New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Stock Solutions
2.2. Fish Maintenance and Egg Production
2.3. Prolonged Fish Embryo Toxicity Test
2.4. Determination of Aqueous and Internal 3,4-DCA Concentrations Using LC-DAD
2.5. Data Analysis
3. Results and Discussion
3.1. Survival of Zebrafish Larvae Exposed to 3,4-DCA
3.2. Dissipation of 3,4-DCA from Exposure Solutions
3.3. Bioconcentration of 3,4-DCA in Exposed Zebrafish Larvae
3.4. Conclusions and Relevance for the FET Test
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarzman, M.R.; Wilson, M.P. New science for chemicals policy. Science 2009, 326, 1065–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation, E.C. No. 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorization and Restriction of Chemicals; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Hommen, U.; Baveco, J.M.; Galic, N.; van den Brink, P.J. Potential application of ecological models in the European environmental risk assessment of chemicals I: Review of protection goals in EU directives and regulations. Integr. Environ. Assess. Manag. 2010, 6, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 3, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hallare, A.V.; Seiler, T.-B.; Hollert, H. The versatile, changing, and advancing roles of fish in sediment toxicity assessment—A review. J. Soils Sediments 2011, 1, 141–173. [Google Scholar] [CrossRef]
- Organisation de coopération et de développement économiques (OECD). Validation Report (Phase 1) for the Zebrafish Embryo Toxicity Test; No. 157; Series on Testing and Assessment; ENV/JM/MONO(2011)37; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Braunbeck, T.; Boettcher, M.; Hollert, H.; Kosmehl, T.; Lammer, E.; Leist, E.; Rudolf, M.; Seitz, N. Towards an alternative for the acute fish LC(50) test in chemical assessment: The fish embryo toxicity test goes multi-species—An update. Altex 2005, 22, 87–102. [Google Scholar] [PubMed]
- Peddinghaus, S.; Brinkmann, M.; Bluhm, K.; Sagner, A.; Hinger, G.; Braunbeck, T.; Eisentrager, A.; Tiehm, A.; Hollert, H.; Keiter, S.H. Quantitative assessment of the embryotoxic potential of NSO-heterocyclic compounds using zebrafish (Danio rerio). Reprod. Toxicol. 2012, 33, 224–232. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The European Parliament and the European Council Directive 2010/63/EU of the 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 53, 33–79. [Google Scholar]
- Organisation de coopération et de développement économiques (OECD) 236. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Schiwy, S.; Bräunig, J.; Alert, H.; Hollert, H.; Keiter, S.H. A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2015, 22, 16305–16318. [Google Scholar] [CrossRef] [PubMed]
- Mundt, M.; Hollender, J. Simultaneous determination of NSO-heterocycles, homocycles and their metabolites in groundwater of tar oil contaminated sites using LC with diode array UV and fluorescence detection. J. Chromatogr. A 2005, 1065, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Lin, Y.J.; Li, M.W.; Chuang, H.N.; Chou, C.C. Aquatic toxicity assessment of single-walled carbon nanotubes using zebrafish embryos. J. Phys. Conf. Ser. 2011, 304, 012–026. [Google Scholar] [CrossRef]
- Vincze, K.; Graf, K.; Scheil, V.; Köhler, H.R.; Triebskorn, R. Embryotoxic and proteotoxic effects of water and sediment from the Neckar River (Southern Germany) to zebrafish (Danio rerio) embryos. Environ. Sci. Eur. 2014, 26, 3. [Google Scholar] [CrossRef] [Green Version]
- Hertl, J.; Nagel, R. Bioconcentration and metabolism of 3,4-dichloroaniline in different life stages of guppy and zebrafish. Chemosphere 1993, 27, 2225–2234. [Google Scholar] [CrossRef]
- Organisation de coopération et de développement économiques (OECD) 305. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure; OECD Publishing: Paris, France, 2012. [Google Scholar]


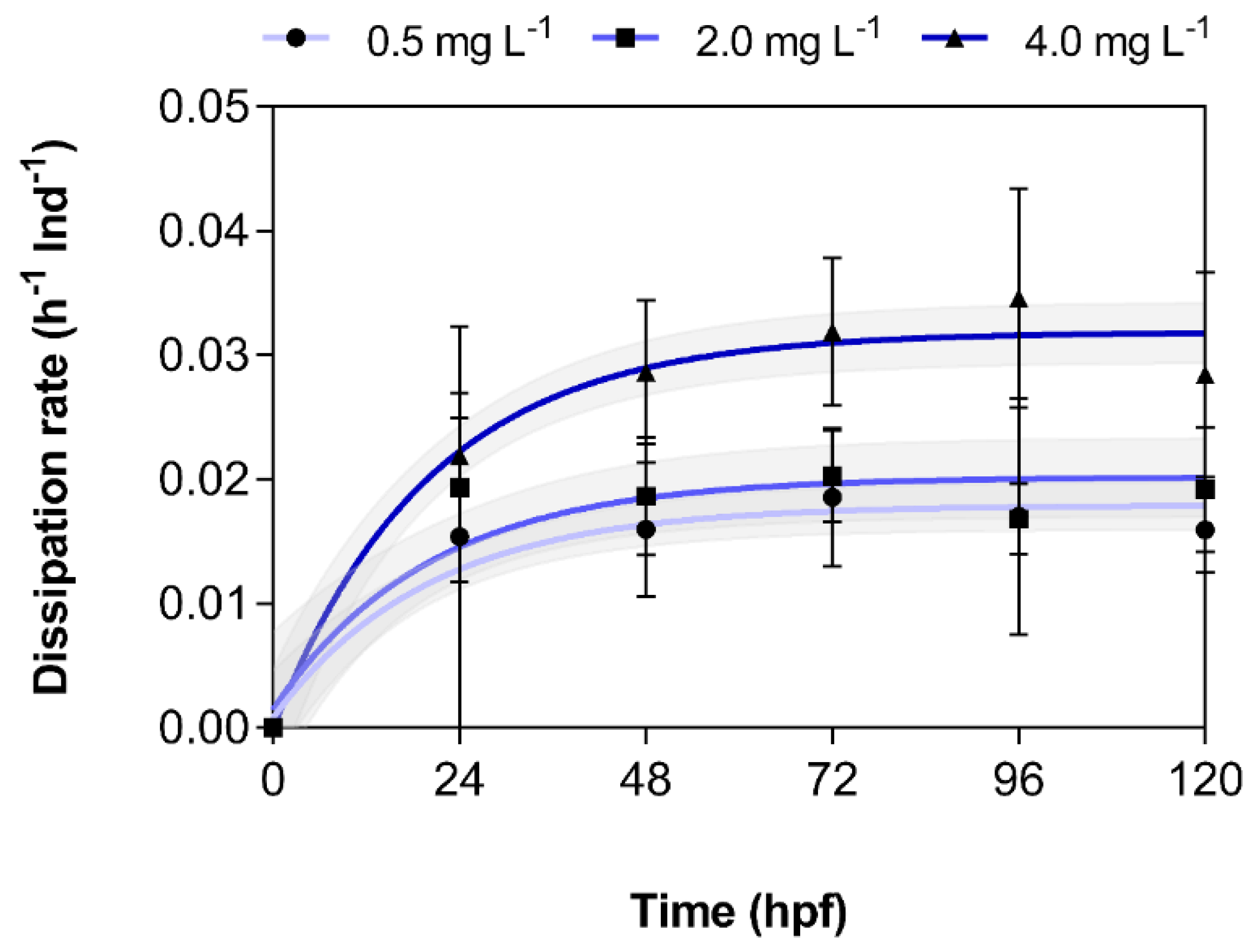
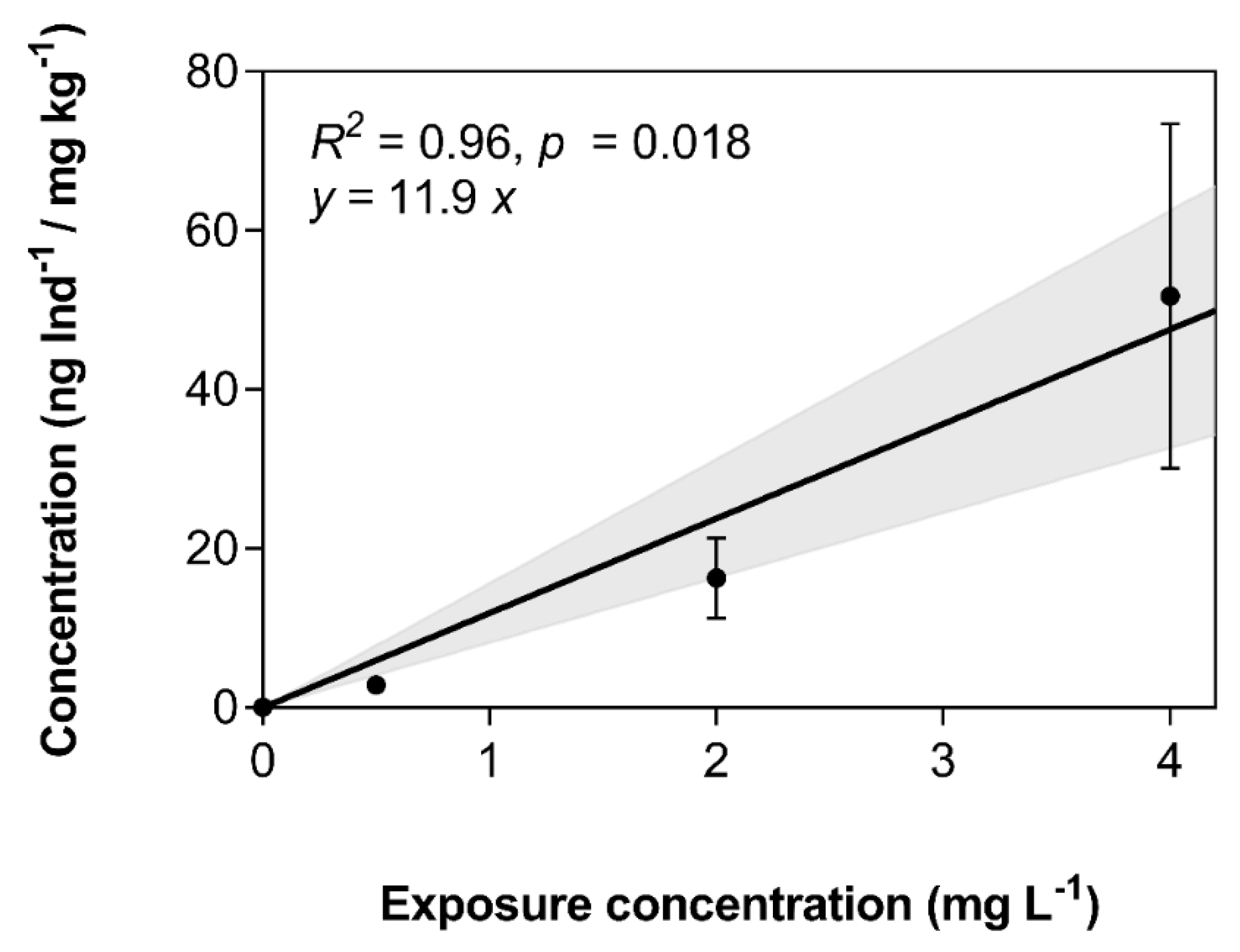
| Nominal Concentration (mg L−1) | Time-Weighted Average Concentration (mg L−1) | Internal Concentration (ng Ind−1/ mg kg−1) | Bioconcentration Factor (Nominal) (L kg) | Bioconcentration Factor (TWA) (L kg) | Maximum Dissipation Rate (h−1 Ind−1) |
|---|---|---|---|---|---|
| 0.50 | 0.25 | 2.83 ± 0.57 | 5.66 ± 1.13 | 11.3 ± 2.27 | 0.018 ± 0.001 |
| 2.00 | 1.03 | 16.3 ± 5.12 | 8.13 ± 2.56 | 15.8 ± 4.97 | 0.020 ± 0.002 |
| 4.00 | 2.25 | 51.7 ± 21.7 | 12.9 ± 5.42 | 23.0 ± 9.64 | 0.032 ± 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiwy, S.; Herber, A.-K.; Hollert, H.; Brinkmann, M. New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio rerio). Toxics 2020, 8, 16. https://doi.org/10.3390/toxics8010016
Schiwy S, Herber A-K, Hollert H, Brinkmann M. New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio rerio). Toxics. 2020; 8(1):16. https://doi.org/10.3390/toxics8010016
Chicago/Turabian StyleSchiwy, Sabrina, Ann-Kathrin Herber, Henner Hollert, and Markus Brinkmann. 2020. "New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio rerio)" Toxics 8, no. 1: 16. https://doi.org/10.3390/toxics8010016
APA StyleSchiwy, S., Herber, A.-K., Hollert, H., & Brinkmann, M. (2020). New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio rerio). Toxics, 8(1), 16. https://doi.org/10.3390/toxics8010016






