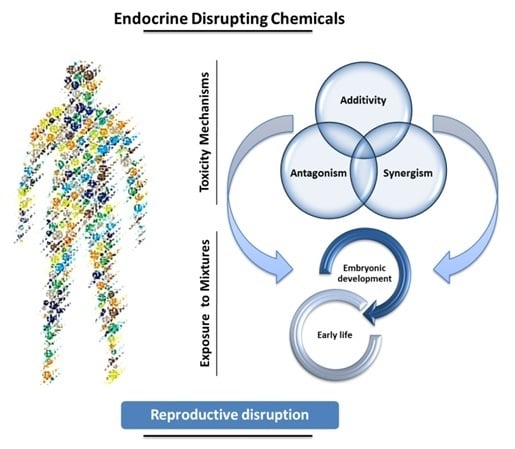
EDCs Mixtures: A Stealthy Hazard for Human Health?
Abstract
:1. Introduction
2. EDCs State of the Art
- −
- EDCs with bioaccumulation ability (e.g., polychlorinated biphenyls -PCBs-, polybrominated flame retardants, perfluorinated chemicals);
- −
- Compounds utilized in food production (e.g., pesticides);
- −
- Chemicals present in food due to contact materials, processing aids, etc. (e.g., BPA); and
- −
- Endocrine-active substances naturally present in food (e.g., genistein).
3. Materials and Methods
4. EDCs Mixtures Toxicity Mechanisms
5. Critical Windows of Exposure
5.1. Embryonic Development
5.2. Early Life
6. Male Vulnerability to EDCs
7. In Vitro Mixtures Effects
8. Discussion
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Toppari, J. Showcase for endocrine disruption. Mol. Cell. Endocrinol. 2012, 355, 191. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Nagel, S.C.; Vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, S56–S69. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Martin, O.V.; Faust, M.; Kortenkamp, A. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci. Total Environ. 2016, 543, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Faust, M.; Scholze, M.; Backhaus, T. Low-level exposure to multiple chemicals: Reason for human health concerns? Environ. Health Perspect. 2007, 115, 106–114. [Google Scholar] [CrossRef]
- Kortenkamp, A. Low dose mixture effects of endocrine disrupters: Implications for risk assessment and epidemiology. Int. J. Androl. 2008, 31, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef] [PubMed]
- International Programme on Chemical Safety (IPCS). Global Assessment of the State-of-the-Science of Endocrine Disruptors; WHO, 2002. Available online: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ (accessed on 10 December 2016).
- Shanle, E.K.; Xu, W. Endocrine Disrupting Chemicals Targeting Estrogen Receptor Signaling: Identification and Mechanisms of Action. Chem. Res. Toxiciol. 2011, 24, 6–19. [Google Scholar] [CrossRef]
- World Health Organization (WHO). State of the Science of Endocrine Disrupting Chemicals 2012. An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme (UNEP) and WHO (2013). Available online: http://www.who.int/ceh/publications/endocrine/en/ (accessed on 10 December 2016).
- Webster, T.F. Mixtures of endocrine disruptors: How similar must mechanisms be for concentration addition to apply? Toxicology 2013, 314, 129–133. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Endocrine Disrupters and the Safety of Food Chains. Horm. Res. Paediatr. 2016, 86, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Tetsu Akiyama, H.O. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991, 201, 362–370. [Google Scholar]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [PubMed]
- Nagaraju, G.P.; Zafar, S.F.; El-Rayes, B.F. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr. Rev. 2013, 71, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Andersen, Z.J.; Frederiksen, M.; Specht, I.O.; Hougaard, K.S.; Ebbehøj, N.; Bailey, J.; Giwercman, A.; Steenland, K.; Longnecker, M.P.; et al. Health Effects of PCBs in Residences and Schools (HESPERUS): PCB—Health Cohort Profile. Sci. Rep. 2016, 6, 24571. [Google Scholar] [CrossRef] [PubMed]
- Gaum, P.M.; Lang, J.; Esser, A.; Schettgen, T.; Neulen, J.; Kraus, T.; Gube, M. Exposure to polychlorinated biphenyls and the thyroid gland - examining and discussing possible longitudinal health effects in humans. Environ. Res. 2016, 148, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Welshons, W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol. Cell. Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Państw. Zakł. Hig. 2015, 66, 5–11. [Google Scholar] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Roosens, L.; Neels, H.; Covaci, A. Assessment of human exposure to Bisphenol-A, Triclosan and Tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 2009, 76, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Zoeller, R.T.; Vom Saal, F.S. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ. Health Perspect. 2009, 117, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Kjærstad, M.B.; Taxvig, C.; Andersen, H.R.; Nellemann, C. Mixture effects of endocrine disrupting compounds in vitro. Int. J. Androl. 2010, 33, 425–433. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC). Technical Report no.115: Effect of Chemicals Co-Exposures at Doses Relevant for Human Safety Assessment; ECETOC: Brussels, Belgium, 2012; Available online: http://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-115-Effects-of-chemical-co-exposures-at-doses-relevant-for-human-safety-assessments.pdf (accessed on 10 December 2016).
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jørgensen, N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’ s Second Scientific Statement on Endocrine-disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Cassee, F.R.; Groten, J.P.; van Vliet, P.W.; van Zorge, J.A. International issues on human health effects of exposure to chemical mixtures. Environ. Health Perspect. 2002, 110, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.F.; Christoph, G.R.; Julien, E.; Kelley, J.M.; Kronenberg, J.; McCarthy, J.; Reiss, R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: How to cumulate? Regul. Toxicol. Pharmacol. 2000, 31, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.S.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol. Appl. Pharmacol. 2013, 272, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Fischer, B.; Navarrete Santos, A. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol a, diethylhexylphthalate, and tributyltin. Obes. Facts 2014, 7, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, H.; Shen, Y.; Qiu, W.; Yang, M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011, 30, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Buechse, A.; Dammann, M.; Melching-Kollmuß, S.; Woitkowiak, C.; van Ravenzwaay, B. Effect of estrogenic binary mixtures in the yeast estrogen screen (YES). Regul. Toxicol. Pharmacol. 2014, 70, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Katchy, A.; Pinto, C.; Jonsson, P.; Nguyen-vu, T.; Pandelova, M.; Riu, A.; Schramm, K.W.; Samarov, D.; Gustafsson, J.Å.; Bondesson, M.; et al. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol. Sci. 2014, 138, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Kang, N.H.; Yi, B.R.; Lee, H.R.; Park, M.A.; Choi, K.C. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17β-estradiol or bisphenol A via the inhibition of cell cycle progression. Int. J. Oncol. 2013, 42, 733–740. [Google Scholar] [PubMed]
- Hugo, E.R.; Brandebourg, T.D.; Woo, J.G.; Loftus, J.; Alexander, J.W.; Ben-Jonathan, N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008, 116, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcikova, A.; Macho, L.; Fikova, M. Bisphenol a alone or in combination with estradiol modulates cell cycle- and apoptosis-related proteins and genes in mcf7 cells. Endocr. Regul. 2013, 47, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bolli, A.; Bulzomi, P.; Galluzzo, P.; Acconcia, F.; Marino, M. Bisphenol a impairs estradiol-induced protective effects against DLD-1 colon cancer cell growth. IUBMB Life 2010, 62, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Zare, A.; Jackson, L.J.; Habibi, H.R.; Weljie, A.M. Environmental contaminant mixtures at ambient concentrations invoke a metabolic stress response in goldfish not predicted from exposure to individual compounds alone. J. Proteome Res. 2012, 11, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Berntsen, H.F.; Zimmer, K.E.; Frizzell, C.; Verhaegen, S.; Ropstad, E.; Connolly, L. Effects of defined mixtures of persistent organic pollutants (POPs) on multiple cellular responses in the human hepatocarcinoma cell line, HepG2, using high content analysis screening. Toxicol. Appl. Pharmacol. 2016, 294, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Peng, T.; Liu, F.; Ren, L.; Peng, Z.; Ji, G.; Zhou, Y.; Fu, Z. Transcriptional responses in male Japanese medaka exposed to antiandrogens and antiandrogen/androgen mixtures. Environ. Toxicol. 2016, 31, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Jansson, I.; Schenkman, J.B.; Sarfarazi, M.; Stoilov, I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch. Biochem. Biophys. 2003, 414, 91–100. [Google Scholar] [CrossRef]
- De Wildt, S.N.; Kearns, G.L.; Leeder, J.S.; van den, A.J. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin. Pharmacokinet. 1999, 36, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.; Fowler, P.A.; Amezaga, M.R.; Rhind, S.M.; Cotinot, C.; Mandon-Pepin, B.; Sharpe, R.M.; Evans, N.P. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ. Health Perspect. 2009, 117, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Bellingham, M.; Sinclair, K.D.; Evans, N.P.; Pocar, P.; Fischer, B.; Schaedlich, K.; Schmidt, J.S.; Amezaga, M.R.; Bhattacharya, S.; et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 2012, 355, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Missmer, S.A.; Altshul, L.; Vitonis, A.F.; Ryan, L.; Cramer, D.W.; Hauser, R. Serum and follicular fluid organochlorine concentrations among women undergoing assisted reproduction technologies. Environ. Health 2009, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederiksen, M.; Vorkamp, K.; Mathiesen, L.; Mose, T.; Knudsen, L.E. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: An experimental study. Environ. Health 2010, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Yunus, M.; Rahman, M.; Graziano, J.H.; et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE 2012, 7, e37147. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Loganath, A.; Chong, Y.S.; Obbard, J.P. Exposure to persistent organic pollutants in utero and related maternal characteristics on birth outcomes: A multivariate data analysis approach. Chemosphere 2009, 74, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol a accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, 703–707. [Google Scholar] [CrossRef]
- Ginsberg, G.; Rice, D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Ritter, L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ. Health Perspect. 2009, 117, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.W.; Twaddle, N.C.; Vanlandingham, M.; Doerge, D.R. Pharmacokinetic modeling: Prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol. Appl. Pharmacol. 2011, 257, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Bustamante, M.; Byun, H.M.; Fernandez, M.F.; Santa Marina, L.; Basterrechea, M.; Ballester, F.; Murcia, M.; Tardón, A.; Fernández-Somoano, A.; et al. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ. Int. 2014, 71, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Bustamante, M.; Morales, E.; Motta, V.; Fernandez, M.F.; Salas, L.A.; Escaramis, G.; Ballester, F.; Murcia, M.; Tardon, A.; et al. Prenatal exposure to mixtures of xenoestrogens and genome-wide DNA methylation in human placenta. Epigenomics 2016, 8, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Yuan, W.; Zhu, G.; He, X.; Li, D.K. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod. Toxicol. 2011, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Yuan, W.; He, Y.; Zhou, Z.; Wang, J.; Gao, E.; Li, G.; Li, D.K. In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. Part A—Clin. Mol. Teratol. 2011, 91, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.K.L.; Jacobsen, P.R.; Hass, U.; Svingen, T.; Vinggaard, A.M.; Isling, L.K.; Axelstad, M.; Christiansen, S.; Boberg, J. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod. Toxicol. 2016, 61, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzio, A.; Monteiro, S.M.; Rocha, E.; Fontaínhas-Fernandes, A.A.; Coimbra, A.M. Development and recovery of histopathological alterations in the gonads of zebrafish (Danio rerio) after single and combined exposure to endocrine disruptors (17α-ethinylestradiol and fadrozole). Aquat. Toxicol. 2016, 175, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Dover, G.J. The Barker hypothesis: How pediatricans will diagnose and prevent common adult-onset diseases. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 199–207. [Google Scholar] [PubMed]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef] [PubMed]
- European food safety authority (EFSA). Scientific opinion on polybrominated diphenyl ethers (pbdes) in food. EFSA J. 2011, 5, 2156. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/2156 (accessed on 10 December 2016). [Google Scholar]
- World Health Organization (WHO). Toxicological and health aspects of bisphenol A. In Proceedings of the Joint FAO/WHO Expert Meeting, Ottawa, ON, Canada, 2–5 November 2010; Available online: http://apps.who.int/iris/bitstream/10665/44624/1/97892141564274_eng.pdf (accessed on 10 December 2016).
- European food safety authority (EFSA). Bisphenol A. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 10 December 2016).
- European Commission. Commission Directive 2011/8/eu of 28 January 2011 Amending Directive 2002/72/ec as Regards the Restriction of Use of Bisphenol a in Plastic Infant Feeding Bottles; European Commission, 2011. Available online: https://www.fsai.ie/uploadedFiles/Dir2011_8.pdf (accessed on 10 December 2016).
- Neal-Kluever, A.; Aungst, J.; Gu, Y.; Hatwell, K.; Muldoon-Jacobs, K.; Liem, A.; Ogungbesan, A.; Shackelford, M. Infant toxicology: State of the science and considerations in evaluation of safety. Food Chem. Toxicol. 2014, 70, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Skakkebaek, N.E.; Hass, U.; Toppari, J.; Juul, A.; Andersson, A.M.; Kortenkamp, A.; Heindel, J.J.; Trasanda, L. Male reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howdeshell, K.L.; Wilson, V.S.; Furr, J.; Lambright, C.R.; Rider, C.V.; Blystone, C.R.; Hotchkiss, A.K.; Gray, L.E., Jr. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008, 105, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.V.; Wilson, V.S.; Howdeshell, K.L.; Hotchkiss, A.K.; Furr, J.R.; Lambright, C.R.; Gray, L.E., Jr. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol. Pathol. 2009, 37, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Crettaz, P.; Oberli-Schrämmli, A.; Fent, K. Antiandrogenic activity of phthalate mixtures: Validity of concentration addition. Toxicol. Appl. Pharmacol. 2012, 259, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Song, W.T.; Wang, Z.J.; Liu, H.C. Effects of individual and binary mixtures of estrogens on male goldfish (Carassius auratus). Fish Physiol. Biochem. 2014, 40, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Conrad, K.; Allen, J.L.; Weston, H.; Martin, K.; Lawrence, B.P.; Cory-Slechta, D.A. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology 2014, 45, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Wu, K.; Wang, Y.; Zhu, H.; Deng, Z.; Peng, L.; Zhu, G. Association of Serum Bisphenol-A Concentration and Male Reproductive Function among Exposed Workers. Arch. Environ. Contam. Toxicol. 2015, 68, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Cabré, R.; Moreno, R.D. Contribution of environmental pollutants to male infertily: A working model of germ cell apoptosis induced by plasticizers. Biol. Res. 2012, 45, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine bisphenol-a (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miao, M.; Zhou, Z.; Gao, E.; Chen, J.; Wang, J.; Sun, F.; Yuan, W.; Li, D.K. Exposure to bisphenol-A and reproductive hormones among male adults. Environ. Toxicol. Pharmacol. 2015, 39, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, Z.; Qing, D.; He, Y.; Wu, T.; Miao, M.; Wang, J.; Weng, X.; Ferber, J.R.; Herrinton, L.J.; et al. Occupational exposure to bisphenol-A (BPA) and the risk of Self-Reported Male Sexual Dysfunction. Hum. Reprod. 2010, 25, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ruiz, P. Toxicol Ind Health Polychlorinated biphenyls: New evidence from the last decade. Toxicol Ind. Health 2015. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yan, J.D.; Yang, S.Q.; Guo, J.P.; Zhang, X.; Sun, X.X.; Na, X.L.; Dai, S.C. Maternal Genistein Intake Can Reduce Body Weight in Male Offspring. Biomed. Environ. Sci. 2015, 28, 769–772. [Google Scholar] [PubMed]
- Mumford, S.L.; Kim, S.; Chen, Z.; Barr, D.B.; Louis, G.M.B. Urinary Phytoestrogens Are Associated with Subtle Indicators of Semen Quality among Male Partners of Couples Desiring Pregnancy. J. Nutr. 2015, 145, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Yum, T.; Lee, S.; Kim, Y. Association between precocious puberty and some endocrine disruptors in human plasma. J. Environ. Sci Health A Tox. Hazard Subst. Environ. Eng. 2013, 48, 912–917. [Google Scholar]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Ciênc. Saúde Colet. 2012, 17, 407–434. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, Á. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell. Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef] [PubMed]
- NTP National Toxicology Program, U.S. Department of Health and Human Services. Endocrine Disruptors Low-Dose Peer Review Final Report. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/liason/LowDosePeerFinalRpt.pdf (accessed on 10 December 2016).
- Sekizawa, J. Low-dose effects of bisphenol A: A serious threat to human health? J. Toxicol. Sci. 2008, 33, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ide, K.; Ishida, M. Response of MCF-7 human breast cancer cells to some binary mixtures of oestrogenic compounds in vitro. J. Pharm. Pharmacol. 2001, 53, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Couleau, N.; Falla, J.; Beillerot, A.; Battaglia, E.; D’Innocenzo, M.; Plancon, S.; Laval-Gilly, P.; Bennasroune, A. Effects of Endocrine Disruptor Compounds, Alone or in Combination, on Human Macrophage-Like THP-1 Cell Response. PLoS ONE 2015, 10, e0131428. [Google Scholar] [CrossRef] [PubMed]
- Charles, G.D.; Gennings, C.; Zacharewski, T.R.; Gollapudi, B.B.; Carney, E.W. Assessment of Interactions of Diverse Ternary Mixtures in an Estrogen Receptor-α Reporter Assay. Toxicol. Appl. Pharmacol. 2002, 180, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Charles, G.D.; Gennings, C.; Zacharewski, T.R.; Gollapudi, B.B.; Carney, E.W. An approach for assessing estrogen receptor-mediated interactions in mixtures of three chemicals: A pilot study. Toxicol. Sci. 2002, 68, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Silva, E.; Scholze, M.; Kortenkamp, A. Deviation from additivity with estrogenic mixtures containing 4-nonylphenol and 4-tert-octylphenol detected in the E-SCREEN assay. Env. Sci Technol 2004, 38, 6343–6352. [Google Scholar] [CrossRef]
- Silva, E.; Rajapakse, N.; Scholze, M.; Backhaus, T.; Ermler, S.; Kortenkamp, A. Joint effects of heterogeneous estrogenic chemicals in the E-Screen-exploring the applicability of concentration addition. Toxicol. Sci. 2011, 122, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Scholze, M.; Kortenkamp, A. Mixtures of four organochlorines enhance human breast cancer cell proliferation. Environ. Health Perspect. 2001, 109, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, M.; Muusse, M.; Van Den Berg, M.; Sanderson, J.T. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol. Appl. Pharmacol. 2005, 208, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.; Müller, A.; Egeberg, D.L.; Alvarez, L.; Brenker, C.; Rehfeld, A.; Frederiksen, H.; Wäschle, B.; Kaupp, U.B.; Balbach, M.; et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014, 15, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Zhou, M.; Xiang, Z.; Han, X.; Li, D. Combined effects of nonylphenol and bisphenol a on the human prostate epithelial cell line RWPE-1. Int. J. Environ. Res. Public Health 2015, 12, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Jacobs, D.R., Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev. Endocr. Metab. Disord. 2016, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Colnot, T.; Csanady, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, C.; Ietta, F.; Avanzati, A.M.; Skarzynski, D.; Paulesu, L. Biological Tools to Study the Effects of Environmental Contaminants at the Feto-Maternal Interface. Dose Response 2015, 13, 1559325815611902. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.K.; Heger, S. Endocrine disrupting chemicals affect the Gonadotropin releasing hormone neuronal network. Reprod. Toxicol. 2014, 44, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Varandas, E.; Pereira, H.S.; Monteiro, S.; Neves, E.; Brito, L.; Ferreira, R.B.; Viegas, W.; Delgado, M. Bisphenol a disrupts transcription and decreases viability in aging vascular endothelial cells. Int. J. Mol. Sci. 2014, 15, 15791–15805. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Varandas, E.; Viegas, W.; Sofia Pereira, H.; Delgado, M. Bisphenol A at concentrations found in human serum induces aneugenic effects in endothelial cells. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2013, 751, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, W.; Prieto, P.; Dekant, W.; Jennings, P.; Blaauboer, B.J. The Predict-IV project: Towards predictive toxicology using in vitro techniques. Toxicol. In Vitro 2015, 30, 1–3. [Google Scholar] [CrossRef] [PubMed]
| EDCs Mixtures | Concentrations | Cell Lines | Results | References |
|---|---|---|---|---|
| o,p′-DDT, p,p′-DDT, p,p′-DDE, and β-hexachlorocyclohexane | 1–10 nM | MCF7 breast cancer cells | Concentration additive effects | [91] |
| E2, estrone, BPA, butyl benzylphthalate, endosulfan, methoxychlor, and pentachlorophenol | 10–400 nM | MCF7 breast cancer cells | Additive, Antagonistic and synergistic effects | [90] |
| Benzo[a]pyrene, 1,2-benzanthracene, chrysene, methoxychlor, o,p′-DDT, dieldrin, E2, and genistein | low range (individual chemical thresholds) and a high range (2–10 higher) | MCF7 breast cancer cells | Concentration additivity; antagonistic effects | [92] |
| 17beta-estradiol (E2), ethinyl estradiol, diethylstilbestrol, epidermal growth factor, insulin-like growth factor-I | E2/DES (0–10−9 M); EE (0–10−10 M); E2 (0–10−10 M); EGF/IGF-I (0–10−9 M) | MCF7 breast cancer cells | Additive and greater-than-additive interaction | [93] |
| E2, EE2, genistein, BPA, 4-nonylphenol, and 4-tert-octylphenol | - | MCF7 breast cancer cells | Additive and Antagonistic effects | [94] |
| 2-hydroxy-4-methoxy-benzophenone (BP-3), 2,4-dihydroxy benzophenone (BP-1), octyl methoxy cinnamate (OMC) and 3-(4-methylbenzylidene) camphor (4-MBC) | 100 nM–1 µM | MCF7 breast cancer cells | Additive interaction; Estrogen-regulated transcription | [97] |
| BPA, Butylparaben, Coumestrol, o,p′-DDT, DES, Dienestrol, Endosulfan α (I), Endosulfan β (II), 17β-estradiol, Estriol, Estrone, 17α-Ethinylestradiol, genistein, β-HCH, Hexestrol, Kepone, Mestranol, Methoxychlor, Propyl paraben, Zearalenone | 10 pM–10 nM | MCF-7 breast cancer cells | Normal and overestimated concentration additivity | [95] |
| E2, BPA, genistein | GN 1.0, 2.5, 5.0, 7.5 and 10 × 10−5 M in the presence of 10−9 M of E2 or 10−5 M of BPA. | Ovarian cancer cell line BG-1 | Suppression of BPA ERα mediated proliferation by GN | [39] |
| Bitertanol, propiconazole, cypermethrin, terbuthylazine, malathion | 10−10–10−5 M | Human breast carcinoma MVLN cells | ER, AR endocrine-disruption | [34] |
| Homosalate, nonylparaben, padimate O, benzophenone-3, chlorophenothane, triclosan, 3-(4-methylbenzylidene) camphor, benzal camphor, α-zearalenol, 4-octylphenol, dibutyl phthalate. | 0.1, 1, and 10 μM | Sperm cells | Pronounced Ca2+ response. | [98] |
| BPA, genistein and daidzein. | 1 μM | MCF7 and HeLa | Additive effects | [38] |
| Nonylphenol (NP) and BPA | NP (0–100 μM) BPA (0–5000 μM) | Human Prostate Epithelial Cell Line RWPE-1 | Synergistic effects | [96] |
| BPA, di-ethylhexyl-phthalate (DEHP), dibutyl phthalate (DBP) and 4-tert-octylphenol (4-OP) | 0.001–10 μM | Human Macrophage-Like THP-1 Cell | Reduction of phagocytosis; disturbance ER-dependent effects | [99] |
| Perfluorinated and brominated | 10.000, 5000, 1000 and 500 times the serum levels | Human hepatocarcinoma (HepG2) cells | Synergistic effects | [44] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics 2017, 5, 5. https://doi.org/10.3390/toxics5010005
Ribeiro E, Ladeira C, Viegas S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics. 2017; 5(1):5. https://doi.org/10.3390/toxics5010005
Chicago/Turabian StyleRibeiro, Edna, Carina Ladeira, and Susana Viegas. 2017. "EDCs Mixtures: A Stealthy Hazard for Human Health?" Toxics 5, no. 1: 5. https://doi.org/10.3390/toxics5010005
APA StyleRibeiro, E., Ladeira, C., & Viegas, S. (2017). EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics, 5(1), 5. https://doi.org/10.3390/toxics5010005