Review of Health Hazards in High-Entropy Alloy Processing Under Laboratory Conditions and Risk Assessment Using a Simple Risk Scoring Model
Abstract
1. Introduction
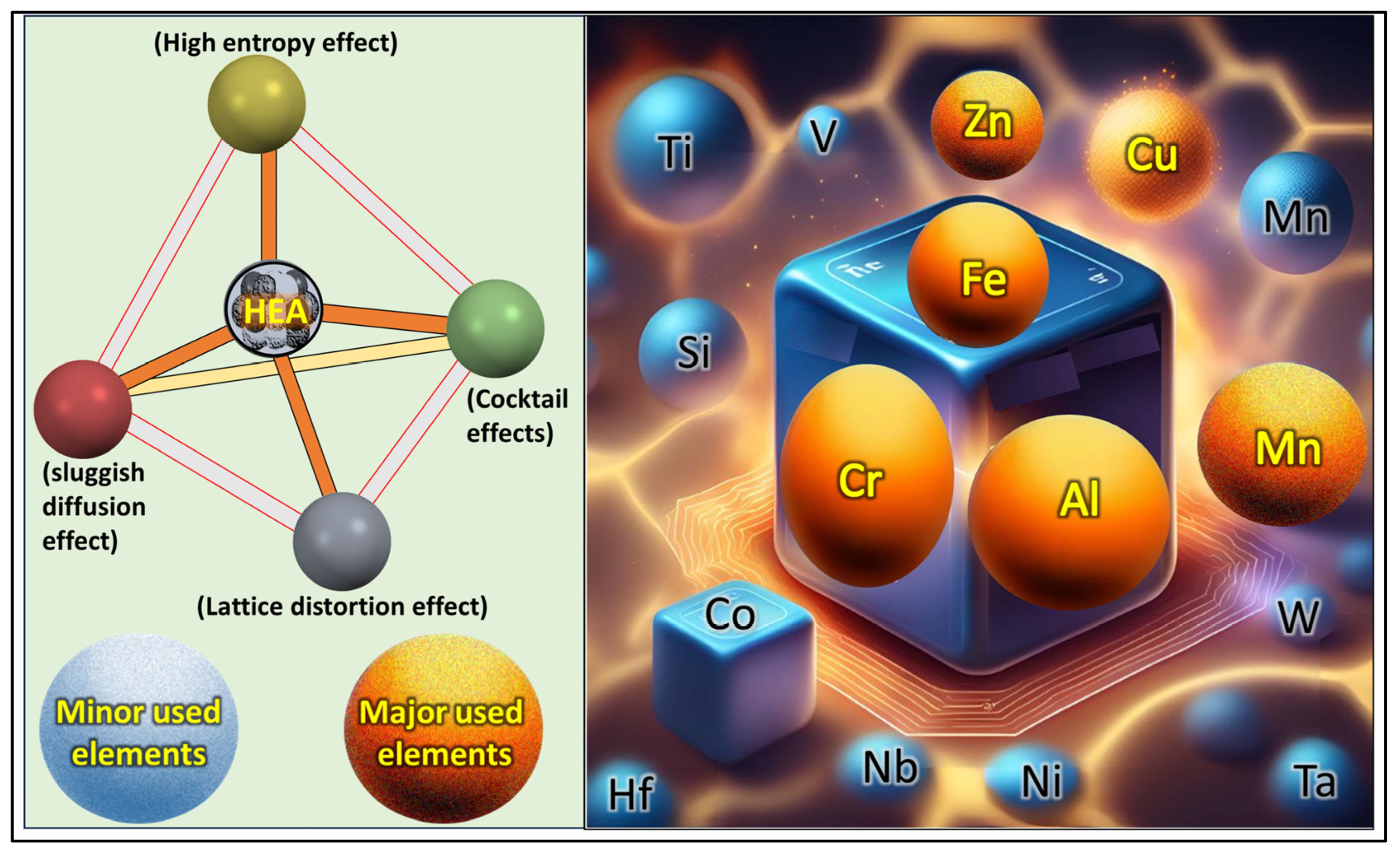
2. Background of HEAs
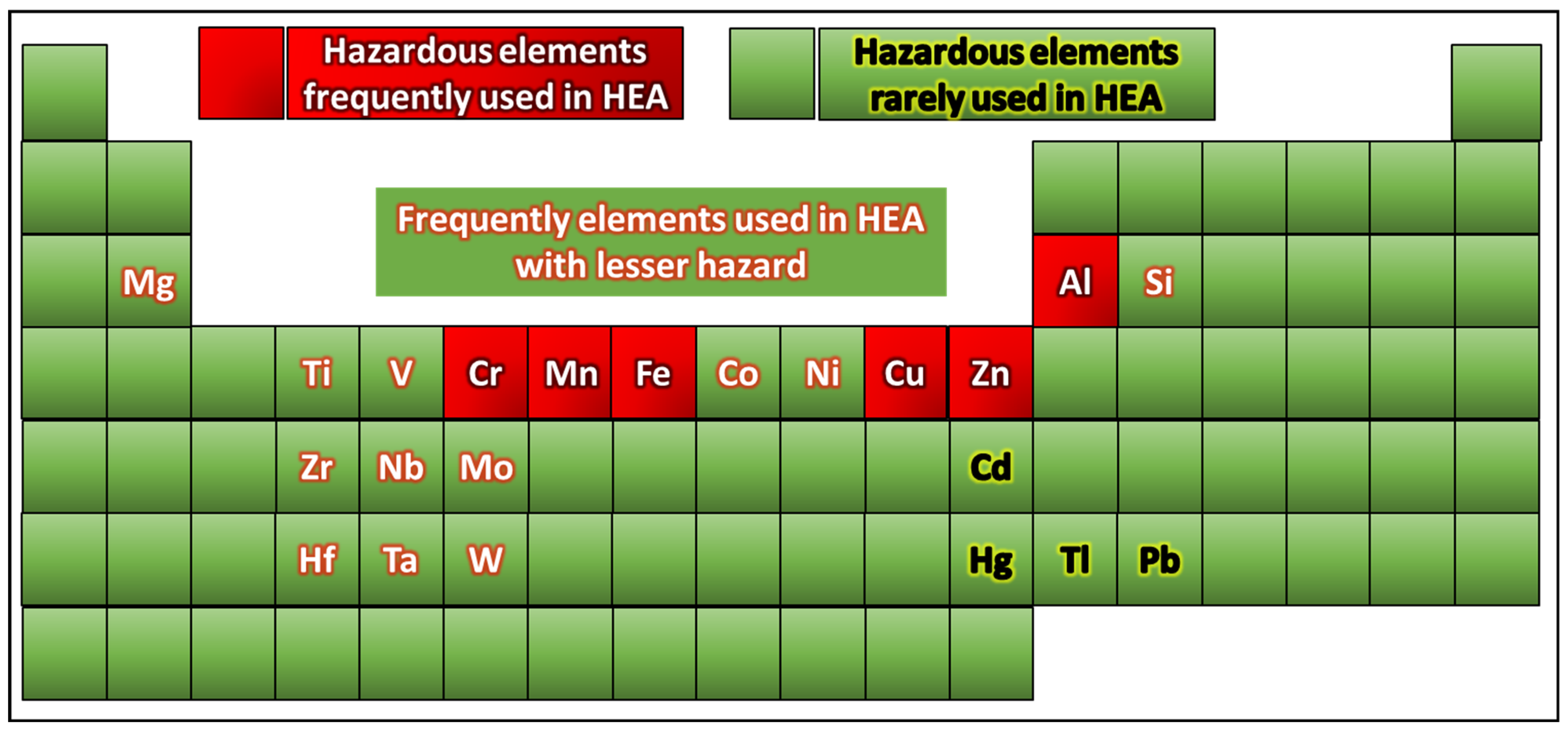
Classes of Elements Used in HEAs
- (i).
- Reactive metals: Reactive metals constitute a category of metallic elements characterized by a pronounced inclination to engage in chemical reactions with other substances, notably oxygen and moisture, resulting in the formation of oxides. This reactivity stems from low electronegativity and a distinct tendency to attain a stable electron configuration. Examples of reactive metals include alkali metals (e.g., sodium and potassium), alkaline earth metals (e.g., calcium and magnesium), as well as transition metals such as titanium and zirconium [53]. Major properties of hazardous reactive metals such as Ti, Zr, Hf, Mg, and Al, which exhibit high affinity for oxygen/nitrogen and can cause fire or explosion hazards in fine powder form in Figure 5.
- (ii).
- Toxic elements: Toxic elements refer to chemical elements that, when present in specific amounts or configurations, can harm living organisms, including humans, animals, and plants. These elements can have detrimental effects on both human health and ecosystems by disrupting biological processes and interfering with typical physiological functions. The influence of toxic elements varies based on factors such as concentration, exposure duration, and the chemical state in which they are present. Prior studies have examined toxic elements, exploring their origins and impacts and proposing approaches to mitigate their adverse effects [54]. Major properties of hazardous toxic elements (e.g., Ni, Co, Cr, V) associated with respiratory disorders, organ damage, and carcinogenicity upon occupational exposure are shown in Figure 6.
- (iii).
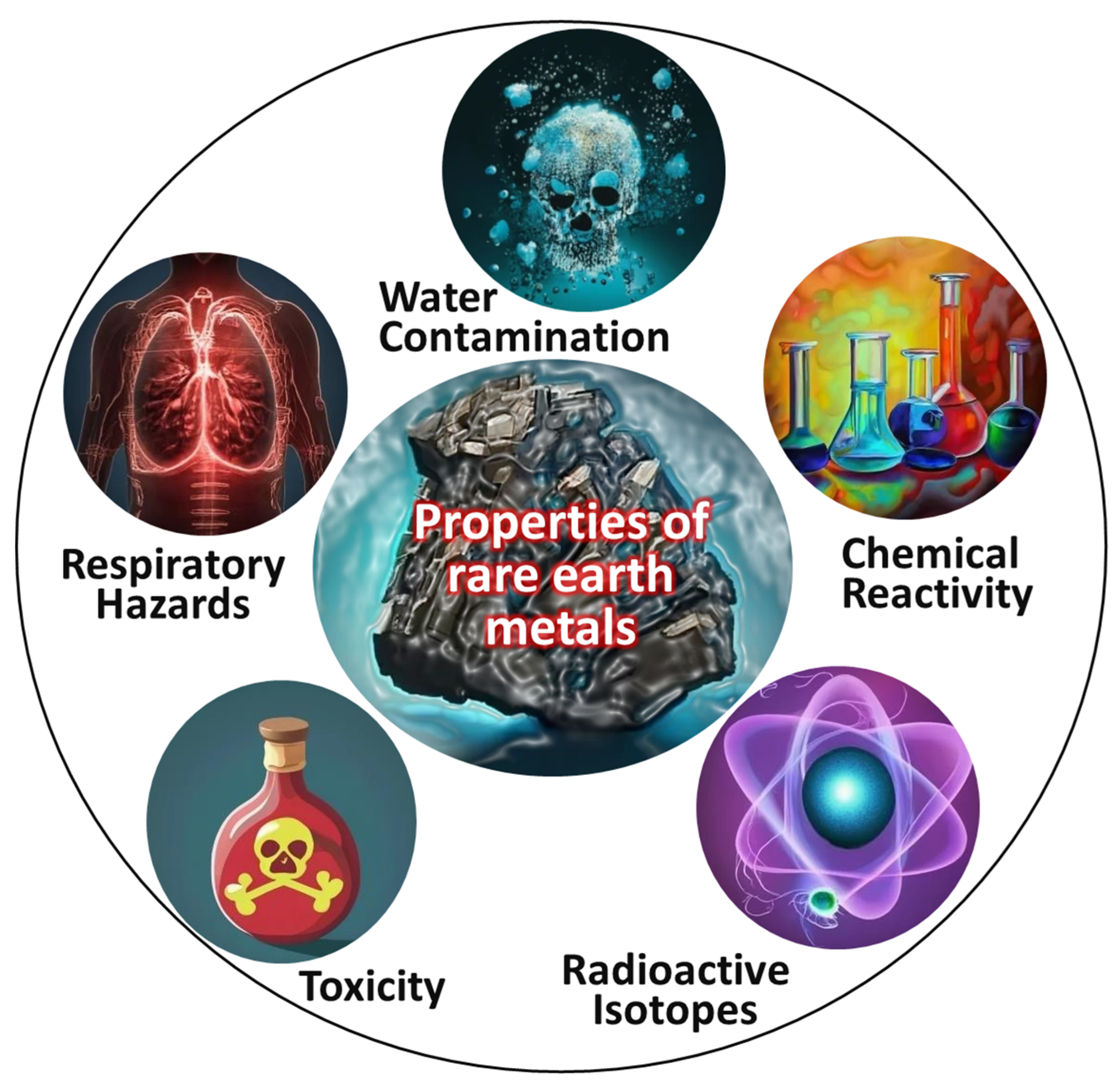
- Rare-earth elements: Rare-earth elements possess hazardous properties, including the presence of radioactive isotopes (such as thorium and uranium), toxicity at high concentrations (notably europium and terbium), the release of toxic or flammable gases due to chemical reactivity, respiratory hazards due to fine-particle generation during mining and processing, and the potential for water contamination through runoff from industrial activities. Effective safety measures, environmentally responsible practices, and proper waste management are crucial for mitigating these hazards and minimizing the adverse effects of these elements on human health and the environment [55,56,57]. Major properties of rare-earth elements (e.g., Ce, Y, La) that may induce lung fibrosis, oxidative stress, and bioaccumulation, posing long-term health hazards in laboratory environments, are shown in Figure 7.
3. Hazardous Effects and Related Diseases
4. Risk Assessment Methodology
4.1. Simple Risk Scoring Model
4.2. Methodology
4.3. Discussion on Risk Assessment of Metallic Elements
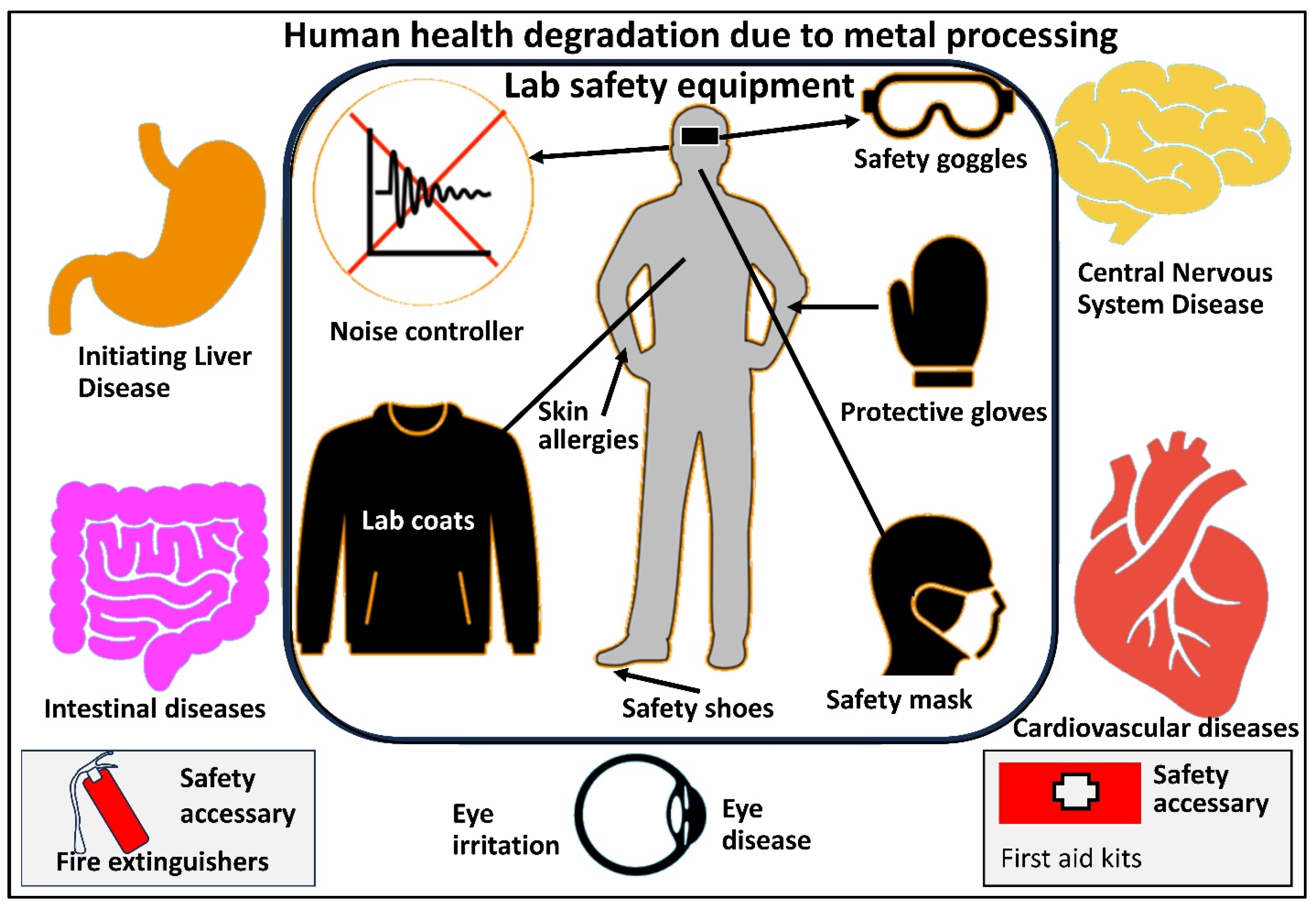
5. Essential Precautions and Prevention Measures
5.1. Prevention Measures
5.2. Health Monitoring
- (i).
- MSDS: MSDS or safety data sheets must be maintained for all metal powders used in the workplace, and they should be readily available to research scholars.
- (ii).
- Regulatory compliance: Individuals must comply with department rules, university authorities, and standards related to the handling and disposal of hazardous materials.
6. Knowledge Gaps and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- About the GHS|UNECE. Available online: https://unece.org/about-ghs (accessed on 19 March 2024).
- Mridha, S. Metallic Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- International Atomic Energy Agency. Guidelines for Integrated Risk Assessment and Management in Large Industrial Areas; International Atomic Energy Agency: Vienna, Austria, 1998; pp. 1–274. [Google Scholar]
- Nordheim, E.; Newson, T. Risk Assessment of Alloys. In Risk Management of Complex Inorganic Materials: A Practical Guide; Academic Press: Cambridge, MA, USA, 2018; pp. 219–243. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kipling, M.D. Health Hazards and Powder Metallurgy. Powder Metall. 1976, 19, 8–11. [Google Scholar] [CrossRef]
- Singer, A.R.E. New areas for development in metal powder and particle technology. Powder Metall. 1976, 19, 4–7. [Google Scholar] [CrossRef]
- Sprince, N.L.; Oliver, L.C.; Eisen, E.A.; Greene, R.E.; Chamberlin, R.I. Cobalt exposure and lung disease in tungsten carbide production. A cross-sectional study of current workers. Am. Rev. Respir. Dis. 1988, 138, 1220–1226. [Google Scholar] [CrossRef]
- Swennen, B.; Buchet, J.P.; Stanescu, D.; Lison, D.; Lauwerys, R. Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Occup. Environ. Med. 1993, 50, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Auchincloss, J.H.; Abraham, J.L.; Gilbert, R.; Lax, M.; Henneberger, P.K.; Heitzman, E.R.; Peppi, D.J. Health hazard of poorly regulated exposure during manufacture of cemented tungsten carbides and cobalt. Occup. Environ. Med. 1992, 49, 832–836. [Google Scholar] [CrossRef][Green Version]
- Kennedy, S.M.; Chan-Yeung, M.; Marion, S.; Lea, J.; Teschke, K. Maintenance of stellite and tungsten carbide saw tips: Respiratory health and exposure-response evaluations. Occup. Environ. Med. 1995, 52, 185–191. [Google Scholar] [CrossRef]
- Willhite, C.C.; Karyakina, N.A.; Yokel, R.A.; Yenugadhati, N.; Wisniewski, T.M.; Arnold, I.M.F.; Momoli, F.; Krewski, D. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol. 2014, 44, 1–80. [Google Scholar] [CrossRef]
- Bhasin, P.; Singla, N.; Dhawan, D.K. Protective role of zinc during aluminum-induced hepatotoxicity. Environ. Toxicol. 2014, 29, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Lantzy, R.J.; Mackenzie, F.T. Atmospheric trace metals: Global cycles and assessment of man’s impact. Geochim. Cosmochim. Acta 1979, 43, 511–523. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef]
- Fergusson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Vallabani, N.V.S.; Wang, X.; Assenhöj, M.; Ljunggren, S.; Karlsson, H.; Odnevall, I. Health hazards of particles in additive manufacturing: A cross-disciplinary study on reactivity, toxicity and occupational exposure to two nickel-based alloys. Sci. Rep. 2023, 13, 20846. [Google Scholar] [CrossRef]
- Wang, X.; Wallinder, I.O.; Hedberg, Y. Bioaccessibility of Nickel and Cobalt Released from Occupationally Relevant Alloy and Metal Powders at Simulated Human Exposure Scenarios. Ann. Work. Expo. Health 2020, 64, 659. [Google Scholar] [CrossRef]
- Occupational Exposure to Hexavalent Chromium|Occupational Safety and Health Administration. Available online: https://www.osha.gov/laws-regs/federalregister/2006-02-28-0 (accessed on 19 March 2024).
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health Part B 2007, 10, 1–269. [Google Scholar] [CrossRef]
- Rosenstock, L.; Cullen, M.; Brodkin, C.; Redlich, C. Textbook of Clinical Occupational and Environmental Medicine, 2nd ed.; U.S. Department of Energy: Washington, DC, USA, 2004.
- Cummings, K.J.; Stefaniak, A.B.; Virji, M.A.; Kreiss, K. A Reconsideration of Acute Beryllium Disease. Environ. Health Perspect. 2009, 117, 1250. [Google Scholar] [CrossRef]
- Clinical Findings Among Hard Metal Workers on JSTOR. Available online: https://www.jstor.org/stable/27727367 (accessed on 19 March 2024).
- Respiratory Diseases in Hard Metal Workers: An Occupational Hygiene Study in a Factory on JSTOR. Available online: https://www.jstor.org/stable/27726227 (accessed on 19 March 2024).
- Issue Paper on the Human Health Effects of Metals|Request pdf. Available online: https://www.researchgate.net/publication/255671769_issue_paper_on_the_human_health_effects_of_metals (accessed on 19 March 2024).
- World Economic Forum. Preface-Global Risks Report 2024. Available online: https://www.weforum.org/publications/global-risks-report-2024/in-full (accessed on 19 March 2024).
- Sakai, M.; Kato, G.; Fujioka, T.; Sugihara, M.; Koyanagi, K.; Kato, O.; Tominaga, M.; Kawabata, Y. A case of hard metal lung disease in a man who worked as an iron grinder. Nihon Kokyuki Gakkai Zasshi 2010, 48, 282–287. [Google Scholar]
- Chiba, Y.; Kido, T.; Tahara, M.; Oda, K.; Noguchi, S.; Kawanami, T.; Yokoyama, M.; Yatera, K. Hard metal lung disease with favorable response to corticosteroid treatment: A case report and literature review. Tohoku J. Exp. Med. 2019, 247, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Kobayashi, M.; Takada, T.; Shimizu, T.; Terada, M.; Narita, J.I.; Maruyama, M.; Watanabe, K.; Suzuki, E.; Gejyo, F. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am. J. Respir. Crit. Care Med. 2007, 176, 70–77. [Google Scholar] [CrossRef]
- Sakamoto, O.; Kosai, S.; Kohrogi, H. A case of hard metal lung disease presenting a type of chronic hypersensitivity pneumonitis. Nihon Kokyuki Gakkai Zasshi 2008, 46, 535–541. [Google Scholar]
- Nemery, B.; Abraham, J.L. Hard metal lung disease: Still hard to understand. Am. J. Respir. Crit. Care Med. 2007, 176, 2–3. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- OSHA.gov. Occupational Chemical Database. Available online: https://www.osha.gov/chemicaldatabase (accessed on 10 September 2025).
- NIOSH. Pocket Guide to Chemical Hazards Introduction|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/npg/pgintrod.html?utm_source=chatgpt.com (accessed on 10 February 2025).
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef]
- Yang, F.; Fu, Q.; Antonietti, M. Anthropogenic, Carbon-Reinforced Soil as a Living Engineered Material. Chem. Rev. 2023, 123, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Stern, P.; Dawood, I.; Cheema, S. Metals and Disease: A Global Primary Health Care Perspective. J. Toxicol. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Li, R.; Song, X.; Duan, Z.; Hao, Z.; Yang, Y.; Han, Y.; Ran, X.; Liu, Y. Improving the high-temperature ductility of γ-TiAl matrix composites by incorporation of AlCoCrFeNi high entropy alloy particles. J. Alloys Compd. 2025, 1012, 178515. [Google Scholar] [CrossRef]
- Modupeola, D.; Popoola, P. Safety practices and occupational hazards of the additive manufacturing of high entropy alloys. Saf. Extrem. Environ. 2024, 6, 139–146. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Nagarjuna, C.; Lee, H.; Sharma, A.; Ahn, B. Surface morphology transformation and densification behaviour of conventionally sintered AlFeCoNiSi high entropy alloys. Powder Metall. 2023, 66, 650–661. [Google Scholar] [CrossRef]
- Rao, K.R.; Dewangan, S.K.; Seikh, A.H.; Sinha, S.K.; Ahn, B. Microstructure and Mechanical Characteristics of AlCoCrFeNi-Based ODS High-Entropy Alloys Consolidated by Vacuum Hot Pressing. Met. Mater. Int. 2023, 30, 726–734. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar Dewangan, S.; Singh, S.; Chopkar, M.; Sreesha, R.B. Chapter 2 Classification of processing routes. In High-Entropy Alloys; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2023; pp. 31–40. [Google Scholar]
- Dewangan, S.K.; Mangish, A.; Kumar, S.; Sharma, A.; Ahn, B.; Kumar, V. A review on High-Temperature Applicability: A milestone for high entropy alloys. Eng. Sci. Technol. Int. J. 2022, 35, 101211. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.; Ahn, B. Effect of Additive Elements (x = Cr, Mn, Zn, Sn) on the Phase Evolution and Thermodynamic Complexity of AlCuSiFe-x High Entropy Alloys Fabricated via Powder Metallurgy. Met. Mater. Int. 2022, 28, 2216–2224. [Google Scholar] [CrossRef]
- Chae, M.J.; Sharma, A.; Oh, M.C.; Ahn, B. Lightweight AlCuFeMnMgTi High Entropy Alloy with High Strength-to-Density Ratio Processed by Powder Metallurgy. Met. Mater. Int. 2021, 27, 629–638. [Google Scholar] [CrossRef]
- Nagarjuna, C.; Dewangan, S.K.; Sharma, A.; Lee, K.; Hong, S.-J.; Ahn, B. Application of Artificial Neural Network to Predict the Crystallite Size and Lattice Strain of CoCrFeMnNi High Entropy Alloy Prepared by Powder Metallurgy. Met. Mater. Int. 2023, 29, 1968–1975. [Google Scholar] [CrossRef]
- Laboratory Safety|Safety Services. Available online: https://safetyservices.ucdavis.edu/units/ehs/research/laboratory (accessed on 19 March 2024).
- Kumari, S.; Mishra, A.; Kumari, S.; Mishra, A. Heavy Metal Contamination. In Soil Contamination-Threats and Sustainable Solutions; IntechOpen: London, UK, 2021. [Google Scholar]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Malhotra, N.; Hsu, H.S.; Liang, S.T.; Roldan, M.J.M.; Lee, J.S.; Ger, T.R.; Hsiao, C.D. An Updated Review of Toxicity Effect of the Rare Earth Elements (REEs) on Aquatic Organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672. [Google Scholar] [CrossRef] [PubMed]
- Alijagic, A.; Engwall, M.; Särndahl, E.; Karlsson, H.; Hedbrant, A.; Andersson, L.; Karlsson, P.; Dalemo, M.; Scherbak, N.; Färnlund, K.; et al. Particle Safety Assessment in Additive Manufacturing: From Exposure Risks to Advanced Toxicology Testing. Front. Toxicol. 2022, 4, 836447. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Rajak, C.; Singh, N.; Parashar, P. Metal toxicity and natural antidotes: Prevention is better than cure. Environ. Sci. Pollut. Res. 2020, 27, 43582–43598. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.C.; Shi, H.; Gu, X. Occupational health and safety risk assessment: A systematic literature review of models, methods, and applications. Saf. Sci. 2022, 160, 106050. [Google Scholar] [CrossRef]
- Gobas, F.A.P.C.; Mayer, P.; Parkerton, T.F.; Burgess, R.M.; van de Meent, D.; Gouin, T. A chemical activity approach to exposure and risk assessment of chemicals. Environ. Toxicol. Chem. 2018, 37, 1235–1251. [Google Scholar] [CrossRef]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans–INTERNATIONAL AGENCY FOR RESEARCH ON CANCER. Available online: https://monographs.iarc.who.int/ (accessed on 12 February 2025).
- Jordan, J.; Maki, R.G. The Weighted Toxicity Score: Confirmation of a Simple Metric to Communicate Toxicity in Randomized Trials of Systemic Cancer Therapy. Oncologist 2024, 29, 67–74. [Google Scholar] [CrossRef]
- Chen, Z.; Krailo, M.D.; Azen, S.P.; Tighiouart, M. A novel toxicity scoring system treating toxicity response as a quasi-continuous variable in Phase I clinical trials. Contemp. Clin. Trials 2010, 31, 473–482. [Google Scholar] [CrossRef] [PubMed]
- METALS-International Occupational Safety & Health Information Centre. Available online: https://www.ilo.org/static/english/protection/safework/cis/products/safetytm/metals.htm (accessed on 20 March 2024).
- Akbar-Khanzadeh, F.; Bisesi, M.S.; Rivas, R.D. Comfort of personal protective equipment. Appl. Ergon. 1995, 26, 195. [Google Scholar] [CrossRef]
- Golmohammadi, R.; Darvishi, E. The combined effects of occupational exposure to noise and other risk factors-a systematic review. Noise Health 2020, 21, 125–141. [Google Scholar]
- Turcot, A.; Girard, S.A.; Courteau, M.; Baril, J.; Larocque, R. Noise-induced hearing loss and combined noise and vibration exposure. Occup. Med. 2015, 65, 238–244. [Google Scholar] [CrossRef]
- Lie, A.; Skogstad, M.; Johannessen, H.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.-C.; Engdahl, B.; Tambs, K. Occupational noise exposure and hearing: A systematic review. Int. Arch. Occup. Environ. Health 2015, 89, 351–372. [Google Scholar] [CrossRef]
- Smagowska, B. Ultrasonic noise sources in a work environment. Arch. Acoust. 2013, 38, 169–176. [Google Scholar] [CrossRef]
- Sabyrov, N.; Jahan, M.P.; Bilal, A.; Perveen, A. Ultrasonic Vibration Assisted Electro-Discharge Machining (EDM)—An Overview. Materials 2019, 12, 522. [Google Scholar] [CrossRef]
- 3D Printing with Metal Powders: Health and Safety Questions to Ask 2020. Available online: https://www.cdc.gov/niosh/docs/2020-114/default.html (accessed on 10 September 2025).
- Health Monitoring|WorkSafe.qld.gov.au. Available online: https://www.worksafe.qld.gov.au/safety-and-prevention/hazards/hazardous-chemicals/managing-hazchem-risks/health-monitoring (accessed on 20 March 2024).
- Rahimpoor, R.; Jalilian, H.; Mohammadi, H.; Rahmani, A. Biological exposure indices of occupational exposure to benzene: A systematic review. Heliyon 2023, 9, e21576. [Google Scholar] [CrossRef] [PubMed]
- DeBord, D.G.; Burgoon, L.; Edwards, S.W.; Haber, L.T.; Kanitz, M.H.; Kuempel, E.; Thomas, R.S.; Yucesoy, B. Systems Biology and Biomarkers of Early Effects for Occupational Exposure Limit Setting. J. Occup. Environ. Hyg. 2015, 12, S41–S54. [Google Scholar] [CrossRef]
- Schulte, P.A.; Hauser, J.E. The use of biomarkers in occupational health research, practice, and policy. Toxicol. Lett. 2012, 213, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, A.H.; Ghobakhloo, S.; Gruszecka-Kosowska, A. Inhalational exposure to heavy metals: Carcinogenic and non-carcinogenic risk assessment. J. Hazard. Mater. Adv. 2024, 16, 100485. [Google Scholar] [CrossRef]







| Metal | OSHA PEL | NIOSH REL | Primary Health Effects |
|---|---|---|---|
| Aluminum (Al) | 15 mg/m3 (total dust); 5 mg/m3 (respirable fraction) | 10 mg/m3 (total dust); 5 mg/m3 (respirable fraction) | Respiratory issues, lung fibrosis |
| Iron (Fe) | 10 mg/m3 (as iron oxide fume) | 5 mg/m3 (as iron oxide fume) | Respiratory irritation, benign pneumoconiosis |
| Manganese (Mn) | 5 mg/m3 (ceiling limit) | 1 mg/m3 (TWA); 3 mg/m3 (STEL) | Neurological effects, manganism |
| Zinc (Zn) | 5 mg/m3 (fume); 15 mg/m3 (total dust); 5 mg/m3 (respirable fraction) | 5 mg/m3 (TWA); 15 mg/m3 (ceiling, 15 min) | Metal fume fever, respiratory irritation |
| Tungsten (W) | 5 mg/m3 (insoluble compounds) | 5 mg/m3 (TWA); 10 mg/m3 (STEL) | Respiratory irritation |
| Silicon (Si) | 15 mg/m3 (total dust); 5 mg/m3 (respirable fraction) | Not established | Respiratory issues with prolonged exposure |
| Copper (Cu) | 1 mg/m3 (dusts and mists); 0.1 mg/m3 (fume) | 1 mg/m3 (dusts and mists); 0.1 mg/m3 (fume) | Respiratory irritation, gastrointestinal distress |
| Titanium (Ti) | Not established | Not established | Generally considered low toxicity, titanium dioxide dust may cause respiratory irritation. |
| Hafnium (Hf) | 0.5 mg/m3 | 0.5 mg/m3 | Eye and skin irritation, respiratory issues |
| Niobium (Nb) | Not established | Not established | Limited data; potential respiratory effects |
| Tantalum (Ta) | 5 mg/m3 | 5 mg/m3 | Lung effects, fibrosis with prolonged exposure |
| Disease | Description |
|---|---|
| (i) Respiratory concerns | The utilization of metal powders in various industries can lead to health issues among lab personnel. Inhaling metal powders, especially fine particles, may result in respiratory complications, such as coughing, shortness of breath, and lung diseases, including fibrosis. Certain metals, such as aluminum and cobalt, have been linked to severe lung diseases. |
| (ii) Systemic and organ-specific toxicity risks | Metal powders, particularly those containing lead and cadmium, can have toxic effects when they enter the body. Lead, in particular, can cause lead poisoning, which impacts the nervous system and various other organs. |
| (iii) Allergic reactions | Working with certain metal powders can trigger allergic responses in susceptible individuals. For instance, cobalt exposure has been associated with acute asthma in sensitive individuals. |
| (iv) Skin sensitization | Contact with metal powders can result in skin irritation, rashes, and dermatitis, especially under prolonged or recurrent exposure. |
| (v) Cancer susceptibility | Prolonged exposure to specific metal powders, such as nickel and chromium, is linked to an increased risk of cancer, particularly in industrial environments. |
| (vi) Cardiovascular implications | Fine particulate matter, including metal powders, can enter the bloodstream and cause cardiovascular problems. |
| (vii) Digestive disturbances | Inadvertently ingesting metal powders, often through hand-to-mouth contact, can lead to gastrointestinal issues. |
| (viii) Radiographic abnormalities | Certain metals, when deposited in the lungs, may not cause immediate harm but may create distinct patterns on chest radiographs. |
| Key Parameters | Description | |
|---|---|---|
| 1 | Toxicity Score (TS) | Represents the inherent toxicity of a metal, assigned based on known toxicological effects. A higher score indicates greater toxicity. |
| 2 | Carcinogenicity Factor (CF) | Based on the International Agency for Research on Cancer (IARC) classification, carcinogenicity is categorized as follows [64]. Group 1 (Carcinogenic to humans) = 5 Group 2A (Probably carcinogenic) = 4 Group 2B (Possibly carcinogenic) = 3 Not classified = 1 |
| Metal | Toxicity Score | Carcinogenicity | Risk Score | TLV (mg/m3) | Explanation |
|---|---|---|---|---|---|
| Mg | 1 | Not classified | 1 | 10.0 |
|
| Ti | 1 | Not classified | 1 | 10.0 | |
| V | 4 | Not classified | 4 | 0.05 | |
| Cr | 5 | Group 1 | 25 | 0.01 | |
| Mn | 3 | Not classified | 3 | 0.2 | |
| Fe | 2 | Not classified | 2 | 5.0 | |
| Co | 4 | Group 2A | 16 | 0.02 | |
| Ni | 4 | Group 1 | 20 | 0.015 | |
| Cu | 2 | Not classified | 2 | 1.0 | |
| Zn | 1 | Not classified | 1 | 5.0 | |
| Al | 1 | Not classified | 1 | 10.0 | |
| Si | 1 | Not classified | 1 | 5.0 | |
| Zr | 1 | Not classified | 1 | 5.0 | |
| Nb | 1 | Not classified | 1 | 5.0 | |
| Mo | 1 | Not classified | 1 | 10.0 | |
| Hf | 1 | Not classified | 1 | 0.5 | |
| Ta | 1 | Not classified | 1 | 5.0 | |
| W | 1 | Not classified | 1 | 5.0 | |
| Cd | 5 | Group 1 | 25 | 0.002 | |
| Hg | 5 | Group 2A | 20 | 0.01 | |
| Pb | 5 | Group 2A | 20 | 0.05 |
| Personal Protective Equipment | Purpose |
|---|---|
| Safety glasses or goggles | Protect the eyes from splashes, dust, and particles. |
| Lab coats or protective Clothing | Prevent skin contact with powders. |
| Respiratory protection | Depending on the particle size and toxicity of the metal powders, use appropriate respirators (e.g., N95 or higher) to prevent inhalation of airborne particles. |
| Gloves | Use gloves resistant to the specific metal being handled. |
| Disposable coveralls or dust suits | These can provide additional protection when working with highly hazardous or toxic powders. |
| Control Measures | Safe Practices for Handling Metal Powders |
|---|---|
| Local exhaust ventilation | Install fume hoods or local exhaust systems to capture and remove airborne dust or fumes at the source. |
| Isolation and containment | Keep metal-powder handling areas separated from other workspaces to prevent contamination. |
| Dust-collection systems | Use dust collectors or industrial vacuum systems to capture and contain loose powders. |
| Safe handling practices | Handle all the equipment with patience and mindfulness. |
| Minimize dust generation | Use techniques such as wetting the powders or working in glove boxes with controlled atmospheres to reduce dust generation. |
| Avoid spills | Handle powders with care to prevent spills or releases. |
| No smoking or eating | Prohibit smoking, eating, or drinking in areas where metal powders are handled. |
| Proper labeling | Clearly label containers with the type of metal powder and any associated hazards. |
| Regular cleaning | Maintain a clean work environment and regularly clean surfaces to prevent dust buildup. |
| Training and Education | Ensure that lab personnel are trained in the safe handling of metal powders, including the proper use of PPE and the recognition of potential hazards. |
| Emergency response | Establish emergency response procedures, including response to spillage and evacuation plans. |
| Process/Equipment | Noise Level (dB) | Vibration Frequency (Hz) | Limited by OSHA | Remark |
|---|---|---|---|---|
| Ball Milling | 80–90 | 5–15 | <85 dB | Noise levels can vary depending on the mill design and operational parameters. Vibration frequencies are typically low, contributing to the overall noise environment. |
| Sintering | 60–70 | Minimal | Sintering furnaces generally produce low noise and minimal vibration, primarily from auxiliary equipment like fans or conveyors. | |
| Ultrasonication | 70–85 | 20,000–40,000 | Ultrasonic processors operate at high frequencies (20 kHz and above), with noise levels depending on power settings and enclosure effectiveness [72]. | |
| Cutting/Polishing | 85–95 | 50–60 | Mechanical cutting and polishing tools generate significant noise and vibration, varying with material hardness and equipment type. | |
| Wire EDM | 75–85 | Variable | Wire Electrical Discharge Machining produces moderate noise; vibration levels depend on machine settings and workpiece material [73]. |
| Aspect | Recommendation | Details/Indicators | Testing Frequency | Ref. |
|---|---|---|---|---|
| Initial Medical Examination | Baseline assessment | Comprehensive medical and occupational history | Once, before exposure | [39,71] |
| Complete Blood Count (CBC) | Monitoring hematological changes | Detect systemic toxicity | High risk: Quarterly Moderate risk: Semi-annual Low risk: Annual | [71,76] |
| Liver Function Tests (LFTs) | Monitor hepatic function | Early detection of liver damage | High risk: Quarterly Moderate risk: Semi-annual Low risk: Annual | [71,76] |
| Kidney Function Tests | Monitor renal function | Early detection of nephrotoxicity | High risk: Quarterly Moderate risk: Semi-annual Low risk: Annual | [71,76] |
| Blood Metal Levels | Measure specific metals (e.g., Pb, Cd, Hg) | Assess internal exposure | High risk: Quarterly Moderate risk: Semi-annual Low risk: Annual | [71,77] |
| Urinary Metal Excretion | Measure metal content in urine | Provides body burden information | High risk: Quarterly Moderate risk: Semi-annual Low risk: Annual | [71,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewangan, S.K. Review of Health Hazards in High-Entropy Alloy Processing Under Laboratory Conditions and Risk Assessment Using a Simple Risk Scoring Model. Toxics 2025, 13, 777. https://doi.org/10.3390/toxics13090777
Dewangan SK. Review of Health Hazards in High-Entropy Alloy Processing Under Laboratory Conditions and Risk Assessment Using a Simple Risk Scoring Model. Toxics. 2025; 13(9):777. https://doi.org/10.3390/toxics13090777
Chicago/Turabian StyleDewangan, Sheetal Kumar. 2025. "Review of Health Hazards in High-Entropy Alloy Processing Under Laboratory Conditions and Risk Assessment Using a Simple Risk Scoring Model" Toxics 13, no. 9: 777. https://doi.org/10.3390/toxics13090777
APA StyleDewangan, S. K. (2025). Review of Health Hazards in High-Entropy Alloy Processing Under Laboratory Conditions and Risk Assessment Using a Simple Risk Scoring Model. Toxics, 13(9), 777. https://doi.org/10.3390/toxics13090777







