Toxicology of Airborne Inorganic Arsenic: Oxidative Stress, Molecular Mechanisms, and Organ-Specific Pathologies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Quality Assessment
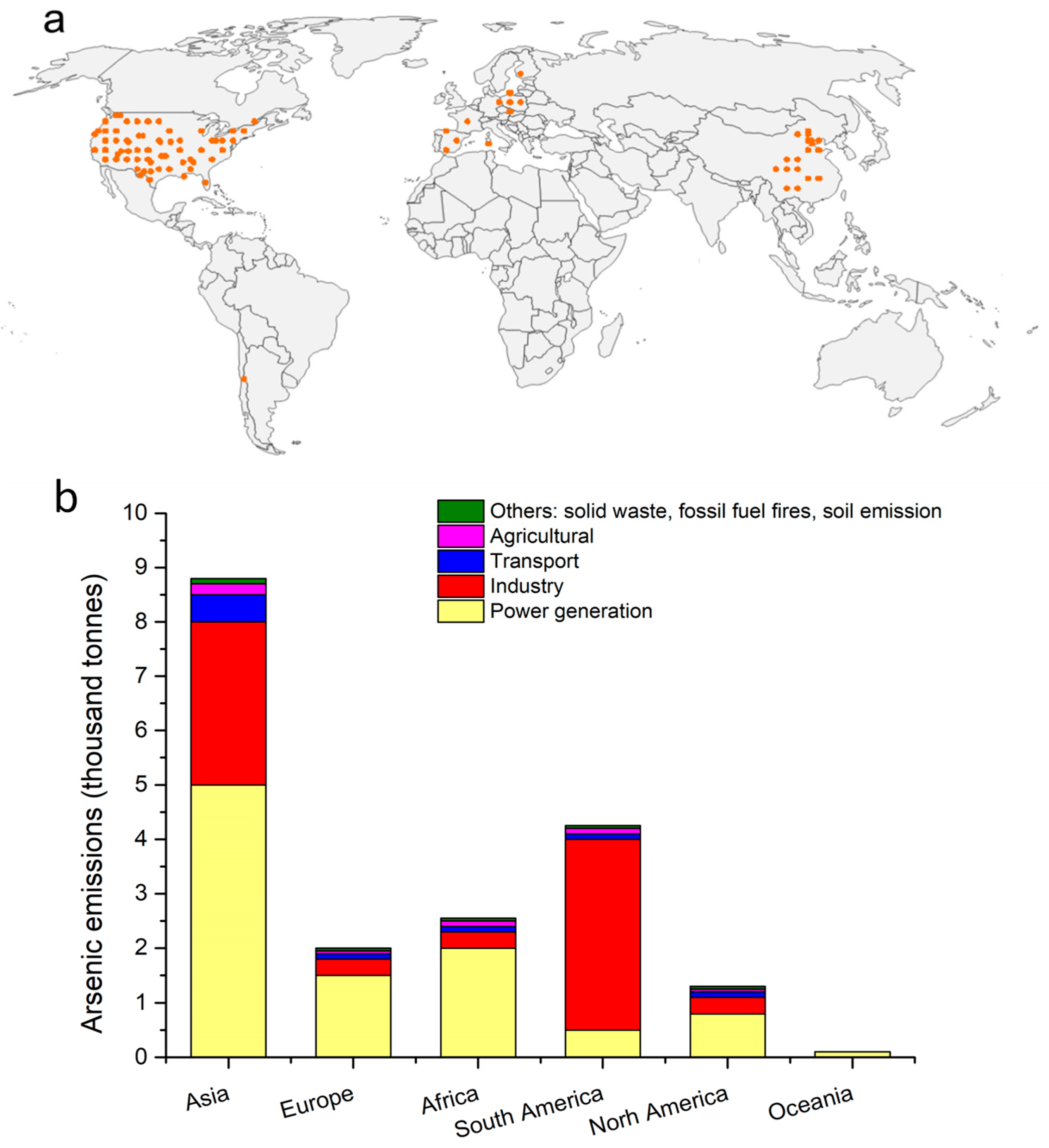
3. Levels of Airborne Arsenic
3.1. Global Distribution
3.2. Chemical Forms
3.3. Particle Size-Dependent Deposition
3.4. Intervention Effects on Temporal Trends
4. Biological Transport Mechanisms and Metabolic Fate
5. Molecular Mechanisms of Arsenic Toxicity
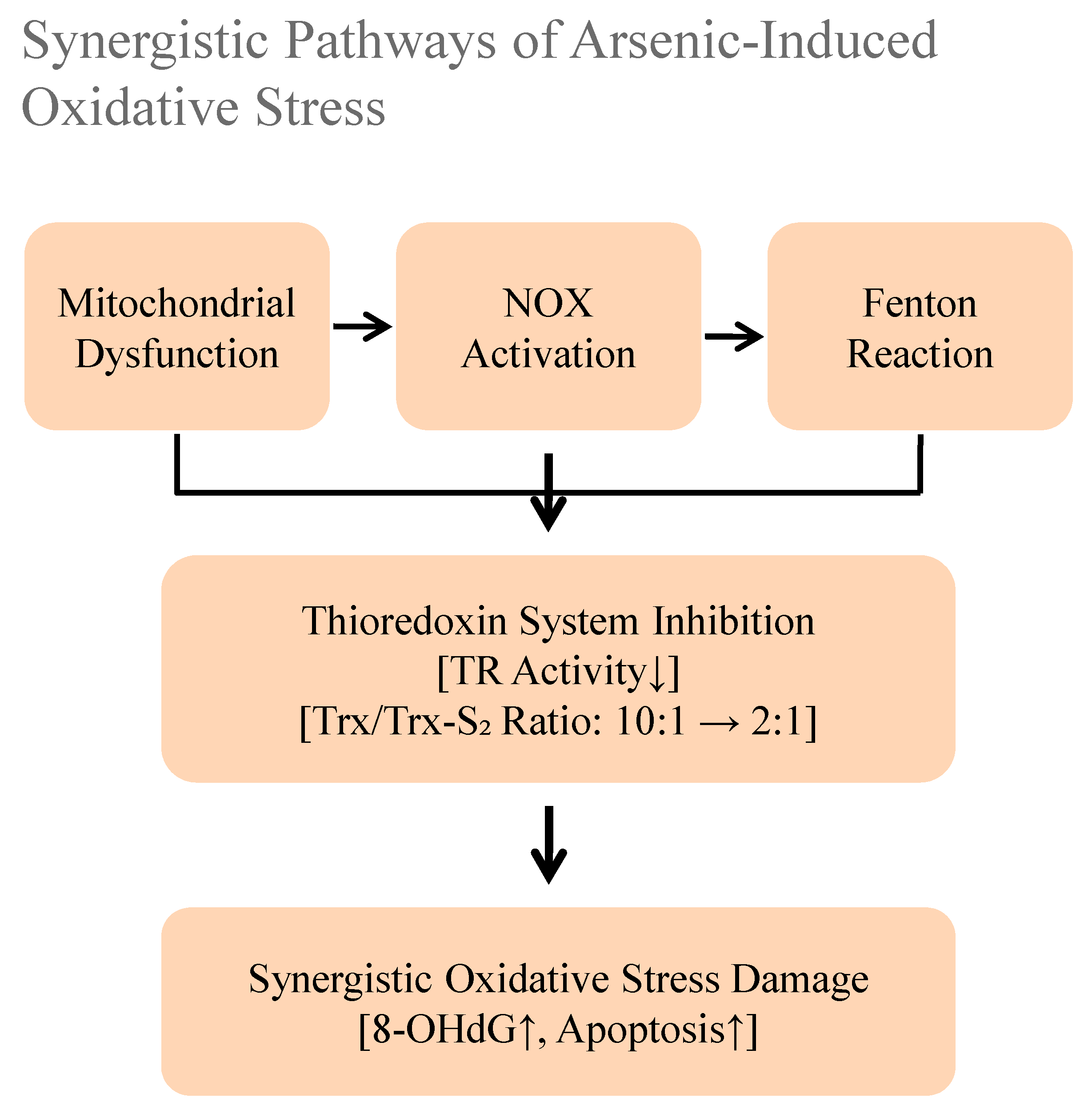
5.1. Multiple Pathways of Reactive Oxygen Species (ROS) Generation
5.2. Collapse of Antioxidant Defense Systems
5.3. Molecular Consequences of Oxidative Damage
6. Signaling Pathway Disorders
6.1. Abnormal DNA Methylation
6.2. Reprogramming of Histone Modifications
6.3. Dysregulation of Non-Coding RNA Networks
6.4. Transgenerational Epigenetic Effects
7. Regulatory Disorders
7.1. Disorder of MAPK Signaling Network
7.2. Dysregulation of PI3K/AKT/mTOR Signaling
7.3. Activation of NF-κB Inflammatory Signaling
7.4. Calcium Signaling and Cytoskeletal Regulation
8. Organ-Specific Toxicity Mechanisms
8.1. Neurotoxicity
8.2. Hepatotoxicity
8.3. Nephrotoxicity
8.4. Cutaneous Toxicity
8.5. Cardiovascular Toxicity
8.6. Reproductive Toxicity
9. Health Risk Assessment and Regulatory Challenges
9.1. Controversies in Carcinogenic Risk Assessment Models
9.2. Current Regulatory Standards and Limitations
9.3. Scientific Challenges in Standard Setting
9.4. Biological Monitoring and Risk Early Warning
10. Future Research Directions
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migaszewski, Z.M.; Gałuszka, A. Primary Fe-(hydr)oxides and pyrite as carriers of arsenic and antimony: An overlooked problem in acid mine drainage areas. Sci. Total Environ. 2025, 977, 179400. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.W.; Qureshi, F.; Ahmed, S.; Kamyab, H.; Rajendran, S.; Ibrahim, H.; Yusuf, M. A comprehensive review on arsenic contamination in groundwater: Sources, detection, mitigation strategies and cost analysis. Environ. Res. 2025, 265, 120457. [Google Scholar] [CrossRef]
- Pei, K.L.; Gailer, J. Probing the interaction of arsenobetaine with blood plasma constituents in vitro: An SEC-ICP-AES study. Metallomics 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Yager, J.W.; Greene, T.; Schoof, R.A. Arsenic relative bioavailability from diet and airborne exposures: Implications for risk assessment. Sci. Total Environ. 2015, 536, 368–381. [Google Scholar] [CrossRef]
- Li, G.; Lv, M.; Zhang, H.; Zhang, D.; Yu, H.; Li, Q.; Wang, L. Toxic effects of co-exposure to polystyrene nanoplastics and arsenic in zebrafish (Danio rerio): Oxidative stress, physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2025, 298, 118286. [Google Scholar] [CrossRef]
- Nield, C.P.; Sleeth, D.K.; Larson, R.R.; Thiese, M.S. Particle size selective sampling of airborne arsenic during electroplating operations. J. Chem. Health Saf. 2014, 21, 15–20. [Google Scholar] [CrossRef]
- Hama, T.; Ito, H.; Kawagoshi, Y.; Nakamura, K.; Kubota, T. Natural attenuation and remobilization of arsenic in a small river contaminated by the volcanic eruption of Mount Iou in southern Kyushu Island, Japan. J. Hazard. Mater. 2023, 455, 131576. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.D.G.; Robey, N.M.; Smallwood, T.J.; Spreadbury, C.J.; Townsend, T.G. Landfill gas as a source of anthropogenic antimony and arsenic release. Chemosphere 2022, 307, 135739. [Google Scholar] [CrossRef]
- Chanda, S.; Dasgupta, U.B.; Guhamazumder, D.; Gupta, M.; Chaudhuri, U.; Lahiri, S.; Das, S.; Ghosh, N.; Chatterjee, D. DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol. Sci. 2006, 89, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, L.; Wu, M.; Liu, Q.; Cao, H. Non-coding RNA therapeutics: Towards a new candidate for arsenic-induced liver disease. Chem.-Biol. Interact. 2023, 382, 110626. [Google Scholar] [CrossRef]
- Rychlik, K.A.; Illingworth, E.J.; Sanchez, I.F.; Attreed, S.E.; Sinha, P.; Casin, K.M.; Taube, N.; Loube, J.; Tasneen, R.; Kabir, R.; et al. Long-term effects of prenatal arsenic exposure from gestational day 9 to birth on lung, heart, and immune outcomes in the C57BL/6 mouse model. Toxicol. Lett. 2023, 383, 17–32. [Google Scholar] [CrossRef]
- Sassano, M.; Seyyedsalehi, M.S.; Siea, A.C.; Boffetta, P. Occupational arsenic exposure and digestive and head and neck cancers: A systematic review and meta-analysis. Environ. Res. 2024, 260, 119643. [Google Scholar] [CrossRef]
- Jirasit, C.; Navasumrit, P.; Chaisatra, K.; Chompoobut, C.; Waraprasit, S.; Parnlob, V.; Ruchirawat, M. Genotoxicity and fibrosis in human hepatocytes in vitro from exposure to low doses of PBDE-47, arsenic, or both chemicals. Chem.-Biol. Interact. 2025, 410, 111410. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, E.J.; Rychlik, K.A.; Maertens, A.; Sillé, F.C.M. Sex-specific transcriptomic effects of low-dose inorganic arsenic exposure on bone marrow-derived macrophages. Toxicology 2025, 510, 153988. [Google Scholar] [CrossRef] [PubMed]
- Serbula, S.M.; Milosavljevic, J.S.; Radojevic, A.A.; Kalinovic, J.V.; Kalinovic, T.S. Extreme air pollution with contaminants originating from the mining–metallurgical processes. Sci. Total Environ. 2017, 586, 1066–1075. [Google Scholar] [CrossRef]
- Wai, K.-M.; Wu, S.; Li, X.; Jaffe, D.A.; Perry, K.D. Global Atmospheric Transport and Source-Receptor Relationships for Arsenic. Environ. Sci. Technol. 2016, 50, 3714–3720. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Wu, S.; Zhang, S.; Smith, K.R.; Yao, X.; Gao, H. Global impact of atmospheric arsenic on health risk: 2005 to 2015. Proc. Natl. Acad. Sci. USA 2020, 117, 13975–13982. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.-S.; Liu, C.-W.; López, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef]
- Genaidy, A.M.; Sequeira, R.; Tolaymat, T.; Kohler, J.; Wallace, S.; Rinder, M. Integrating science and business models of sustainability for environmentally-challenging industries such as secondary lead smelters: A systematic review and analysis of findings. J. Environ. Manag. 2010, 91, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Yadav, G.; Yadav, B.; Maharjan, M. History and assessment of household Arsenic Biosand Filter interventions in Nepal: Over two decades of efforts and challenges. Groundw. Sustain. Dev. 2025, 29, 101444. [Google Scholar] [CrossRef]
- Barkhordari, M.S.; Qi, C. Prediction of zinc, cadmium, and arsenic in european soils using multi-end machine learning models. J. Hazard. Mater. 2025, 490, 137800. [Google Scholar] [CrossRef]
- Schwanck, F.; Simões, J.C.; Handley, M.; Mayewski, P.A.; Bernardo, R.T.; Aquino, F.E. Anomalously high arsenic concentration in a West Antarctic ice core and its relationship to copper mining in Chile. Atmos. Environ. 2016, 125, 257–264. [Google Scholar] [CrossRef]
- Guo, J.; Cao, W.; Li, X.; Ren, Y.; Lu, C.; Wang, Y.; Song, L.; Liu, Y.; Sun, X. Comparative study on genesis mechanism of high arsenic groundwater in typical alluvial plain of the Upper and lower Yellow River, China. Sci. Total Environ. 2024, 957, 177694. [Google Scholar] [CrossRef]
- Deng, S.; Luo, S.; Lin, Q.; Shen, L.; Gao, L.; Zhang, W.; Chen, J.; Li, C. Analysis of heavy metal and arsenic sources in mangrove surface sediments at Wulishan Port on Leizhou Peninsula, China, using the APCS-MLR model. Ecotoxicol. Environ. Saf. 2024, 283, 116788. [Google Scholar] [CrossRef] [PubMed]
- Csavina, J.; Landázuri, A.; Wonaschütz, A.; Rine, K.P.; Rheinheimer, P.; Barbaris, B.; Conant, W.; Sáez, A.E.; Betterton, E.A. Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water Air Soil Pollut. 2011, 221, 145–157. [Google Scholar] [CrossRef]
- Ramirez-Andreotta, M.D.; Brusseau, M.L.; Beamer, P.; Maier, R.M. Home Gardening Near a Mining Site in an Arsenic-Endemic Region of Arizona: Assessing Arsenic Exposure Dose and Risk via Ingestion of Home Garden Vegetables, Soils, and Water. Sci. Total Environ. 2013, 454–455, 373–382. [Google Scholar] [CrossRef]
- Hoover, J.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Van Horne, Y.O.; Chief, K.; Charley, P.H.; Begay, M.G.; Lothrop, N.; Bell, M.L.; Canales, R.A.; Teufel-Shone, N.I.; Beamer, P.I. A Community-Based Health Risk Assessment Following the Gold King Mine Spill: Results from the Gold King Mine Spill Diné Exposure Project. Expo. Health 2024, 16, 643–660. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sunar, S.; Saha, P.; Saha, S.; Dutta, S.; Yakub Ali, S. An integrated approach towards groundwater quality and human health risk assessment in the Indo-Gangetic plains of West Bengal, India. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101022. [Google Scholar] [CrossRef]
- Serbula, S.M.; Milosavljevic, J.S.; Kalinovic, J.V.; Kalinovic, T.S.; Radojevic, A.A.; Trujic, T.L.A.; Tasic, V.M. Arsenic and SO2 hotspot in South-Eastern Europe: An overview of the air quality after the implementation of the flash smelting technology for copper production. Sci. Total Environ. 2021, 777, 145981. [Google Scholar] [CrossRef]
- Vishwakarma, Y.K.; Tiwari, S.; Mohan, D.; Singh, R.S. A review on health impacts, monitoring and mitigation strategies of arsenic compounds present in air. Clean. Eng. Technol. 2021, 3, 100115. [Google Scholar] [CrossRef]
- González-Castanedo, Y.; Sanchez-Rodas, D.; Sánchez de la Campa, A.M.; Pandolfi, M.; Alastuey, A.; Cachorro, V.E.; Querol, X.; de la Rosa, J.D. Arsenic species in atmospheric particulate matter as tracer of the air quality of Doñana Natural Park (SW Spain). Chemosphere 2015, 119, 1296–1303. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Zheng, Y.; Yang, J.; Guo, G.; Wang, J.; Chen, T. Effects of environmental governance in mining areas: The trend of arsenic concentration in the environmental media of a typical mining area in 25 years. Chemosphere 2019, 235, 849–857. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, C.; Chen, Z.; Huang, W.; Dong, Y.; Liu, Q.; Ning, X.; Wang, S. Assessing the effectiveness of atmospheric pollution control policies by monitoring the changes in trace elements in atmospheric deposition, Lanzhou: 2010–2021. J. Clean. Prod. 2025, 498, 145137. [Google Scholar] [CrossRef]
- Dellise, M.; Villot, J.; Gaucher, R.; Amardeil, A.; Laforest, V. Challenges in assessing Best Available Techniques (BATs) compliance in the absence of industrial sectoral reference. J. Clean. Prod. 2020, 263, 121474. [Google Scholar] [CrossRef]
- Leclerc, A.; Sala, S.; Secchi, M.; Laurent, A. Building national emission inventories of toxic pollutants in Europe. Environ. Int. 2019, 130, 104785. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, V.; Varum, C.; Madaleno, M. How economic growth affects emissions? An investigation of the environmental Kuznets curve in Portuguese and Spanish economic activity sectors. Energy Policy 2017, 106, 326–344. [Google Scholar] [CrossRef]
- Ghosh, S.; Igwegbe, C.A.; Malloum, A.; Elmakki, M.A.E.; Onyeaka, H.; Fahmy, A.H.; Aquatar, M.O.; Ahmadi, S.; Alameri, B.M.; Ghosh, S.; et al. Sustainable technologies for removal of arsenic from water and wastewater: A comprehensive review. J. Mol. Liq. 2025, 427, 127412. [Google Scholar] [CrossRef]
- Smith, A.H.; Ercumen, A.; Yuan, Y.; Steinmaus, C.M. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 343–348. [Google Scholar] [CrossRef]
- Stýblo, M.; Venkatratnam, A.; Fry, R.C.; Thomas, D.J. Origins, fate, and actions of methylated trivalent metabolites of inorganic arsenic: Progress and prospects. Arch. Toxicol. 2021, 95, 1547–1572. [Google Scholar] [CrossRef]
- Hirano, S. Biotransformation of arsenic and toxicological implication of arsenic metabolites. Arch. Toxicol. 2020, 94, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Huang, H.W.; Lee, C.H.; Yu, H.S. Arsenic-Induced Carcinogenesis and Immune Dysregulation. Int. J. Environ. Res. Public Health 2019, 16, 2746. [Google Scholar] [CrossRef]
- Cantoni, O.; Zito, E.; Guidarelli, A.; Fiorani, M.; Ghezzi, P. Mitochondrial ROS, ER Stress, and Nrf2 Crosstalk in the Regulation of Mitochondrial Apoptosis Induced by Arsenite. Antioxidants 2022, 11, 1034. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.L.; Rockenbauer, A.; Villamena, F.A. Radical model of arsenic(III) toxicity: Theoretical and EPR spin trapping studies. Chem. Res. Toxicol. 2014, 27, 765–774. [Google Scholar] [CrossRef]
- Sun, H.J.; Ding, S.; Guan, D.X.; Ma, L.Q. Nrf2/Keap1 pathway in countering arsenic-induced oxidative stress in mice after chronic exposure at environmentally-relevant concentrations. Chemosphere 2022, 303, 135256. [Google Scholar] [CrossRef] [PubMed]
- Garla, R.; Sharma, N.; Shamli; Kaushal, N.; Garg, M.L. Effect of Zinc on Hepatic and Renal T tissues of Chronically Arsenic Exposed Rats: A Biochemical and Histopathological Study. Biol. Trace Elem. Res. 2021, 199, 4237–4250. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Li, J.; Zhao, S.; Li, W.; Zhao, L.; Li, W.; Nie, H.; Sun, G.; Li, B. Inorganic Arsenic Induces NRF2-Regulated Antioxidant Defenses in Both Cerebral Cortex and Hippocampus In Vivo. Neurochem. Res. 2016, 41, 2119–2128. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Piao, F.; Ma, N.; Hiraku, Y.; Murata, M.; Oikawa, S.; Cheng, F.; Zhong, L.; Yamauchi, T.; Kawanishi, S.; Yokoyama, K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J. Occup. Health 2005, 47, 445–449. [Google Scholar] [CrossRef]
- Pandey, R.; Rai, V.; Mishra, J.; Mandrah, K.; Kumar Roy, S.; Bandyopadhyay, S. From the Cover: Arsenic Induces Hippocampal Neuronal Apoptosis and Cognitive Impairments via an Up-Regulated BMP2/Smad-Dependent Reduced BDNF/TrkB Signaling in Rats. Toxicol. Sci. 2017, 159, 137–158. [Google Scholar] [CrossRef]
- Samikkannu, T.; Chen, C.H.; Yih, L.H.; Wang, A.S.; Lin, S.Y.; Chen, T.C.; Jan, K.Y. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chem. Res. Toxicol. 2003, 16, 409–414. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Ro, S.H.; Bae, J.; Jang, Y.; Myers, J.F.; Chung, S.; Yu, J.; Natarajan, S.K.; Franco, R.; Song, H.S. Arsenic Toxicity on Metabolism and Autophagy in Adipose and Muscle Tissues. Antioxidants 2022, 11, 689. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ghosh, S.; Biswas, B.; Pramanik, S.; Nriagu, J.; Bhowmick, S. Epigenetic modifications from arsenic exposure: A comprehensive review. Sci. Total Environ. 2022, 810, 151218. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Paul, S.; Sau, T.J.; Das, J.K.; Bandyopadhyay, A.; Banerjee, S.; Giri, A.K. Epigenetic modifications of DAPK and p16 genes contribute to arsenic-induced skin lesions and nondermatological health effects. Toxicol. Sci. 2013, 135, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Xu, H.; Chang, D.; Wu, Z.; Yao, X.; Zhang, S.; Li, Z.; Bai, J.; Cai, Q.; Zhang, W. Arsenic exposure is associated with DNA hypermethylation of the tumor suppressor gene p16. J. Occup. Med. Toxicol. 2014, 9, 42. [Google Scholar] [CrossRef]
- Intarasunanont, P.; Navasumrit, P.; Waraprasit, S.; Chaisatra, K.; Suk, W.A.; Mahidol, C.; Ruchirawat, M. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ. Health 2012, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.; Hernández-Sánchez, J.; Dipp, V.R.; Huerta-González, D.; Olivares-Bañuelos, T.N.; González-Fraga, J.; Bardullas, U. Exposure to low doses of inorganic arsenic induces transgenerational changes on behavioral and epigenetic markers in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2020, 396, 115002. [Google Scholar] [CrossRef] [PubMed]
- Arita, A.; Shamy, M.Y.; Chervona, Y.; Clancy, H.A.; Sun, H.; Hall, M.N.; Qu, Q.; Gamble, M.V.; Costa, M. The effect of exposure to carcinogenic metals on histone tail modifications and gene expression in human subjects. J. Trace Elem. Med. Biol. 2012, 26, 174–178. [Google Scholar] [CrossRef]
- Pournara, A.; Kippler, M.; Holmlund, T.; Ceder, R.; Grafström, R.; Vahter, M.; Broberg, K.; Wallberg, A.E. Arsenic alters global histone modifications in lymphocytes in vitro and in vivo. Cell Biol. Toxicol. 2016, 32, 275–284. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Paul, S.; Bhattacharjee, P. Understanding the mechanistic insight of arsenic exposure and decoding the histone cipher. Toxicology 2020, 430, 152340. [Google Scholar] [CrossRef]
- Ge, Y.; Zhu, J.; Wang, X.; Zheng, N.; Tu, C.; Qu, J.; Ren, X. Mapping dynamic histone modification patterns during arsenic-induced malignant transformation of human bladder cells. Toxicol. Appl. Pharmacol. 2018, 355, 164–173. [Google Scholar] [CrossRef]
- Cantone, L.; Nordio, F.; Hou, L.; Apostoli, P.; Bonzini, M.; Tarantini, L.; Angelici, L.; Bollati, V.; Zanobetti, A.; Schwartz, J.; et al. Inhalable metal-rich air particles and histone H3K4 dimethylation and H3K9 acetylation in a cross-sectional study of steel workers. Environ. Health Perspect. 2011, 119, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Wang, X.; Li, D.; Zeng, Q.; Xing, X.; Li, C.; Xie, L.; Chen, L.; Chen, W.; et al. Modifications of H3K9me2, H3K36me3 and H4K20me2 may be involved in arsenic-induced genetic damage. Toxicol. Res. 2016, 5, 1380–1387. [Google Scholar] [CrossRef]
- Banerjee, N.; Bandyopadhyay, A.K.; Dutta, S.; Das, J.K.; Roy Chowdhury, T.; Bandyopadhyay, A.; Giri, A.K. Increased microRNA 21 expression contributes to arsenic induced skin lesions, skin cancers and respiratory distress in chronically exposed individuals. Toxicology 2017, 378, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Xu, Y.; Ling, M.; Zhao, Y.; Xu, W.; Liang, X.; Jiang, R.; Wang, B.; Bian, Q.; Liu, Q. Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2013, 273, 27–34. [Google Scholar] [CrossRef]
- Ngalame, N.N.; Makia, N.L.; Waalkes, M.P.; Tokar, E.J. Mitigation of arsenic-induced acquired cancer phenotype in prostate cancer stem cells by miR-143 restoration. Toxicol. Appl. Pharmacol. 2016, 312, 11–18. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.Y.; Li, J.; Sun, H.; Wu, F.; Zhu, Y.; Kluz, T.; Jordan, A.; DesMarais, T.; Zhang, X.; Murphy, A.; et al. Role of miR-31 and SATB2 in arsenic-induced malignant BEAS-2B cell transformation. Mol. Carcinog. 2018, 57, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zou, Z.; Wang, Q.; Sun, B.; Liu, Y.; Liang, B.; Liu, Q.; Zhang, A. Association and risk of five miRNAs with arsenic-induced multiorgan damage. Sci. Total Environ. 2019, 680, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiot. 2022, 12, 214–222. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Medda, N.; De, S.K.; Maiti, S. Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol. Environ. Saf. 2021, 208, 111752. [Google Scholar] [CrossRef]
- Mao, J.; Yang, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; Nie, X.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol. Appl. Pharmacol. 2016, 303, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Jiang, B.H. Roles of EGFR, PI3K, AKT, and mTOR in heavy metal-induced cancer. Curr. Cancer Drug Targets 2013, 13, 252–266. [Google Scholar] [CrossRef]
- Felix, K.; Manna, S.K.; Wise, K.; Barr, J.; Ramesh, G.T. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J. Biochem. Mol. Toxicol. 2005, 19, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef]
- Jimi, S.; Uchiyama, M.; Takaki, A.; Suzumiya, J.; Hara, S. Mechanisms of cell death induced by cadmium and arsenic. Ann. N. Y. Acad. Sci. 2004, 1011, 325–331. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Jiménez-Capdeville, M.E.; Giordano, M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003, 145, 1–18. [Google Scholar] [CrossRef]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef] [PubMed]
- Bardullas, U.; Limón-Pacheco, J.H.; Giordano, M.; Carrizales, L.; Mendoza-Trejo, M.S.; Rodríguez, V.M. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol. Appl. Pharmacol. 2009, 239, 169–177. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Carrizales, L.; Mendoza, M.S.; Fajardo, O.R.; Giordano, M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol. Teratol. 2002, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Biswas, A.; Dhali, G.K.; Chowdhury, A.; Boyer, J.L.; Santra, A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol. Appl. Pharmacol. 2011, 251, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Kumar, V. Chronic Arsenic Exposure-Induced Oxidative Stress is Mediated by Decreased Mitochondrial Biogenesis in Rat Liver. Biol. Trace Elem. Res. 2016, 173, 87–95. [Google Scholar] [CrossRef]
- Renu, K.; Saravanan, A.; Elangovan, A.; Ramesh, S.; Annamalai, S.; Namachivayam, A.; Abel, P.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; et al. An appraisal on molecular and biochemical signalling cascades during arsenic-induced hepatotoxicity. Life Sci. 2020, 260, 118438. [Google Scholar] [CrossRef]
- Robles-Osorio, M.L.; Sabath-Silva, E.; Sabath, E. Arsenic-mediated nephrotoxicity. Ren. Fail. 2015, 37, 542–547. [Google Scholar] [CrossRef]
- Li, Z.; Piao, F.; Liu, S.; Wang, Y.; Qu, S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp. Toxicol. Pathol. 2010, 62, 543–547. [Google Scholar] [CrossRef]
- Reshi, M.S.; Yadav, D.; Uthra, C.; Shrivastava, S.; Shukla, S. Acetaminophen-induced renal toxicity: Preventive effect of silver nanoparticles. Toxicol. Res. 2020, 9, 406–412. [Google Scholar] [CrossRef]
- Rajiv, S.V.; George, M.; Nandakumar, G. Dermatological manifestations of arsenic exposure. J. Ski. Sex. Transm. Dis. 2022, 5, 14–21. [Google Scholar] [CrossRef]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A Review of Arsenic Poisoning and its Effects on Human Health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Da Cunha Martins, A., Jr.; Carneiro, M.F.H.; Grotto, D.; Adeyemi, J.A.; Barbosa, F., Jr. Arsenic, cadmium, and mercury-induced hypertension: Mechanisms and epidemiological findings. J. Toxicol. Environ. Health Part B Crit. Rev. 2018, 21, 61–82. [Google Scholar] [CrossRef]
- Martinez, V.D.; Lam, W.L. Health Effects Associated with Pre- and Perinatal Exposure to Arsenic. Front. Genet. 2021, 12, 664717. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.L.; Wei, H.J.; Ho, H.Y.; Liao, K.W.; Chien, L.C. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: A cross-sectional study from Taiwan. BMC Public Health 2015, 15, 1220. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Chatterjee, D.; Singh, K.K.; Giri, A.K. Systems biology approaches to evaluate arsenic toxicity and carcinogenicity: An overview. Int. J. Hyg. Environ. Health 2013, 216, 574–586. [Google Scholar] [CrossRef]
- Wang, J.S.; Wai, C.M. Arsenic in Drinking Water—A Global Environmental Problem. J. Chem. Educ. 2004, 81, 207. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Santos, A.C.; Fernandes, C.S.; Ng, J.C. Arsenic contamination assessment in Brazil—Past, present and future concerns: A historical and critical review. Sci. Total Environ. 2020, 730, 138217. [Google Scholar] [CrossRef] [PubMed]
- Caussy, E. A Field Guide for Detection, Management and Surveillance of Arsenicosis Cases; World Health Organization: New Delhi, India, 2005. [Google Scholar]
- GB 3095-2012; Ambient Air Quality Standard. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2012.
- Sett, A.; Sarkar, L.; Tripathy, S.; Karmakar, G.; Nimisha; Kumari, A.; Bhattacharyya, T.K. Functionalized Reduced Graphene Oxide Sheets: An Efficient Resistive Sensing Platform for Arsenic. IEEE Sens. J. 2023, 23, 24160–24168. [Google Scholar] [CrossRef]
- Perreault, F.; Fonseca de Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Tamil Elakkiya, V.; Rajeswari, R. Graphene-based materials for environmental applications: A review. Environ. Chem. Lett. 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, D.; Qian, Y.; Nie, Z.; Long, Y.; Shen, D.; Fang, C.; Yao, J. Microbes drive changes in arsenic species distribution during the landfill process. Environ. Pollut. 2022, 292 Pt A, 118322. [Google Scholar] [CrossRef] [PubMed]

| Standard/Region | Limit/Guideline | Legal Status | Notes |
|---|---|---|---|
| European Union (2004/107/EC) | Annual target: 6 ng/m3 (As in PM10) | Non-binding | 2017 monitoring: Only 7/645 sites exceeded the limit (max: 550 ng/m3 near Bor copper plant, Serbia). |
| United States | No federal air arsenic standard | / | / |
| OSHA | Workplace PEL-TWA: 10 μg/m3 (inorganic As) | Binding (occupational) | Does not cover general public exposure. |
| California OEHHA | Chronic REL-TWA: 0.015 μg/m3 (developmental toxicity) | Non-binding (advisory) | Health-based reference exposure level. |
| China (GB3095-2012) [101] | Annual limit: 6 ng/m3 | Binding | E-waste dismantling areas measured up to 200 ng/m3 (33× above limit). |
| WHO | Unit Risk Factor (URF): 1.5 × 10−3 (μg/m3)−1 (lung cancer risk) | Guideline | Corresponds to 6.6 ng/m3 for 1:105 lifetime risk; high-pollution areas (≥30 ng/m3) should assess inhaled–oral dose equivalence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q. Toxicology of Airborne Inorganic Arsenic: Oxidative Stress, Molecular Mechanisms, and Organ-Specific Pathologies. Toxics 2025, 13, 753. https://doi.org/10.3390/toxics13090753
Liu Q. Toxicology of Airborne Inorganic Arsenic: Oxidative Stress, Molecular Mechanisms, and Organ-Specific Pathologies. Toxics. 2025; 13(9):753. https://doi.org/10.3390/toxics13090753
Chicago/Turabian StyleLiu, Qingyang. 2025. "Toxicology of Airborne Inorganic Arsenic: Oxidative Stress, Molecular Mechanisms, and Organ-Specific Pathologies" Toxics 13, no. 9: 753. https://doi.org/10.3390/toxics13090753
APA StyleLiu, Q. (2025). Toxicology of Airborne Inorganic Arsenic: Oxidative Stress, Molecular Mechanisms, and Organ-Specific Pathologies. Toxics, 13(9), 753. https://doi.org/10.3390/toxics13090753









