A Scoping Review on Male-Mediated Developmental Toxicity
Abstract
1. Introduction
2. Methods
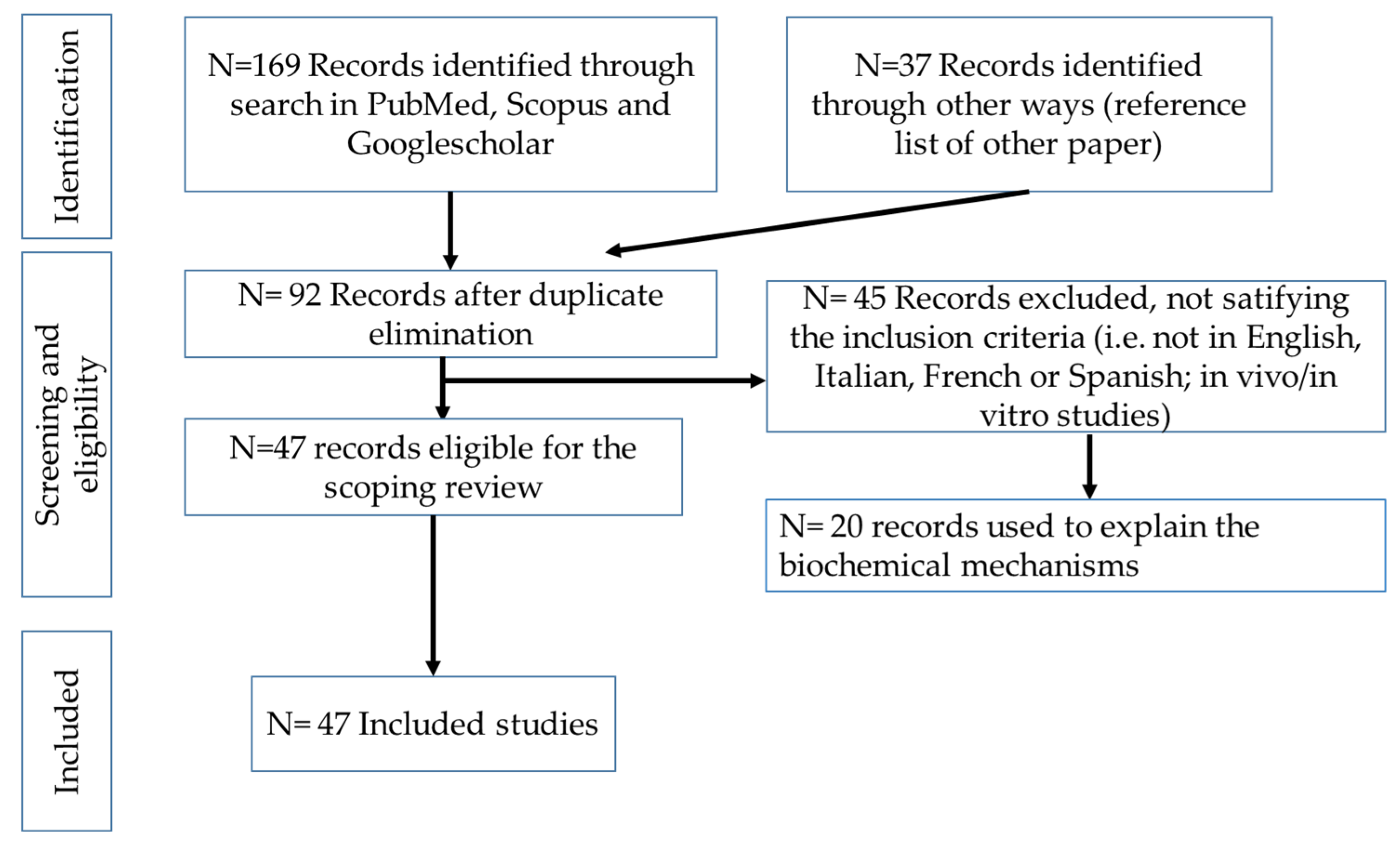
2.1. Information Sources and Literature Search
2.2. Inclusion Criteria
2.3. Screening Process
3. Possible Biological Mechanisms
- Exposure to toxic contaminants in seminal fluid may directly affect the ovum or embryo. The blood–testis barrier is broken by many industrial chemicals, which can be detected in seminal plasma [27]. However, the real significance of this pathway remains unclear. In vitro fertilization assays could be useful to understand this pathway of toxicity; for instance, some studies [28] have reported that paternal exposure to certain chemicals, such as flame retardants, is associated with impaired fertilization of the oocyte.
- Direct DNA damage in the germ line can be caused by chemicals, particularly affecting spermatogonia (point mutations), spermatocytes (aneuploidy), and spermatids (DNA strand breaks and chromosomal aberrations) [29]. Considering that a sperm cell’s ability to repair DNA fades during the last stage of spermatogenesis, it is not surprising that these effects have been demonstrated for many chemicals [26].
- Development in subsequent generations may be directly or indirectly affected by paternal chemical exposure through interference with gene expression via imprinting and disruption of the epigenome. A proposed molecular mechanism involves DNA methylation, typically occurring at cytosine residues adjacent to guanine (CpG sites) [30], which can lead to the silencing of gene transcription in specific genomic regions. Another suggested mechanism is histone modification [17,31]. Although the replacement of most histones by smaller protamines occurs during spermatogenesis, approximately 5 to 10% of human histones persist in the sperm nucleus and remain unaltered [32,33]. Consequently, these histone modifications can be transmitted through spermatozoa. For example, paternal exposure to valproic acid in mice has been shown to induce behavioral deficits such as decreased social interaction, impaired pre-pulse inhibition, and non-spatial memory deficits. These effects have been linked to altered acetylation of histone H3 in the prefrontal cortex and hippocampus of the offspring [34].
4. Evidence About Substances of Abuse: Alcohol, Tobacco Smoke, Opioids, Cannabis
5. Occupational and Environmental Exposure
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone; |
| DNA | Deoxyribonucleic acid |
| THC | ∆9-Tetrahydrocannabinol; 5GRH- Growth hormone-releasing hormone |
| OR | Odds ratio |
| CI | Confidence interval |
References
- Organization for Economic Cooperation and Development—OECD. Series on testing and assessment, number 43. In Guidance Document on Mammalian Reproductive Toxicity Testing and Assessment; ENV/JM/MONO 16; OECD: Paris, France, 2008. [Google Scholar]
- Hass, U. The need for developmental neurotoxicity studies in risk assessment for developmental toxicity. Reprod. Toxicol. 2006, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ellekilde Bonde, J.P.; Søgaard Tøttenborg, S.; Sørig Hougaard, K. Paternal environmental exposure and offspring health. Curr. Opin. Endocr. Metab. Res. 2019, 7, 14–20. [Google Scholar] [CrossRef]
- Scialli, A.R. Paternally mediated effects and political correctness. Reprod. Toxicol. 1993, 7, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.X.W.; Huang, X.; Oon, H.H. Review of dermatologic medications and impact on male fertility, sexual dysfunction and teratogenicity. Andrology 2022, 10, 1272–1285. [Google Scholar] [CrossRef]
- Kumar, P.; Das, A.; Lal, N.R.; Jain, S.; Ghosh, A. Safety of important dermatological drugs (retinoids, immune suppressants, anti androgens and thalidomide) in reproductively active males with respect to pregnancy outcome: A brief review of literature. Indian J. Dermatol Vener. Vener. Leprol 2018, 84, 539–546. [Google Scholar] [CrossRef]
- Garvik, O.S.; Jølving, L.R.; Lund, K.; Friedman, S.; Nørgård, B.M. Paternal use of selective serotonin reuptake inhibitors and adverse health outcomes: A nationwide cohort study on 13,547 exposed children. Andrology 2025, 13, 259–267. [Google Scholar] [CrossRef]
- De Mello, T.F.; Goedert, A.B.; Sengl de Souza, J.S.; da Cruz, J.V.R.; Santos da Silva, A.; Knorst, J.K.; Muller, Y.M.R.; Barreto Silva, F.R.M.; Araujo Leite, G.A. Prolonged exposure to rosuvastatin from pre-puberty to adulthood impairs sperm quality in mice and leads to paternally mediated developmental toxicity. Reprod. Toxicol. 2024, 130, 108717. [Google Scholar] [CrossRef]
- Xu, N.; Lei, I.; Lin, Y.; Ju, L.S.; Morey, T.E.; Gravenstein, N.; Yang, J.; Martynyuk, A.E. A methyltransferase inhibitor (Decitabine) alleviates intergenerational effects of paternal neonatal exposure to anesthesia with sevoflurane. Anesth. Analg. 2020, 131, 1291–1299. [Google Scholar] [CrossRef]
- Adams, P.M.; Fabricant, J.D.; Legator, M.S. Active avoidance behavior in the F1 progeny of male rats exposed to cyclophosphamide prior to fertilization. Neurobehav. Toxicol. Teratol. 1982, 4, 531–534. [Google Scholar]
- Hsu, L.L.; Adams, P.M.; Legator, M.S. Cyclophosphamide- effects of paternal exposure on the brain chemistry of the F1 progeny. J. Toxicol. Environ. Health 1987, 21, 471–481. [Google Scholar] [CrossRef]
- Yang, F.; Chen, J.; Miao, M.H.; Yuan, W.; Li, L.; Liang, H.; Ehrenstein, V.; Li, J. Risk of autism spectrum disorder in offspring following paternal use of selective serotonin reuptake inhibitors before conception: A population-based cohort study. BMJ Open 2017, 7, e016368. [Google Scholar] [CrossRef]
- Yang, F.; Liang, H.; Chen, J.; Miao, M.; Yuan, W.; Norgaard, M.; Li, J. Prenatal paternal selective serotonin reuptake inhibitors use and risk of ADHD in offspring. Pediatrics 2018, 141, e20171081. [Google Scholar] [CrossRef]
- Tagne-Fotso, R.; Leroyer, A.; Howsam, M.; Dehon, B.; Richeval, C.; Nisse, C. Current sources of lead exposure and their relative contributions to the blood lead levels in the general adult population of northern France: The IMEPOGE Study, 2008–2010. J. Toxicol. Environ. Health 2016, 79, 245–265. [Google Scholar] [CrossRef]
- Nybo Andersen, A.M.; Urhoj, S.K. Is advanced paternal age a health risk for the offspring? Fertil. Steril. 2017, 107, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, Y.S.; Baker, V.L.; Shaw, G.M.; Stevenson, D.K.; Lu, Y.; Eisenberg, M.L. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: Population based cohort study. BMJ Clin. Res. Ed. 2018, 363, k4372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and origins of paternal effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef]
- Hanson, M.A.; Skineer, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenetics 2016, 2, dvw002. [Google Scholar] [CrossRef]
- Poynor, D.H.; Lupkiewicz, S.; Lubs, H.A.; Williams, C.A. Paternal exposures and the question of birth defects. J. Fla. Med. Assoc. 1997, 84, 323–326. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive (EU) 2022/431 of the European Parliament and of the Council of 9 March 2022 Amending Directive 2004/37/EC on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work; European Union: Brussels, Belgium, 2022. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Dobrzynska, M.M.; Tyrkiel, E.J.; Pachocki, K.A. Developmental toxicity in mice following paternal exposure to di-n-butyl-phthalate (DBP). Biomed. Environ. Sci. 2011, 24, 569–578. [Google Scholar]
- Reshma Anjum, M.; Sainath, S.B.; Suneetha, Y.; Sreenivasula Reddy, P. Lead acetate induced reproductive and paternal mediated developmental toxicity in rats. Ecotoxicol Environ. Saf. 2011, 74, 793–799. [Google Scholar] [CrossRef]
- Cordier, S. Evidence for a role of paternal exposures in developmental toxicity. Basic Clin. Pharmacol. Toxicol. 2008, 102, 176–181. [Google Scholar] [CrossRef]
- Anderson, D.; Schmid, T.E.; Baumgartner, A. Male-mediated developmental toxicity. Asian J. Androl. 2014, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, L.; Scialli, A.R. The transport of chemicals in semen. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2005, 74, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Carignan, C.C.; Mínguez-Alarcón, L.; Williams, P.L.; Meeker, J.D.; Stapleton, H.M.; Butt, C.M.; Toth, T.L.; Ford, J.B.; Hauser, R. EARTH Study Team Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures and pregnancy outcomes among couples undergoing in vitro fertilization. Environ. Int. 2018, 111, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.A.; Yauk, C.L.; Marchetti, F. From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat. Res. 2017, 773, 26–50. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Erkek, S.; Hisano, M.; Liang, C.Y.; Gill, M.; Murr, R.; Dieker, J.; Schubeler, D.; van der Vlag, J.; Stadler, M.B.; Peters, A.H. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat. Struct. Mol. Biol. 2013, 20, 868–875. [Google Scholar] [CrossRef]
- Hammond, S.S.; Nix, D.A.; Zhang, H.; Purwar, J.; Carrell, D.T.; Cairns, B.R. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009, 460, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Ibi, D.; Fujiki, Y.; Koide, N.; Nakasai, G.; Takaba, R.; Hiramatsu, M. Paternal valproic acid exposure in mice triggers behavioral alterations in offspring. Neurotoxicol. Teratol. 2019, 76, 106837. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Schuster, A.; Tang, C.; Yu, T.; Ortogero, N.; Bao, J.; Zheng, H.; Yan, W. Sperm-borne miRNAs and endo-siRNA are important for fertilization and preimplantation embryonic development. Development 2016, 143, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, S.A.; Kruger, A.; Lalancette, C.; Tagett, R.; Anton, E.; Draghici, S.; Diamond, M.P. A survey of small RNAs in human sperm. Hum. Reprod. 2011, 26, 3401–3412. [Google Scholar] [CrossRef]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A decade of exploring the mammalian sperm epigenome: Paternal epigenetic and transgenerational inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Donkin, I.; Barres, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Lo, J.O.; Hedges, J.C.; Chou, W.H.; Tager, K.R.; Bachli, I.D.; Hagen, O.L.; Murphy, S.K.; Hanna, C.B.; Easley, C.A. Influence of substance use on male reproductive health and offspring outcomes. Nat. Rev. 2024, 21, 534–564. [Google Scholar] [CrossRef]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of alcohol consumption on male fertility potential: A narrative review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef]
- Lo, J.O.; Hedges, J.C.; Girardi, G. Impact of cannabinoids on pregnancy, reproductive health, and offspring outcomes. Am. J. Obstet. Gynecol 2022, 227, 571–581. [Google Scholar] [CrossRef]
- Ryan, K.S.; Bash, J.C.; Hanna, C.B.; Hedges, J.C.; Lo, J.O. Effects of marijuana on reproductive health: Preconception and gestational effects. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.O.; D’Mello, R.J.; Watch, L.; Schust, D.J.; Murphy, S.K. An epigenetic synopsis of parental substance use. Epigenomics 2023, 15, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Easey, K.E.; Sharp, G.C. The impact of paternal alcohol, tobacco, caffeine use and physical activity on offspring mental health: A systematic review and meta-analysis. Reprod. Health 2021, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.O.; Shaw, B.; Robalino, S.; Ayers, C.K.; Durbin, S.; Rushkin, M.C.; Olyaei, A.; Kansagara, D.; Harrod, C.S. Cannabis use in pregnancy and neonatal outcomes: A systematic review and meta-analysis. Cannabis Cannabinoid Res. 2024, 9, 470–485. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Killinger, C.E.; Robinson, S.; Stanwood, G.D. Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse 2012, 66, 902–908. [Google Scholar] [CrossRef]
- Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sadri-Vakili, G.; Pierce, R.C. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 2013, 16, 42–47. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef]
- Salonen, I.; Pakarinen, P.; Huhtaniemi, I. Effect of chronic ethanol diet on expression of gonadotropin genes in the male rat. J. Pharmacol. Exp. Ther. 1992, 260, 463–467. [Google Scholar] [CrossRef]
- Salonen, I.; Huhtaniemi, I. Effects of chronic ethanol diet on pituitary-testicular function of the rat. Biol. Reprod. 1990, 42, 55–62. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Balercia, G.; Vicari, E.; Calogero, A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J. Androl. 2013, 15, 221–225. [Google Scholar] [CrossRef]
- Talebi, A.R.; Sarcheshmeh, A.A.; Khalili, M.A.; Tabibnejad, N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011, 45, 403–409. [Google Scholar] [CrossRef]
- Adler, R.A. Clinical review 33: Clinically important effects of alcohol on endocrine function. J. Clin. Endocrinol. Metab. 1992, 74, 957–960. [Google Scholar]
- Emanuele, M.A.; Emanuele, N.V. Alcohol’s effects on male reproduction. Alcohol Health Res. World 1998, 22, 195–201. [Google Scholar]
- Grover, S.; Mattoo, S.K.; Pendharkar, S.; Kandappan, V. Sexual dysfunction in patients with alcohol and opioid dependence. Indian J. Psychol. Med. 2014, 36, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.; Wang, H.; Bedi, Y.; Golding, M.C. Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.N.; Srikanth, N.; Bhadsavle, S.S.; Thomas, K.R.; Zimmel, K.N.; Basel, A.; Roach, A.N.; Mehta, N.A.; Bedi, Y.S.; Golding, M.C. Preconception paternal ethanol exposures induce alcohol-related craniofacial growth deficiencies in fetal offspring. J. Clin. Investig. 2023, 133, e167624. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, L.; Chen, J.; Wang, Q.; Shen, H.; Zhang, S.; Li, X. Association of preconception paternal alcohol consumption with increased fetal birth defect risk. JAMA Pediatr. 2021, 175, 742–743. [Google Scholar] [CrossRef]
- Liang, F.; Diao, L.; Liu, J.; Jiang, N.; Zhang, J.; Wang, H.; Zhou, W.; Huang, G.; Ma, D. Paternal ethanol exposure and behavioral abnormities in offspring: Associated alterations in imprinted gene methylation. Neuropharmacology 2014, 81, 126–133. [Google Scholar] [CrossRef]
- Meek, L.R.; Myren, K.; Sturm, J.; Burau, D. Acute paternal alcohol use affects offspring development and adult behavior. Physiol. Behav. 2007, 91, 154–160. [Google Scholar] [CrossRef]
- Hayer, S.; Mandelbaum, A.D.; Watch, L.; Ryan, K.S.; Hedges, M.A.; Manuzak, J.A.; Easley, C.A.; Schust, D.J.; Lo, J.O. Cannabis and pregnancy: A review. Obs. Gynecol. Surv. 2023, 78, 411–428. [Google Scholar] [CrossRef]
- Lo, J.O.; Hedges, J.C.; Metz, T.D. Cannabis use and perinatal health research. JAMA 2023, 330, 913–914. [Google Scholar] [CrossRef]
- Rubinstein, A.L.; Carpenter, D.M. Association between commonly prescribed opioids and androgen deficiency in men: A retrospective cohort analysis. Pain Med. 2017, 18, 637–644. [Google Scholar] [CrossRef]
- Eshraghi, Y.; Hanks, N.; Rooney, S.; Yousefi Ata, F.M.; Velasco, C.; Guirguis, M.; Uwaifo, G. Establishing a dose-response relationship between opioid use and hypogonadism: A retrospective case-control study. Ochsner J. 2021, 21, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.R.; Gould, T.J. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur. J. Neurosci. 2019, 50, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Jalali, Z.; Bahrampour, S.; Khalili, P.; Khademalhosseini, M.; Esmaeili Nadimi, A. Cohort based analysis of paternal opioid use in relation to offspring’s BMI and plasma lipid profile. Sci. Rep. 2021, 11, 9462. [Google Scholar] [CrossRef] [PubMed]
- Pachenari, N.; Azizi, H.; Ghasemi, E.; Azadi, M.; Semnanian, S. Exposure to opiates in male adolescent rats alters pain perception in the male offspring. Behav. Pharmacol. 2018, 29, 255–260. [Google Scholar] [CrossRef]
- Azadi, M.; Moazen, P.; Wiskerke, J.; Semnanian, S.; Azizi, H. Preconception paternal morphine exposure leads to an impulsive phenotype in male rat progeny. Psychopharmacology 2021, 238, 3435–3446. [Google Scholar] [CrossRef]
- Ellis, A.S.; Toussaint, A.B.; Knouse, M.C.; Thomas, A.S.; Bongiovanni, A.R.; Mayberry, H.L.; Bhakta, S.; Peer, K.; Bangasser, D.A.; Wimmer, M.E. Paternal morphine self-administration produces object recognition memory deficits in female, but not male offspring. Psychopharmacology 2020, 237, 1209–1221. [Google Scholar] [CrossRef]
- Mitra, A.; Chakraborty, B.; Mukhopadhay, D.; Pal, M.; Mukherjee, S.; Banerjee, S.; Chaudhuriet, K. Effect of smoking on semen quality, FSH, testosterone level, and CAG repeat length in androgen receptor gene of infertile men in an Indian city. Syst. Biol. Reprod. Med. 2012, 58, 255–262. [Google Scholar] [CrossRef][Green Version]
- Tweed, J.O.; Hsia, S.H.; Lutfy, K.; Friedman, T.C. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metab. 2012, 23, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Du Fossé, N.A.; Petronella van der Hoorn, M.L.; Buisman, N.H.; vaqn Lith, I.N.N.; le Cessie, S.; Lashley, L.E.E.L.O. Paternal smoking is associated with an increased risk of pregnancy loss in a dose-dependent manner: A systematic review and meta-analysis. FS Rev. 2021, 2, 227–238. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.; Wang, Q.; Shen, H.; Zhang, Y.; Tian, W.; Li, X. Association between preconception paternal smoking and birth defects in offspring: Evidence from the database of the National Free Preconception Health Examination Project in China. BJOG 2020, 127, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.E.; Aitke, R.J. Male-mediated developmental defects and childhood disease. Reprod. Med. Rev. 2000, 8, 107–126. [Google Scholar] [CrossRef]
- Baldacci, S.; Gorini, F.; Santoro, M.; Pierini, A.; Minichilli, F.; Bianchi, F. Environmental and individual exposure and the risk of congenital anomalies: A review of recent epidemiological evidence. Epidemiol. Prev. 2018, 42 (Suppl. S1), 1–34. [Google Scholar]
- Savits, D.A.; Sonnefeld, N.L.; Olshan, A.F. Review of epidemiologic studies of paternal occupational exposure and spontaneous abortion. Am. J. Ind. Med. 1994, 25, 361–383. [Google Scholar] [CrossRef]
- Logman, J.F.S.; de Vries, L.E.; Hemels, M.E.H.; Khattak, S.; Einarson, T.R. Paternal organic solvent exposure and adverse pregnancy outcomes: A meta-analysis. Am. J. Ind. Med. 2005, 47, 37–44. [Google Scholar] [CrossRef]
- Hooiveld, M.; Haveman, W.; Roskes, K.; Bretveld, R.; Burstyn, I.; Roeleveld, N. Adverse reproduction outcomes among male painters with occupational exposure to organic solvents. Occup. Environ. Med. 2006, 63, 538–544. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J.; Dadvand, P.; Grellier, J.; Martinez, D.; Vrijheid, M. Environmental risk factors of pregnancy outcomes: A summary of recent meta-analysis of epidemiological studies. Environ. Health 2013, 12, 6. [Google Scholar] [CrossRef]
- Jørgensen, K.T.; Jensen, M.S.; Toft, G.V.; Larsen, A.D.; Bonde, J.P.; Hougaard, K.S. Risk of cryptorchidism among sons of horticultural workers and farmers in Denmark. Scand. J. Work Environ. Health 2014, 40, 323–330. [Google Scholar] [CrossRef]
- Snijder, C.A.; Vlot, I.J.; Burdorf, A.; Obermann-Borst, S.A.; Helbing, W.A.; Wildhagen, M.F.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Congenital heart defects and parental occupational exposure to chemicals. Hum. Reprod. 2012, 27, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Dimich-Ward, H.; Hertzman, C.; Teschke, K.; Hershler, R.; Marion, S.A.; Ostry, A.; Kelly, S. Reproductive effects of paternal exposure to chlorophenate wood preservatives in the sawmill industry. Scand. J. Work. Environ. Health 1996, 22, 267–273. [Google Scholar] [CrossRef]
- Mirilas, P.; Mentessidou, A.; Kontis, E.; Asimakidou, M.; Moxham, B.J.; Petropoulos, A.S.; Emmanouil-Nikolousi, E.N. Parental exposures and risk of nonsyndromic orofacial clefts in offspring: A case-control study in Greece. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 695–699. [Google Scholar] [CrossRef]
- PLoS Medicine Editors. Best practice in systematic reviews: The importance of protocols and registration. PLoS Med. 2011, 8, e1001009. [Google Scholar]
| Type of Substance Use | Target of Reprotoxicity | In Detail | Effects on Offspring |
|---|---|---|---|
| Alcohol | Alters male reproductive hormones [41,51,52] Alters semen parameters [42,53,54] Testicular volume [55,56] Erectile and sexual function [57] | Higher: LH 1, FSH 2, DNA 3 fragmentation; lower: testosterone, seminal volume and sperm count, motility and morphology | Increased intrauterine growth restriction Increased birth defects, decreased birthweight Increased risk of cancers (leukemia and brain tumors) [41,58,59,60,61,62] |
| Cannabis (in particular THC 4) | Alters male reproductive hormones, semen parameters, libido, erectile and sexual function [42,43] | Lower: LH and sperm count, motility and morphology; higher: sperm DNA fragmentation. Testosterone levels were found to be both higher than lower | Increased pregnancy loss, increased congenital cardiac anomalies Increased behavioral issues [43,63,64] |
| Opioids | Chronic opioid use was linked with an increased risk of androgen suppression [65,66] | Lower: GRH 5 secretion, testosterone, sperm motility and morphology; higher sperm DNA fragmentation | Decreased fetal weight Increased withdrawal-like behaviors Increased risk of opioid addiction, delayed learning, and impulsive behaviors [67,68,69,70,71] |
| Tobacco smoke (nicotine in particular) | Alters sexual hormones, testis, and sperm [72,73] | Higher: testosterone levels; lower: sperm count, motility and morphology. Both higher and lower LH and/or FSH | Increased pregnancy loss Increased testosterone levels in child < 1 years Decreased sperm count and increased risk of neurodivergent behavior in childhood and adolescent [74,75] |
| Ref. | Chemicals | Effects for Offspring | Results |
|---|---|---|---|
| [81] | Solvents | Neural tube defects | OR 1 = 1.86, 95% CI 2 1.40–2.46 |
| Solvents | Anencephaly | OR = 2.18, 95% CI 1.52–3.11 | |
| Solvents | Spina bifida | OR = 1.59, 95% CI 0.99–2.56 | |
| Pesticides | Hypospadias | RR = 1.19, 95% CI 1.00–1.41 | |
| [82] | Pesticides | Cryptorchidism | OR = 1.04, 95% CI 0.96–1.12 |
| [83] | Phthalates and polychlorinated compounds | Congenital heart defects | OR = 2.08, 95% CI 1.27–3.40 |
| Phthalates | Perimembranous ventricular septal defect | OR = 2.84, 95% CI 1.37–5.92 | |
| Bisphenols | Atrial ventricular septal defects | OR = 4.22, 95% CI 1.23–14.42 | |
| Alkylphenols | Coarctation of aorta | OR = 3.85, 95% CI 1.17–12.67 | |
| [84] | Chlorophenate wood preservatives | Eye malformations | OR = 2.87, 95% CI 1.5–5.5 |
| Cataracts | OR = 5.68, 95% CI 1.4–22.6 | ||
| Undescended testicles | OR = 1.16, 95% CI 0.8–1.6 | ||
| Genital organs in general | OR = 1.29 95% CI 0.9–1.5 | ||
| Spina bifida | OR = 1.32 95% CI 0.2–2.1 | ||
| [85] | Pesticides | Cleft lip | OR = 3.00 95% CI 1.03–8.70 |
| [79] | Solvents in painting activity | Birth defects | OR = 1.86 95% CI 1.4–2.5 |
| [80] | Solvents in painting activity | Birth defects | OR = 6.2 95% CI 1.4–28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporossi, L.; Castellano, P.; Paci, E.; Pigini, D. A Scoping Review on Male-Mediated Developmental Toxicity. Toxics 2025, 13, 707. https://doi.org/10.3390/toxics13090707
Caporossi L, Castellano P, Paci E, Pigini D. A Scoping Review on Male-Mediated Developmental Toxicity. Toxics. 2025; 13(9):707. https://doi.org/10.3390/toxics13090707
Chicago/Turabian StyleCaporossi, Lidia, Paola Castellano, Enrico Paci, and Daniela Pigini. 2025. "A Scoping Review on Male-Mediated Developmental Toxicity" Toxics 13, no. 9: 707. https://doi.org/10.3390/toxics13090707
APA StyleCaporossi, L., Castellano, P., Paci, E., & Pigini, D. (2025). A Scoping Review on Male-Mediated Developmental Toxicity. Toxics, 13(9), 707. https://doi.org/10.3390/toxics13090707










