Latency Period Among Asbestosis Cases in South Korea by Demographic and Asbestos Exposure Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Asbestos Exposure Assessment
2.3. Latency Period
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ANCOVA | Analysis of covariance |
References
- Gulati, M.; Redlich, C.A. Asbestosis and environmental causes of usual interstitial pneumonia. Curr. Opin. Pulm. Med. 2015, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sporn, T.A.; Roggli, V.L. Asbestosis. In Pathology of Asbestos-Associated Diseases; Springer: Berlin/Heidelberg, Germany, 2013; pp. 53–80. [Google Scholar]
- Ki, Y.-H.; Kim, J.-M.; Roh, Y.-M.; Chung, L.; Kim, Y.-S.; Sim, S.-H. A survey for some asbestos containing products in Korea. J. Environ. Health Sci. 2008, 34, 108–115. [Google Scholar] [CrossRef][Green Version]
- Kang, D.-M.; Kim, J.-E.; Lee, Y.-J.; Lee, H.-H.; Lee, C.-Y.; Moon, S.-J.; Kang, M.-S. Environmental health centers for asbestos and their health impact surveys and activities. Ann. Occup. Environ. Med. 2016, 28, 68. [Google Scholar] [CrossRef]
- Huh, D.-A.; Chae, W.-R.; Choi, Y.-H.; Kang, M.-S.; Lee, Y.-J.; Moon, K.-W. Disease latency according to asbestos exposure characteristics among malignant mesothelioma and asbestos-related lung cancer cases in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 15934. [Google Scholar] [CrossRef]
- Guerreschi, E.; Dragoni, L.; Sisinni, A.; Fabrizi, S.; Miceli, G.; Nante, N. Asbestos is still paid for dearly. Eur. J. Public Health 2024, 34 (Suppl. 3), ckae144.1455. [Google Scholar] [CrossRef]
- Marinaccio, A.; Binazzi, A.; Cauzillo, G.; Cavone, D.; De Zotti, R.; Ferrante, P.; Gennaro, V.; Gorini, G.; Menegozzo, M.; Mensi, C.; et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur. J. Cancer 2007, 43, 2722–2728. [Google Scholar] [CrossRef]
- Magnani, C.; Ferrante, D.; Barone-Adesi, F.; Bertolotti, M.; Todesco, A.; Mirabelli, D.; Terracini, B. Cancer risk after cessation of asbestos exposure: A cohort study of Italian asbestos cement workers. Occup. Environ. Med. 2008, 65, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Baur, X. Asbestos-related disorders in Germany: Background, politics, incidence, diagnostics and compensation. Int. J. Environ. Res. Public Health 2018, 15, 143. [Google Scholar] [CrossRef]
- Petrof, O.; Neyens, T.; Nuyts, V.; Nackaerts, K.; Nemery, B.; Faes, C. On the impact of residential history in the spatial analysis of diseases with a long latency period: A study of mesothelioma in Belgium. Stat. Med. 2020, 39, 3840–3866. [Google Scholar] [CrossRef]
- Huh, D.-A.; Choi, Y.-H.; Kim, L.; Park, K.; Lee, J.; Hwang, S.H.; Moon, K.W.; Kang, M.-S.; Lee, Y.-J. Air pollution and survival in patients with malignant mesothelioma and asbestos-related lung cancer: A follow-up study of 1591 patients in South Korea. Environ. Health 2024, 23, 56. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-C.; Kim, Y.; Hong, W.-H. Predicting the mortality from asbestos-related diseases based on the amount of asbestos used and the effects of slate buildings in Korea. Sci. Total Environ. 2016, 542, 1–11. [Google Scholar] [CrossRef]
- Kwak, K.M.; Paek, D.; Hwang, S.-S.; Ju, Y.-S. Estimated future incidence of malignant mesothelioma in South Korea: Projection from 2014 to 2033. PLoS ONE 2017, 12, e0183404. [Google Scholar] [CrossRef]
- Kwak, K.; Cho, S.-I.; Paek, D. Future incidence of malignant mesothelioma in South Korea: Updated projection to 2038. Int. J. Environ. Res. Public Health 2021, 18, 6614. [Google Scholar] [CrossRef]
- Frost, G. The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005). Br. J. Cancer 2013, 109, 1965–1973. [Google Scholar] [CrossRef]
- Nielsen, L.S.; Baelum, J.; Rasmussen, J.; Dahl, S.; Olsen, K.E.; Albin, M.; Hansen, N.C.; Sherson, D. Occupational asbestos exposure and lung cancer—A systematic review of the literature. Arch. Environ. Occup. Health 2014, 69, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Buncher, C.R. Latent period for malignant mesothelioma of occupational origin. J. Occup. Med. 1992, 34, 718–721. [Google Scholar]
- Fisher, S.A.; Patrick, K.; Hoang, T.; Marcq, E.; Behrouzfar, K.; Young, S.; Miller, T.J.; Robinson, B.W.S.; Bueno, R.; Nowak, A.K.; et al. The MexTAg collaborative cross: Host genetics affects asbestos related disease latency, but has little influence once tumours develop. Front. Toxicol. 2024, 6, 1373003. [Google Scholar] [CrossRef]
- Ferri, G.M.; Guastadisegno, C.M.; Intranuovo, G.; Luisi, V.; Cavone, D.; Licchelli, B.; Buononato, E.V.; Macinagrossa, L.; Molinini, R. Relationship between the Asbestos Cumulative Exposure Index (ACEI) and the Latency Period of Asbestos Related Diseases (ARD) within an Italian Study Group of Ex-Asbestos Workers. Occup. Med. Health Aff. 2016, 4, 1000243. [Google Scholar]
- Dominguez, C.E.; González, M.M.; Molina, A.H.; Ramírez, I.M.; Andreu, A.M.; Valero, F.R.; Doña, J.A.C.; Jiménez, A.L. Characteristics of Patients Evaluated in Specialized Post-Asbestos Exposure Outpatient Clinic. Eur. Respir. Soc. 2020, 56, 3165. [Google Scholar]
- Lazarus, A.; Massoumi, A.; Hostler, J.; Hostler, D.C. Asbestos–Related Pleuropulmonary Diseases: Benign and Malignant. Postgrad. Med. 2012, 124, 116–130. [Google Scholar] [CrossRef]
- Baur, X.; Manuwald, U.; Wilken, D. Does long-term asbestos exposure cause an obstructive ventilation pattern? Pneumologie 2010, 64, 736–744. [Google Scholar] [CrossRef]
- Henderson, I.; Sterman, D.H.; Smith, R.L.; Rom, W.N. Asbestosis. In Modern Occupational Diseases: Diagnosis, Epidemiology, Management and Prevention; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 41–57. [Google Scholar]
- Liddell, D. Asbestos in the occupational environment. In Mineral Fibers and Health; CRC Press: Boca Raton, FL, USA, 2024; pp. 79–87. [Google Scholar]
- Kurunthachalam, S. Asbestos and Its Toxicological Concern. Hydrol. Curr. Res. 2013, 4, 1000e110. [Google Scholar] [CrossRef]
- Brody, A.R. OCCUPATIONAL DISEASES | Asbestos-Related Lung Disease. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 216–226. [Google Scholar]
- Larrouy, C.; Tandjaoui-Lambiotte, H.; Mellat, M.; Fabre, C.; Defrejacques, C.; Adotti, F.; Piquet, J. Environmental interstitial pneumonia caused by asbestos. Study of a Turkish family exposed to tremolite. Rev. Pneumol. Clin. 1990, 46, 78–82. [Google Scholar]
- Luo, S.; Liu, X.; Mu, S.; Tsai, S.; Wen, C. Asbestos related diseases from environmental exposure to crocidolite in Da-yao, China. I. Review of exposure and epidemiological data. Occup. Environ. Med. 2003, 60, 35–42. [Google Scholar] [CrossRef]
- Banks, D.E.; Morris, M.J.; Jindal, S.K. Asbestos Fibers: Mechanisms of Injury. In Studies on Respiratory Disorders; Springer: Berlin/Heidelberg, Germany, 2014; pp. 203–224. [Google Scholar]
- Popper, H. Pneumoconiosis and Environmentally Induced Lung Diseases. In Pathology of Lung Disease: Morphology–Pathogenesis–Etiology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 291–320. [Google Scholar]
- Ministry of Employment and Labor (MOEL). Investigation on the Actual Condition of Hazardous Environment at Asbestos Handling Workplaces; MOEL: Seoul, Republic of Korea, 1984; pp. 254–263. (In Korean) [Google Scholar]
- Kim, S. Clinical Characteristics and Long-Term Follow-Up of Asbestos in Workers at One Asbestos Textile Factory in Busan; Inje University: Gimhae-si, Republic of Korea, 2019. [Google Scholar]
- Choi, J.K.; Paek, D.M.; Paik, N.W.; Hisanaga, N.; Sakai, K. A study on several minerals contaminated with asbestiform fibers in Korea. J. Korean Soc. Occup. Environ. Hyg. 1998, 8, 254–263. [Google Scholar]
- Smith, C.M.; Kelsey, K.T.; Wiencke, J.K.; Leyden, K.; Levin, S.; Christiani, D.C. Inherited glutathione-S-transferase deficiency is a risk factor for pulmonary asbestosis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1994, 3, 471–477. [Google Scholar]
- Kukkonen, M.K.; Hämäläinen, S.; Kaleva, S.; Vehmas, T.; Huuskonen, M.S.; Oksa, P.; Vainio, H.; Piirilä, P.; Hirvonen, A. Genetic susceptibility to asbestos-related fibrotic pleuropulmonary changes. Eur. Respir. J. 2011, 38, 672–678. [Google Scholar] [CrossRef]
- Franko, A.; Dolžan, V.; Arneric, N.; Dodic-Fikfak, M. The influence of genetic polymorphisms of GSTP1 on the development of asbestosis. J. Occup. Environ. Med. 2008, 50, 7–12. [Google Scholar] [CrossRef]
- Kuzmina, L.P.; Khotuleva, A.G.; Kovalevsky, E.V.; Anokhin, N.N.; Tskhomariya, I.M.; Хoтулева, А. Association of genetic polymorphism of cytokines and antioxidant enzymes with the development of asbestosis. Meditsina Tr. I Promyshlennaya Ekol. 2020, 60, 898–903. [Google Scholar] [CrossRef]
- Kukkonen, M.K.; Vehmas, T.; Piirilä, P.; Hirvonen, A. Genes involved in innate immunity associated with asbestos-related fibrotic changes. Occup. Environ. Med. 2014, 71, 48–54. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, L.; Huh, D.-A.; Moon, K.W.; Kang, M.-S.; Lee, Y.-J. Association between oil spill clean-up work and thyroid cancer: Nine years of follow-up after the Hebei Spirit oil spill accident. Mar. Pollut. Bull. 2024, 199, 116041. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, Y.-J.; Choi, Y.-H.; Huh, D.-A.; Kang, M.-S.; Moon, K.W. Long-term effects of the Hebei Spirit oil spill on the prevalence and incidence of allergic disorders. Sci. Total Environ. 2024, 912, 168801. [Google Scholar] [CrossRef]
- Kim, L.; Huh, D.-A.; Kang, M.-S.; Park, K.; Lee, J.; Hwang, S.H.; Choi, H.J.; Lim, W.; Moon, K.W.; Lee, Y.-J. Chemical exposure from the Hebei spirit oil spill accident and its long-term effects on mental health. Ecotoxicol. Environ. Saf. 2024, 284, 116938. [Google Scholar] [CrossRef]
- Lee, J.; Huh, D.-A.; Kim, L.; Park, K.; Choi, Y.-H.; Hwang, S.-H.; Lim, W.; Choi, H.J.; Moon, K.W.; Kang, M.-S.; et al. The short-term and long-term effects of oil spill exposure on dyslipidemia: A prospective cohort study of Health Effects Research on the Hebei Spirit Oil Spill. Mar. Pollut. Bull. 2025, 219, 118321. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Huh, D.-A.; Kim, L.; ji Lee, S.; Moon, K.W. Health risks of pest control and disinfection workers after the COVID-19 outbreak in South Korea. J. Environ. Sci. 2024, 139, 350–363. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Choi, Y.-H.; Huh, D.-A.; Kim, L.; Park, K.; Lee, J.; Choi, H.J.; Lim, W.; Moon, K.W. Per-and polyfluoroalkyl substances exposures are associated with non-alcoholic fatty liver disease, particularly fibrosis. Environ. Pollut. 2025, 372, 126085. [Google Scholar] [CrossRef]
- Park, K.; Huh, D.-A.; Kim, L.; Choi, Y.-H.; Lee, J.; Hwang, S.H.; Choi, H.J.; Lim, W.; Moon, K.W. Levels of serum per-and polyfluoroalkyl substances and association with dyslipidemia in the Korean population. Ecotoxicol. Environ. Saf. 2025, 302, 118633. [Google Scholar] [CrossRef]
- Zheng, P.-F.; Shu, L.; Si, C.-J.; Zhang, X.-Y.; Yu, X.-L.; Gao, W. Dietary patterns and chronic obstructive pulmonary disease: A meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 515–522. [Google Scholar] [CrossRef]
- Kim, L.; Choi, Y.-H.; Huh, D.-A.; Moon, K.W. Associations of minimally processed and ultra-processed food intakes with cardiovascular health in Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES VI), 2013–2015. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 1045–1053. [Google Scholar] [CrossRef]
- Kim, L.; Huh, D.-A.; Park, K.; Lee, J.; Hwang, S.-H.; Choi, H.J.; Lim, W.; Moon, K.W. Dietary exposure to environmental phenols and phthalates in Korean adults: Data analysis of the Korean National Environmental Health Survey (KoNEHS) 2018–2020. Int. J. Hyg. Environ. Health 2025, 267, 114597. [Google Scholar] [CrossRef]
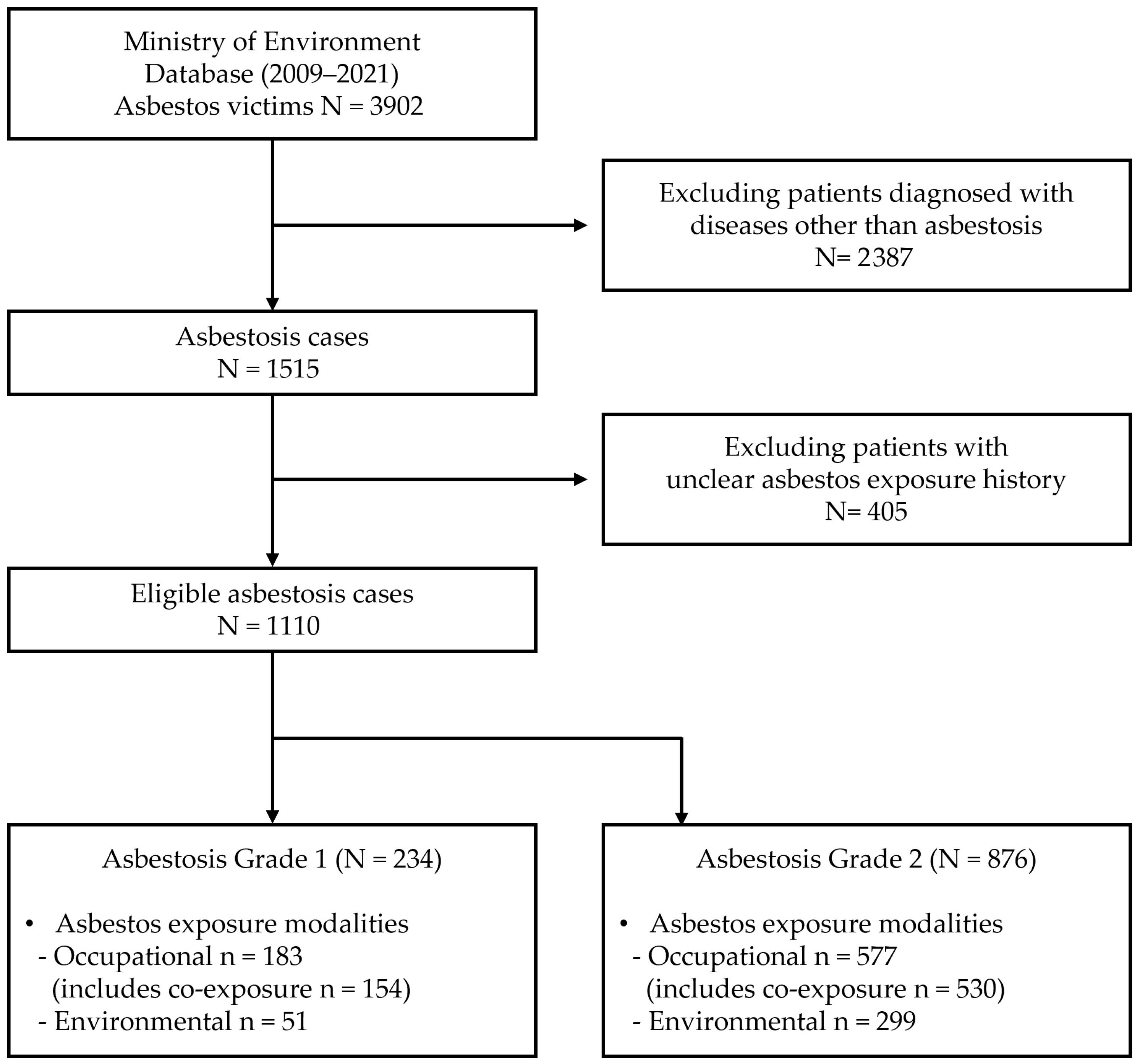
| Variables | Asbestosis Grade 1 (Severe) | Asbestosis Grade 2 (Moderate) |
|---|---|---|
| n (%) | n (%) | |
| Total | 234 (100.0) | 876 (100.0) |
| Sex | ||
| Male | 198 (84.6) | 632 (72.1) |
| Female | 36 (15.4) | 244 (27.9) |
| Age | ||
| <70 | 29 (12.4) | 127 (14.5) |
| 70–79 | 73 (31.2) | 328 (37.4) |
| 80–89 | 97 (41.5) | 335 (38.2) |
| ≥90 | 35 (15.0) | 86 (9.8) |
| Smoking status | ||
| Never | 43 (18.4) | 174 (19.9) |
| Past smoker | 22 (9.4) | 88 (10.0) |
| Current smoker | 4 (1.7) | 10 (1.1) |
| Unknown | 165 (70.5) | 604 (68.9) |
| Exposure modalities | ||
| Occupational | 183 (78.2) | 577 (65.9) |
| Environmental | 51 (21.8) | 299 (34.1) |
| Diagnosis year | ||
| <2010 | 7 (3.0) | 5 (0.6) |
| 2010–2014 | 96 (41.0) | 289 (33.0) |
| 2015–2019 | 97 (41.5) | 349 (39.8) |
| ≥2020 | 34 (14.5) | 233 (26.6) |
| Variables | n | Mean Latency 1 (±SD) | p-Value | Median Latency | Range (Min–Max) | 5–95 Percentile |
|---|---|---|---|---|---|---|
| Asbestosis (Grade 1) | ||||||
| Total | 234 | 45.3 (±14.4) | 44.0 | 11.0–83.0 | 20.0–72.3 | |
| Sex | ||||||
| Male | 198 | 45.1 (±14.5) | 0.656 | 44.0 | 11.0–83.0 | 20.0–73.0 |
| Female | 36 | 46.3 (±14.1) | 43.5 | 15.0–78.0 | 19.3–72.9 | |
| Age | ||||||
| <70 | 29 | 30.9 a (±10.5) | <0.001 | 33.0 | 11.0–62.0 | 14.5–52.5 |
| 70–79 | 73 | 42.4 b (±11.6) | 43.0 | 16.0–67.0 | 19.7–61.3 | |
| 80–89 | 97 | 49.1 c (±13.2) | 49.0 | 14.0–81.0 | 28.6–74.2 | |
| ≥90 | 35 | 52.7 c (±16.1) | 46.0 | 25.0–83.0 | 31.4–82.2 | |
| Asbestosis (Grade 2) | ||||||
| Total | 876 | 46.3 (±14.9) | 45.0 | 6.0–86.0 | 20.9–74.2 | |
| Sex | ||||||
| Male | 632 | 45.6 (±13.9) | 0.041 | 45.0 | 6.0–86.0 | 22.0–71.0 |
| Female | 244 | 48.1 (±17.0) | 46.0 | 6.0–86.0 | 20.0–79.0 | |
| Age | ||||||
| <70 | 127 | 36.3 a (±12.5) | <0.001 | 37.0 | 6.0–67.0 | 11.8–58.0 |
| 70–79 | 328 | 44.3 b (±12.5) | 45.0 | 6.0–74.0 | 20.5–68.0 | |
| 80–89 | 335 | 49.9 c (±15.1) | 48.0 | 11.0–86.0 | 27.0–77.2 | |
| ≥90 | 86 | 54.6 d (±15.9) | 51.0 | 23.0–86.0 | 28.4–84.7 |
| Variables | Asbestosis (Grade 1) | Asbestosis (Grade 2) | ||||
|---|---|---|---|---|---|---|
| n | Estimates (95% CI) | p-Value | n | Estimates (95% CI) | p-Value | |
| Sex | ||||||
| Male | 198 | 44.9 (44.3, 45.6) | 0.533 | 632 | 46.4 (46.2, 46.7) | 0.859 |
| Female | 36 | 44.5 (43.3, 45.7) | 244 | 46.5 (46.1, 46.9) | ||
| Age | ||||||
| <70 | 29 | 30.7 (29.0, 32.3) | <0.001 | 127 | 33.7 (33.0, 34.5) | <0.001 |
| 70–79 | 73 | 40.7 (39.6, 41.9) | 328 | 43.6 (43.1, 44.1) | ||
| 80–89 | 97 | 49.1 (48.1, 50.2) | 335 | 51.1 (50.7, 51.6) | ||
| ≥90 | 35 | 56.2 (54.7, 57.8) | 86 | 57.7 (56.8, 58.6) | ||
| Smoking status | ||||||
| Never | 43 | 45.4 (43.8, 46.9) | 0.117 | 174 | 47.0 (45.4, 48.5) | 0.170 |
| Past smoker | 22 | 45.3 (43.6, 47.0) | 88 | 47.2 (45.5, 48.9) | ||
| Current smoker | 4 | 44.1 (42.0, 46.2) | 10 | 46.5 (44.4, 48.6) | ||
| Unknown | 165 | 44.3 (43.5, 45.1) | 604 | 46.7 (45.9, 47.5) | ||
| Exposure modalities | ||||||
| Occupational | 183 | 44.4 (43.6, 45.2) | 0.010 | 577 | 45.0 (44.7, 45.3) | <0.001 |
| Environmental | 51 | 46.0 (44.9, 47.0) | 299 | 47.0 (46.6, 47.3) | ||
| Type of job | ||||||
| Building 2 | 39 | 43.8 (42.2, 45.3) | 0.214 | 86 | 44.5 (43.7, 45.2) | <0.001 |
| Production 3 | 30 | 42.0 (40.6, 43.5) | 90 | 42.3 (41.8, 42.8) | ||
| Maintenance 4 | 27 | 43.2 (41.8, 44.5) | 94 | 44.4 (43.7, 45.1) | ||
| Mining 5 | 65 | 44.1 (42.4, 45.9) | 191 | 45.0 (44.4, 45.6) | ||
| Others | 22 | 44.3 (42.6, 46.1) | 116 | 43.1 (42.5, 43.8) | ||
| Type of exposure source | ||||||
| Asbestos mines | 27 | 52.5 (49.3, 55.6) | 0.348 | 174 | 53.8 (52.6, 55.0) | 0.001 |
| Asbestos industries | 16 | 47.4 (41.5, 53.3) | 76 | 50.4 (48.8, 52.1) | ||
| Shipyards | 4 | 49.3 (48.1, 50.5) | 28 | 51.1 (50.6, 51.5) | ||
| Asbestos containing building | 3 | 49.0 (45.5, 52.4) | 9 | 51.5 (50.8, 52.2) | ||
| Others | 1 | 49.9 (48.4, 51.4) | 12 | 52.9 (50.9, 54.8) | ||
| Variables | Asbestosis (Grade 1) | Asbestosis (Grade 2) | ||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Occupational exposure | ||||
| Exposure duration (years) | −0.051 (−0.109, 0.007) | 0.061 | −0.159 (−0.288, −0.030) | 0.039 |
| Age of first exposure (years) | −0.993 (−1.044, −0.943) | <0.001 | −0.952 (−0.977, −0.928) | <0.001 |
| Pack-year | −0.050 (−0.122, 0.022) | 0.127 | −0.044 (−0.125, 0.037) | 0.205 |
| Environmental exposure | ||||
| Distance (km) | 1.131 (0.001, 2.260) | 0.048 | 0.997 (0.035, 1.959) | 0.036 |
| Exposure duration (years) | −0.050 (−0.210, 0.111) | 0.971 | −0.031 (−0.178, 0.116) | 0.710 |
| Age of first exposure (years) | −0.935 (−0.986, −0.884) | <0.001 | −0.937 (−0.956, −0.918) | <0.001 |
| Pack-year | −0.053 (−0.119, 0.013) | 0.091 | −0.047 (−0.122, 0.028) | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, W.Y.; Kang, M.-S.; Hwangbo, Y.; Lee, M.-R. Latency Period Among Asbestosis Cases in South Korea by Demographic and Asbestos Exposure Characteristics. Toxics 2025, 13, 775. https://doi.org/10.3390/toxics13090775
So WY, Kang M-S, Hwangbo Y, Lee M-R. Latency Period Among Asbestosis Cases in South Korea by Demographic and Asbestos Exposure Characteristics. Toxics. 2025; 13(9):775. https://doi.org/10.3390/toxics13090775
Chicago/Turabian StyleSo, Won Young, Min-Sung Kang, Young Hwangbo, and Mee-Ri Lee. 2025. "Latency Period Among Asbestosis Cases in South Korea by Demographic and Asbestos Exposure Characteristics" Toxics 13, no. 9: 775. https://doi.org/10.3390/toxics13090775
APA StyleSo, W. Y., Kang, M.-S., Hwangbo, Y., & Lee, M.-R. (2025). Latency Period Among Asbestosis Cases in South Korea by Demographic and Asbestos Exposure Characteristics. Toxics, 13(9), 775. https://doi.org/10.3390/toxics13090775







