Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl3 via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Study Design
2.4. Morris Water Maze (MWM) Test
2.5. Sample Collection and Preparation
2.6. Preparation of Brain Homogenate
2.7. Protein Estimation
2.8. Enzyme Linked Immunosorbent Assay (ELISA)
2.8.1. AChE, P-tau, and SOD
2.8.2. MDA
2.9. Transmission Electron Microscopy (TEM)
2.9.1. Tissue Preparation and Qualitative Analysis
2.9.2. Mitochondrial Abnormalities
2.9.3. Abnormalities of the Nucleus
2.9.4. Synaptic Abnormalities
2.10. Statistical Analysis
3. Results
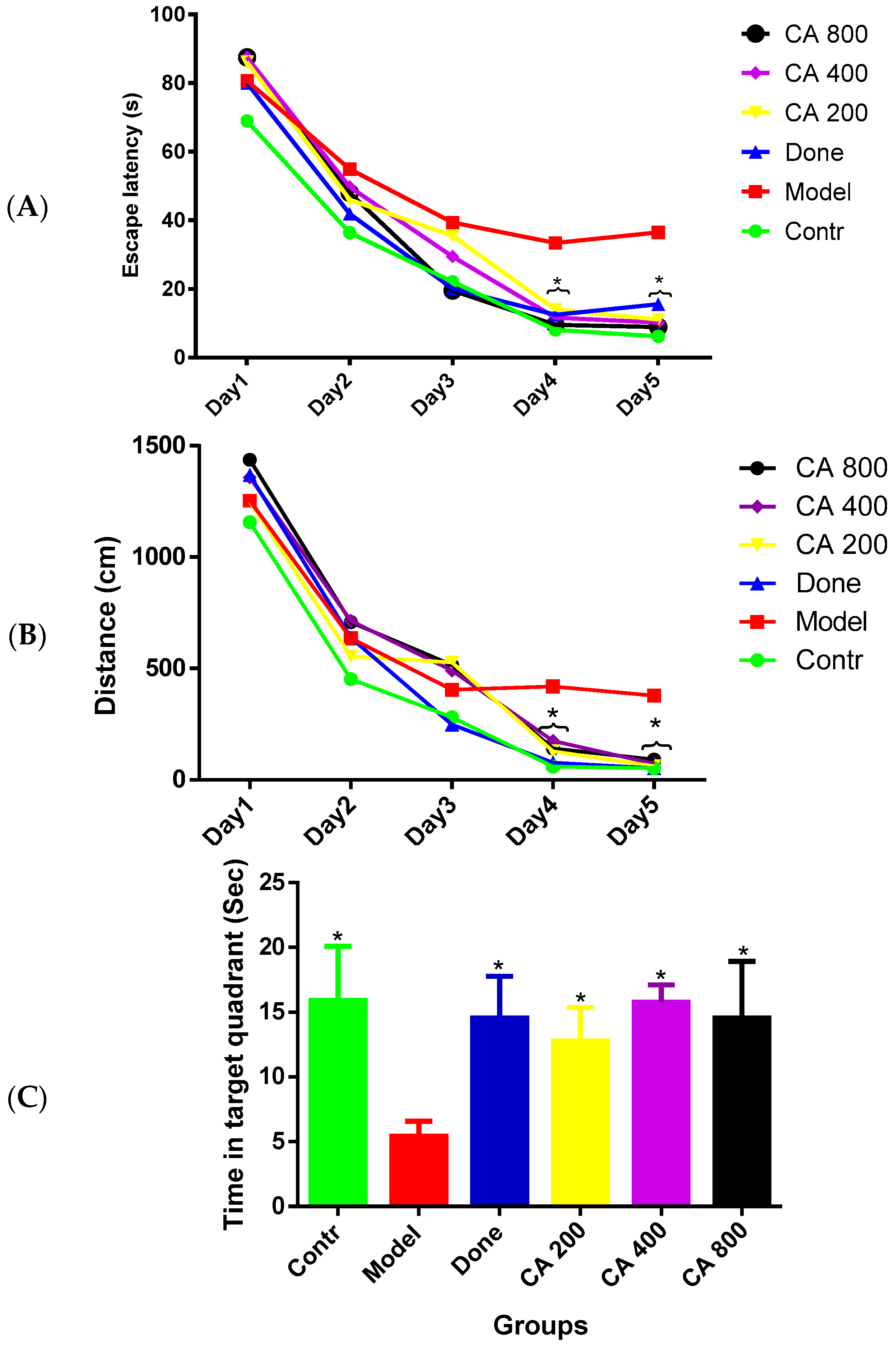
3.1. Protective Effects of CA on Cognitive Functions
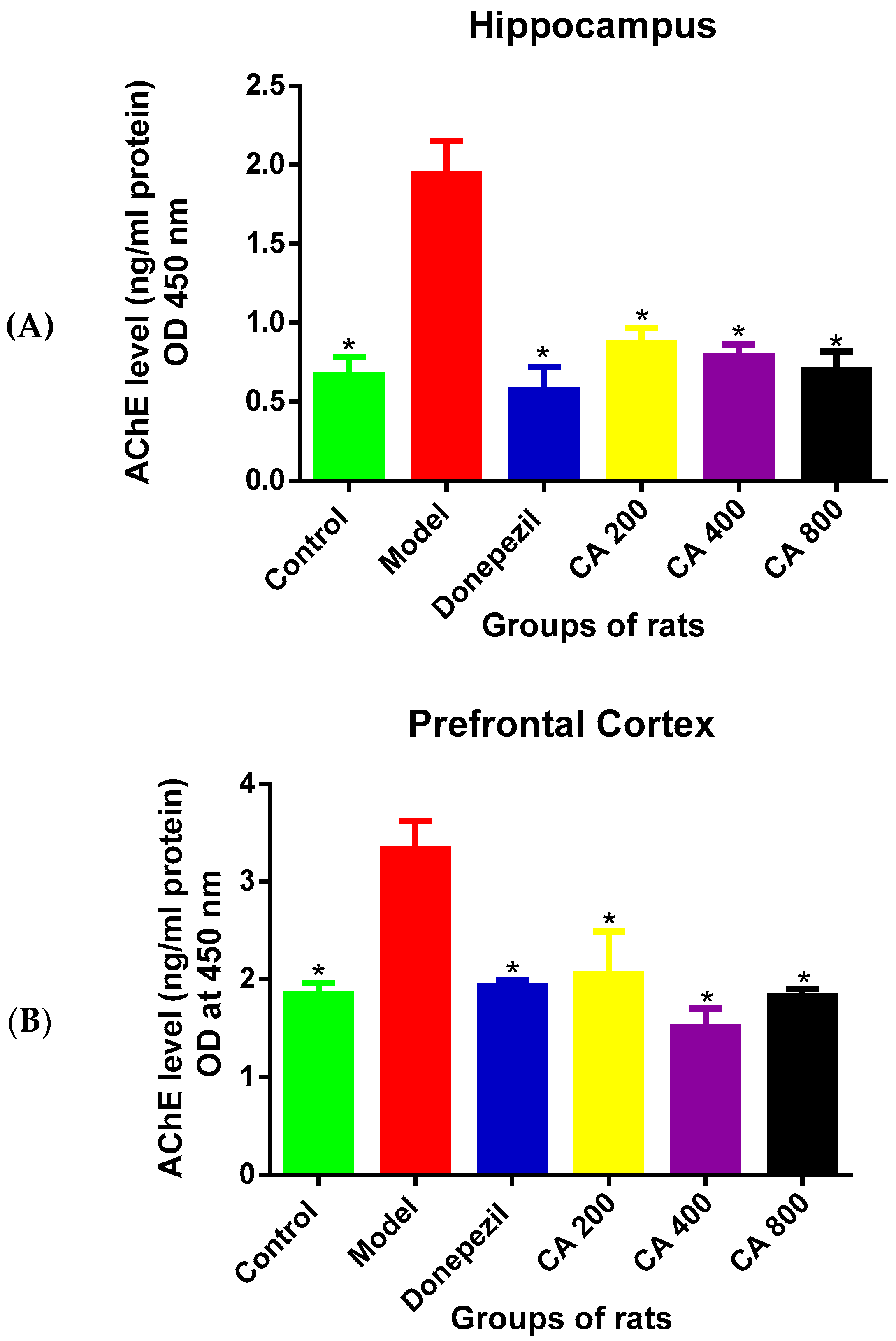
3.2. Effects of CA on AChE Level
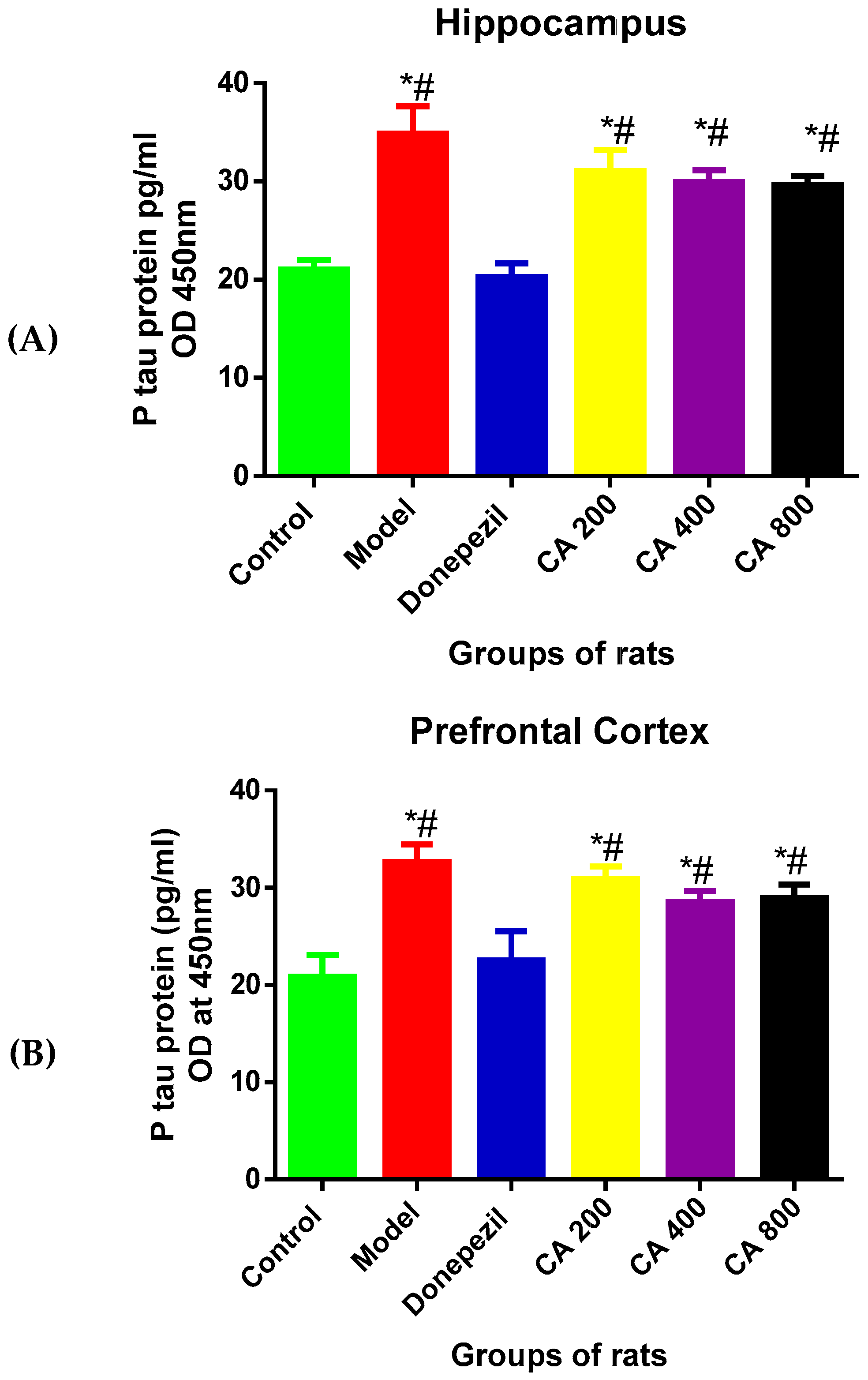
3.3. Effects of CA on P-Tau Level
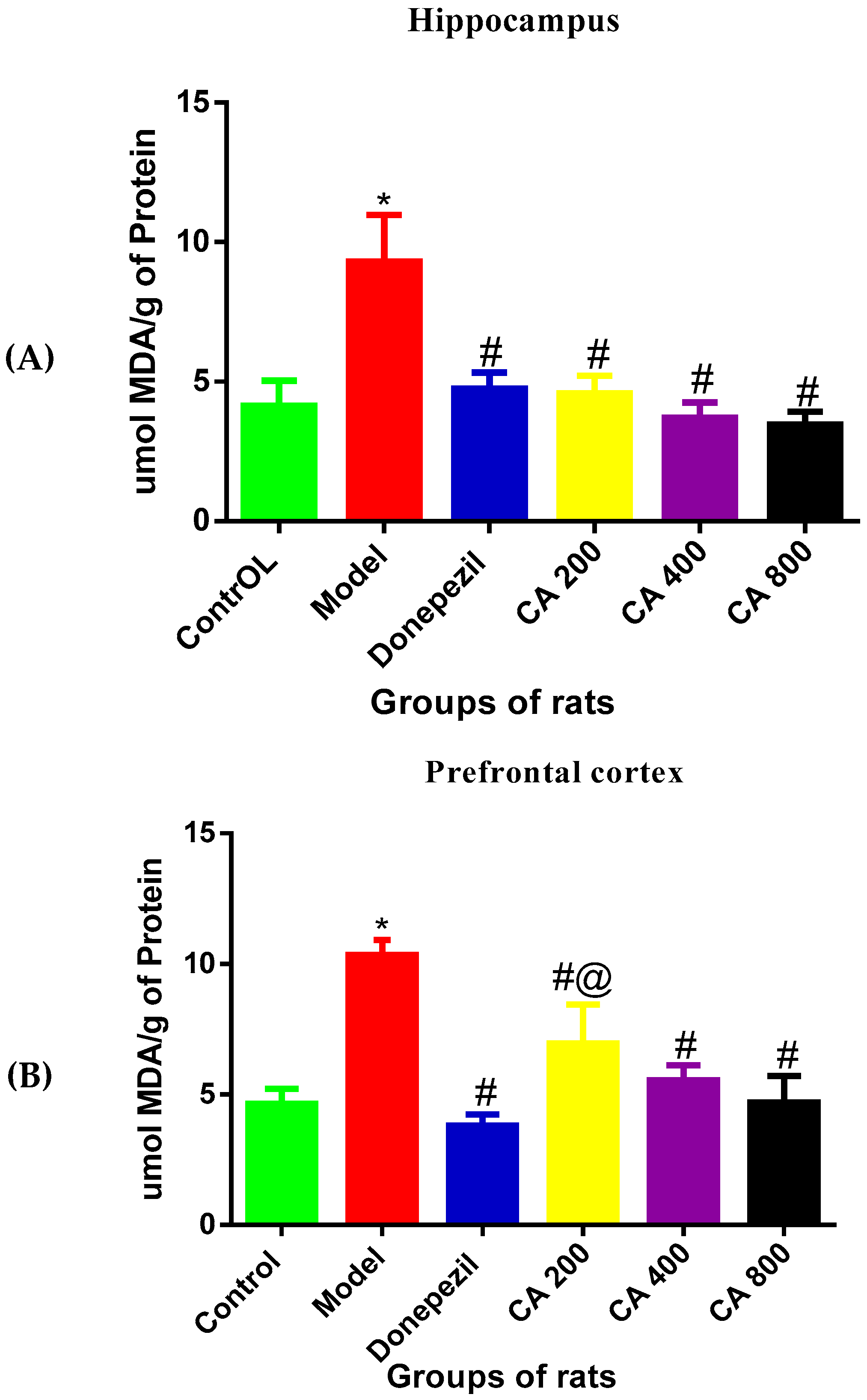
3.4. Effects of CA on MDA Level
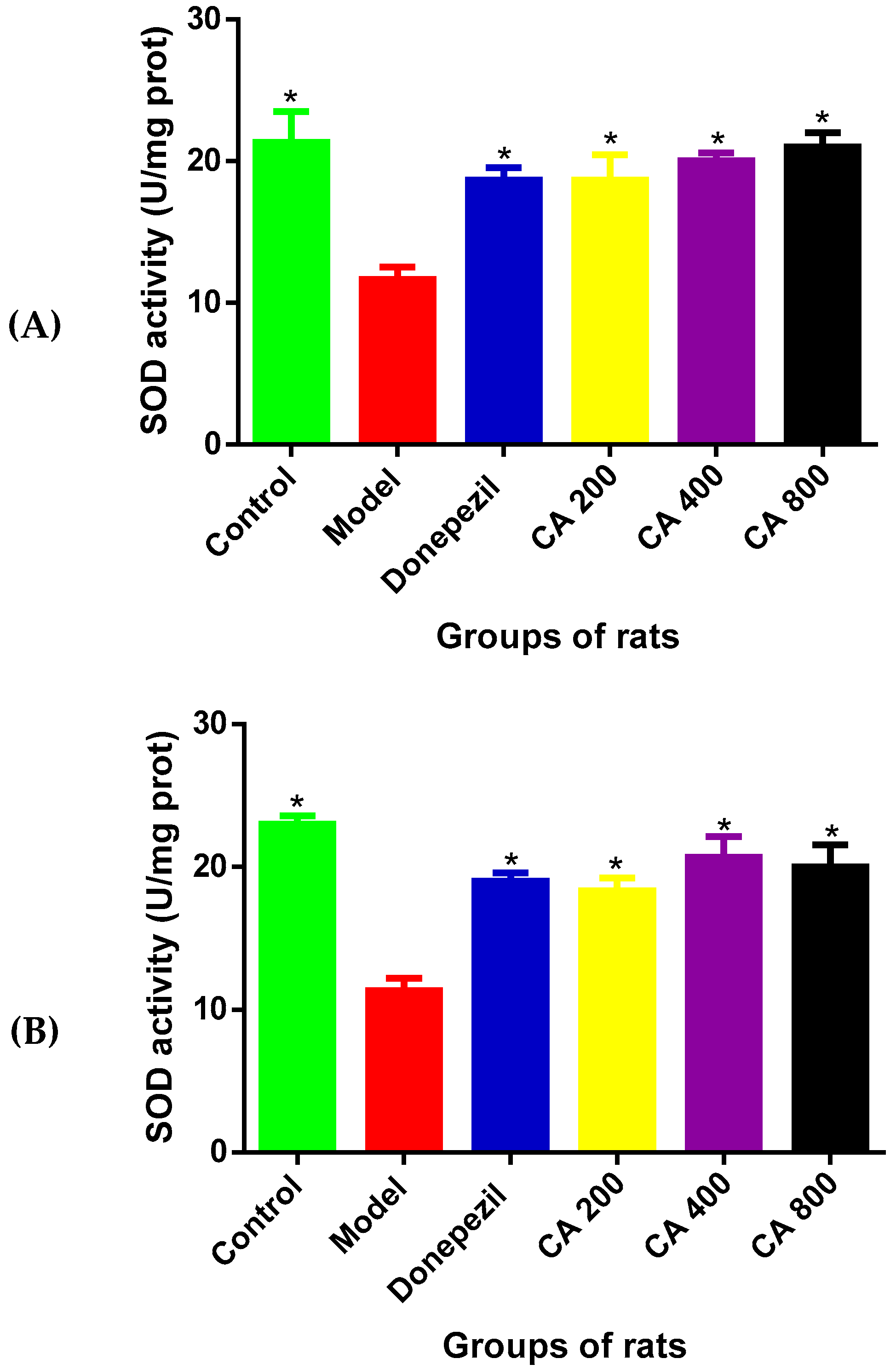
3.5. The Effects of CA on SOD Activities
3.6. Ultrastructural Changes
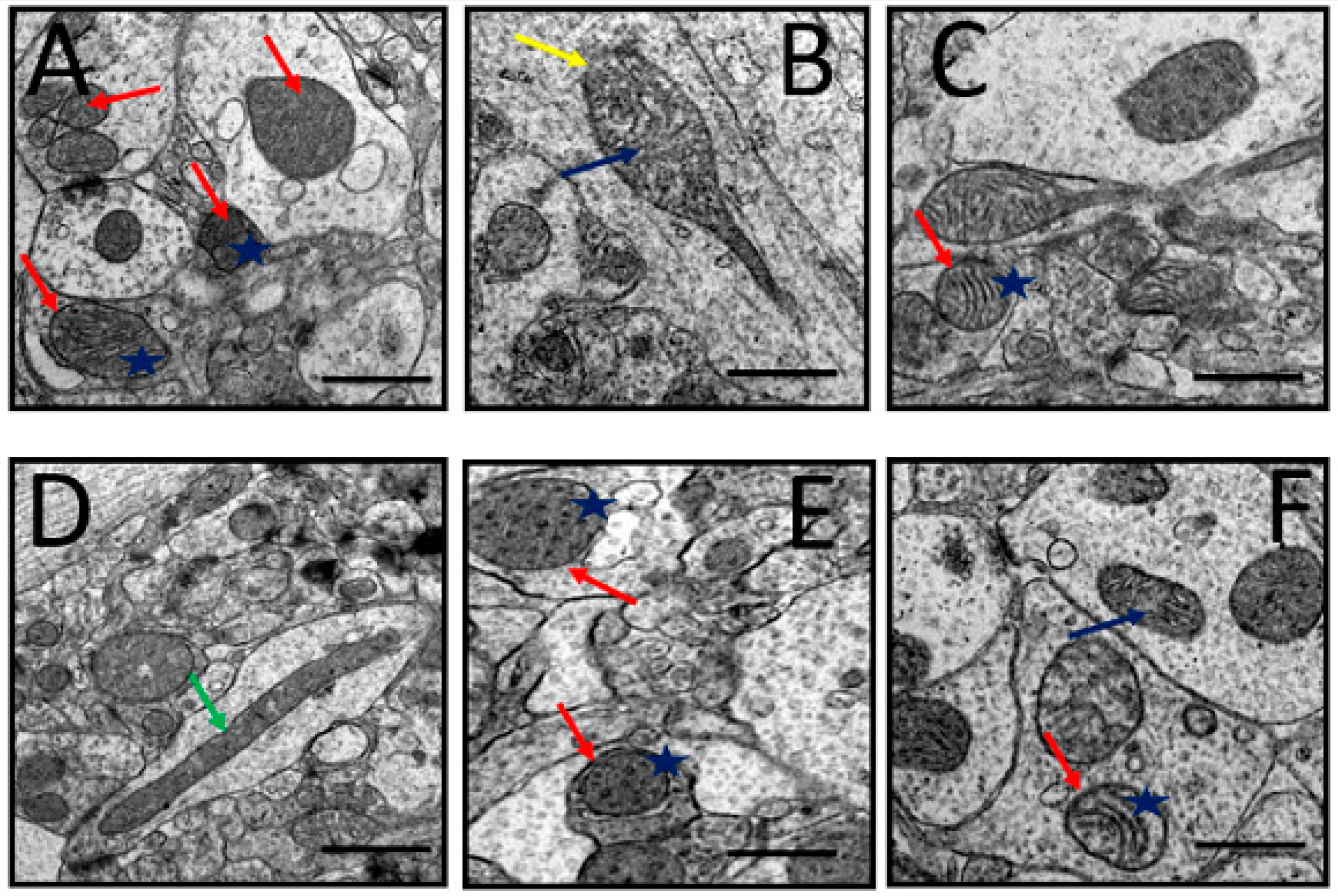
3.6.1. Mitochondria
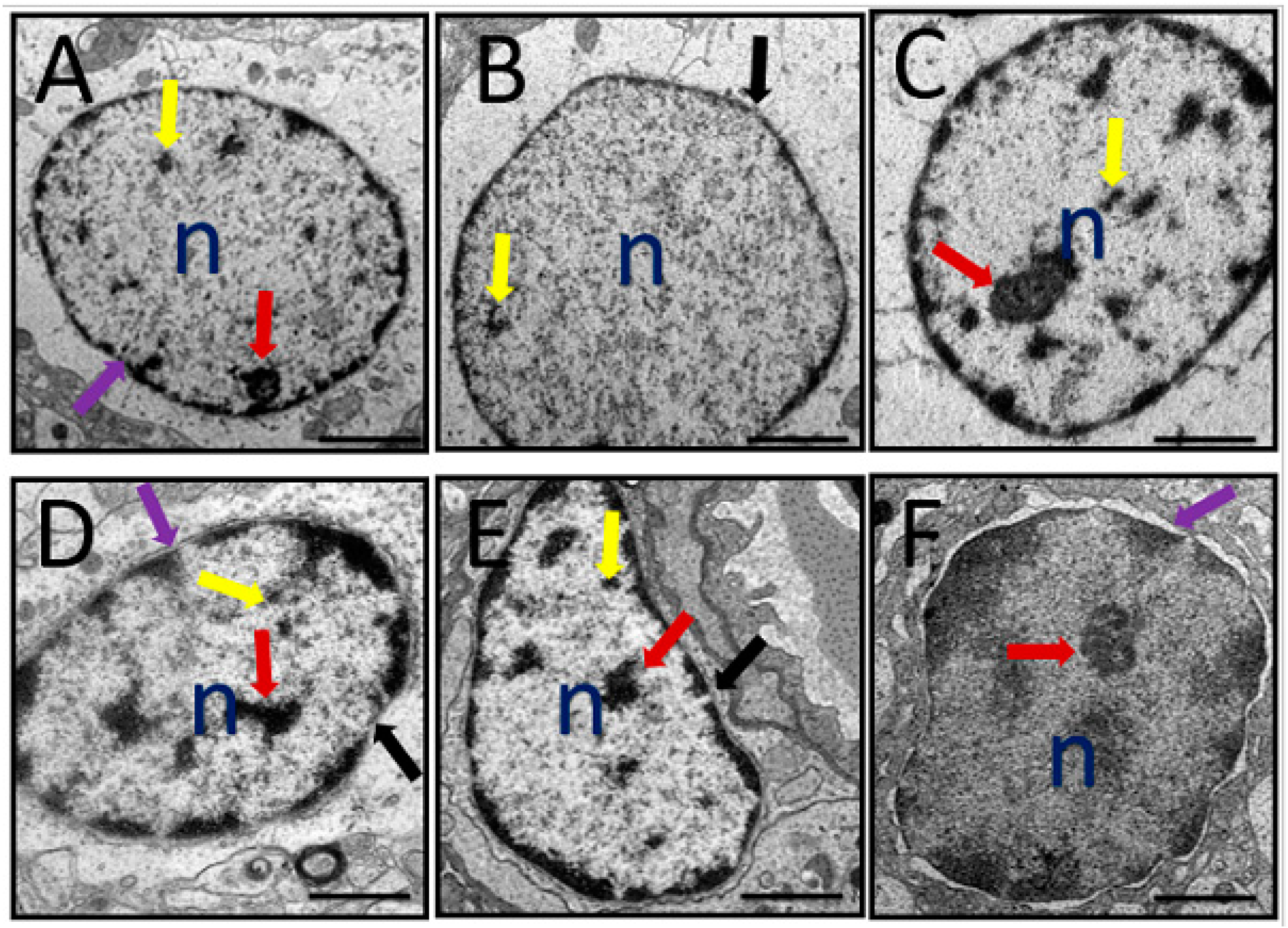
3.6.2. Nucleus
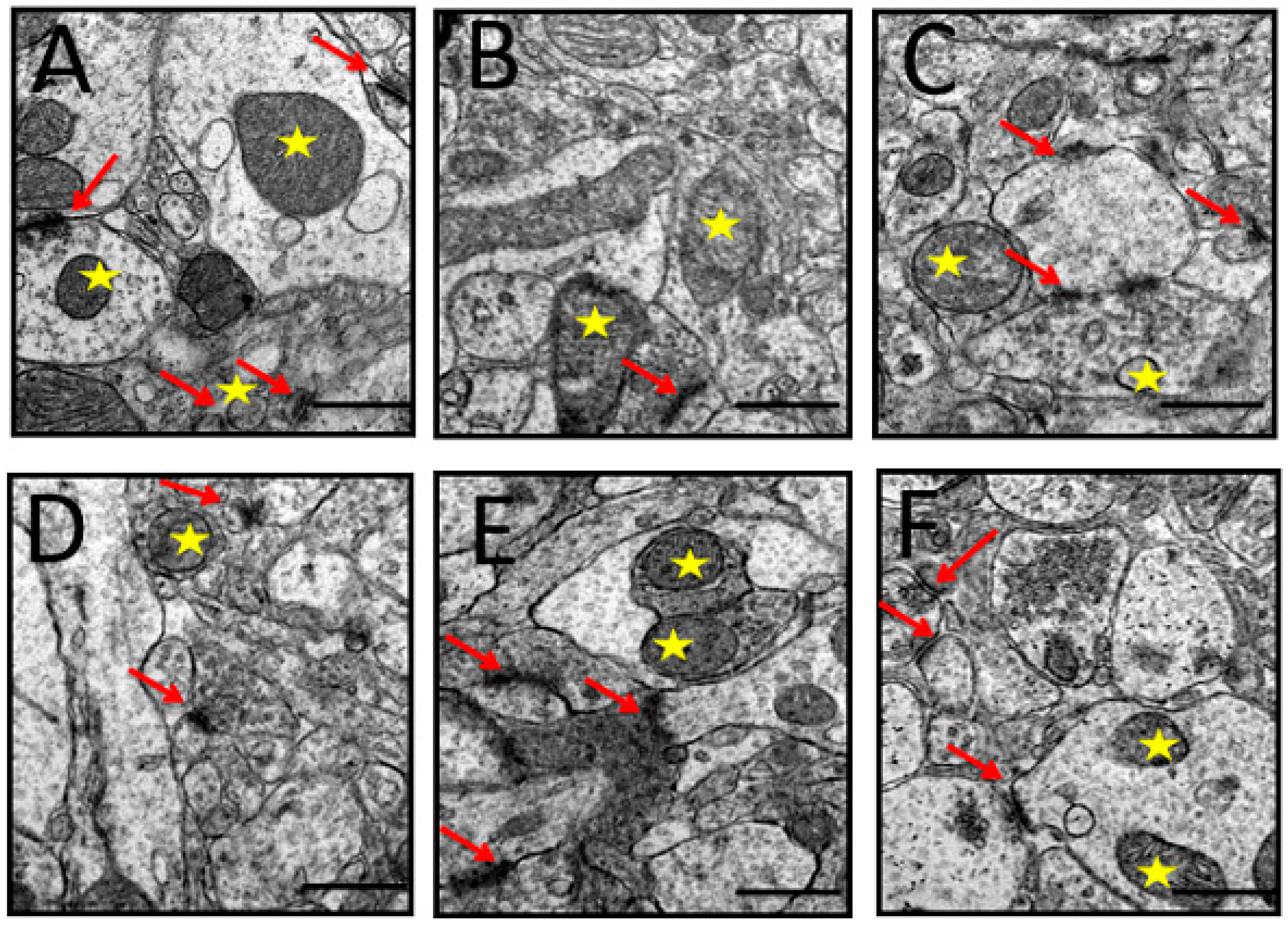
3.6.3. Synapses
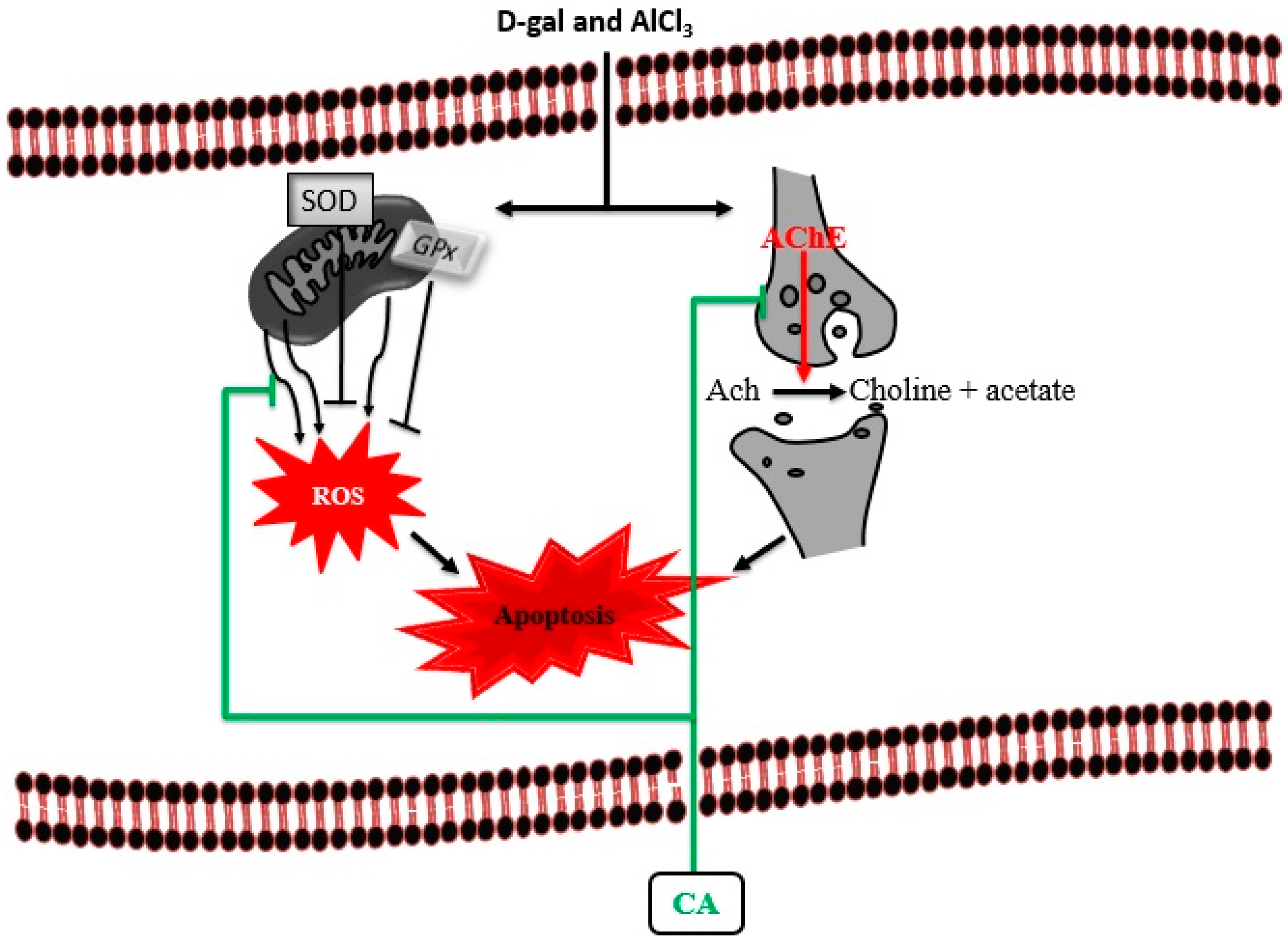
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atukeren, P.; Cengiz, M.; Yavuzer, H.; Gelisgen, R.; Altunoglu, E.; Oner, S.; Erdenen, F.; Yuceakın, D.; Derici, H.; Cakatay, U.; et al. The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer’s disease. Biomed. Pharmacother. 2017, 90, 786–795. [Google Scholar] [CrossRef]
- Muñoz-Torrero, D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors stabilize Alzheimer disease. Neurochem. Res. 2000, 25, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Iacobazzi, R.M.; Catto, M.; Rullo, M.; Farina, R.; Denora, N.; Cellamare, S.; Altomare, C.D. Investigating alkyl nitrates as nitric oxide releasing precursors of multitarget acetylcholinesterase-monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2019, 161, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Reddy, A.P.; Reddy, P.H. Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer’s disease. Drug Discov. Today 2018, 24, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.; Pottier, C.; Nicolas, G.; Le Guennec, K.; Rovelet-Lecrux, A. Alzheimer disease: Modeling an Aβ-centered biological network. Mol. Psychiatry 2016, 21, 861. [Google Scholar] [CrossRef]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49. [Google Scholar] [CrossRef]
- Puri, R.; Suzuki, T.; Yamakawa, K.; Ganesh, S. Hyperphosphorylation and aggregation of Tau in laforin-deficient mice, an animal model for Lafora disease. J. Biol. Chem. 2009, 284, 22657–22663. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Lei, Y.; Fu, W.; Chen, J.; Xiong, C.; Wu, G.; Wei, H.; Ruan, J. Neuroprotective effects of Abacopterin e from Abacopteris penangiana against oxidative stress-induced neurotoxicity. J. Ethnopharmacol. 2011, 134, 275–280. [Google Scholar] [CrossRef]
- Yang, W.N.; Han, H.; Hu, X.D.; Feng, G.F.; Qian, Y.H. The effects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol. Biochem. Behav. 2013, 114, 31–36. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, R.; You, X.; Luo, F.; He, H.; Chang, X.; Zhu, L.; Ding, X.; Yan, T. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer’s disease via SIRT1/NF-κB pathway. Metab. Brain Dis. 2016, 31, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, R.R.; Corrêa, M.C.; Gris, L.R.S.; Da Rosa, C.S.; Bohrer, D.; Morsch, V.M.; Schetinger, M.R.C. Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem. Res. 2008, 33, 2294–2301. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef]
- Li, H.; Kang, T.; Qi, B.; Kong, L.; Jiao, Y.; Cao, Y.; Zhang, J.; Yang, J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 179, 162–169. [Google Scholar] [CrossRef]
- Chiroma, S.M.; Mohd Moklas, M.A.; Mat Taib, C.N.; Baharuldin, M.T.H.; Amon, Z. D-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother. 2018, 103, 1602–1608. [Google Scholar] [CrossRef]
- Nepovimova, E.; Korabecny, J.; Dolezal, R.; Babkova, K.; Ondrejicek, A.; Jun, D.; Sepsova, V.; Horova, A.; Hrabinova, M.; Soukup, O. Tacrine–trolox hybrids: A novel class of centrally active, nonhepatotoxic multi-target-directed ligands exerting anticholinesterase and antioxidant activities with low in vivo toxicity. J. Med. Chem. 2015, 58, 8985–9003. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-S.; Lan, J.-S.; Wang, X.-B.; Jiang, N.; Dong, G.; Li, Z.-R.; Wang, K.D.G.; Guo, P.-P.; Kong, L.-Y. Multifunctional tacrine–trolox hybrids for the treatment of Alzheimer’s disease with cholinergic, antioxidant, neuroprotective and hepatoprotective properties. Eur. J. Med. Chem. 2015, 93, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gaijar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Syed Umesalma, S.A. Protective Effect of Centella asiatica against Aluminium-Induced Neurotoxicity in Cerebral Cortex, Striatum, Hypothalamus and Hippocampus of Rat Brain- Histopathological, and Biochemical Approach. J. Mol. Biomark. Diagn. 2015, 06, 1–7. [Google Scholar] [CrossRef]
- Qureshi, M.; Jahan, N.; Muhammad, S.; Mohani, N.; Wazir, A.; Baig, I.A.; Ahmad, M. Evaluation of neuropharmacological, analgesic and anti-inflammatory effects of the extract of Centella asiatica (Gotu kola) in mice. African J. Pharm. Pharmacol. 2015, 9, 995–1001. [Google Scholar]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N.H. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Veerendra Kumar, M.H.; Gupta, Y.K. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J. Ethnopharmacol. 2002, 79, 253–260. [Google Scholar] [CrossRef]
- Chiroma, S.M.; Baharuldin, M.T.H.; Taib, C.N.M.; Amom, Z.; Jagadeesan, S.; Adenan, M.I.; Moklas, M.A.M. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: Behavioral and ultrastructural approaches. Biomed. Pharmacother. 2019, 109, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Muthuraju, S.; Reza, F.; Senik, M.H.; Zhang, J.; Mohd Yusuf Yeo, N.A.B.; Chuang, H.G.; Jaafar, H.; Yusof, S.R.; Mohamad, H.; et al. Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica. Biomed. Pharmacother. 2019, 110, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Binti Mohd Yusuf Yeo, N.A.; Muthuraju, S.; Wong, J.H.; Mohammed, F.R.; Senik, M.H.; Zhang, J.; Yusof, S.R.; Jaafar, H.; Adenan, M.L.; Mohamad, H.; et al. Hippocampal amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid GluA1 (AMPA GluA1) receptor subunit involves in learning and memory improvement following treatment with Centella asiatica extract in adolescent rats. Brain Behav. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Devkota, A.; Jha, P.K. Variation in growth of Centella asiatica along different soil composition. Bot. Res. Int. 2009, 2, 55–60. [Google Scholar]
- Kabir, A.U.; Samad, M.B.; D’Costa, N.M.; Akhter, F.; Ahmed, A.; Hannan, J.M.A. Anti-hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Complement. Altern. Med. 2014, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Masola, B.; Oguntibeju, O.O.; Oyenihi, A.B. Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed. Pharmacother. 2018, 101, 447–457. [Google Scholar] [CrossRef]
- van Rijn, C.M.; Krijnen, H.; Menting-Hermeling, S.; Coenen, A.M.L. Decapitation in Rats: Latency to Unconsciousness and the ‘Wave of Death’. PLoS ONE 2011, 6, e16514. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.M. Spatial localisation does not depend on the presence of local cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Tucker, L.B.; Velosky, A.G.; McCabe, J.T. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci. Biobehav. Rev. 2018, 88, 187–200. [Google Scholar] [CrossRef]
- Gao, J.; Yao, H.; Pan, X.D.; Xie, A.M.; Zhang, L.; Song, J.H.; Ma, A.J.; Liu, Z.C. Alteration of mitochondrial function and ultrastructure in the hippocampus of pilocarpine-treated rat. Epilepsy Res. 2014, 108, 162–170. [Google Scholar] [CrossRef]
- Castejón, O.J.; De Castejón, H.V. Structural patterns of injured mitochondria in human oedematous cerebral cortex. Brain Inj. 2004, 18, 1107–1126. [Google Scholar] [CrossRef]
- Xiao, F.; Li, X.-G.; Zhang, X.-Y.; Hou, J.-D.; Lin, L.-F.; Gao, Q.; Luo, H.-M. Combined administration of D-galactose and aluminium induces Alzheimerlike lesions in brain. Neurosci. Bull. 2011, 27, 143–155. [Google Scholar] [CrossRef]
- Lei, M.; Hua, X.; Xiao, M.; Ding, J.; Han, Q.; Hu, G. Impairments of astrocytes are involved in the d-galactose-induced brain aging. Biochem. Biophys. Res. Commun. 2008, 369, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ishibashi, K.; Ishibashi, K.; Reiser, K.; Grebe, R.; Biswal, S.; Gehlbach, P.; Handa, J.T. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: A comprehensive transcriptional response. Proc. Natl. Acad. Sci. USA 2005, 102, 11846–11851. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.J.; Frye, C.A. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction 2008, 136, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Dussaubat, N.; Díaz-Véliz, G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 1996, 21, 609–620. [Google Scholar] [CrossRef]
- Lovick, T.A. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: Implications for premenstrual syndrome in women. Exp. Physiol. 2006, 91, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, D.; Zheng, Y.; Li, H.; Hao, C.; Ouyang, W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res. Bull. 2017, 134, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate D-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Kříž, Z.; Kuča, K.; Jun, D.; Koča, J. Acetylcholinesterases–the structural similarities and differences. J. Enzyme Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Mahtaj, A.K. Ameliorative effect of rosmarinic acid on scopolamine-induced memory impairment in rats. Neurosci. Lett. 2015, 585, 23–27. [Google Scholar] [CrossRef]
- Mathiyazahan, D.B.; Justin Thenmozhi, A.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Dunnett, S.B.; Everitt, B.J.; Robbins, T.W. The basal forebrain-cortical cholinergic system: Interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991, 14, 494–501. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, J.; Yang, H.; Huo, L.; Gao, J.; Chen, H.; Gao, W. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiol. Behav. 2016, 154, 114–125. [Google Scholar] [CrossRef]
- Arora, R.; Kumar, R.; Agarwal, A.; Reeta, K.H.; Gupta, Y.K. Comparison of three different extracts of Centella asiatica for anti-amnesic, antioxidant and anticholinergic activities: In vitro and in vivo study. Biomed. Pharmacother. 2018, 105, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Tota, S.; Kamat, P.K.; Saxena, G.; Hanif, K.; Najmi, A.K.; Nath, C. Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav. Brain Res. 2012, 226, 317–330. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Antioxidant treatment in Alzheimer’s disease: Current state. J. Mol. Neurosci. 2003, 21, 1–11. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Perkins, G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim. Biophys. Acta-Bioenerg. 2009, 1787, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Opazo, C.; Larrondo, L.F.; Muñoz, F.J.; Ruiz, F.; Leighton, F.; Inestrosa, N.C. The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648. [Google Scholar] [CrossRef]
- Mattson, M.P.; Duan, W. “Apoptotic” biochemical cascades in synaptic compartments: Roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res. 1999, 58, 152–166. [Google Scholar] [CrossRef]
- Swomley, A.M.; Butterfield, D.A. Oxidative stress in Alzheimer disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015, 89, 1669–1680. [Google Scholar] [CrossRef]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 45, 1594–1601. [Google Scholar] [CrossRef]
- Gray, N.E.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016, 180, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Zweig, J.A.; Matthews, D.G.; Caruso, M.; Quinn, J.F.; Soumyanath, A. Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Aβ-Exposed Hippocampal Neurons. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Dogra, S. Centella asiatica Attenuates D-Galactose-Induced Cognitive Impairment, Oxidative and Mitochondrial Dysfunction in Mice. Int. J. Alzheimers Dis. 2011, 2011. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrong, P.; Tantisira, M.H. Acute and sub-chronic toxicity studies of a standardized extract of Centella asiatica ECa 233. Thai J. Pharm. Sci. 2011, 35, 55–64. [Google Scholar]
- Deshpande, P.O.; Mohan, V.; Thakurdesai, P. Preclinical Safety Assessment of Standardized Extract of Centella asiatica (L.) Urban Leaves. Toxicol. Int. 2015, 22, 10–20. [Google Scholar]
- Gadahad, M.R.K.; Rao, M.; Rao, G. Enhancement of hippocampal CA3 neuronal dendritic arborization by Centella asiatica (Linn) fresh leaf extract treatment in adult rats. J. Chin. Med. Assoc. 2008, 71, 6–13. [Google Scholar] [CrossRef]
- Soumyanath, A.; Zhong, Y.-P.; Henson, E.; Wadsworth, T.; Bishop, J.; Gold, B.G.; Quinn, J.F. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int. J. Alzheimers Dis. 2012, 2012. [Google Scholar] [CrossRef]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice. Mol. Cell. Neurosci. 2018, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Groups | Description | Treatment i.p | Treatment p.o |
|---|---|---|---|
| I | Control | Saline | Distilled water |
| II | Model | D-gal 60 mg/kg.bwt | AlCl3 200 mg/kg.bwt |
| III | Donepezil | D-gal 60 mg/kg.bwt | AlCl3 200 mg/kg.bw + Done 1 mg/kg.bwt |
| IV | CA 200 | D-gal 60 mg/kg.bwt | AlCl3 200 mg/kg.bw + CA 200 mg/kg.bwt |
| V | CA 400 | D-gal 60 mg/kg.bwt | AlCl3 200 mg/kg.bw + CA 400 mg/kg.bwt |
| VI | CA 800 | D-gal 60 mg/kg.bwt | AlCl3 200 mg/kg.bw + CA 800 mg/kg.bwt |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiroma, S.M.; Baharuldin, M.T.H.; Mat Taib, C.N.; Amom, Z.; Jagadeesan, S.; Ilham Adenan, M.; Mahdi, O.; Moklas, M.A.M. Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl3 via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level. Toxics 2019, 7, 19. https://doi.org/10.3390/toxics7020019
Chiroma SM, Baharuldin MTH, Mat Taib CN, Amom Z, Jagadeesan S, Ilham Adenan M, Mahdi O, Moklas MAM. Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl3 via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level. Toxics. 2019; 7(2):19. https://doi.org/10.3390/toxics7020019
Chicago/Turabian StyleChiroma, Samaila Musa, Mohamad Taufik Hidayat Baharuldin, Che Norma Mat Taib, Zulkhairi Amom, Saravanan Jagadeesan, Mohd Ilham Adenan, Onesimus Mahdi, and Mohamad Aris Mohd Moklas. 2019. "Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl3 via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level" Toxics 7, no. 2: 19. https://doi.org/10.3390/toxics7020019
APA StyleChiroma, S. M., Baharuldin, M. T. H., Mat Taib, C. N., Amom, Z., Jagadeesan, S., Ilham Adenan, M., Mahdi, O., & Moklas, M. A. M. (2019). Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl3 via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level. Toxics, 7(2), 19. https://doi.org/10.3390/toxics7020019





