In Silico Forensic Toxicology: Is It Feasible?
Highlights
- Computational toxicology tools can effectively predict toxicokinetic and toxicodynamic properties of substances relevant to forensic investigations. These predictions include absorption, distribution, metabolism, and excretion (ADME) profiles, as well as potential toxic effects.
- In silico models provide valuable support in postmortem toxicological interpretation, especially when experimental data are limited or unavailable, by simulating drug interactions, estimating lethal concentrations, and assisting in the reconstruction of exposure scenarios.
- In silico tools enable forensic toxicologists to simulate drug behavior and toxicity even when biological samples are degraded, missing, or insufficient for traditional analysis.
- Predictive modeling supports the development of more informed hypotheses regarding cause of death, timing of exposure, and potential drug interactions. This capability can strengthen expert testimony and enhance case reconstruction efforts.
- By reducing reliance on animal testing and minimizing experimental costs, computational toxicology aligns with ethical standards while offering scalable solutions for both routine and complex forensic cases.
Abstract
1. Introduction
2. Systematic Literature Review
2.1. Primary Studies
2.2. Trails in Forensic Medicine Operated by In Silico Forensic Toxicology
2.3. Case Studies Where In Silico Predictions Have Directly Influenced Forensic Conclusions
Forensic Impact
2.4. Case Reports in In Silico Forensic Toxicology
2.5. Pricing
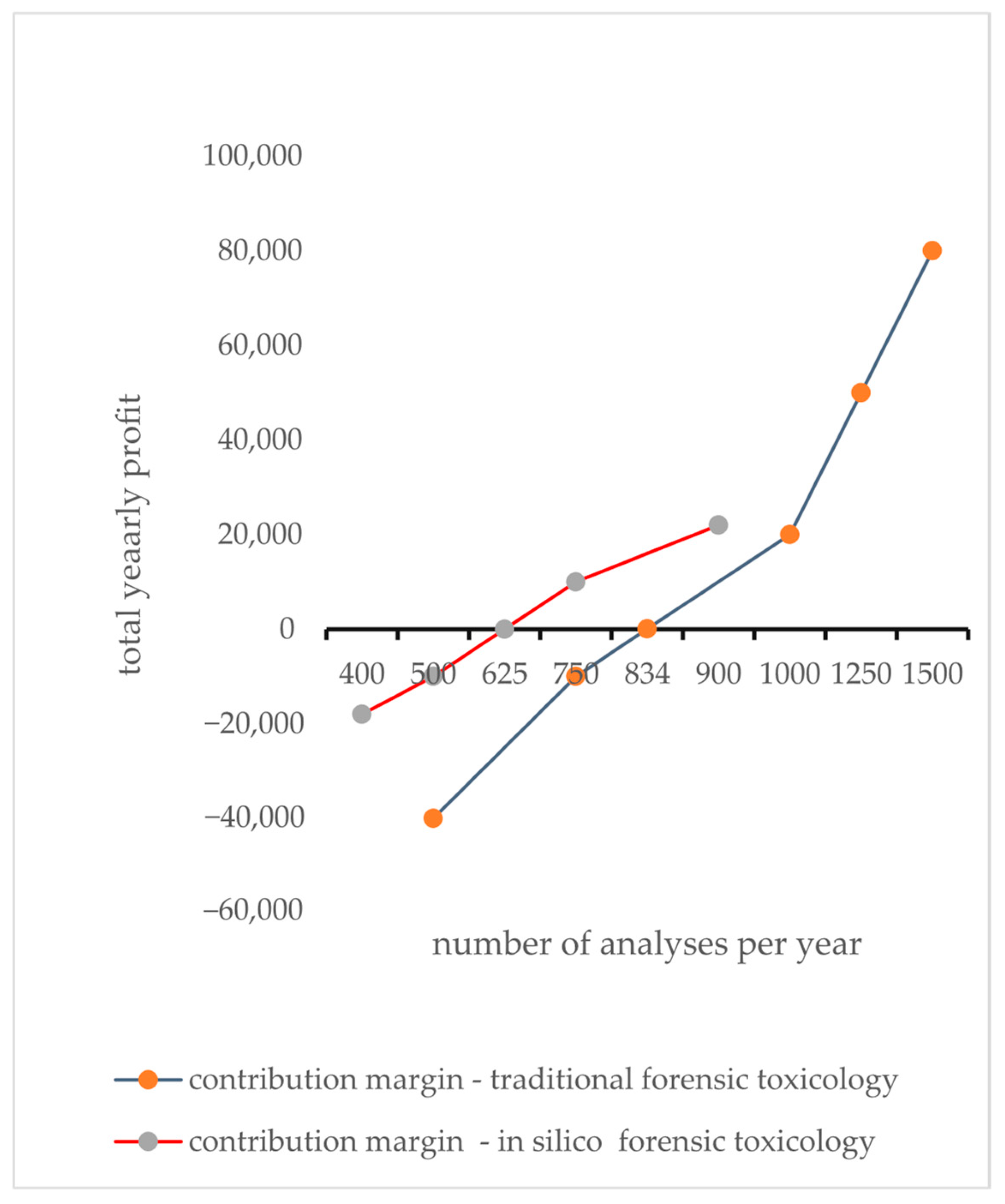
2.6. Break-Even Analysis
- p: Revenue (or price charged) per analysis;
- N: number of analyses;
- F: Annual fixed costs (e.g., software licenses, infrastructure, maintenance);
- v: Variable cost per analysis (e.g., additional materials, labor costs of analysts).
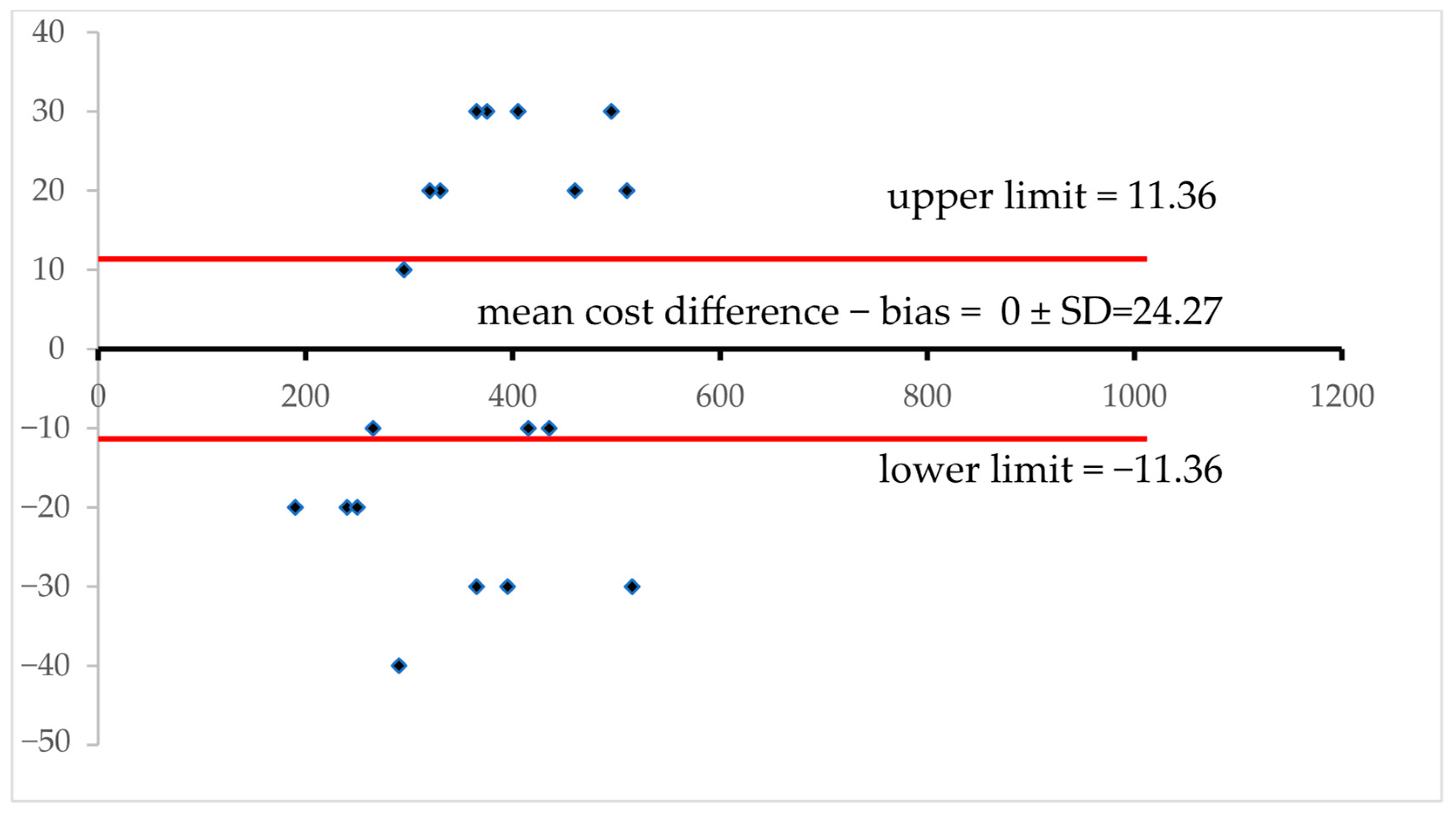
2.7. Bland–Altman Plot
3. Future Insights
Machine Learning, Artificial Intelligence, and In Silico Forensic Toxicology
4. Limitations of In Silico Forensic Toxicology
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3Rs | Replacement, reduction, and refinement (of animal use) |
| 4-Cl-PVP | 4-Chloro-α-pyrrolidinovalerophenone (designer stimulant) |
| 4CMC | 4 Chloromethcathinone (a new psychoactive substance) |
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| AH-7921 | Synthetic analgesic compound AH-7921 |
| AI | Artificial intelligence |
| AP-238 | An identifier for a new synthetic opioid discussed in the text |
| DOI | Digital object identifier |
| GC–MS | Gas chromatography–mass spectrometry |
| hERG | Human Ether-a-go-go-Related Gene |
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| LD50 | Lethal dose for 50% of the test subjects |
| ML | Machine learning |
| NGS | Next-generation sequencing |
| NPS | Novel psychoactive substance |
| OSF | Open Science Framework |
| PBPK | Physiologically based pharmacokinetic (model) |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| SCD | Sudden cardiac death |
| QSAR | Quantitative structure–activity relationship |
References
- Amorim, A.M.B.; Piochi, L.F.; Gaspar, A.T.; Preto, A.J.; Rosario-Ferreira, N.; Moreira, I.S. Advancing Drug Safety in Drug Development: Bridging Computational Predictions for Enhanced Toxicity Prediction. Chem. Res. Toxicol. 2024, 37, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Mekenyan, O. In Silico Toxicology: Principles and Applications; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Peters, F.T.; Wissenbach, D.K.; Busardo, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2017, 23, 5455–5467. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J. Together informing justice: 23rd International ANZFSS Symposium on the Forensic Sciences. Aust. J. Forensic Sci. 2017, 49, 487–488. [Google Scholar] [CrossRef]
- Rim, K.T. In silico prediction of toxicity and its applications for chemicals at work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef]
- Dawidowska, J.; Krzyzanowska, M.; Markuszewski, M.J.; Kaliszan, M. The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation. Metabolites 2021, 11, 801. [Google Scholar] [CrossRef]
- Greco, E. AI Methods for New Psychoactive Substance (NPS) Design and Analysis. Analytica 2025, 6, 17. [Google Scholar] [CrossRef]
- Hemmerich, J.; Ecker, G.F. In silico toxicology: From structure-activity relationships towards deep learning and adverse outcome pathways. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1475. [Google Scholar] [CrossRef]
- Boyce, M.; Favela, K.A.; Bonzo, J.A.; Chao, A.; Lizarraga, L.E.; Moody, L.R.; Owens, E.O.; Patlewicz, G.; Shah, I.; Sobus, J.R.; et al. Identifying xenobiotic metabolites with in silico prediction tools and LCMS suspect screening analysis. Front. Toxicol. 2023, 5, 1051483. [Google Scholar] [CrossRef]
- Wheeler, M.W.; Lim, S.; House, J.; Shockley, K.; Bailer, A.J.; Fostel, J.; Yang, L.; Talley, D.; Raghuraman, A.; Gift, J.S.; et al. ToxicR: A computational platform in R for computational toxicology and dose-response analyses. Comput. Toxicol. 2023, 25, 100259. [Google Scholar] [CrossRef]
- Carstens, K.E.; Dönmez, A.; Hsieh, J.H.; Bartmann, K.; Friedman, K.P.; Koch, K.; Scholze, M.; Fritsche, E. A comparative study of biostatistical pipelines for benchmark concentration modeling of in. Comput. Toxicol. 2025, 34, 100360. [Google Scholar] [CrossRef]
- Ellison, C.M.; Enoch, S.J.; Cronin, M.T. A review of the use of in silico methods to predict the chemistry of molecular initiating events related to drug toxicity. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1481–1495. [Google Scholar] [CrossRef]
- Noga, M.; Jurowski, K. Application of in silico methods to predict the acute toxicity of bicyclic organophosphorus compounds as potential chemical weapon. Arch. Toxicol. 2025, 99, 2507–2528. [Google Scholar] [CrossRef]
- Taylor, K.; Rego Alvarez, L. Regulatory drivers in the last 20 years towards the use of in silico techniques as replacements to animal testing for cosmetic-related substances. Comput. Toxicol. 2020, 13, 100112. [Google Scholar] [CrossRef]
- Cathaoir, Ó.; Katherina, E.G.; Hartlev, M.; Mourby, M.; Lukaseviciene, V. A European Standardization Framework for Data Integration and Data-Driven in Silico Models for Personalized Medicine. In Proceedings of the EU-STANDS4PM Annual Meeting 2022, Virtual, 18–19 May 2022. [Google Scholar]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.B.; Panteri, E. SCCS (Scientific Committee on Consumer Safety) Opinion on the Safety of the Presence of Bisphenol A in Clothing Articles-2, 2-Bis (4-Hydroxyphenyl) Propane (CAS Number 80-05-7), Preliminary Version of 16 October 2020, Final Version of 30–31 March 2021, SCCS/1620/20; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. In Silico Proposal of Screening Strategies for Detecting EU Authorised GMOs. Available online: https://gmo-crl.jrc.ec.europa.eu/doc/JRC127110_finalwebscreening_v10.pdf (accessed on 12 June 2025).
- Macmillan, D.S.; Bergqvist, A.; Burgess-Allen, E.; Callan, I.; Dawick, J.; Carrick, B.; Ellis, G.; Ferro, R.; Goyak, K.; Smulders, C.; et al. The last resort requirement under REACH: From principle to practice. Regul. Toxicol. Pharmacol. 2024, 147, 105557. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Michalska, A.; Jurowski, K. Application of toxicology in silico methods for prediction of acute toxicity (LD(50)) for Novichoks. Arch. Toxicol. 2023, 97, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, N.; Sewell, F. Regulatory acceptance of in silico approaches for the safety assessment of cosmetic-related substances. Comput. Toxicol. 2019, 11, 82–89. [Google Scholar] [CrossRef]
- Lacasse, K. Alternative Approaches to Animal Testing. Available online: https://cefic.org/policy/alternative-approaches-to-animal-testing/ (accessed on 12 June 2025).
- Cronin, M.T.D.; Enoch, S.J.; Madden, J.C.; Rathman, J.F.; Richarz, A.-N.; Yang, C. A review of in silico toxicology approaches to support the safety assessment of cosmetics-related materials. Comput. Toxicol. 2022, 21, 100213. [Google Scholar] [CrossRef]
- Wohlfarth, A.; Scheidweiler, K.B.; Pang, S.; Zhu, M.; Castaneto, M.; Kronstrand, R.; Huestis, M.A. Metabolic characterization of AH-7921, a synthetic opioid designer drug: In vitro metabolic stability assessment and metabolite identification, evaluation of in silico prediction, and in vivo confirmation. Drug Test. Anal. 2016, 8, 779–791. [Google Scholar] [CrossRef]
- Berardinelli, D.; Kutzler, J.; Taoussi, O.; Zaami, S.; Pichini, S.; Basile, G.; Busardo, F.P.; Auwarter, V.; Carlier, J. Dipyanone, a new methadone-like synthetic opioid: In vitro and in vivo human metabolism and pharmacological profiling. Arch. Toxicol. 2025, 99, 2339–2353. [Google Scholar] [CrossRef]
- Noga, M.; Michalska, A.; Jurowski, K. The prediction of acute toxicity (LD(50)) for organophosphorus-based chemical warfare agents (V-series) using toxicology in silico methods. Arch. Toxicol. 2024, 98, 267–275. [Google Scholar] [CrossRef]
- Pampalakis, G. Underestimations in the In Silico-Predicted Toxicities of V-Agents. J. Xenobiot. 2023, 13, 615–624. [Google Scholar] [CrossRef]
- Jurowski, K.; Niznik, L. Toxicity of the New Psychoactive Substance (NPS) Clephedrone (4-Chloromethcathinone, 4-CMC): Prediction of Toxicity Using In Silico Methods for Clinical and Forensic Purposes. Int. J. Mol. Sci. 2024, 25, 5867. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Krosniak, A. Prediction of key toxicity endpoints of AP-238 a new psychoactive substance for clinical toxicology and forensic purposes using in silico methods. Sci. Rep. 2024, 14, 28977. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Le Dare, B.; Ferron, P.J.; Le Bouedec, D.; Kernalleguen, A.; Morel, I.; Gicquel, T. Use of innovative, cross-disciplinary in vitro, in silico and in vivo approaches to characterize the metabolism of chloro-alpha-pyrrolidinovalerophenone (4-Cl-PVP). Arch. Toxicol. 2023, 97, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Nahle, D.; Sarr, M.; Bourdais, A.; Morel, I.; Le Dare, B.; Gicquel, T. Identifying metabolites of new psychoactive substances using in silico prediction tools. Arch. Toxicol. 2025, 99, 2953–2973. [Google Scholar] [CrossRef]
- Busardo, F.P.; Lo Faro, A.F.; Sirignano, A.; Giorgetti, R.; Carlier, J. In silico, in vitro, and in vivo human metabolism of acetazolamide, a carbonic anhydrase inhibitor and common “diuretic and masking agent” in doping. Arch. Toxicol. 2022, 96, 1989–2001. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, L.; Guo, Z.; Zhao, J.; Xiao, Y.; Xiang, P.; Xu, L.; Yan, H. Metabolism study of two phenethylamine—Derived new psychoactive substances using in silico, in vivo, and in vitro approaches. Arch. Toxicol. 2025, 99, 2367–2378. [Google Scholar] [CrossRef]
- Hernandez, A.M.; Bilbrough, G.E.A.; DeNicola, D.B.; Myrick, C.; Edwards, S.; Hammond, J.M.; Myers, A.N.; Heseltine, J.C.; Russell, K.; Giraldi, M.; et al. Comparison of the performance of the IDEXX SediVue Dx(R) with manual microscopy for the detection of cells and 2 crystal types in canine and feline urine. J. Vet. Intern. Med. 2019, 33, 167–177. [Google Scholar] [CrossRef]
- Karanasiou, G.; Edelman, E.; Boissel, F.H.; Byrne, R.; Emili, L.; Fawdry, M.; Filipovic, N.; Flynn, D.; Geris, L.; Hoekstra, A.; et al. Advancing in Silico Clinical Trials for Regulatory Adoption and Innovation. IEEE J. Biomed. Health Inform. 2025, 29, 2654–2668. [Google Scholar] [CrossRef]
- Arsene, S.; Pares, Y.; Tixier, E.; Granjeon-Noriot, S.; Martin, B.; Brueziere, L.; Couty, C.; Courcelles, E.; Kahoul, R.; Pitrat, J.; et al. In Silico Clinical Trials: Is It Possible? Methods Mol. Biol. 2024, 2716, 51–99. [Google Scholar] [CrossRef]
- Pathmanathan, P.; Aycock, K.; Badal, A.; Bighamian, R.; Bodner, J.; Craven, B.A.; Niederer, S. Credibility assessment of in silico clinical trials for medical devices. PLoS Comput. Biol. 2024, 20, e1012289. [Google Scholar] [CrossRef]
- Toennes, S.W.; Schneider, D.; Pogoda, W.; Paulke, A.; Wunder, C.; Theunissen, E.L.; de Sousa Fernandes Perna, E.B.; Ramaekers, J.G. Excretion of 4-fluoroamphetamine and three metabolites in urine after controlled oral ingestion. J. Pharm. Biomed. Anal. 2020, 179, 113008. [Google Scholar] [CrossRef]
- Papaseit, E.; Olesti, E.; Perez-Mana, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; de la Torre, R.; Farre, M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals 2021, 14, 100. [Google Scholar] [CrossRef]
- Losacker, M.; Toennes, S.W.; de Sousa Fernandes Perna, E.B.; Ramaekers, J.G.; Roehrich, J.; Hess, C. Chiral Serum Pharmacokinetics of 4-Fluoroamphetamine after Controlled Oral Administration: Can (R)/(S)-Concentration Ratios Help in Interpreting Forensic Cases? J. Anal. Toxicol. 2021, 45, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Alape-Ariza, J.; Bermudez-Santana, C.I. Complexity of Molecular Analysis by New Generation Sequencing in the Study of Sudden Cardiac Death Within the Forensic Context. In Advances in Forensic Biology and Genetics; Springer: Berlin/Heidelberg, Germany, 2025; pp. 239–265. [Google Scholar]
- Worth, A.; Lapenna, S.; Lo Piparo, E.; Mostrag-Szlichtyng, A.; Serafimova, R. A Framework for Assessing In Silico Toxicity Predictions: Case Studies with Selected Pesticides; EUR 24705 EN; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Nunes, C.; Proenca, S.; Ambrosini, G.; Pamies, D.; Thomas, A.; Kramer, N.I.; Zurich, M.G. Integrating distribution kinetics and toxicodynamics to assess repeat dose neurotoxicity in vitro using human BrainSpheres: A case study on amiodarone. Front. Pharmacol. 2023, 14, 1248882. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Quintanal, L.M.; Matey, J.M.; Perretti, M.D.; Martínez-Ramírez, C.; Hernández-Díaz, F.J. Potential of high-resolution mass spectrometry for identification and structural elucidation of scopolamine metabolomic biomarkers in a confirmed case of Brugmansia intoxication. Specially application in drug-facilitated crimes. Forensic Chem. 2024, 40, 100602. [Google Scholar] [CrossRef]
- Schmeisser, S.; Miccoli, A.; von Bergen, M.; Berggren, E.; Braeuning, A.; Busch, W.; Desaintes, C.; Gourmelon, A.; Grafstrom, R.; Harrill, J.; et al. New approach methodologies in human regulatory toxicology—Not if, but how and when! Environ. Int. 2023, 178, 108082. [Google Scholar] [CrossRef] [PubMed]
- Fuzi, B.; Mathai, N.; Kirchmair, J.; Ecker, G.F. Toxicity prediction using target, interactome, and pathway profiles as descriptors. Toxicol. Lett. 2023, 381, 20–26. [Google Scholar] [CrossRef]
- Sacco, M.A.; Gualtieri, S.; Spiliopoulou, C.; Tarallo, A.P.; Verrina, M.C.; Aquila, I. The Role of Toxicology Investigations in Overdose Deaths. Cureus 2025, 17, e79352. [Google Scholar] [CrossRef]
- Montesano, C.; Vannutelli, G.; Fanti, F.; Vincenti, F.; Gregori, A.; Rita Togna, A.; Canazza, I.; Marti, M.; Sergi, M. Identification of MT-45 Metabolites: In Silico Prediction, In Vitro Incubation with Rat Hepatocytes and In Vivo Confirmation. J. Anal. Toxicol. 2017, 41, 688–697. [Google Scholar] [CrossRef]
- Priani, S.E.; Fakih, T.M.; Wilar, G.; Chaerunisaa, A.Y.; Sopyan, I. Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework. Pharmaceutics 2025, 17, 701. [Google Scholar] [CrossRef]
- Djukić-Ćosić, D.; Baralić, K.; Jorgovanović, D.; Živančević, K.; Javorac, D.; Stojilković, N.; Radović, B.; Marić, D.; Ćurčić, M.; Djordjević, A.B. In silico toxicology methods in drug safety assessment. Arch. Pharm. 2021, 71, 257–278. [Google Scholar]
- Menéndez-Quintanal, L.M.; Matey, J.M.; del Fresno González, V.; Bravo Serrano, B.; Hernández-Díaz, F.J.; Zapata, F.; Montalvo, G.; García-Ruiz, C. The State of the Art in Post-Mortem Redistribution and Stability of New Psychoactive Substances in Fatal Cases: A Review of the Literature. Psychoactives 2024, 3, 525–610. [Google Scholar] [CrossRef]
- Çelik, H.T.; Vural, N.; Kaymak, S. SPME-GC-MS profiling of volatile compounds in Lucilia sericata larva extract and in Silico biotherapeutic analysis. Int. J. Trop. Insect Sci. 2025, 45, 741–749. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- Frühwein, H.; Paul, N.W. “Lost in translation?” Animal research in the era of precision medicine. J. Transl. Med. 2025, 23, 152. [Google Scholar] [CrossRef]
- Morger, A.L. Strategies to Enhance the Applicability of In Silico Toxicity Prediction Methods; Freie Universitaet: Berlin, Germany, 2022. [Google Scholar]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Roney, M.; Aluwi, M.F.F.M. The importance of in-silico studies in drug discovery. Intell. Pharm. 2024, 2, 578–579. [Google Scholar] [CrossRef]
- Price, P.S.; Hubbell, B.J.; Hagiwara, S.; Paoli, G.M.; Krewski, D.; Guiseppi-Elie, A.; Gwinn, M.R.; Adkins, N.L.; Thomas, R.S. A Framework that Considers the Impacts of Time, Cost, and Uncertainty in the Determination of the Cost Effectiveness of Toxicity-Testing Methodologies. Risk Anal. 2022, 42, 707–729. [Google Scholar] [CrossRef]
- Raunio, H. In silico toxicology—Non-testing methods. Front. Pharmacol. 2011, 2, 33. [Google Scholar] [CrossRef]
- Dhanya, S.; Lal, K.; Reena, S. In Silico Toxicology-A Tool for Early Safety Evaluation of Drug. J. Bioinform. Genom. Proteom. 2018, 3, 1030–1041. [Google Scholar]
- Javorac, D.; Baralić, K.; Bulat, Z.; Đukić-Ćosić, D.; Antonijević, B. In silico metodologija u toksikologiji-softveri za predviđanje toksičnosti. Arh. Farm. 2019, 69, 28–38. [Google Scholar] [CrossRef]
- Justice, D. Needs Assessment of Forensic Laboratories and Medical Examiner/Coroner Offices: A Report to Congress; U.S. Department of Justice Office of Justice Programs: Washington, DC, USA, 2019. [Google Scholar]
- Sewell, F.; Alexander-White, C.; Brescia, S.; Currie, R.A.; Roberts, R.; Roper, C.; Vickers, C.; Westmoreland, C.; Kimber, I. New approach methodologies (NAMs): Identifying and overcoming hurdles to accelerated adoption. Toxicol. Res. 2024, 13, tfae044. [Google Scholar] [CrossRef] [PubMed]
- Mirakhori, F.; Niazi, S.K. Harnessing the AI/ML in Drug and Biological Products Discovery and Development: The Regulatory Perspective. Pharmaceuticals 2025, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Masarone, S.; Beckwith, K.V.; Wilkinson, M.R.; Tuli, S.; Lane, A.; Windsor, S.; Lane, J.; Hosseini-Gerami, L. Advancing predictive toxicology: Overcoming hurdles and shaping the future. Digit. Discov. 2025, 4, 303–315. [Google Scholar] [CrossRef]
- Jarantow, S.W.; Pisors, E.D.; Chiu, M.L. Introduction to the Use of Linear and Nonlinear Regression Analysis in Quantitative Biological Assays. Curr. Protoc. 2023, 3, e801. [Google Scholar] [CrossRef]
- Moore, A.R. A review of Bland-Altman difference plot analysis in the veterinary clinical pathology laboratory. Vet. Clin. Pathol. 2024, 53 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Carnesecchi, E.; Toma, C.; Roncaglioni, A.; Kramer, N.; Benfenati, E.; Dorne, J. Integrating QSAR models predicting acute contact toxicity and mode of action profiling in honey bees (A. mellifera): Data curation using open source databases, performance testing and validation. Sci. Total Environ. 2020, 735, 139243. [Google Scholar] [CrossRef]
- De Borja, J.R.; Cabrera, H.S. In Silico Drug Screening for Hepatitis C Virus Using QSAR-ML and Molecular Docking with Rho-Associated Protein Kinase 1 (ROCK1) Inhibitors. Computation 2024, 12, 175. [Google Scholar] [CrossRef]
- Ball, N.; Bars, R.; Botham, P.A.; Cuciureanu, A.; Cronin, M.T.D.; Doe, J.E.; Dudzina, T.; Gant, T.W.; Leist, M.; van Ravenzwaay, B. A framework for chemical safety assessment incorporating new approach methodologies within REACH. Arch. Toxicol. 2022, 96, 743–766. [Google Scholar] [CrossRef]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in silico methods in drug target and lead prediction. Brief. Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Chango, X.; Flor-Unda, O.; Gil-Jiménez, P.; Gómez-Moreno, H. Technology in Forensic Sciences: Innovation and Precision. Technologies 2024, 12, 120. [Google Scholar] [CrossRef]
- Nasnodkar, S.; Cinar, B.; Ness, S. Artificial intelligence in toxicology and pharmacology. J. Eng. Res. Rep. 2023, 25, 192–206. [Google Scholar] [CrossRef]
- Boscolo-Berto, R. Challenges and future trends of forensic toxicology to keep a cut above the rest. Adv. Clin. Exp. Med. 2024, 33, 423–425. [Google Scholar] [CrossRef]
- Rajpoot, K.; Desai, N.; Koppisetti, H.; Tekade, M.; Sharma, M.C.; Behera, S.K.; Tekade, R.K. In silico methods for the prediction of drug toxicity. In Pharmacokinetics and Toxicokinetic Considerations; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 357–383. [Google Scholar]
- Gangwal, A.; Lavecchia, A. Artificial intelligence in preclinical research: Enhancing digital twins and organ-on-chip to reduce animal testing. Drug Discov. Today 2025, 30, 104360. [Google Scholar] [CrossRef]
- Racz, A.; Bajusz, D.; Miranda-Quintana, R.A.; Heberger, K. Machine learning models for classification tasks related to drug safety. Mol. Divers. 2021, 25, 1409–1424. [Google Scholar] [CrossRef]
- Bonetti, A.; Martínez-Sober, M.; Torres, J.C.; Vega, J.M.; Pellerin, S.; Vila-Francés, J. Comparison between Machine Learning and Deep Learning Approaches for the Detection of Toxic Comments on Social Networks. Appl. Sci. 2023, 13, 6038. [Google Scholar] [CrossRef]
- Barbierato, E.; Gatti, A. The Challenges of Machine Learning: A Critical Review. Electronics 2024, 13, 416. [Google Scholar] [CrossRef]
- Schwartz, R.; Schwartz, R.; Vassilev, A.; Greene, K.; Perine, L.; Burt, A.; Hall, P. Towards a Standard for Identifying and Managing Bias in Artificial Intelligence; US Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022; Volume 3. [Google Scholar]
- Chen, P.; Wu, L.N.; Wang, L. AI Fairness in Data Management and Analytics: A Review on Challenges, Methodologies and Applications. Appl. Sci. 2023, 13, 10258. [Google Scholar] [CrossRef]
- Pagano, T.P.; Loureiro, R.B.; Lisboa, F.V.N.; Peixoto, R.M.; Guimaraes, G.A.S.; Cruz, G.O.R.; Araujo, M.M.; Santos, L.L.; Cruz, M.A.S.; Oliveira, E.L.S.; et al. Bias and Unfairness in Machine Learning Models: A Systematic Review on Datasets, Tools, Fairness Metrics, and Identification and Mitigation Methods. Big Data Cogn. Comput. 2023, 7, 15. [Google Scholar] [CrossRef]
- Ajmal, C.S.; Yerram, S.; Abishek, V.; Nizam, V.P.M.; Aglave, G.; Patnam, J.D.; Raghuvanshi, R.S.; Srivastava, S. Innovative Approaches in Regulatory Affairs: Leveraging Artificial Intelligence and Machine Learning for Efficient Compliance and Decision-Making. AAPS J. 2025, 27, 22. [Google Scholar] [CrossRef]
- Huang, L.; Duan, Q.; Liu, Y.; Wu, Y.; Li, Z.; Guo, Z.; Liu, M.; Lu, X.; Wang, P.; Liu, F.; et al. Artificial intelligence: A key fulcrum for addressing complex environmental health issues. Environ. Int. 2025, 198, 109389. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.; Kleinstreuer, N. Challenges and opportunities for validation of AI-based new approach methods. ALTEX 2025, 42, 3–21. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Dall’Olio, D.; Sala, C.; Dall’Olio, L.; Sauta, E.; Zampini, M.; Asti, G.; Lanino, L.; Maggioni, G.; Campagna, A.; et al. Synthetic Data Generation by Artificial Intelligence to Accelerate Research and Precision Medicine in Hematology. JCO Clin. Cancer Inform. 2023, 7, e2300021. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Leveraging Artificial Intelligence and Machine Learning for Characterizing Protein Corona, Nanobiological Interactions, and Advancing Drug Discovery. Bioengineering 2025, 12, 312. [Google Scholar] [CrossRef]
- Kavlock, R.J.; Ankley, G.; Blancato, J.; Breen, M.; Conolly, R.; Dix, D.; Houck, K.; Hubal, E.; Judson, R.; Rabinowitz, J.; et al. Computational toxicology--a state of the science mini review. Toxicol. Sci. 2008, 103, 14–27. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P. Encouraging Safety 4.0 to enhance industrial culture: An extensive study of its technologies, roles, and challenges. Green Technol. Sustain. 2025, 3, 100158. [Google Scholar] [CrossRef]
- Scholz, M. Machine learning in forensic toxicology: Applications, experiences, and future directions. Toxicol. Anal. Clin. 2025, 37, S14–S15. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Suspect and non-target screening workflow for studying the occurrence, fate, and environmental risk of contaminants in wastewater using data-independent acquisition. J. Chromatogr. A 2022, 1667, 462905. [Google Scholar] [CrossRef]
- Imani, S.; Li, X.; Chen, K.; Maghsoudloo, M.; Jabbarzadeh Kaboli, P.; Hashemi, M.; Khoushab, S.; Li, X. Computational biology and artificial intelligence in mRNA vaccine design for cancer immunotherapy. Front. Cell Infect. Microbiol. 2024, 14, 1501010. [Google Scholar] [CrossRef]
- Yordanova, D.; Schultz, T.W.; Kuseva, C.; Tankova, K.; Ivanova, H.; Dermen, I.; Pavlov, T.; Temelkov, S.; Chapkanov, A.; Georgiev, M. Automated and standardized workflows in the OECD QSAR Toolbox. Comput. Toxicol. 2019, 10, 89–104. [Google Scholar] [CrossRef]
- Achar, J.; Firman, J.W.; Cronin, M.T.D.; Oberg, G. A framework for categorizing sources of uncertainty in in silico toxicology methods: Considerations for chemical toxicity predictions. Regul. Toxicol. Pharmacol. 2024, 154, 105737. [Google Scholar] [CrossRef] [PubMed]
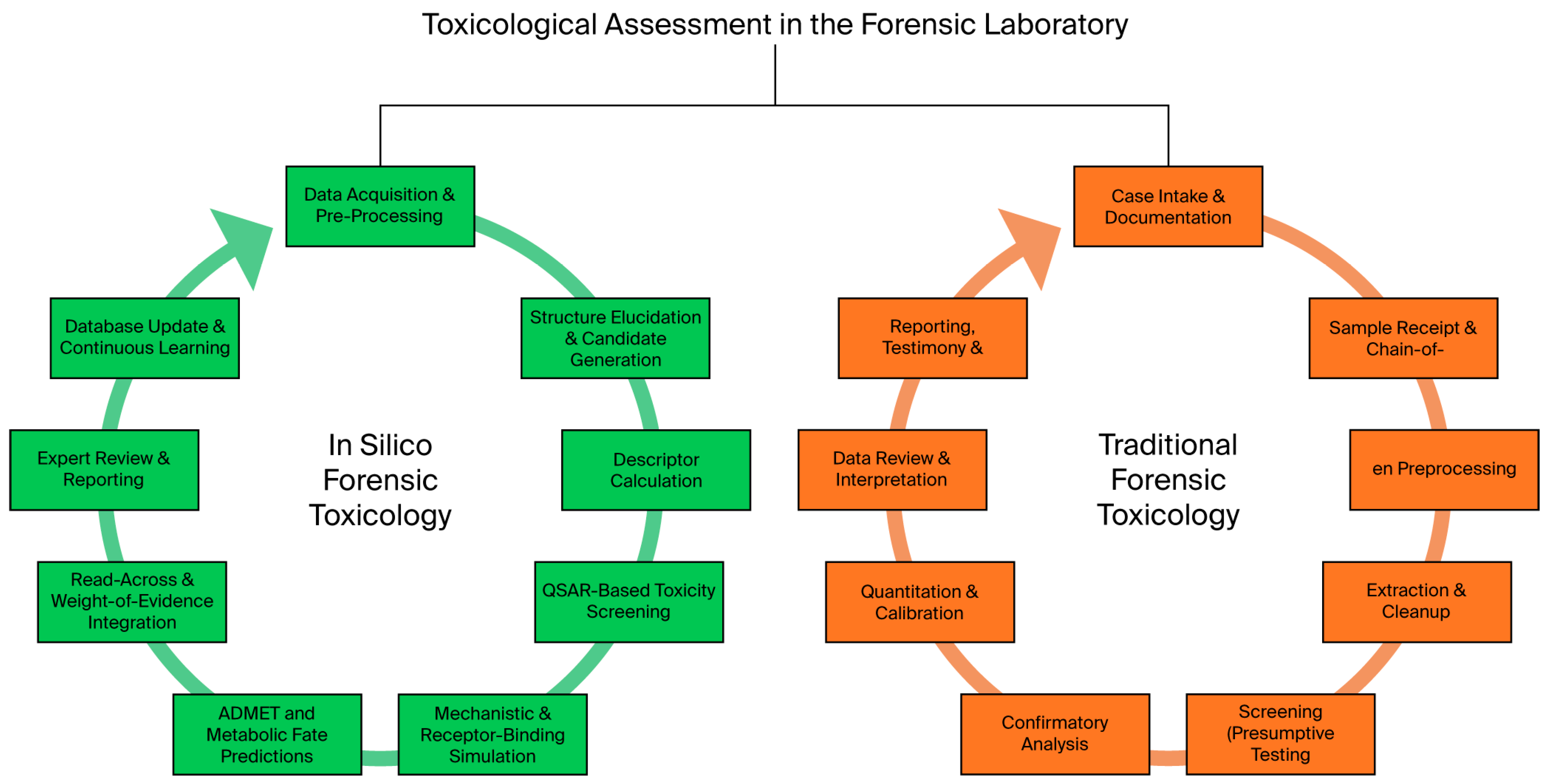
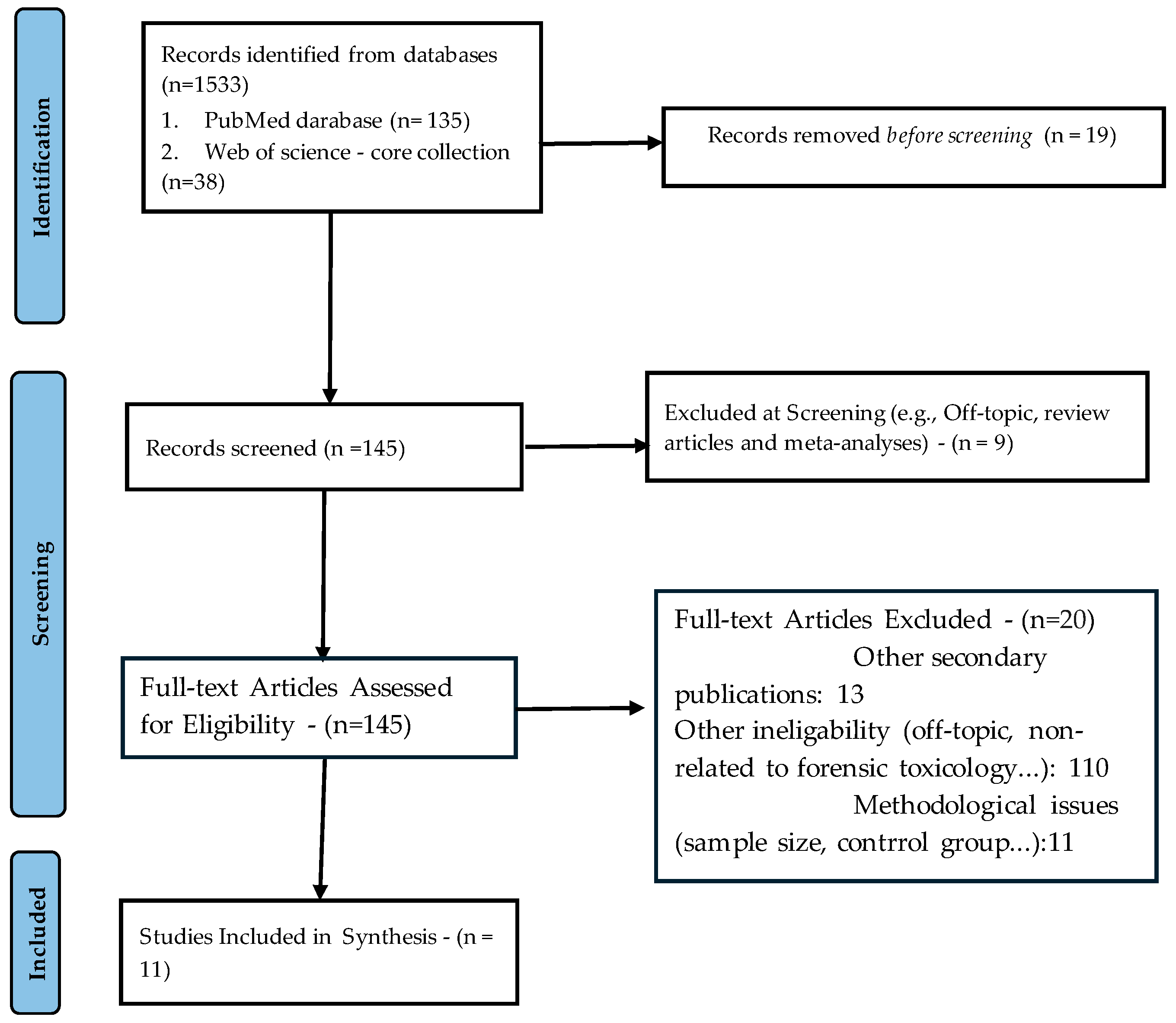
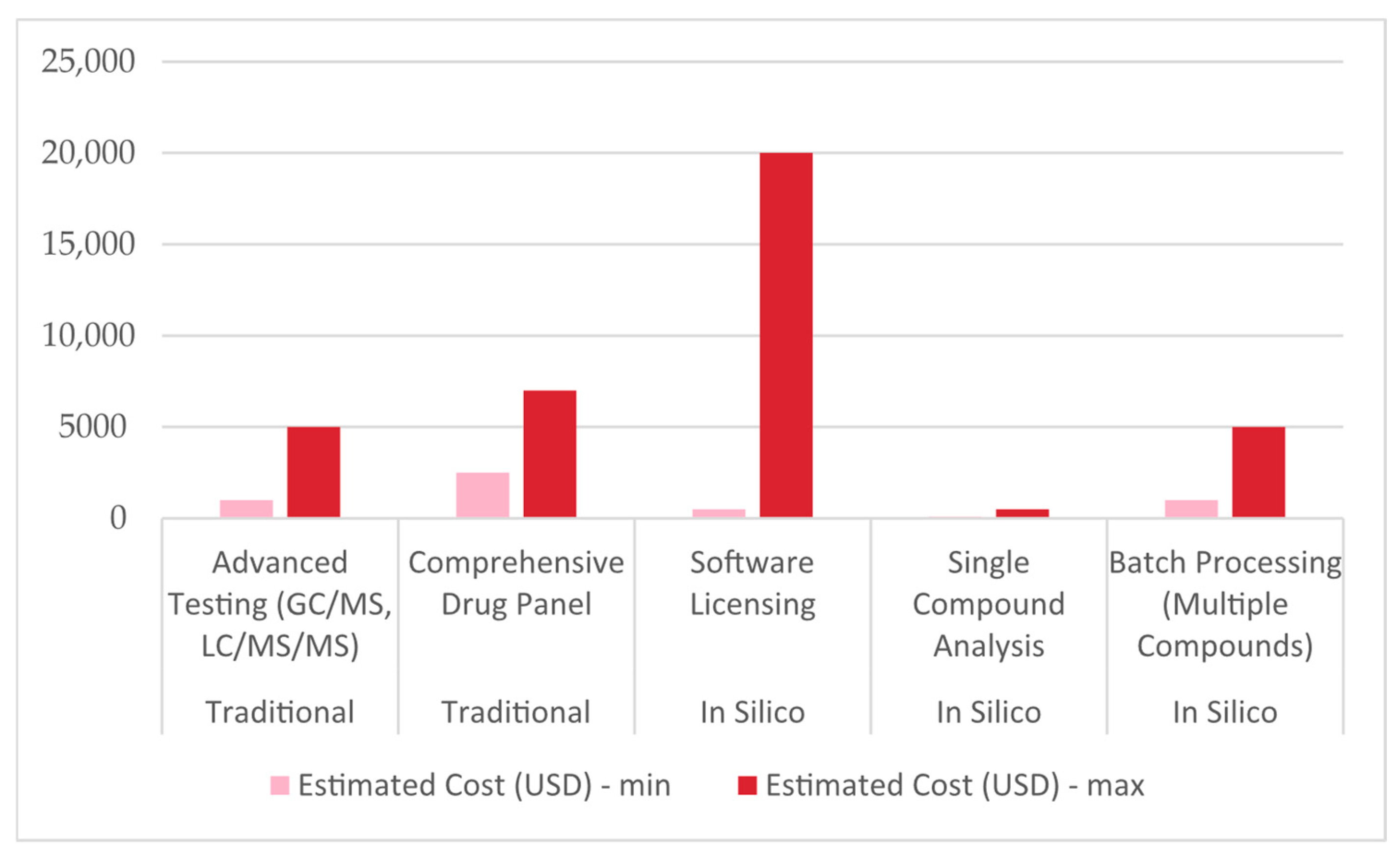
| Study (Authors Year) | P: Population/Substance | I: Intervention/Methods | C: Comparison/Baseline | O: Outcomes | Reference |
|---|---|---|---|---|---|
| Wohlfarth et al., 2016 | Human liver microsomes and reference volunteers exposed to AH-7921 In vitro metabolic stability assays; in silico prediction; in vivo confirmation | In silico predictions vs. observed in vitro/in vivo profiles | Metabolic stability data; full metabolite panel for AH-7921 | Provides validated metabolites to target in forensic screens; improves interpretations of AH-7921 intoxications | [23] |
| Hernandez et al., 2019 | Model chemical mixtures relevant to human exposures Integration of in vitro bioassays; in silico models; epidemiological data | Single-chemical risk assessments | Harmonized risk estimates for mixtures | Framework to interpret mixed-compound toxicology in forensic casework; supports expert testimony on combined exposures | [33] |
| Busardò et al., 2022 | Human subjects and hepatic models given acetazolamide In silico metabolite prediction; in vitro hepatocyte assays; in vivo sampling | Conventional metabolic data for acetazolamide | Comprehensive metabolite list; elimination kinetics | Identifies masking agent metabolites for doping control; guides anti-doping laboratories’ workflows | [31] |
| Pelletier et al., 2023 | Hepatic systems and volunteers exposed to 4-Cl-PVP Cross-disciplinary in vitro assays; in silico docking; limited in vivo profiling | Reference cathinone metabolic profiles | Structural identification of major and minor metabolites | Enables forensic labs to detect 4-Cl-PVP and differentiate it from other cathinones | [29] |
| Jurowski and Krosniak, 2024 | New psychoactive substance AP-238 In silico QSAR and toxicity-prediction algorithms | Published toxicity endpoints of similar NPSs | Predicted LD50, ARfD, genotoxicity, organ toxicity endpoints | Rapid hazard-ranking tool for emergent NPSs; assists forensic toxicologists in triage and risk communication | [28] |
| Noga et al., 2024 | Organophosphorus V-series nerve agents In silico acute-toxicity (LD50) modeling | Historical animal-derived LD50 values | Predicted human LD50 ranges | Supports threat assessments of chemical warfare agents; informs forensic readiness and triage protocols | [25] |
| Berardinelli et al., 2025 | Novel synthetic opioid Dipyanone In vitro human hepatocyte incubations; in vivo volunteer studies; receptor binding assays | Methadone and known opioid metabolic profiles | Metabolite map; pharmacokinetic parameters; μ-opioid affinity | Supplies detection targets and potency data for forensic and clinical toxicology; refines interpretation of Dipyanone overdoses | [24] |
| Pampalakis et al., 2023, | Organophosphorus V-series nerve agents | In silico toxicity predictions (QSAR and computational models) | Empirical toxicity data from animal studies and the literature | Highlights critical limitations of unvalidated computational predictions in forensic assessments Warns clinicians of potential under-triage in V-agent exposures; emphasizes need for empirical confirmation | [26] |
| Noga and Jurowski, 2025 | Bicyclic organophosphorus compounds In silico acute-toxicity and mechanistic models | V-series organophosphonates | Predicted LD50, mechanistic toxicity pathways | Guides forensic identification of emerging OP threats; supports rapid hazard evaluation | [13] |
| Pelletier et al., 2025 | Diverse new psychoactive substances In silico metabolite prediction platforms | Experimental metabolite libraries | Ranked list of likely phase I/II metabolites | Prioritizes compounds for analytical method development in forensic labs; accelerates NPS detection | [30] |
| Tang et al., 2025 | Two phenethylamine-derived NPSs Integrated in silico docking; in vitro microsomal assays; in vivo rodent studies | Standard phenethylamine metabolic pathways | Complete metabolite profiling; metabolic-kinetic parameters | Provides validated biomarkers for forensic screening of new phenethylamines; informs toxicological interpretation | [32] |
| Study | Design and Population | Intervention | Outcomes |
|---|---|---|---|
| Toennes et al. [37] | A controlled dosing study in human volunteers. | Oral administration of 4-fluoroamphetamine | Measurement of urinary metabolites (pharmacokinetics) to support forensic and therapeutic insights. |
| Papaseit et al. [38] | An observational study in humans evaluating acute effects. | Administration of mephedrone via oral and intranasal routes | Assessment of acute pharmacological effects (likely vital sign changes, subjective effects, etc.) in a real-life setting. |
| Losacker et al. [39] | A controlled, interventional pharmacokinetic study involving human subjects. | Controlled oral ingestion of 4-fluoroamphetamine | Measurement of chiral (R)/serum concentration (S) ratios to aid interpretation in forensic toxicology. |
| Parameter | In Silico Toxicology | Traditional Toxicology |
|---|---|---|
| Fixed Annual Costs (F) | EUR 50,000 | EUR 100,000 |
| Variable Cost per Analysis (v) | EUR 20 | EUR 80 |
| Revenue per Analysis (p) | EUR 100 | EUR 200 |
| Contribution margin per Analysis (p—−v) | EUR 80 | EUR 120 |
| Break-Even Analyses | 625 analyses/year | 834 analyses/year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I. In Silico Forensic Toxicology: Is It Feasible? Toxics 2025, 13, 790. https://doi.org/10.3390/toxics13090790
Šoša I. In Silico Forensic Toxicology: Is It Feasible? Toxics. 2025; 13(9):790. https://doi.org/10.3390/toxics13090790
Chicago/Turabian StyleŠoša, Ivan. 2025. "In Silico Forensic Toxicology: Is It Feasible?" Toxics 13, no. 9: 790. https://doi.org/10.3390/toxics13090790
APA StyleŠoša, I. (2025). In Silico Forensic Toxicology: Is It Feasible? Toxics, 13(9), 790. https://doi.org/10.3390/toxics13090790








