The Source and Pathophysiologic Significance of Excreted Cadmium
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Collection of Biological Specimens and Laboratory Analyses
2.3. Estimated Glomerular Filtration Rate (eGFR)
2.4. Normalization of Excretion Rates to Creatinine Clearance (Ccr)
2.5. Statistical Analysis
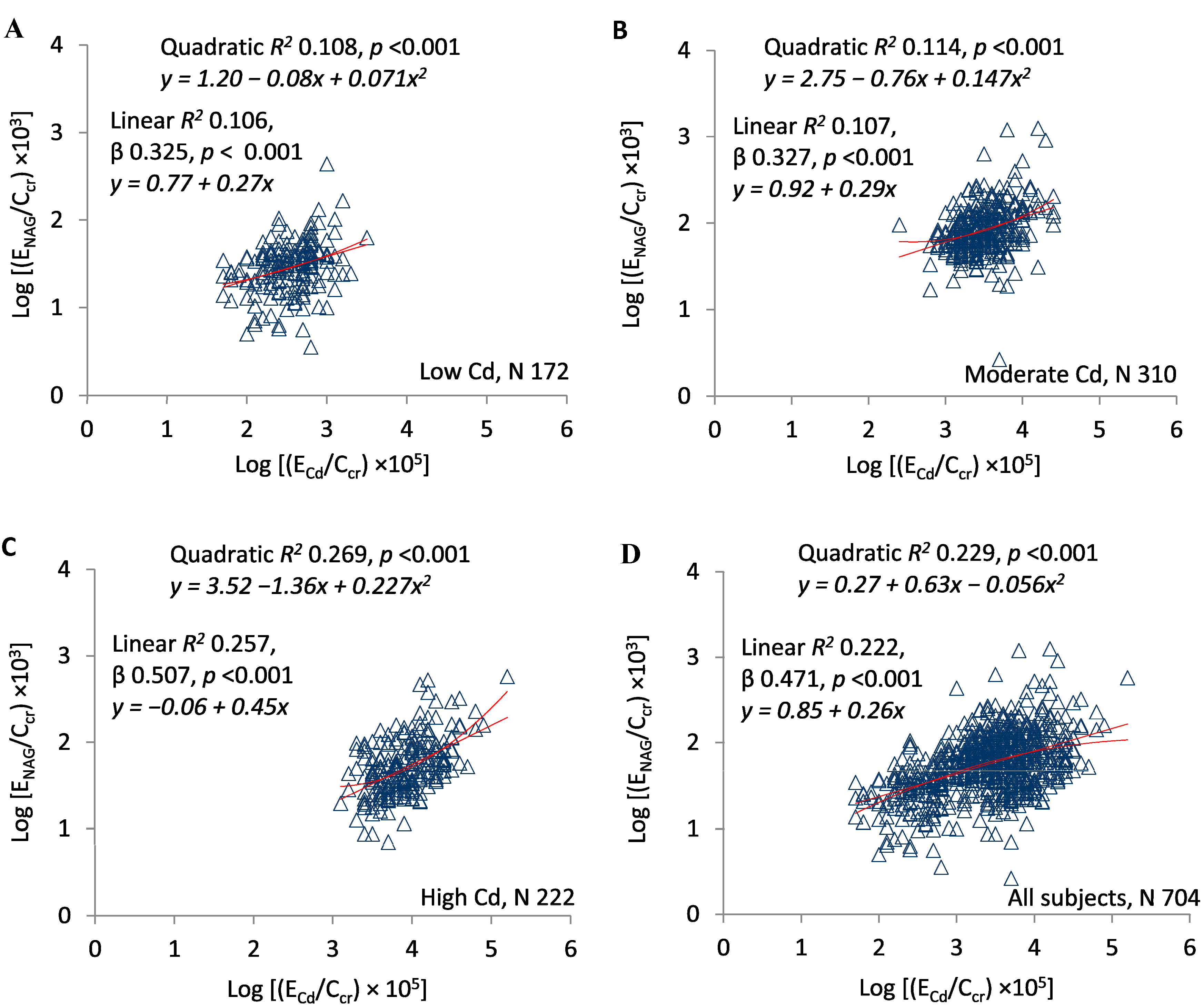
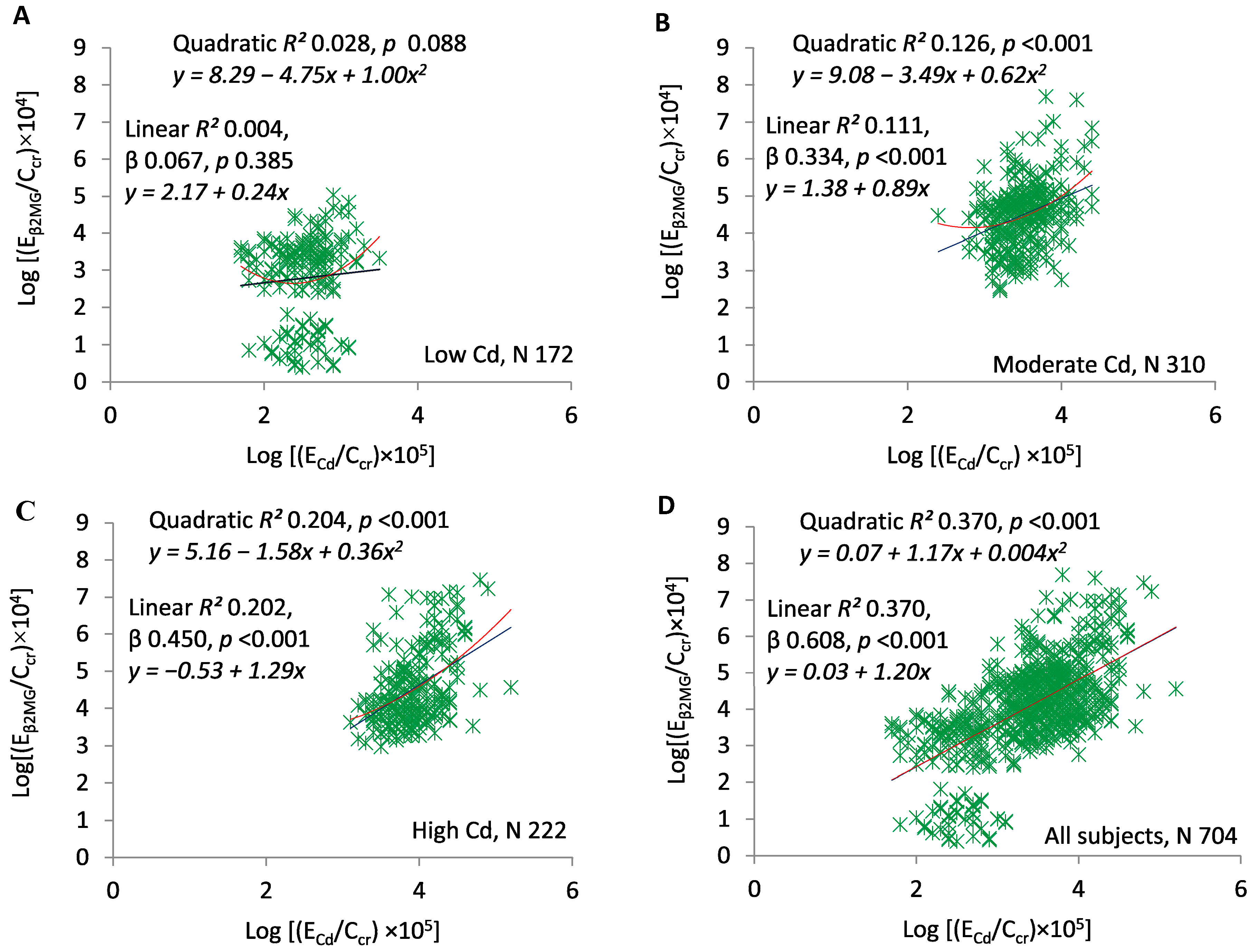
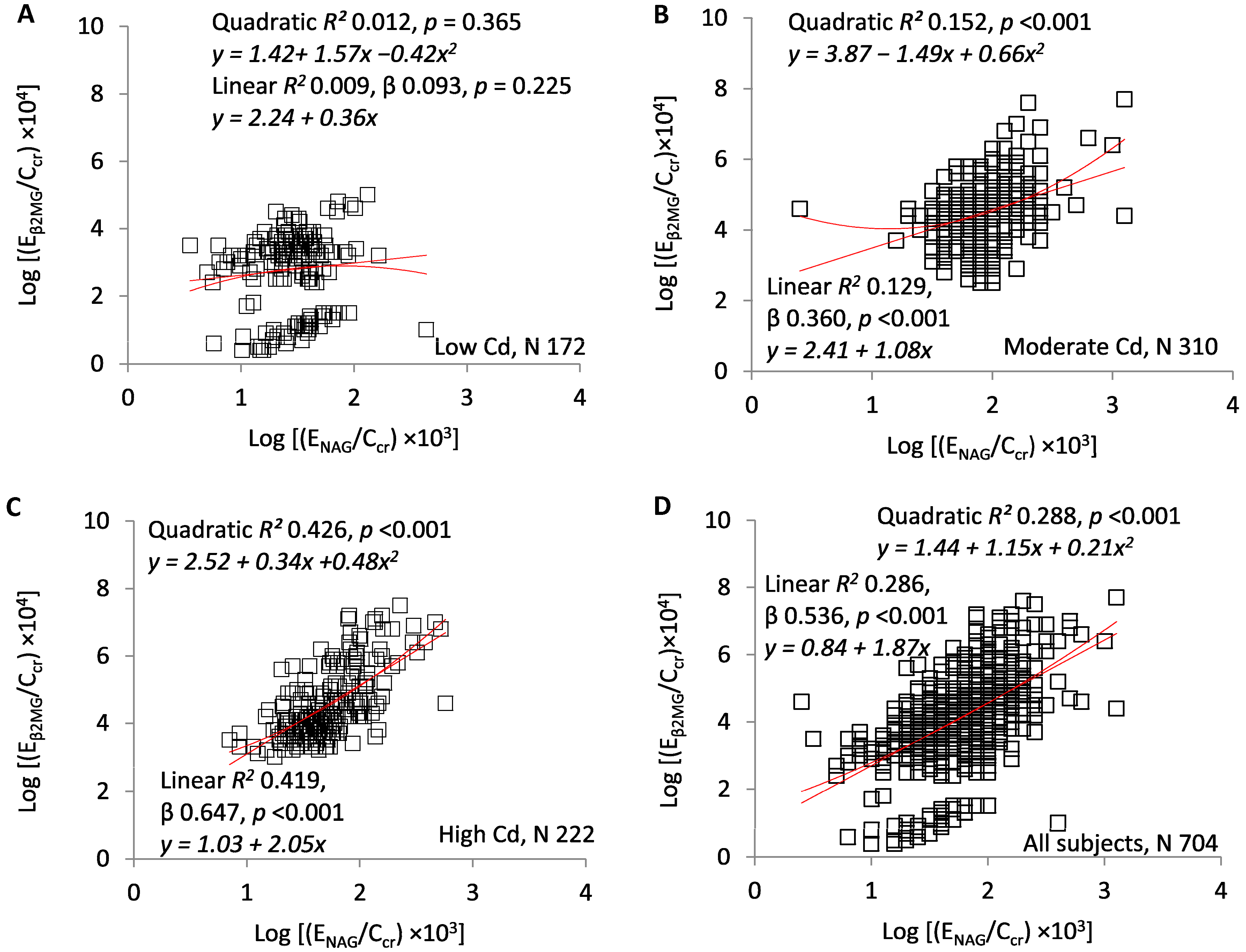
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| Cd | Cadmium |
| GFR | Glomerular filtration rate, units of volume/time |
| eGFR | Estimated glomerular filtration rate, units of mL/min/1.73 m2 |
| CKD-EPI | Chronic kidney disease epidemiology collaboration |
| MT | Metallothionein |
| CdMT | Cadmium–metallothionein complex |
| PC | Phytochelatin |
| CdPC | Cadmium–phytochelatin complex |
| GSH | Glutathione |
| NAG | N-acetyl-β-d-glucosaminidase |
| β2MG | Beta2-microglobulin |
| Ccr | Creatinine clearance, units of volume/time |
| Vu | Urine flow rate, units of volume/time |
| Ex/Ccr | Excretion rate of x per volume of filtrate, units of mass/volume, where x = Cd, NAG, or β2MG |
| TDβ2MG | Rate of tubular degradation of β2MG, units of mass/time |
| TDβ2MG/Ccr | Amount of β2MG degraded per volume of filtrate, units of mass/volume |
References
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Cadmium; Department of Health and Humans Services, Public Health Service, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2012. [Google Scholar]
- WHO. IPCS (International Programme on Chemical Safety) Environmental Health Criteria 134: Cadmium; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Dietary cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Lee, W.-K.; Thevenod, F. Differential transcytosis and toxicity of the hNGAL receptor ligands cadmium-metallothionein and cadmium-phytochelatin in colon-like Caco-2 cells: Implications for cadmium toxicity. Toxicol. Lett. 2014, 226, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Dorian, C.; Gattone, V.H., II; Klaassen, C.D. Discrepancy between the nephrotoxic potencies of cadmium-metallothionein and cadmium chloride and the renal concentration of cadmium in the proximal convoluted tubules. Toxicol. Appl. Pharmacol. 1995, 130, 161–168. [Google Scholar] [CrossRef]
- Sabolic, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Fujita, Y.; ElBelbasi, H.I.; Min, K.-S.; Onosaka, S.; Okada, Y.; Matsumoto, Y.; Mutoh, N.; Tanaka, K. Fate of cadmium bound to phytochelatin in rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 82, 357–365. [Google Scholar]
- Sudo, J.-I.; Hayashi, T.; Soyama, M.; Fukata, M.; Kakuino, K. Kinetics of Cd2+ in plasma, liver and kidneys after single intravenous injection of Cd-metallothionein-II. Eur. J. Pharmacol. 1994, 270, 229–235. [Google Scholar] [CrossRef]
- Chan, H.M.; Zhu, L.F.; Zhong, R.; Grant, D.; Goyer, R.A.; Cherian, M.G. Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicol. Appl. Pharmacol. 1993, 123, 89–96. [Google Scholar] [CrossRef]
- Dudley, R.E.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef]
- Dorian, C.; Gattone, V.H., II; Klaassen, C.D. Renal cadmium deposition and injury as a result of accumulation of cadmium-metallothionein (CdMT) by the proximal convoluted tubules—A light microscopic autoradiography study with 109CdMT. Toxicol. Appl. Pharmacol. 1992, 114, 173–181. [Google Scholar] [CrossRef]
- Barbier, O.; Jacquillet, G.; Tauc, M.; Poujeol, P.; Cougnon, M. Acute study of interaction among cadmium, calcium, and zinc transport along rat nephron in vivo. Am. J. Physiol. 2004, 287, F1067–F1075. [Google Scholar] [CrossRef] [PubMed]
- Felley-Bosco, E.; Diezi, J. Fate of cadmium in rat renal tubules: A micropuncture study. Toxicol. Appl. Pharmacol. 1989, 98, 243–251. [Google Scholar] [CrossRef]
- Thevenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: Myths and facts. Biometals 2019, 32, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Soodvilai, S.; Nantavishit, J.; Muanprasat, C.; Chatsudthipong, V. Renal organic cation transporters mediated cadmium-induced nephrotoxicity. Toxicol. Lett. 2011, 204, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Evidence for basolateral uptake of cadmium in kidneys of rats. Toxicol. Appl. Pharmacol. 2000, 164, 15–23. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Habeebu, S.S.; Klaassen, C.D. Metallothionein protects against the nephrotoxicity produced by chronic CdMT exposure. Toxicol. Sci. 1999, 50, 221–227. [Google Scholar] [CrossRef]
- Goyer, R.A.; Miller, C.R.; Zhu, S.-Y.; Victery, W. Non-metallothionein-bound cadmium in the pathogenesis of cadmium nephrotoxicity in the rat. Toxicol. Appl. Pharmacol. 1989, 101, 232–244. [Google Scholar] [CrossRef]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef]
- Jarup, L.; Hellstrom, L.; Alfven, T.; Carlsson, M.D.; Grubb, A.; Persson, B.; Pettersson, C.; Spang, G.; Schutz, A.; Elinder, C.-G. Low level exposure to cadmium and early kidney damage: The OSCAR study. Occup. Environ. Med. 2000, 57, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.A.; Lauwerys, R.R.; Buchet, J.P.; Bernard, A.M.; Vos, A.; Oversteyns, M. Health significance of cadmium induced renal dysfunction: A five year follow up. Occup. Environ. Med. 1989, 46, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Boonprasert, K.; Gobe, G.C.; Ruenweerayut, R.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin. Kidney J. 2018, 12, 468–475. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahassakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
- Price, R.G. Measurement of N-acetyl-beta-glucosaminidase and its isoenzymes in urine: Methods and clinical applications. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 693–705. [Google Scholar]
- Portman, R.J.; Kissane, J.M.; Robson, A.M. Use of β2 microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986, 30, 91–98. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Peterson, P.A.; Evrin, P.-E.; Berggard, I. Differentiation of glomerular, tubular, and normal proteinuria: Determination of urinary excretion of β2-microglobulin, albumin, and total protein. J. Clin. Investig. 1969, 48, 1189–1198. [Google Scholar] [CrossRef]
- Chaumont, A.; Voisin, C.; Deumer, G.; Haufroid, V.; Annesi-Maesano, I.; Roels, H.; Thijs, L.; Staessen, J.; Bernard, A. Associations of urinary cadmium with age and urinary proteins: Further evidence of physiological variations unrelated to metal accumulation and toxicity. Environ. Health Perspect. 2013, 121, 1047–1053. [Google Scholar] [CrossRef]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 133–142. [Google Scholar]
- Honda, R.; Swaddiwudhipong, W.; Nishijo, M.; Mahasakpan, P.; Teeyakasem, W.; Ruangyuttikarn, W.; Satarug, S.; Padungtod, C.; Nakagawa, H. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol. Lett. 2010, 198, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Phelps, K.R.; Stote, K.S.; Mason, D. Tubular calcium reabsorption and other aspects of calcium homeostasis in primary and secondary hyperparathyroidism. Clin. Nephrol. 2014, 82, 83–91. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Anderson, C.A.; Yang, W.; Zhang, X.; Fischer, M.J.U.; Dember, L.M.; Fink, J.C.; Frydrych, A.; Jensvold, N.; Lustigova, E.; et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 2015, 65, 412–424. [Google Scholar] [CrossRef]
- Staplin, N.; Haynes, R.; Herrington, W.G.; Reith, C.; Cass, A.; Fellstrom, B.; Jiang, L.; Kasiske, B.L.; Krane, V.; Levin, A.; et al. Smoking and adverse outcomes in patients with CKD: The study of heart and renal protection (SHARP). Am. J. Kidney Dis. 2016, 68, 371–380. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liang, M.; Wang, G.; Li, J.; Zhang, Y.; Huo, Y.; Cui, Y.; Xu, X.; Qin, X. Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin. Epidemiol. 2018, 10, 121–132. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, P.; Liang, X.X.; Tan, C.S.; Tan, J.B.; Wang, J.; Huang, Q.; Huang, R.; Li, Z.X.; Chen, W.C.; et al. Associations between urinary excretion of cadmium and renal biomarkers in non-smoking females: A cross-sectional study in rural areas of South China. Int. J. Environ. Res. Public Health 2015, 12, 11988–12001. [Google Scholar] [CrossRef]
- Bernard, A.; Thielemans, N.; Roels, H.; Lauwerys, R. Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup. Environ. Med. 1995, 52, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Li, Y.B.; Zhju, C.S.; Dong, Z.M.; Zhang, K.; Zhao, Y.B.; Xu, Y.L. Benchmark dose for cadmium exposure and elevated N-acetyl-β-d-glucosaminidase: A meta-analysis. Environ. Sci. Pollut. Res. 2016, 23, 20528–20538. [Google Scholar] [CrossRef]
- Kawada, T.; Koyama, H.; Suzuki, S. Cadmium, NAG activity, and β2-microglobulin in the urine of cadmium pigment workers. Occup. Environ. Med. 1989, 46, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Shinmyo, R.R.; Suzuki, S. Urinary cadmium and N-acetyl-β-d-gluosaminidase excretion of inhabitants living in a cadmium-polluted area. Int. Arch. Occup. Environ. Health 1992, 63, 541–546. [Google Scholar] [CrossRef]
- Koyama, H.; Satoh, H.; Suzuki, S.; Tohyama, C. Increased urinary cadmium excretion and its relationship to urinary N-acetyl-β-d-gluosaminidase activity in smokers. Arch. Toxicol. 1992, 66, 598–601. [Google Scholar] [CrossRef]
- Sabolic, I.; Skarica, M.; Ljubojevic, M.; Breljak, D.; Herak-Kramberger, C.M.; Crljen, V.; Ljubesic, N. Expression and immunolocalization of metallothioneins MT1, MT2 and MT3 in rat nephron. J. Trace Elem. Med. Biol. 2018, 46, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.B.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013, 268, 286–293. [Google Scholar] [CrossRef]
- Roels, H.A.; Lauwerys, R.; Dardenne, A.N. The critical level of cadmium in human renal cortex: A reevaluation. Toxicol. Lett. 1983, 15, 357–360. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007-2012. Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Kim, N.-S.; Jaar, B.G.; Schwartz, B.S.; Parsons, P.J.; Steuerwald, A.J.; Todd, A.C.; Simon, D.; Lee, B.-K. Associations of low-level urine cadmium with kidney function in lead workers. Occup. Environ. Med. 2011, 68, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhu, X.; Shrubsole, J.M.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003-2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, K.; Foulkes, E.C. Reabsorption of filtered cadmium-metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med. 1977, 156, 97–99. [Google Scholar] [CrossRef]
- Roels, H.A.; Bernard, A.M.; Buchet, J.P.; Lauwerys, R.R.; Hotter, G.; Ramis, I.U.; Mutti, A.; Franchini, I.; Bundschuh, I.; Stolte, H.; et al. Markers of early renal changes induced by industrial pollutants. III Application to workers exposed to cadmium. Occup. Environ. Med. 1993, 50, 37–48. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.-D.; Yim, D.-H.; Eom, S.-Y.; Moon, S.-I.; Park, C.-H.; Kim, G.-B.; Uy, S.-D.; Choi, B.-S.; Park, J.-D.; Kim, H. Temporal changes in urinary levels of cadmium, N-acetyl-β-d-gluosaminidase and β2-miocroglobuilin in individuals in a cadmium-contaminated area. Environ. Toxicol. Pharmacol. 2015, 39, 35–41. [Google Scholar] [CrossRef]
- Forman, D.T. Beta-2 microglobulin—An immunogenetic marker of inflammatory and malignant origin. Ann. Clin. Lab. Sci. 1982, 12, 447–451. [Google Scholar]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef]
| Descriptors | All Subjects | Locality | ||
|---|---|---|---|---|
| Low Cd | Moderate Cd | High Cd | ||
| Number of subjects | 704 | 172 | 310 | 222 |
| Women (%) | 60.7 | 47.7 | 72.9 | 53.6 * |
| Smoking (%) | 43.6 | 23.8 | 40.6 | 63.1 * |
| Age (years) | 48.34 ± 11.11 | 38.72 ± 10.29 | 47.24 ± 4.72 | 57.34 ± 11.13 † |
| SBP (mmHg) | 122.7 ± 13.5 | 120.1 ± 10.4 | 124.2 ± 14.7 ¶ | − |
| DBP (mmHg) | 78.9 ± 9.5 | 78.1 ± 7.6 | 79.4 ± 10.4 | − |
| MBP (mmHg) | 93.5 ± 9.9 | 92.1 ± 7.9 | 94.3 ± 3.3 ¶¶ | − |
| eGFR (mL/min/1.73 m2) | 90.32 ± 21.84 | 105.46 ± 15.05 | 95.72 ± 16.13 | 71.05 ± 19.65 † |
| CKD prevalence (%) | 9.5 | 0 | 3.5 | 25.2 |
| Kidney disease stage (%) | ||||
| Stage 1 | 54.8 | 84.1 | 66.5 | 16.2 * |
| Stage 2 | 36.6 | 15.9 | 31.0 | 59.9 * |
| Stage 3 | 7.7 | 0 | 2.6 | 20.7 ** |
| Stage 4 | 1.0 | 0 | 0 | 3.2 |
| Serum creatinine (mg/dL) | 0.80 (0.70, 1.0) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 1.0 (0.9, 1.2) † |
| Urine creatinine (mg/dL) | 97 (53, 156) | 52 (32, 106) | 110 (62, 171) | 115 (69, 158) † |
| Urine Cd (μg/L) | 3.7 (1.1, 8.3) | 0.2 (0.1, 0.6) | 3.9 (2.4, 7.2) | 8.3 (4.7, 13.9) † |
| Urine NAG (units/L) | 6.4 (2.4, 16.6) | 1.8 (1.3, 2.9) | 11.9 (7.1, 19) | 5.3 (2.7, 9.2) † |
| Urine β2MG (μg/L) | 154 (33, 778) | 9.5 (0.3, 42) | 400 (134, 1118) | 171 (64, 1368) † |
| ECd/Ccr × 100, µg/L | 3.1 (1.1, 7.0) | 0.3 (0.3, 0.6) | 3.0 (1.7, 5.0) | 8.2 (4.8, 15) † |
| ENAG/Ccr × 100, units/L | 5.6 (3.4, 9.4) | 3.2 (2.0, 4.1) | 8.0 (5.8, 12) | 4.9 (3.1. 8.3) † |
| Eβ2MG/Ccr × 100, µg/L | 137 (29, 604) | 17 (0.4, 36) | 368 (92, 808) | 162 (64, 176) † |
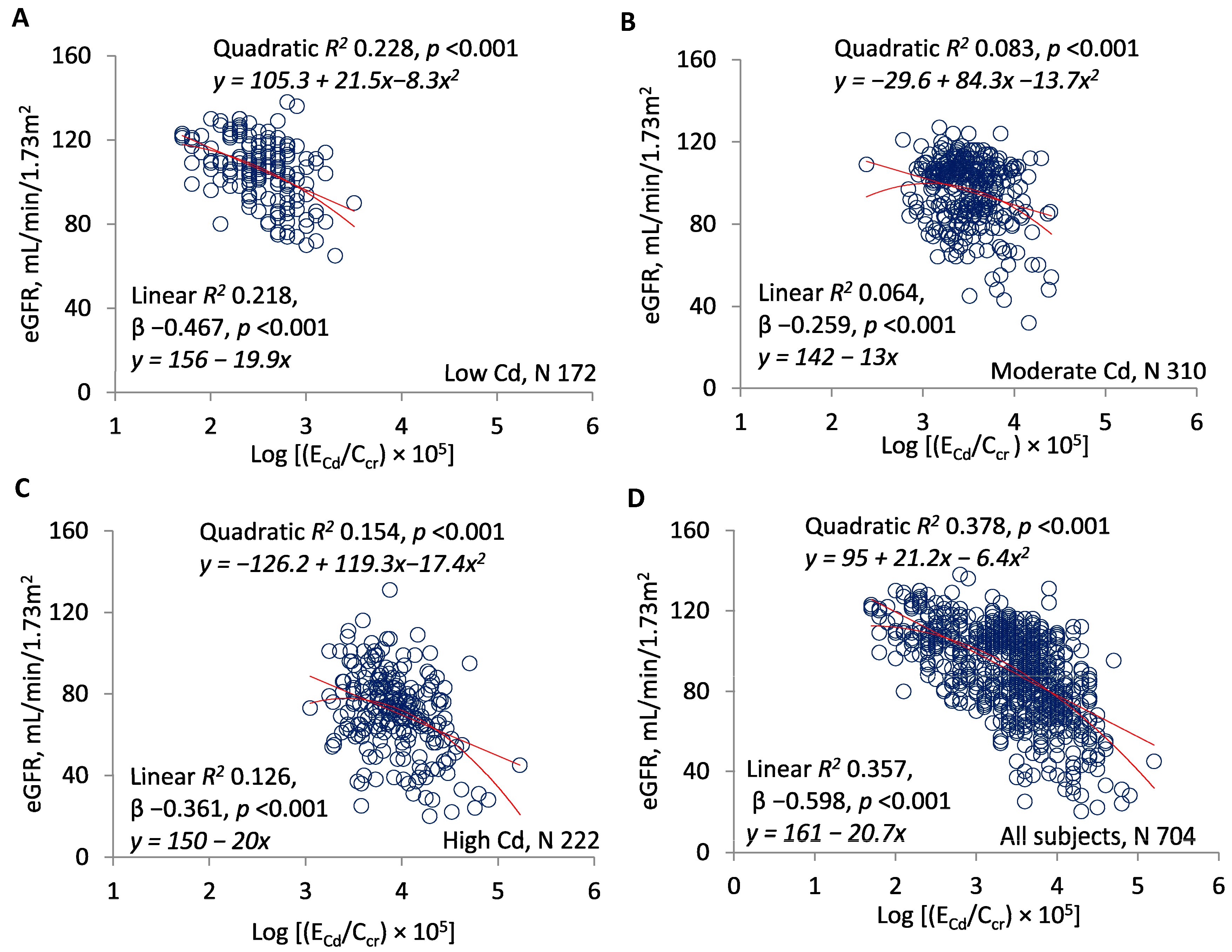
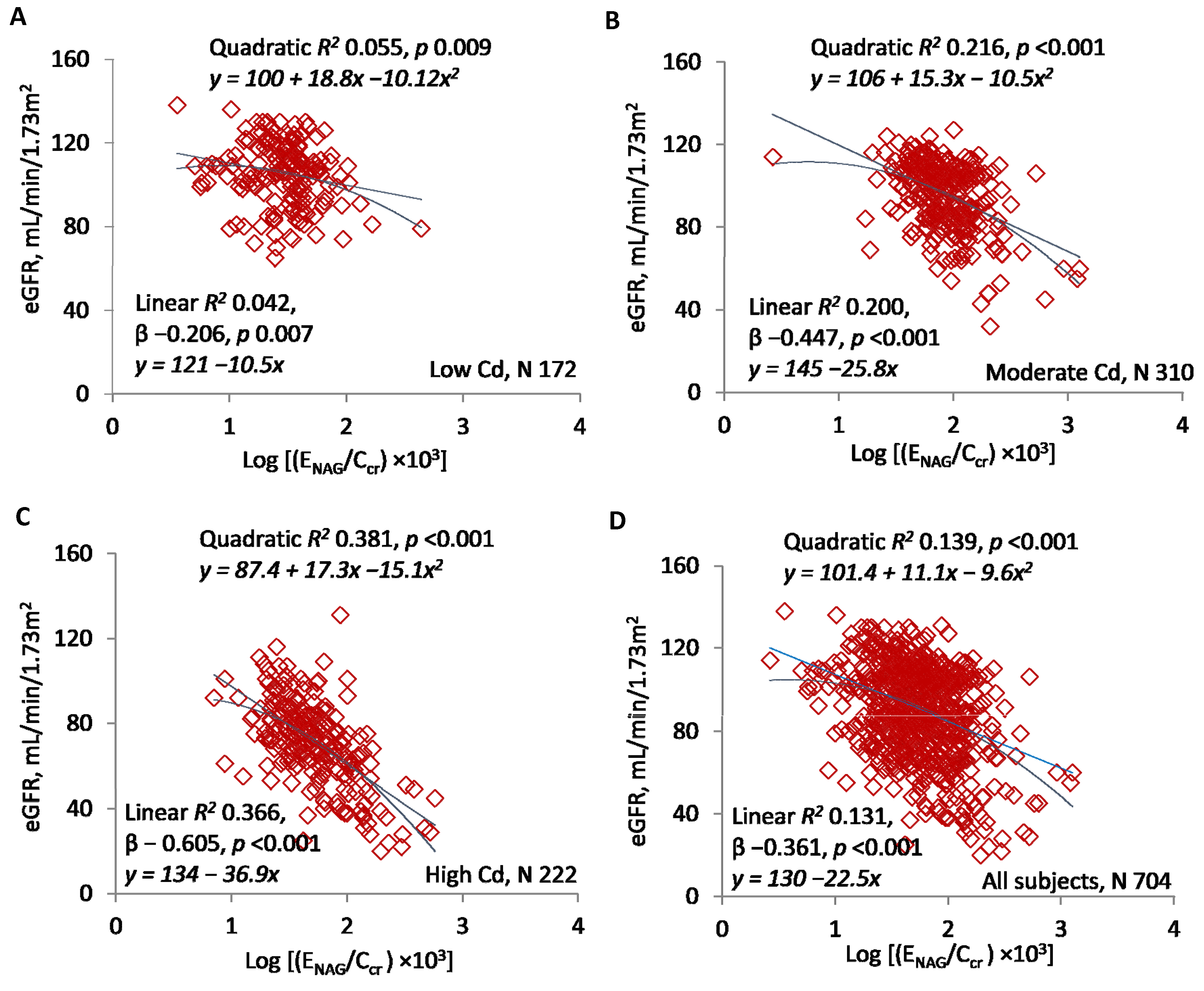
| Excretion Rates of Cd or NAG | Number of Subjects | eGFR vs. log[(ECd/Ccr) × 105] or log[(ENAG/Ccr) × 103] | |||
|---|---|---|---|---|---|
| β Coefficients | R2 | p Value | |||
| Slope (Unstandardized β) ± SE | Standardized β | ||||
| Log[(ECd/Ccr) × 105] | |||||
| <3 | 168 | −17.62 ± 3.20 | −0.392 | 0.154 | <0.001 |
| ≥3 | 536 | −27.71 ± 2.06 | −0.503 | 0.253 | <0.001 |
| All subjects | 704 | −20.86 ± 1.06 | −0.598 | 0.357 | <0.001 |
| Log[(ENAG/Ccr) × 103] | |||||
| <1.5 | 153 | −15.91 ± 7.62 | −0.168 | 0.028 | 0.038 |
| ≥1.5 | 551 | −28.35 ± 3.35 | −0.339 | 0.115 | <0.001 |
| All subjects | 704 | −22.49 ± 2.19 | −0.361 | 0.131 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics 2019, 7, 55. https://doi.org/10.3390/toxics7040055
Satarug S, Vesey DA, Ruangyuttikarn W, Nishijo M, Gobe GC, Phelps KR. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics. 2019; 7(4):55. https://doi.org/10.3390/toxics7040055
Chicago/Turabian StyleSatarug, Soisungwan, David A. Vesey, Werawan Ruangyuttikarn, Muneko Nishijo, Glenda C. Gobe, and Kenneth R. Phelps. 2019. "The Source and Pathophysiologic Significance of Excreted Cadmium" Toxics 7, no. 4: 55. https://doi.org/10.3390/toxics7040055
APA StyleSatarug, S., Vesey, D. A., Ruangyuttikarn, W., Nishijo, M., Gobe, G. C., & Phelps, K. R. (2019). The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics, 7(4), 55. https://doi.org/10.3390/toxics7040055








