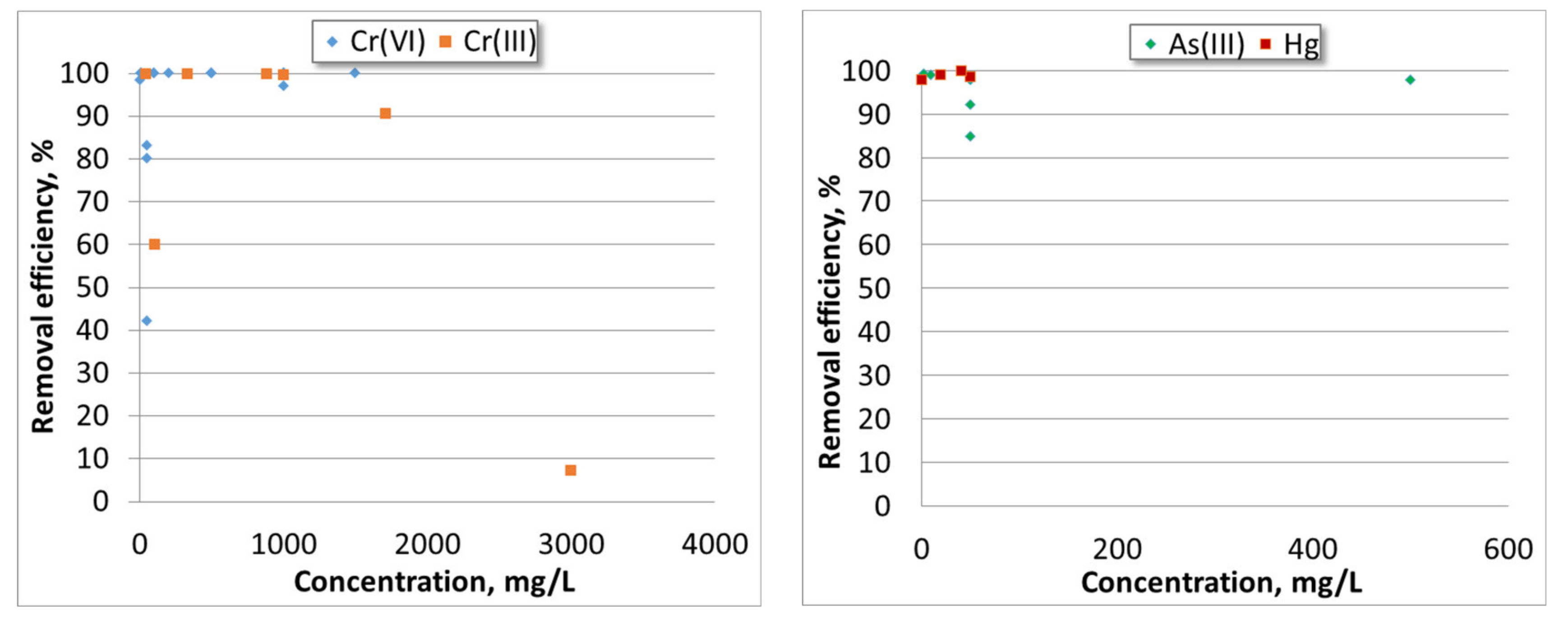

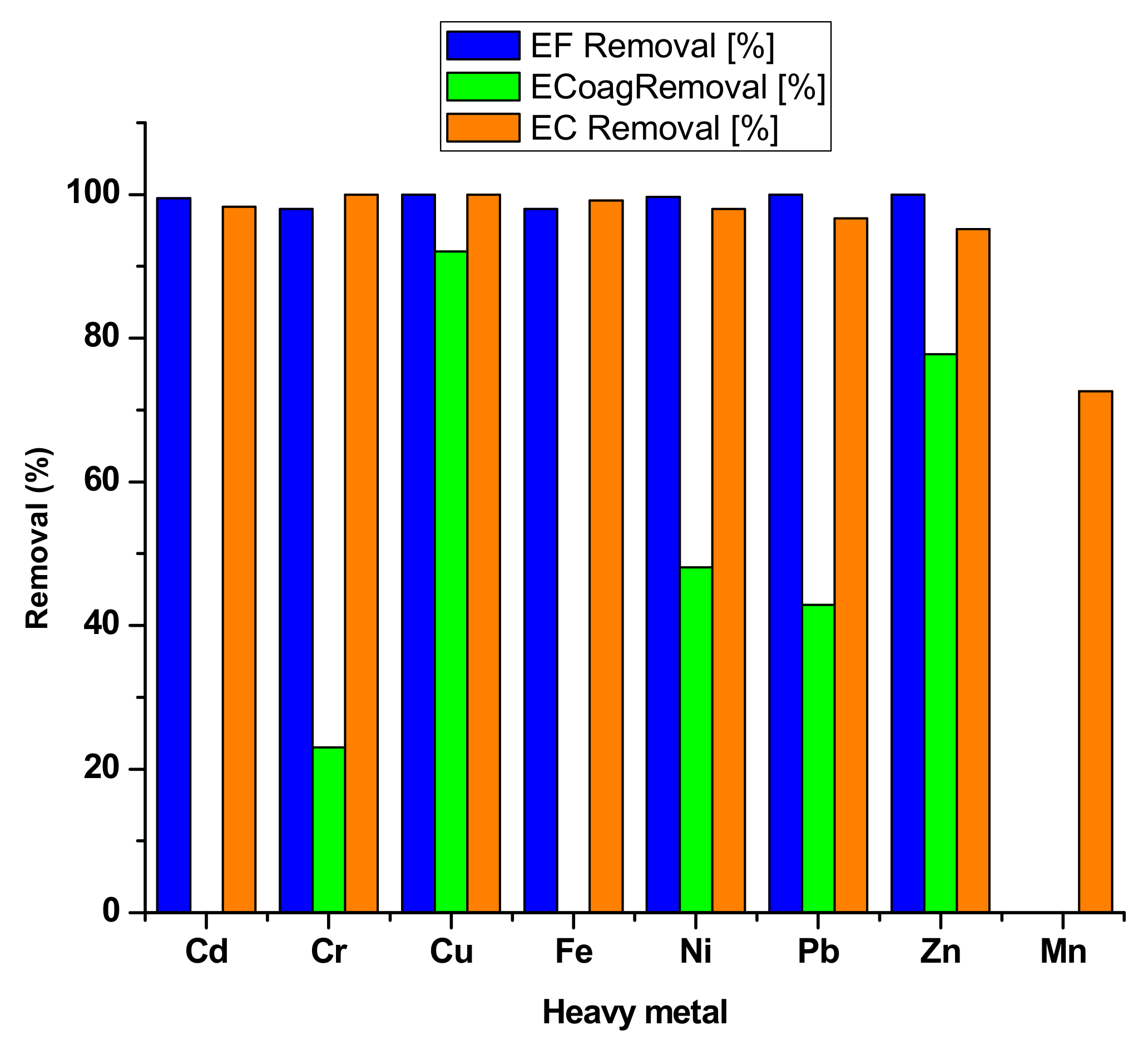
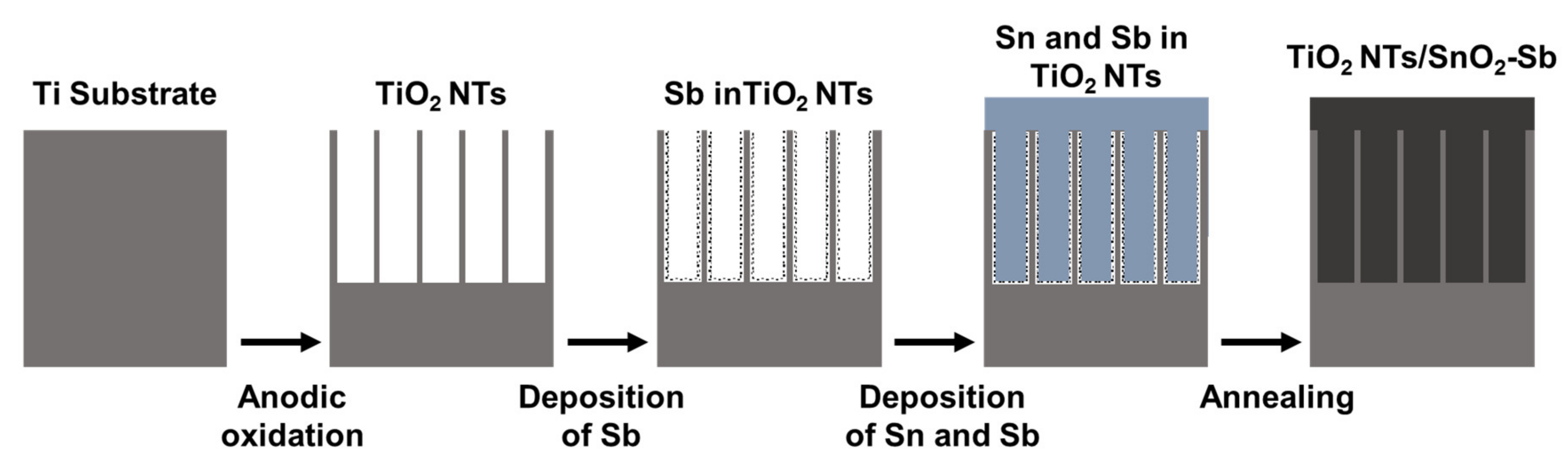
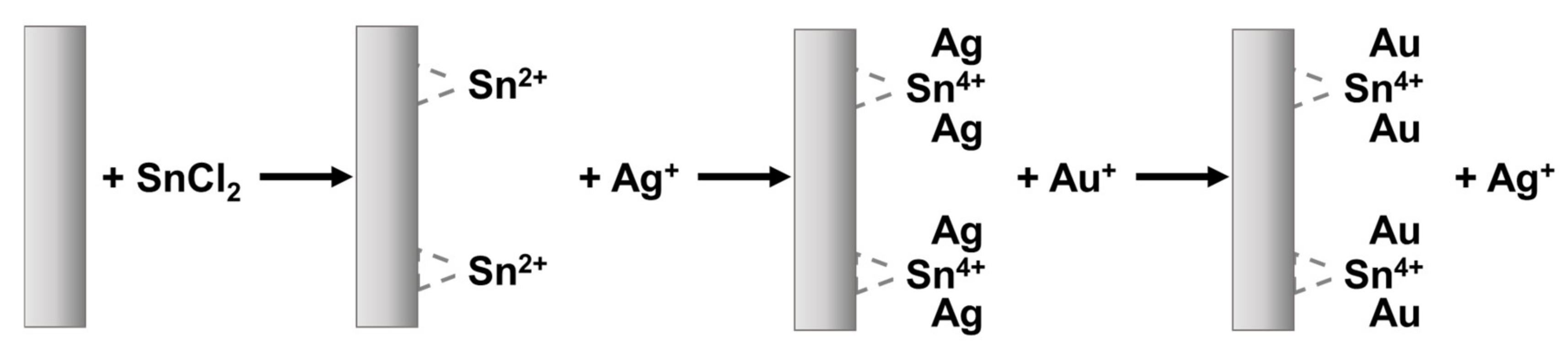
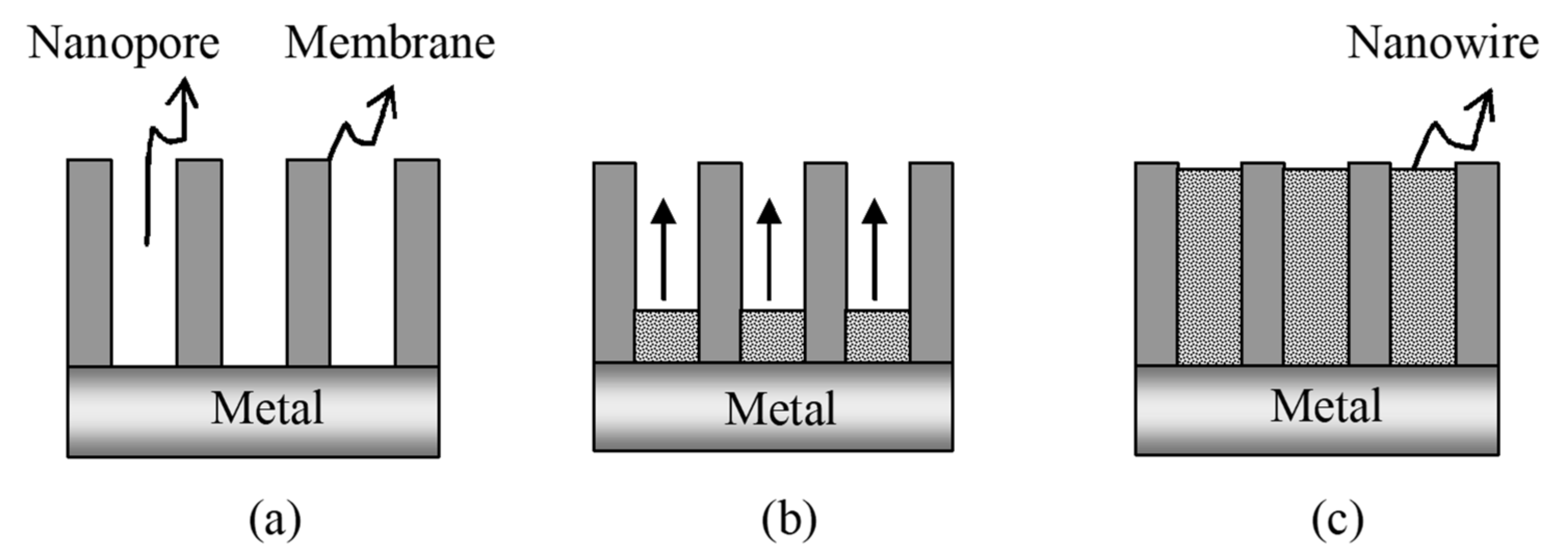
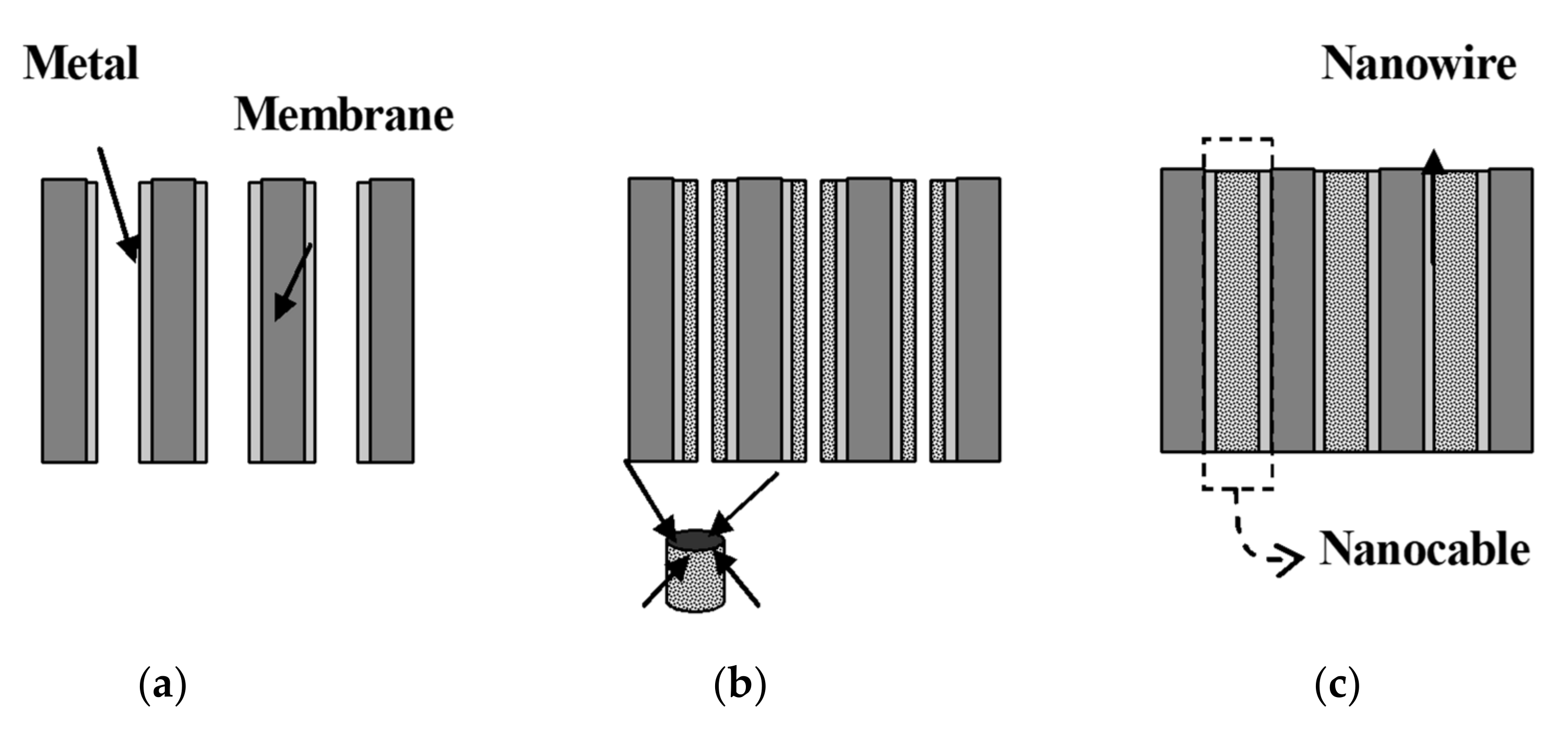
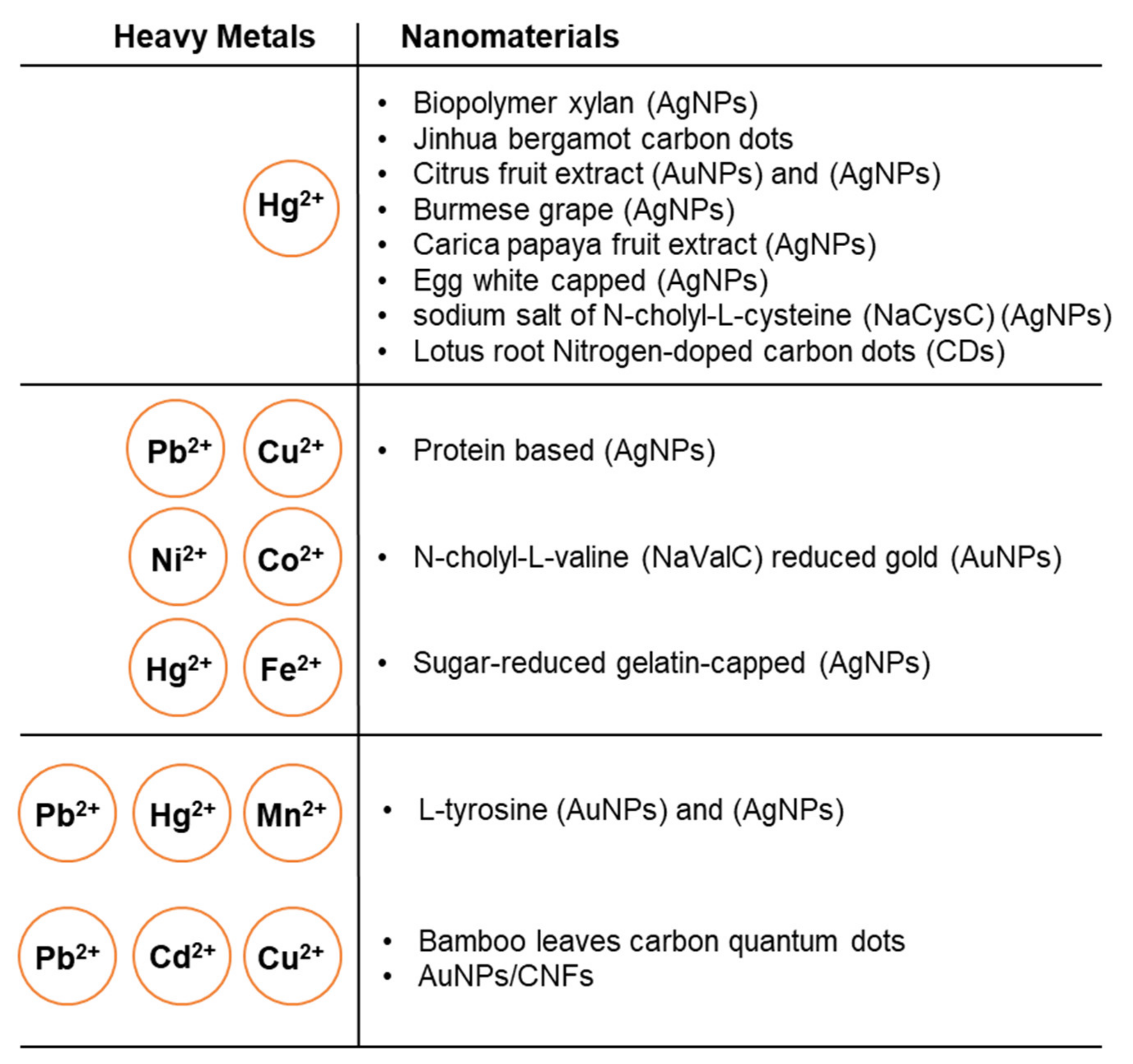
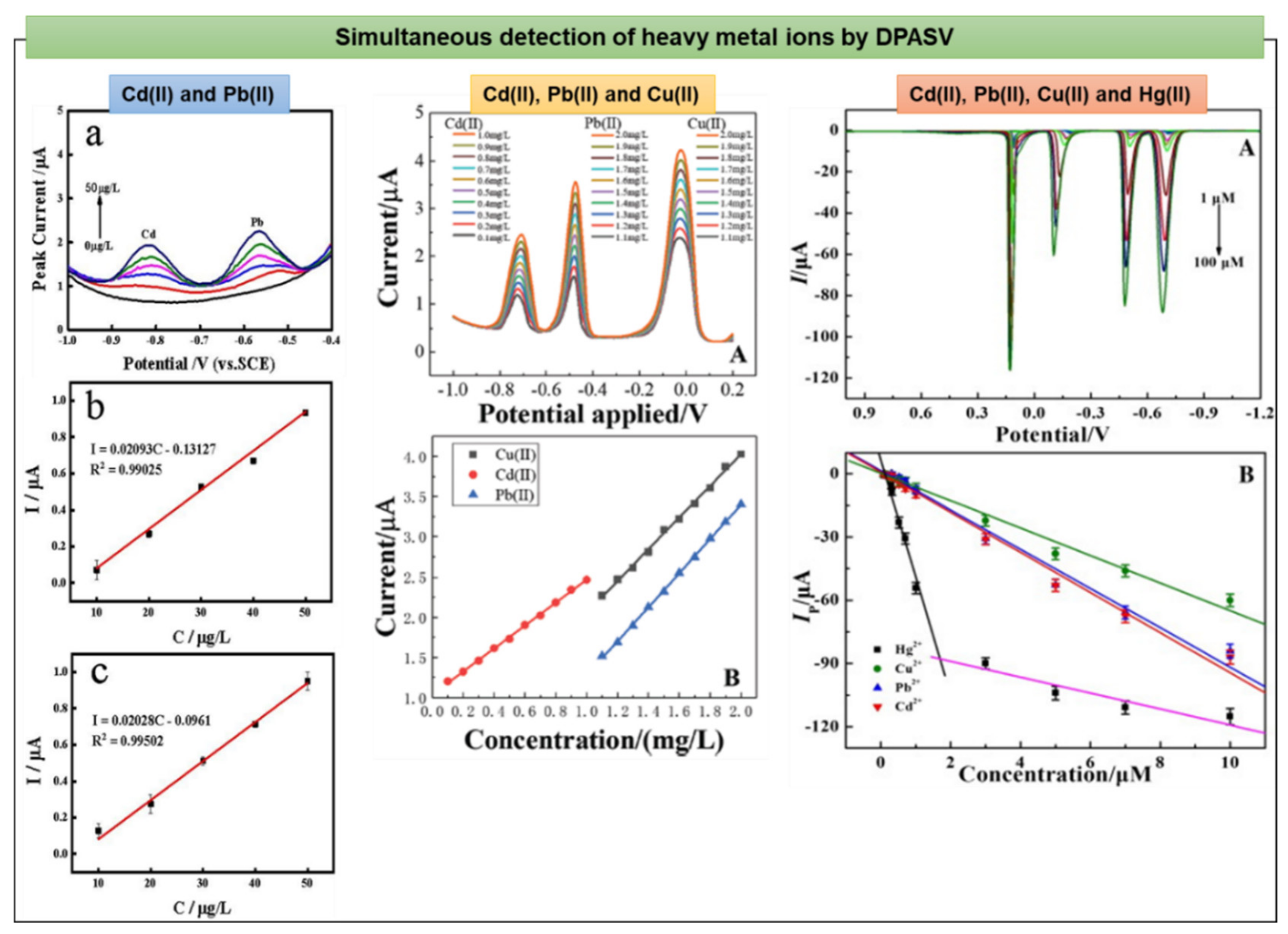
| Heavy Metal | Permissible Limits (WHO) µg/L | Permissible Limits (USEPA) µg/L | Health Hazards |
|---|---|---|---|
| Arsenic | 500 | * | Carcinogenic, producing liver tumors, skin and gastrointestinal effects |
| Mercury | 1 | 0.03 | Corrosive to skin, eyes and muscle membrane, dermatitis, anorexia, kidney damage and severe muscle pain |
| Cadmium | 3 | 10 | Carcinogenic, cause lung fibrosis, dyspnea and weight loss |
| Lead | 10 | 6 | Suspected carcinogen, loss of appetite, anemia, muscle and joint pains, diminishing IQ, cause sterility, kidney problem and high blood pressure |
| Chromium | 50 | 50 | Suspected human carcinogen, producing lung tumors, allergic dermatitis |
| Nickel | 20 | 200 | Causes chronic bronchitis, reduced lung function, cancer of lungs and nasal sinus |
| Zinc | 5000 | * | Causes short-term illness called “metal fume fever” and restlessness |
| Copper | 3000 | * | Long term exposure causes irritation of nose, mouth, eyes, headache, stomachache, dizziness, diarrhea |
| Chemical Precipitation | Advantages | Disadvantages |
|---|---|---|
| Hydroxide precipitation (lime, limestone, CaCO3) | Easy method, simple operation, cheap and broad applications. | Accumulation of large quantity of residual sludge with water content, problematic for dewater and disposal. Not suitable for wastewaters with high heavy metals concentrations. |
| Sulfide precipitation | Less sludge quantity, easier dehydration. | Metallic sulfide precipitate is very small and difficult to settle down. |
| Ferrite co-precipitation | Efficient for heavy metals removal with density higher than 3.8 g/cm3 (Cu, Pb, Zn, Cd, Co, Cr, Mn, Hg, Bi, Sn, As, Mo, Fe, V, Ti). Possible separation of formed precipitates by filtration or magnetic methods. | High temperature, not suitable for large volume of wastewater, high energy consumption. |
| Adsorbate | Adsorbent | Max. Adsorption, mg/g | Initial Concentration, mg/L | pH | Ref. |
|---|---|---|---|---|---|
| Pb2+ | MWCNTs | 15.6 | 10–80 | 3, 5, 7 | [51] |
| AC | 18 | 10–60 | 5 | [52] | |
| Oxidized MWCNTs | 59 | 5 | [52] | ||
| SWCNTs | 33.55 | 7 | [53] | ||
| Cu2+ | MWCNTs | 12.34 | 7 | [54] | |
| Oxidized MWCNTs | 28.49 | 5–30 | 5 | [55] | |
| SWCNTs | 24.29 | 5 | [52] | ||
| Cd2+ | MWCNTs | 4.1 | 4 | 3–12 | [56] |
| AC | 2.9 | 4 | 3–12 | [56] | |
| Oxidized MWCNTs | 10.86 | 2–15 | 5 | [55] | |
| SWCNTs | 24.07 | 7 | [53] | ||
| Ni2+ | MWCNTs | 13.05 | 10–80 | 2–9 | [57] |
| Oxidized MWCNTs | 38.46 | 10–80 | 7 | [58] | |
| oxidized SWCNTs | 47.85 | 10–80 | 7 | [58] | |
| GAC | 26.39 | 10–80 | 7 | [58] | |
| Zn2+ | MWCNTs | 32.68 | 10–80 | [59] | |
| SWCNTs | 43.66 | 10–80 | [59] | ||
| PAC | 13.04 | 10–80 | [59] |
| UF Type | MEUF | PEUF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Heavy Metal | Cd | Ni | Zn | Pb | AsO4 | Cd | Cu | Cr | Ni |
| Membrane | Polysulfone | Polycarbonate | Amicon regenerated cellulose | Ceramic | Ceramic | Polysulfone | Ceramic | Polyethersulfone | Polyethersulfone |
| Removal efficiency | 92% | 98.6% | 99% | 99% | 19% | 99% | 99.5% | 99.5% | 100% |
| Reference | [80] | [81] | [82] | [83] | [83] | [84] | [85] | [86] | [87] |
| Membrane | Reverse Osmosis | Nanofiltration | Reverse Osmosis + Nanofiltration | ||||
|---|---|---|---|---|---|---|---|
| Heavy Metal | Cu | Ni | Zn | As | Cu | Cr | Cu |
| Removal Efficiency | 99.5 | 99.3 | 98.9 | As(V) 91–99%, As(III) 20–55% | 96–98 | 99.5 | 95–99 |
| Reference | [88] | [89] | [89] | [90] | [91] | [92] | [93] |
| Metals or Other Compounds | Concentration, mg/L | Electrodes Anode/Cathode | Removal Efficiency, % | References |
|---|---|---|---|---|
| Cr3+, Cr6+ | 887.2, 1495.2 | Fe/Fe | 100 | [110] |
| Cu2+, Cr, Ni2+ | 45, 44.5, 394 | Al/Fe | 100 | [111] |
| Cd2+ | 20 | Al/Al | AC: 97.5 DC: 96.2 | [112] |
| NO3− | 150 | Fe/Fe, Al/Al | 90, 89.7 | [113] |
| Pb2+, Zn2+, Cd2+ | 170, 50, 1.5 | Al/SS | 95, 68, 66 | [114] |
| As | 150 | Al/Al, Fe/Fe | 93.5, 94 | [115] |
| TOC, Ni2+, Zn2+ | 173, 248, 232 | SS 304-SS 304 | 66, 90, 100 | [116] |
| Humic acid | 20 | Fe/Fe | 92.69 | [117] |
| Water Treatment Method | Metal | Initial Concentration mg/L | Efficiency % | References |
|---|---|---|---|---|
| Reverse Osmosis (pH = 7–9) | Ni2+ | 26 | 99 | [133] |
| Cu2+ | 17 | 99 | [133] | |
| Cr | 167 | 99 | [133] | |
| Ultrafiltration (pH > 7) | Ni2+ | 50 | 99 | [86] |
| Cu2+ | 50 | 98 | [86] | |
| Cr | 50 | 93 | [86] | |
| Ni2+ | 25 | 100 | [134] | |
| Nanofiltration (pH = 4–11) | Cu2+ | 200 | 96 | [135] |
| Electrocoagulation (pH = 8) | Ni2+ | 394 | 99 | [111] |
| Cu2+ | 45 | 100 | [111] | |
| Cr | 44.5 | 100 | [111] | |
| Ni2+, Zn2+ | 248, 270, 282, 217, 232, 236 | 100 | [136] | |
| Chemical Precipitation (pH > 7) | Cu2+, Zn2+, Cr3+, Pb2+ | 100 | 99.3–99.6 | [13] |
| Cu2+, Zn2+, Pb2+ | 0.01, 1.34, 2.3 | 100, >94, >92 | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics 2020, 8, 101. https://doi.org/10.3390/toxics8040101
Vidu R, Matei E, Predescu AM, Alhalaili B, Pantilimon C, Tarcea C, Predescu C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics. 2020; 8(4):101. https://doi.org/10.3390/toxics8040101
Chicago/Turabian StyleVidu, Ruxandra, Ecaterina Matei, Andra Mihaela Predescu, Badriyah Alhalaili, Cristian Pantilimon, Claudia Tarcea, and Cristian Predescu. 2020. "Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications" Toxics 8, no. 4: 101. https://doi.org/10.3390/toxics8040101
APA StyleVidu, R., Matei, E., Predescu, A. M., Alhalaili, B., Pantilimon, C., Tarcea, C., & Predescu, C. (2020). Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics, 8(4), 101. https://doi.org/10.3390/toxics8040101








