Measuring Environmental Chemical Burden with Wristbands: Implications for Kidney Health Among Women in Rural Guatemala
Abstract
1. Introduction
2. Material and Methods
2.1. Study Background and Population
2.2. Study Design
2.2.1. Wristband Deployment and Analysis
2.2.2. Urine and Blood Collection
2.2.3. Questionnaires
2.3. Statistical Analysis
3. Results
3.1. Chemical Measurements
3.2. Chemical Categories Between Groups
3.3. Individual Chemical Detections and Concentrations Between Participant Groups
3.3.1. PAH Exposure
3.3.2. Pesticide Exposure
3.3.3. Phthalates and Chemicals of Commerce Exposure
3.3.4. Other Chemical Exposures
3.4. Determinants of Chemical Concentrations
3.5. Correlations Between Individual Chemicals and Markers of Kidney Function
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKDu | Chronic kidney disease of unknown origin |
| PAH | Polycyclic aromatic hydrocarbons |
| eGFR | Estimated glomerular filtration rate |
| ACR | Albumin to Creatinine ratio |
| BMI | Body mass index |
| PTFE | Polytetrafluoroethylene |
| GC-MS | Gas chromatography–mass spectrometry |
| VOC | Volatile organic chemicals |
| TPP | Triphenyl phosphate |
References
- Claudel, S.E.; Waikar, S.S. Systematic Review of Kidney Injury Biomarkers for the Evaluation of CKD of Uncertain Etiology. Kidney Int. Rep. 2024, 9, 1614–1632. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Crowe, J.; Hogstedt, C.; Jakobsson, K.; Lucas, R.; Wegman, D.H. Resolving the Enigma of the Mesoamerican Nephropathy: A Research Workshop Summary. Am. J. Kidney Dis. 2014, 63, 396–404. [Google Scholar] [CrossRef]
- Ramirez-Rubio, O.; McClean, M.D.; Amador, J.J.; Brooks, D.R. An epidemic of chronic kidney disease in Central America: An overview. J. Epidemiol. Community Health 2013, 67, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; McClean, M.D.; Kaufman, J.S.; Brooks, D.R. The Central American Epidemic of CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 504–511. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Glyphosate, P.S. Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef]
- Reddy, D.V.; Gunasekar, A. Chronic kidney disease in two coastal districts of Andhra Pradesh, India: Role of drinking water. Environ. Geochem. Health 2013, 35, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.; Wesseling, C.; Solano, B.R.; Umaña, M.P.; Ramírez, A.R.; Kjellstrom, T.; Morales, D.; Nilsson, M. Heat exposure in sugarcane harvesters in Costa Rica. Am. J. Ind. Med. 2013, 56, 1157–1164. [Google Scholar] [CrossRef]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef]
- García-Trabanino, R.; Jarquín, E.; Wesseling, C.; Johnson, R.J.; González-Quiroz, M.; Weiss, I.; Glaser, J.; José Vindell, J.; Stockfelt, L.; Roncal, C.; et al. Heat stress, dehydration, and kidney function in sugarcane cutters in EI Salvador—A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2015, 142, 746–755. [Google Scholar] [CrossRef]
- Bodin, T.; García-Trabanino, R.; Weiss, I.; Jarquín, E.; Glaser, J.; Jakobsson, K.; Lucas, R.A.; Wesseling, C.; Hogstedt, C.; Wegman, D.H.; et al. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup. Environ. Med. 2016, 73, 409–416. [Google Scholar] [CrossRef]
- Wesseling, C.; Crowe, J.; Hogstedt, C.; Jakobsson, K.; Lucas, R.; Wegman, D.H. The Epidemic of Chronic Kidney Disease of Unknown Etiology in Mesoamerica: A Call for Interdisciplinary Research and Action. Am. J. Public Health 2013, 103, 1927–1930. [Google Scholar] [CrossRef]
- Butler-Dawson, J.; Krisher, L.; Yoder, H.; Dally, M.; Sorensen, C.; Johnson, R.J.; Asensio, C.; Cruz, A.; Johnson, E.C.; Carlton, E.J.; et al. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. Int. Arch. Occup. Environ. Health 2019, 92, 977–990. [Google Scholar] [CrossRef]
- Sorensen, C.J.; Butler-Dawson, J.; Dally, M.; Krisher, L.; Griffin, B.R.; Johnson, R.J.; Lemery, J.; Asensio, C.; Tenney, L.; Newman, L.S. Risk Factors and Mechanisms Underlying Cross-Shift Decline in Kidney Function in Guatemalan Sugarcane Workers. J. Occup. Environ. Med. 2019, 61, 239–250. [Google Scholar] [CrossRef]
- Dally, M.; Butler-Dawson, J.; Cruz, A.; Krisher, L.; Johnson, R.J.; Asensio, C.; Pilloni, W.D.; Asturias, E.J.; Newman, L.S. Longitudinal trends in renal function among first time sugarcane harvesters in Guatemala. PLoS ONE 2020, 15, e0229413. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.S.M.; De Silva, P.M.C.S.; Ekanayake, E.M.D.V.; Thakshila, W.A.K.G.; Gunarathna, S.D.; Gunasekara, T.D.K.S.C.; Jayasinghe, S.S.; Asanthi, H.B.; Chandana, E.P.S.; Chaminda, G.G.T.; et al. Occupational Paraquat and Glyphosate Exposure May Decline Renal Functions among Rural Farming Communities in Sri Lanka. Int. J. Environ. Res. Public Health 2021, 18, 3278. [Google Scholar] [CrossRef]
- Ulrich, J.C.; Hoffman, K.; Gunasekara, T.D.K.S.C.; Sandamini, P.M.M.A.; Jackson, B.P.; De Silva, P.M.C.S.; Jayasundara, N.; Ferguson, P.L. Glyphosate and Fluoride in High-Hardness Drinking Water Are Positively Associated with Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Environ. Sci. Technol. Lett. 2023, 10, 916–923. [Google Scholar] [CrossRef]
- Uthayarajan, N.; Jayawardene, K.L.T.D.; Weerasekara, I. Quality and sources of food and water consumed by people with chronic kidney disease of unknown etiology in Sri Lanka: A systematic review. J. Nephrol. 2025, 38, 1287–1311. [Google Scholar] [CrossRef]
- Johnson, R.J.; Wesseling, C.; Newman, L.S. Epidemic Chronic Kidney Disease in Agricultural Communities. N. Engl. J. Med. 2018, 380, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Kannan, K.; Lee, S.; Ma, J.; Warady, B.A.; Furth, S.; Trachtman, H.; Trasande, L. Urinary Polycyclic Aromatic Hydrocarbons in a Longitudinal Cohort of Children with CKD: A Case of Reverse Causation? Kidney360 2022, 3, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Chen, Y.; Trachtman, H.; Trasande, L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ. Res. 2016, 144, 149–157. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.Y.; Wang, X.X.; Shi, Z.; Zhang, Y.; Wu, G.; Zhang, S.Y. Identification of hepatotoxicity and renal dysfunction of pyrene in adult male rats. Environ. Toxicol. 2018, 33, 1304–1311. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; He, Q. Sex-specific impact of polycyclic aromatic hydrocarbons and metals on renal function in U.S. adults: Mediating roles of inflammation, oxidative stress and aging. Ecotoxicol. Environ. Saf. 2025, 299, 118380. [Google Scholar] [CrossRef] [PubMed]
- Adgate, J.L.; Erlandson, G.; Butler-Dawson, J.; Calvimontes-Barrientos, L.; Amezquita, L.; Seidel, J.; Barnoya, J.; Castro, C.; Coyoy, M.; Pérez, M.; et al. Airborne Particulate Matter Exposure in Male Sugarcane Workers at Risk for Chronic Kidney Disease in Guatemala. Ann. Work. Expo. Health 2025, 69, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Herrera Valdés, R.; Orantes, C.M.; Almaguer López, M.; López Marín, L.; Arévalo, P.A.; Smith González, M.J.; Morales, F.E.; Bacallao, R.; Bayarre, H.D.; Vela Parada, X.F. Clinical characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities in El Salvador. Clin. Nephrol. 2015, 83, S56–S63. [Google Scholar] [CrossRef]
- Orantes Navarro, C.M.; Herrera Valdés, R.; López, M.A.; Calero, D.J.; Fuentes de Morales, J.; Alvarado Ascencio, N.P.; Vela Parada, X.F.; Zelaya Quezada, S.M.; Granados Castro, D.V.; Orellana de Figueroa, P. Epidemiological characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities of El Salvador. Clin. Nephrol. 2015, 83, S24–S31. [Google Scholar] [CrossRef]
- Gonzalez-Quiroz, M.; Smpokou, E.T.; Silverwood, R.J.; Camacho, A.; Faber, D.; Garcia, B.R.; Oomatia, A.; Hill, M.; Glaser, J.; Le Blond, J. Decline in Kidney Function among Apparently Healthy Young Adults at Risk of Mesoamerican Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2020–2212. [Google Scholar] [CrossRef]
- Gonzalez-Quiroz, M.; Smpokou, E.T.; Silverwood, R.J.; Camacho, A.; Faber, D.; Garcia, B.R.; Oomatia, A.; Hill, M.; Glaser, J.; Le Blond, J.; et al. A work and off-work evaluation of female workers’ heat and particulate matter exposures and kidney health in Guatemala. J. Clim. Change Health 2025, 22, 100408. [Google Scholar] [CrossRef]
- Butler-Dawson, J.; Erlandson, G.; Jaramillo, D.; Calvimontes, L.; Pilloni, D.; Seidel, J.; Castro, C.; Villarreal Hernandez, K.; Krisher, L.; Brindley, S.; et al. Concurrent Particulate Matter and Heat Exposure in Working and Non-Working Women in Rural Guatemala. Atmosphere 2024, 15, 1175. [Google Scholar] [CrossRef]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef]
- Orantes, C.M.; Herrera, R.; Almaguer, M.; Brizuela, E.G.; Núñez, L.; Alvarado, N.P.; Fuentes, E.J.; Bayarre, H.D.; Amaya, J.C.; Calero, D.J.; et al. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. Medicc Rev. 2014, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Scammell, M.K.; Sennett, C.M.; Petropoulos, Z.E.; Kamal, J.; Kaufman, J.S. Environmental and Occupational Exposures in Kidney Disease. Semin. Nephrol. 2019, 39, 230–243. [Google Scholar] [CrossRef]
- Lee, J.; Oh, S.; Kang, H.; Kim, S.; Lee, G.; Li, L.; Kim, C.T.; An, J.N.; Oh, Y.K.; Lim, C.S.; et al. Environment-Wide Association Study of CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 766–775. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. EPA ExpoBox: Exposure Assessment Tools by Chemical Classes—Other Organics; US EPA: Washington, DC, USA, 2025.
- Tfouni, S.A.V.; Souza, N.G.; Neto, M.B.; Loredo, I.S.D.; Leme, F.M.; Furlani, R.P.Z. Polycyclic aromatic hydrocarbons (PAHs) in sugarcane juice. Food Chem. 2009, 116, 391–394. [Google Scholar] [CrossRef]
- Pope, D.; Diaz, E.; Smith-Sivertsen, T.; Lie, R.T.; Bakke, P.; Balmes, J.R.; Smith, K.R.; Bruce, N.G. Exposure to Household Air Pollution from Wood Combustion and Association with Respiratory Symptoms and Lung Function in Nonsmoking Women: Results from the RESPIRE Trial, Guatemala. Environ. Health Perspect. 2015, 123, 285–292. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Terra-Filho, M.; de Albuquerque, A.L.; Di Giorgi, D.; Grupi, C.; Negrão, C.E.; Rondon, M.U.; Martinez, D.G.; Marcourakis, T.; dos Santos, F.A.; et al. Burnt Sugarcane Harvesting—Cardiovascular Effects on a Group of Healthy Workers, Brazil. PLoS ONE 2012, 7, e46142. (In English) [Google Scholar] [CrossRef]
- Prado, G.F.; Zanetta, D.M.; Arbex, M.A.; Braga, A.L.; Pereira, L.A.; de Marchi, M.R.; de Melo Loureiro, A.P.; Marcourakis, T.; Sugauara, L.E.; Gattás, G.J.; et al. Burnt sugarcane harvesting: Particulate matter exposure and the effects on lung function, oxidative stress, and urinary 1-hydroxypyrene. Sci. Total Environ. 2012, 437, 200–208. [Google Scholar] [CrossRef]
- Arbex, M.A.; Martins, L.C.; de Oliveira, R.C.; Pereira, L.A.; Arbex, F.F.; Cançado, J.E.; Saldiva, P.H.; Braga, A.L. Air pollution from biomass burning and asthma hospital admissions in a sugar cane plantation area in Brazil. J. Epidemiol. Community Health 2007, 61, 395–400. [Google Scholar] [CrossRef]
- Le Blond, J.S.; Woskie, S.; Horwell, C.J.; Williamson, B. Particulate matter produced during commercial sugarcane harvesting and processing: A respiratory health hazard? Atmos. Environ. 2017, 149, 34–46. [Google Scholar] [CrossRef]
- Stuveling, E.M.; Hillege, H.L.; Bakker, S.J.L.; Gans, R.O.B.; de Jong, P.E.; de Zeeuw, D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003, 63, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Huckins, J.N.; Petty, J.D.; Booij, K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices; Springer: New York, NY, USA, 2010. [Google Scholar]
- Dixon, H.M.; Scott, R.P.; Holmes, D.; Calero, L.; Kincl, L.D.; Waters, K.M.; Camann, D.E.; Calafat, A.M.; Herbstman, J.B.; Anderson, K.A. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 2018, 410, 3059–3071. [Google Scholar] [CrossRef]
- Dixon, H.M.; Armstrong, G.; Barton, M.; Bergmann, A.J.; Bondy, M.; Halbleib, M.L.; Hamilton, W.; Haynes, E.; Herbstman, J.; Hoffman, P.; et al. Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci. 2019, 6, 181836. [Google Scholar] [CrossRef]
- Doherty, B.T.; Pearce, J.L.; Anderson, K.A.; Karagas, M.R.; Romano, M.E.; Program, I. Collaborators Environm Assessment of Multipollutant Exposures During Pregnancy Using Silicone Wristbands. Front. Public Health 2020, 8, 547239. [Google Scholar] [CrossRef]
- Hammel, S.C.; Hoffman, K.; Webster, T.F.; Anderson, K.A.; Stapleton, H.M. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 2016, 50, 4483–4491. [Google Scholar] [CrossRef]
- Hammel, S.C.; Phillips, A.L.; Hoffman, K.; Stapleton, H.M. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 2018, 52, 11875–11885. [Google Scholar] [CrossRef] [PubMed]
- Harley, G.; Parra, K.L.; Camacho, J.; Bradman, A.; Nolan, J.E.S.; Lessard, C.; Anderson, K.A.; Poutasse, C.M.; Scott, R.P.; Lazaro, G.; et al. Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ. 2019, 652, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Romanak, K.A.; Tarallo, S.; Francavilla, A.; Viviani, M.; Vineis, P.; Rothwell, J.A.; Mancini, F.R.; Cordero, F.; Naccarati, A.; et al. The use of silicone wristbands to evaluate personal exposure to semi-volatile organic chemicals (SVOCs) in France and Italy. Environ. Pollut. 2020, 267, 115490. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, J.L.; Hammel, S.C.; Hoffman, K.; Phillips, A.L.; Zhang, S.; Ye, X.; Calafat, A.M.; Webster, T.F.; Stapleton, H.M. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 2021, 147, 106317. [Google Scholar] [CrossRef]
- Goin, D.E.; Abrahamsson, D.; Wang, M.; Park, J.S.; Sirota, M.; Morello-Frosch, R.; DeMicco, E.; Trowbridge, J.; August, L.; O’Connell, S.; et al. Investigating geographic differences in environmental chemical exposures in maternal and cord sera using non-targeted screening and silicone wristbands in California. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 548–557. [Google Scholar] [CrossRef]
- Samon, S.M.; Hammel, S.C.; Stapleton, H.M.; Anderson, K.A. Silicone wristbands as personal passive sampling devices: Current knowledge, recommendations for use, and future directions. Environ. Int. 2022, 169, 107339. [Google Scholar] [CrossRef]
- Young, A.S.; Herkert, N.; Stapleton, H.M.; Coull, B.A.; Hauser, R.; Zoeller, T.; Behnisch, P.A.; Felzel, E.; Brouwer, A.; Allen, J.G. Hormone receptor activities of complex mixtures of known and suspect chemicals in personal silicone wristband samplers worn in office buildings. Chemosphere 2023, 315, 137705. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.H.; Day, D.B.; Hazlehurst, M.F.; Herkert, N.J.; Stapleton, H.M.; Sathyanarayana, S. Associations of environmental chemical exposures measured in personal silicone wristbands with sociodemographic factors, COVID-19 restrictions, and child respiratory health. Environ. Res. 2024, 262, 119776. [Google Scholar] [CrossRef] [PubMed]
- MyEposome, I. Personal Environmental Monitoring. Available online: https://www.myexposome.com/ (accessed on 2 August 2025).
- Anderson, K.A.; Points 3rd, G.L.; Donald, C.E.; Dixon, H.M.; Scott, R.P.; Wilson, G.; Tidwell, L.G.; Hoffman, P.D.; Herbstman, J.B.; O’Connell, S.G. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.E.; Elie, M.R.; Smith, B.W.; Hoffman, P.D.; Anderson, K.A. Transport stability of pesticides and PAHs sequestered in polyethylene passive sampling devices. Environ. Sci. Pollut. Res. 2016, 23, 12392–12399. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Kincl, L.D.; Anderson, K.A. Silicone Wristbands as Personal Passive Samplers. Environ. Sci. Technol. 2014, 48, 3327–3335. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Pesticide Registration—Inert Ingredients Overview and Guidance; US EPA: Washington, DC, USA, 2025.
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J.P. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef]
- Mendelsohn, E.; Hagopian, A.; Hoffman, K.; Butt, C.M.; Lorenzo, A.; Congleton, J.; Webster, T.F.; Stapleton, H.M. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 2016, 86, 45–51. [Google Scholar] [CrossRef]
- Jia, C.R.; Batterman, S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Wu, X.; Cao, X.; Lintelmann, J.; Peters, A.; Koenig, W.; Zimmermann, R.; Schneider, A.; Wolf, K. KORA-Study group, Assessment of the association of exposure to polycyclic aromatic hydrocarbons, oxidative stress, and inflammation: A cross-sectional study in Augsburg, Germany. Int. J. Hyg. Environ. Health 2022, 244, 113993. [Google Scholar] [CrossRef]
- Montano, L.; Baldini, G.M.; Piscopo, M.; Liguori, G.; Lombardi, R.; Ricciardi, M.; Esposito, G.; Pinto, G.; Fontanarosa, C.; Spinelli, M.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Occupational Exposure, Health Risks and Fertility Implications. Toxics 2025, 13, 151. [Google Scholar] [CrossRef]
- Li, J.; Fan, H.; Liu, K.; Li, X.; Fan, D.; Lu, X.; Xia, Y.; Cao, Y.; Xiao, C. Associations of urinary polycyclic aromatic hydrocarbons with albuminuria in US adults, NHANES 2003–2014. Ecotoxicol. Environ. Saf. 2020, 195, 110445. [Google Scholar] [CrossRef]
- Yuan, T.H.; Ke, D.Y.; Wang, J.E.; Chan, C.C. Associations between renal functions and exposure of arsenic and polycyclic aromatic hydrocarbon in adults living near a petrochemical complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef]
- Begum, M.A.M.; Siddiquee, M.N.A.; Islam, M.S.; Alam, K.S.; Alam, M.N.; Rahman, M.H. Efficacy of Some Fipronil Insecticides in Controlling Sugarcane Termites. Bangladesh Sugarcane Res. Inst. 2013, 33 & 34, 152–155. [Google Scholar]
- Shearer, J.J.; Sandler, D.P.; Andreotti, G.; Murata, K.; Shrestha, S.; Parks, C.G.; Liu, D.; Alavanja, M.C.; Landgren, O.; Beane Freeman, L.E.; et al. Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study. Environ. Res. 2021, 199, 111276. [Google Scholar] [CrossRef]
- Lebov, J.F.; Engel, L.S.; Richardson, D.; Hogan, S.L.; Sandler, D.P.; Hoppin, J.A. Pesticide exposure and end-stage renal disease risk among wives of pesticide applicators in the Agricultural Health Study. Environ. Res. 2015, 143, 198–210. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Zafeer, M.F.; Javed, M.; Ahmad, M. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci. Rep. 2018, 8, 17139. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.F.; Butt, A.M.; Ashraf, W.; Matti, N.; Farooq, M.A.; bin Nasim, M.; Siddique, M.I.; Khan, T.M. The Impact of Fluid and Electrolyte Imbalance on the Severities of Diseases and Their Management in Developing Countries. In Handbook of Medical and Health Sciences in Developing Countries: Education, Practice, and Research; Al-Worafi, Y.M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–20. [Google Scholar]
- Young, A.S.; Herkert, N.; Stapleton, H.M.; Cedeño Laurent, J.G.; Jones, E.R.; MacNaughton, P.; Coull, B.A.; James-Todd, T.; Hauser, R.; Lahaie Luna, M.; et al. Chemical contaminant exposures assessed using silicone wristbands among occupants in office buildings in the USA, UK, China, and India. Environ. Int. 2021, 156, 106727. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.J.; North, P.E.; Vasquez, L.; Bello, H.; Ruiz, M.D.G.; Anderson, K.A. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Donald, E.; Scott, R.P.; Blaustein, K.L.; Halbleib, M.L.; Sarr, M.; Jepson, P.C.; Anderson, K.A. Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci. 2016, 3, 160433. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.C.; Aga, D.S.; Queirolo, E.I.; Olson, J.R.; Daleiro, M.; Kordas, K. Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ. 2020, 740, 140136. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.; Staskal, D. Brominated Flame Retardants: Cause for Concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Pinto, F.C.M.; De-Carvalho, R.R.; De-Oliveira, A.; Delgado, I.F.; Paumgartten, F.J.R. Study on the developmental toxicity of β-ionone in the rat. Regul. Toxicol. Pharmacol. 2018, 97, 110–119. [Google Scholar] [CrossRef]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Di Giacomo, S.; Pagano, E.; Borrelli, F.; Mazzanti, G. Genotoxicity assessment of some cosmetic and food additives. Regul. Toxicol. Pharmacol. 2014, 68, 16–22. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Date, M.; Dekant, W.; et al. Update to RIFM fragrance ingredient safety assessment, ethylene brassylate, CAS Registry Number 105-95-3. Food Chem. Toxicol. 2022, 169, 113459. [Google Scholar] [CrossRef] [PubMed]
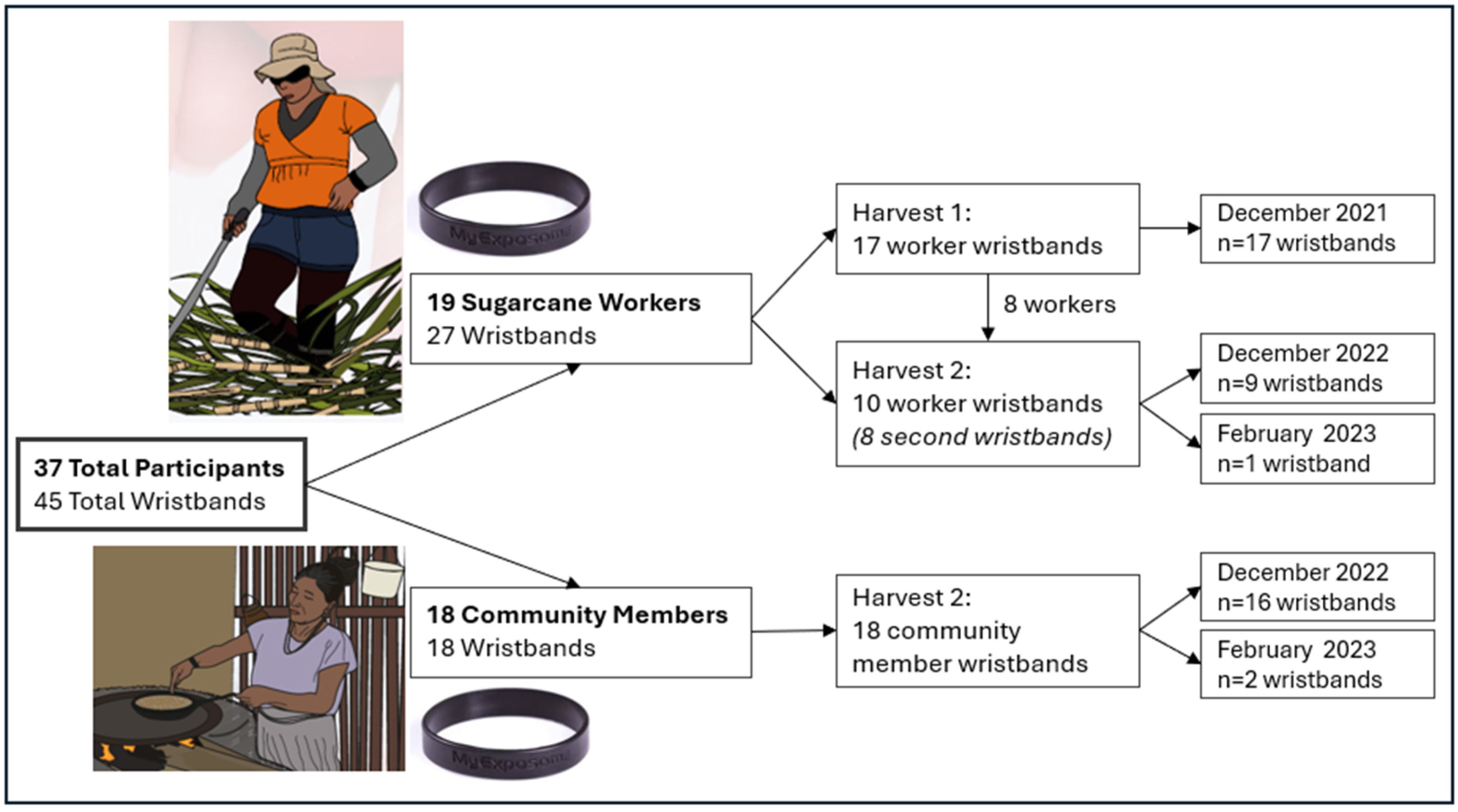
| Characteristics | Workers (n = 19, 51%) | Community Member (n = 18, 49%) |
|---|---|---|
| Age, years, mean (SD) | 38 (8.4) | 31 (9.9) |
| Current work | ||
| 19 (100%) | N/A |
| N/A | 17 (94%) |
| 0 (0%) | 1 (6%) |
| Department of residence | ||
| 18 (100%) | 8 (44%) |
| 0 (0%) | 10 (56%) |
| Education level | ||
| 7 (39%) | 6 (33%) |
| 3 (17%) | 7 (39%) |
| 8 (44%) | 5 (28%) |
| Race | ||
| 2 (11%) | 2 (11%) |
| 11 (58%) | 16 (89%) |
| 6 (31%) | 0 (0%) |
| Smoking status | ||
| 14 (78%) | 13 (75%) |
| 3 (17%) | 0 (0%) |
| 1 (6%) | 1 (6%) |
| 0 (0%) | 3 (18%) |
| Primary cook in household | 12 (67%) | 16 (94%) |
| Type of fuel used to cook | ||
| 8 (44%) | 8 (47%) |
| 10 (56%) | 9 (53%) |
| Stove location in compound | ||
| 8 (44%) | 6 (35%) * |
| 3 (17%) | 10 (59%) |
| 7 (39%) | 1 (6%) |
| Activities during wristband wearing (7 days) | ||
| Tobacco product use | 1 (5%) | 0 (0%) |
| Household member used tobacco product | 1 (5%) | 1 (6%) |
| NSAID use | 0 (0%) | 4 (22%) |
| Lotion/Body oil use | ||
| 1 (8%) | 4 (24%) |
| 15 (79%) | 9 (53%) |
| 3 (16%) | 4 (23%) |
| Nail polish use | ||
| 15 (79%) | 17 (100%) |
| 4 (21%) | 0 (0%) |
| Detection Frequency, n (%) | Number of Chemicals, Mean (SD), Range | |||||
|---|---|---|---|---|---|---|
| Chemical Groups A | Worker Wristbands | CM Wristbands | p-Value ^ | Worker Wristbands | CM Wristbands | p-Value ^ |
| Pesticides | 25 (93%) | 18 (100%) | - | 3.08 (1.12), 1–5 | 2.11 (1.08), 1–5 | 0.007 |
| PAHs | 27 (100%) | 18 (100%) | - | 6.48 (4.44), 2–17 | 10.44 (3.54), 3–17 | 0.002 |
| VOCs | 14 (52%) | 11 (61%) | 0.54 | 1.29 (0.61), 1–3 | 1.27 (0.65), 1–3 | 0.91 |
| Pharmacological | 6 (22%) | 0 (0%) | - | 1 (0), 1–1 | - | - |
| Flame retardants | 27 (100%) | 17 (94%) | - | 1.26 (0.53), 1–3 | 1.24 (0.44), 1–2 | 0.98 |
| Chemicals in commerce | 27 (100%) | 18 (100%) | - | 10.90 (1.86), 8–16 | 11.33 (2.14), 8–17 | 0.71 |
| Consumer products | 27 (100%) | 15 (83%) | - | 2.14 (0.99), 1–5 | 1.93 (1.22), 1–5 | 0.28 |
| Personal care products | 27 (100%) | 18 (100%) | - | 9.74 (2.53), 6–15 | 8.44 (1.42), 5–11 | 0.09 |
| Worker Wristbands (n = 27) | Community Member Wristbands (n = 18) | MyExposome Classification A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical | % Detected | Median (IQR) | % Detected | Median (IQR) | Pesticide | PAH | VOC | Flame Retardant | Chemical in Commerce | Consumer Product | Personal Care Product |
| Benzyl salicylate | 100 | 2550 (1310, 4440) | 100 | 3290 (930, 9340) | X | ||||||
| Diisobutyl phthalate | 100 | 1930 (995, 4110) | 100 | 1465 (785, 3000) | X | ||||||
| Galaxolide | 100 | 6330 (4110, 8230) | 100 | 8775 (4670, 11,500) | X | X | |||||
| Lilial | 100 | 668 (323, 1210) | 100 | 910.5 (318, 1420) | X | ||||||
| Pyrene | 100 | 68.7 (41.1, 106) | 100 | 116.55 (57.3, 264) ^ | X | X | |||||
| Tonalide | 100 | 283 (141, 668) | 100 | 280.5 (112, 467) | X | ||||||
| Triphenyl phosphate | 100 | 144 (113, 249) | 94 | 297 (102, 4260) ^ | X | X | |||||
| Diethyl phthalate | 96 | 774 (213, 2180) | 94 | 465 (182, 658) | X | ||||||
| Bis(2-ethylhexyl) phthalate | 93 | 422 (283, 985) | 100 | 503 (233, 1050) | X | ||||||
| Anthracene | 85 | 56.6 (30.4, 99) | 83 | 62.0 (42.6, 116) | X | ||||||
| Caffeine | 85 | 1360 (739, 2130) | 44 ^ | 352 (197.5, 406.5) ^ | X | ||||||
| Ethylene brassylate | 81 | 1090 (404, 2370) | 78 | 1260 (340, 4240) | X | ||||||
| Di-n-butyl phthalate | 78 | 3090 (2350, 6680) | 100 * | 5870 (2350, 10,900) | X | X | |||||
| Di-n-nonyl phthalate | 74 | 490.5 (329, 1018) | 61 | 966 (504, 1550) | X | ||||||
| Diuron metabolite B | 63 | 219 (126, 283) | 0 | 0 (0, 0) | X | ||||||
| Pendimethalin | 63 | 463 (226, 591) | 0 | 0 (0, 0) | X | ||||||
| Benzophenone | 56 | 30.9 (21.3, 59.1) | 33 | 90.75 (10.2, 205) | X | X | |||||
| β-Ionone | 56 | 147 (108, 249) | 83 * | 202 (65.8, 548) | X | ||||||
| Amyl cinnamal | 48 | 206 (118, 334) | 28 | 76.4 (62, 110) | X | ||||||
| Benzyl benzoate | 44 | 1235 (629.5, 5805) | 39 | 773 (140, 9690) | X | ||||||
| Butyl benzyl phthalate | 44 | 116 (62.65, 261) | 72 * | 91.2 (72.2, 158) | X | ||||||
| Naphthalene | 41 | 5.66 (4.63, 6.43) | 11 ^ | 13.37 (4.24, 22.5) | X | X | |||||
| 2-Methylphenanthrene | 41 | 16.0 (12.9, 28.3) | 61 | 31.8 (20.5, 50.4) | X | ||||||
| Benz[a]anthracene | 37 | 21.0 (14.9, 36.0) | 94 ^ | 36.1 (18.4, 47.7) | X | ||||||
| Musk ketone | 37 | 360 (334, 488) | 11 * | 834.5 (279, 1390) | X | ||||||
| Fluorene | 33 | 16.0 (11.1, 16.5) | 72 ^ | 16.7 (10.9, 27.6) | X | ||||||
| Cyclopenta[cd]pyrene | 33 | 22.5 (16.5, 30.9) | 56 | 44.85 (38.2, 65.9) ^ | X | ||||||
| Permethrin | 33 | 77.1 (74.6, 309) | 33 | 234.5 (59.4, 580) | X | ||||||
| Citral A | 33 | 46.3 (38.4, 64.3) | 6 ^ | 22.7 (22.7, 22.7) | X | X | |||||
| 1-Methylphenanthrene | 30 | 16.8 (9, 22.9) | 44 | 19.6 (17.2, 28.45) | X | ||||||
| B-citronellol | 30 | 98.5 (58.65, 247) | 22 | 43.5 (30.75, 98.6) | X | X | |||||
| Fipronil | 30 | 114 (83.55, 136) | 0 | 0 (0, 0) | X | ||||||
| Benzo[a]pyrene | 26 | 6.94 (5.14, 15.7) | 61 ^ | 21.2 (10.8, 28.6) | X | ||||||
| Fluoranthene | 26 | 38.6 (17.5, 141) | 61 ^ | 119 (67.7, 322) * | X | ||||||
| Biphenyl | 26 | 9.67 (8.44, 15.8) | 67 ^ | 8.09 (5.09, 10.36) | X | X | |||||
| Chrysene | 22 | 38.65 (25.3, 64.3) | 50 * | 50.4 (24.8, 89.1) | X | ||||||
| 9-Fluorenone | 22 | 36.75 (21.1, 54.5) | 39 | 29.8 (15.5, 58.1) | X | X | |||||
| 1-Methylnaphthalene | 19 | 6.3 (5.3, 6.6) | 56 ^ | 3.8 (3.2, 4.9) | X | X | X | ||||
| Benzo[a]fluorene | 19 | 22.6 (11.7, 25.5) | 44 * | 26.95 (22, 35.3) | X | ||||||
| Phenanthrene | 19 | 72 (51.4, 74.6) | 44 * | 211 (76.85, 325.5) ^ | X | ||||||
| Benzo[ghi]perylene | 11 | 8.48 (3.34, 70.4) | 33 * | 12.74 (8.06, 27.4) | X | ||||||
| Workers (n = 19, 51%) | Community Members (n = 18, 49%) | |
|---|---|---|
| Diastolic blood pressure, mmHg | 72 (70, 80) | 74 (69, 77) |
| Systolic blood pressure, mmHg | 110 (110, 120) | 109 (104, 117) |
| Hypertension A, n (%) | 0 | 2 (11%) |
| Body mass index, kg/m2 | 26.9 (23.4, 29.2) | 26.4 (22.7, 32.1) |
| HbA1c, % | 4.7 (4.4, 4.9) | 5.2 (5.1, 5.5) * |
| Hematocrit, % | 36.0 (34, 39) | 39.5 (38, 41) * |
| Hemoglobin, g/dL | 12.2 (11.6, 13.3) | 13.6 (13.3, 14.0) * |
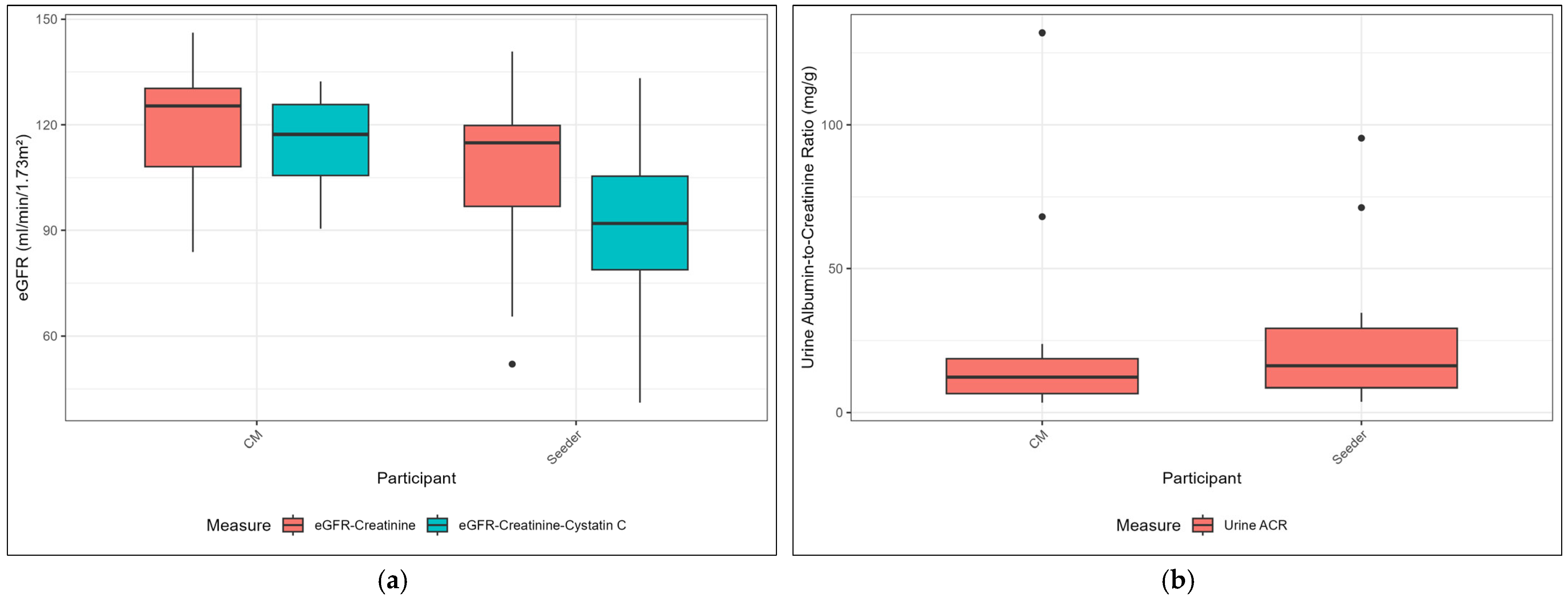
| eGFR-creatinine B | 114.88 (93.70, 119.99) | 125.34 (106.77, 130.94) * |
| 3 (17%) | 1 (6%) |
| 1 (6%) | 0 |
| eGFR-creatinine-cystatin C B | 91.97 (78.66, 105.91) | 117.27 (105.22, 125.95) * |
| 8 (44%) | 0 |
| 1 (6%) | 0 |
| Urine ACR, mg/g | 16.2 (8.6, 29.2) | 12.3 (6.6, 18.7) |
| 4 (21%) | 2 (11%) |
| eGFR-Creatinine | eGFR-Creatinine-Cystatin C | ACR | |
|---|---|---|---|
| Diethyl phthalate | Community Member | ||
| r = 0.44, p = 0.09 | |||
| Butyl benzyl phthalate | Worker | ||
| r = −0.57, p = 0.05 | |||
| Pyrene | Worker | ||
| r = 0.57, p = 0.003 | |||
| β-ionone | Worker | Worker | Worker |
| r = −0.40, p = 0.14 | r = −0.36, p = 0.18 | r = 0.43, p = 0.14 | |
| Benzyl salicylate | Worker | ||
| r = 0.34, p = 0.10 | |||
| Pendimethalin | Worker | ||
| r = 0.55, p = 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler-Dawson, J.; Erlandson, G.; Jaramillo, D.; Villarreal Hernandez, K.; Calvimontes, L.; Krisher, L.; Dally, M.; Brindley, S.; Pilloni, D.; Cruz, A.; et al. Measuring Environmental Chemical Burden with Wristbands: Implications for Kidney Health Among Women in Rural Guatemala. Toxics 2025, 13, 761. https://doi.org/10.3390/toxics13090761
Butler-Dawson J, Erlandson G, Jaramillo D, Villarreal Hernandez K, Calvimontes L, Krisher L, Dally M, Brindley S, Pilloni D, Cruz A, et al. Measuring Environmental Chemical Burden with Wristbands: Implications for Kidney Health Among Women in Rural Guatemala. Toxics. 2025; 13(9):761. https://doi.org/10.3390/toxics13090761
Chicago/Turabian StyleButler-Dawson, Jaime, Grant Erlandson, Diana Jaramillo, Karely Villarreal Hernandez, Laura Calvimontes, Lyndsay Krisher, Miranda Dally, Stephen Brindley, Daniel Pilloni, Alex Cruz, and et al. 2025. "Measuring Environmental Chemical Burden with Wristbands: Implications for Kidney Health Among Women in Rural Guatemala" Toxics 13, no. 9: 761. https://doi.org/10.3390/toxics13090761
APA StyleButler-Dawson, J., Erlandson, G., Jaramillo, D., Villarreal Hernandez, K., Calvimontes, L., Krisher, L., Dally, M., Brindley, S., Pilloni, D., Cruz, A., Bauer, A. K., Johnson, R. J., Newman, L. S., Schaeffer, J., Adgate, J. L., Anderson, K. A., & James, K. A. (2025). Measuring Environmental Chemical Burden with Wristbands: Implications for Kidney Health Among Women in Rural Guatemala. Toxics, 13(9), 761. https://doi.org/10.3390/toxics13090761









