Associations Between Prenatal Phthalate Exposure and Atopic Symptoms in Childhood: Effect Modification by Child Sex
Highlights
- Prenatal exposure to phthalate mixtures was examined in relation to atopic disease among children in Mexico City.
- Second trimester phthalate exposure was associated with atopic dermatitis symptoms in a sex-specific manner.
- This effect was greater in males.
- The main finding highlights the need for long-term follow-up to explore whether early atopic symptoms evolve into chronic allergic conditions.
- It underscores the second trimester as a period of heightened susceptibility to endocrine-disrupting chemicals, such as phthalates, which may inform the timing of protective interventions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Prenatal Urinary Phthalate Metabolite Concentrations
2.3. Atopic Symptoms Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Sex-Stratified WQS Analysis
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutowska-Ślesik, J.; Samoliński, B.; Krzych-Fałta, E. The increase in allergic conditions based on a review of literature. Postep. Dermatol. Alergol. 2023, 40, 1–7. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Jomha, F.; Ehlayel, M. Allergic diseases among children: Nutritional prevention and intervention. Ther. Clin. Risk Manag. 2016, 12, 361–372. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef]
- Silverwood, R.J.; Mansfield, K.E.; Mulick, A.; Wong, A.Y.; Schmidt, S.A.; Roberts, A.; Smeeth, L.; Abuabara, K.; Langan, S.M. Atopic eczema in adulthood and mortality: UK population-based cohort study, 1998–2016. J. Allergy Clin. Immunol. 2021, 147, 1753–1763. [Google Scholar]
- Meltzer, E.O.; Bukstein, D.A. The economic impact of allergic rhinitis and current guidelines for treatment. Ann. Allergy Asthma. Immunol. 2011, 106 (Suppl. S2), S12–S16. [Google Scholar]
- Bølling, A.K.; Sripada, K.; Becher, R.; Bekö, G. Phthalate exposure and allergic diseases: Review of epidemiological and experimental evidence. Environ. Int. 2020, 139, 105706. [Google Scholar] [CrossRef]
- Redlich, C.A. Skin exposure and asthma: Is there a connection? Proc. Am. Thorac. Soc. 2010, 7, 134–137. [Google Scholar] [CrossRef]
- Searing, D.A.; Rabinovitch, N. Environmental pollution and lung effects in children. Curr. Opin. Pediatr. 2011, 23, 314–318. [Google Scholar] [CrossRef]
- Dick, S.; Friend, A.; Dynes, K.; AlKandari, F.; Doust, E.; Cowie, H.; Ayres, J.G.; Turner, S.W. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open 2014, 4, e006554. [Google Scholar] [CrossRef]
- Becerril-Ángeles, M.; Vargas, M.H.; Medina-Reyes, I.S.; Rascón-Pacheco, R.A. Trends (2007–2019) of major atopic diseases throughout the life span in a large Mexican population. World Allergy Organ. J. 2023, 16, 100732. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma. Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Matheson, M.C.; Gurrin, L.; Burgess, J.A.; Osborne, N.; Lowe, A.J.; Morrison, S.; Mészáros, D.; Giles, G.G.; Abramson, M.J.; et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: A prospective cohort study over 4 decades. J. Allergy Clin. Immunol. 2011, 127, 1473–1479.e1. [Google Scholar] [CrossRef]
- Jurewicz, J.; Hanke, W. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2011, 24, 115–141. [Google Scholar] [CrossRef]
- Wu, H.; Kupsco, A.J.; Deierlein, A.L.; Just, A.C.; Calafat, A.M.; Oken, E.; Braun, J.M.; Mercado-Garcia, A.; Cantoral, A.; Téllez-Rojo, M.M.; et al. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ. Sci. Technol. 2020, 54, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shao, H.; Ying, X.; Huang, W.; Hua, Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Front. Public Health 2020, 8, 366. [Google Scholar] [CrossRef]
- Mose, T.; Mortensen, G.K.; Hedegaard, M.; Knudsen, L.E. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol. 2007, 23, 83–91. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.-I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Luo, J.; Pan, J.; Luo, T.; Yang, W. Association of prenatal exposure to phthalates with risks of asthma, wheeze, and allergic diseases during childhood: A systematic review and meta-analysis. J. Environ. Health Sci. Eng. 2025, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.V.; Till, C.; Berghuis, S.; Braun, J.M.; Muckle, G.; Chen, A.; Oulhote, Y.; Lanphear, B.; Ashley-Martin, J.; Arbuckle, T.E. Prenatal exposure to phthalates and preschool children’s intellectual scores: Effect modification by child sex. Environ. Int. 2025, 202, 109701. [Google Scholar] [CrossRef]
- Hu, C.Y.; Alcala, C.S.; Lamadrid-Figueroa, H.; Tamayo-Ortiz, M.; Mercado-Garcia, A.; Rivera, N.R.; Just, A.C.; Gennings, C.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Associations of prenatal exposure to phthalates and their mixture with lung function in Mexican children. J. Hazard. Mater. 2024, 475, 134863. [Google Scholar] [CrossRef]
- Alcala, C.S.; Lamadrid-Figueroa, H.; Tamayo-Ortiz, M.; Mercado-Garcia, A.; Midya, V.; Just, A.C.; Foppa-Pedretti, N.; Colicino, E.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Prenatal exposure to phthalates and childhood wheeze and asthma in the PROGRESS cohort. Sci. Total. Environ. 2024, 954, 176311. [Google Scholar] [CrossRef]
- Jøhnk, C.; Høst, A.; Husby, S.; Schoeters, G.; Timmermann, C.A.G.; Kyhl, H.B.; Beck, I.H.; Andersson, A.M.; Frederiksen, H.; Jensen, T.K. Maternal phthalate exposure and asthma, rhinitis and eczema in 552 children aged 5 years; a prospective cohort study. Environ. Health 2020, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K.G.; Kalayci, Ö. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr. Allergy Immunol. 2019, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, C.; Zhang, J.; Qi, X.; Lv, S.; Jiang, S.; Zhou, T.; Lu, D.; Feng, C.; Chang, X.; et al. Prenatal exposure to mixture of heavy metals, pesticides and phenols and IQ in children at 7 years of age: The SMBCS study. Environ. Int. 2020, 139, 105692. [Google Scholar] [CrossRef]
- Lazarevic, N.; Barnett, A.G.; Sly, P.D.; Knibbs, L.D. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ. Health Perspect. 2019, 127, 26001. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Palanza, P.; Paterlini, S.; Brambilla, M.M.; Ramundo, G.; Caviola, G.; Gioiosa, L.; Parmigiani, S.; Saal, F.S.V.; Ponzi, D. Sex-biased impact of endocrine disrupting chemicals on behavioral development and vulnerability to disease: Of mice and children. Neurosci. Biobehav. Rev. 2021, 121, 29–46. [Google Scholar] [CrossRef]
- Burris, H.H.; Braun, J.M.; Byun, H.M.; Tarantini, L.; Mercado, A.; Wright, R.J.; Schnaas, L.; Baccarelli, A.A.; Wright, R.O.; Tellez-Rojo, M.M. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 2013, 5, 271–281. [Google Scholar] [CrossRef]
- Kupsco, A.; Wu, H.; Calafat, A.M.; Kioumourtzoglou, M.-A.; Tamayo-Ortiz, M.; Pantic, I.; Cantoral, A.; Tolentino, M.; Oken, E.; Braun, J.M.; et al. Prenatal maternal phthalate exposures and child lipid and adipokine levels at age six: A study from the PROGRESS cohort of Mexico City. Environ. Res. 2021, 192, 110341. [Google Scholar] [CrossRef]
- Silva, M.; Samandar, E.; Preaujr, J.; Reidy, J.; Needham, L.; Calafat, A. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 860, 106–112. [Google Scholar] [CrossRef]
- Braun-Fahrländer, C.; Wüthrich, B.; Gassner, M.; Grize, L.; Sennhauser, F.H.; Varonier, H.S.; Vuille, J. Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school children visiting the school health services. SCARPOL-team. Swiss Study on Childhood Allergy and Respiratory Symptom with respect to Air Pollution and Climate. International Study of Asthma and Allergies in Childhood. Pediatr. Allergy Immunol. 1997, 8, 75–82. [Google Scholar]
- Ferris, B.G. Epidemiology Standardization Project (American Thoracic Society). Am. Rev. Respir. Dis. 1978, 118 Pt 2, 1–120. [Google Scholar]
- Lai, C.K.; Chan, J.K.; Chan, A.; Wong, G.; Ho, A.; Choy, D.; Lau, J.; Leung, R. Comparison of the ISAAC video questionnaire (AVQ3.0) with the ISAAC written questionnaire for estimating asthma associated with bronchial hyperreactivity. Clin. Exp. Allergy 1997, 27, 540–545. [Google Scholar] [CrossRef]
- Rhodes, H.L.; Thomas, P.; Sporik, R.; Holgate, S.T.; Cogswell, J.J. A birth cohort study of subjects at risk of atopy: Twenty-two-year follow-up of wheeze and atopic status. Am. J. Respir. Crit. Care. Med. 2002, 165, 176–180. [Google Scholar] [CrossRef]
- Solé, D.; Vanna, A.T.; Yamada, E.; Rizzo, M.C.; Naspitz, C.K. International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire: Validation of the asthma component among Brazilian children. J. Investig. Allergol. Clin. Immunol. 1998, 8, 376–382. [Google Scholar] [PubMed]
- Venables, K.M.; Farrer, N.; Sharp, L.; Graneek, B.J.; Taylor, A.J.N. Respiratory symptoms questionnaire for asthma epidemiology: Validity and reproducibility. Thorax 1993, 48, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; The ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of sy mptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Hartert, T.V.; Carroll, K.; Gebretsadik, T.; Woodward, K.; Minton, P. The Tennessee Children’s Respiratory Initiative: Objectives, design and recruitment results of a prospective cohort study investigating infant viral respiratory illness and the development of asthma and allergic diseases. Respirology 2010, 15, 691–699. [Google Scholar] [CrossRef]
- Soria-Contreras, D.C.; Trejo-Valdivia, B.; Cantoral, A.; Pizano-Zárate, M.L.; Baccarelli, A.A.; Just, A.C.; Colicino, E.; Deierlein, A.L.; Wright, R.O.; Oken, E.; et al. Patterns of Weight Change One Year after Delivery Are Associated with Cardiometabolic Risk Factors at Six Years Postpartum in Mexican Women. Nutrients 2020, 12, 170. [Google Scholar] [CrossRef]
- Butz, A.M.; Breysse, P.; Rand, C.; Curtin-Brosnan, J.; Eggleston, P.; Diette, G.B.; Williams, D.; Bernert, J.T.; Matsui, E.C. Household smoking behavior: Effects on indoor air quality and health of urban children with asthma. Matern. Child Health J. 2011, 15, 460–468. [Google Scholar] [CrossRef]
- Busgang, S.A.; Spear, E.A.; Andra, S.S.; Narasimhan, S.; Bragg, J.B.; Renzetti, S.; Curtin, P.; Bates, M.; Arora, M.; Gennings, C.; et al. Application of growth modeling to assess the impact of hospital-based phthalate exposure on preterm infant growth parameters during the neonatal intensive care unit hospitalization. Sci. Total. Environ. 2022, 850, 157830. [Google Scholar] [CrossRef]
- Bennett, D.H.; A Busgang, S.; Kannan, K.; Parsons, P.J.; Takazawa, M.; Palmer, C.D.; Schmidt, R.J.; Doucette, J.T.; Schweitzer, J.B.; Gennings, C.; et al. Environmental exposures to pesticides, phthalates, phenols and trace elements are associated with neurodevelopment in the CHARGE study. Environ. Int. 2022, 161, 107075. [Google Scholar] [CrossRef]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- Casas, M.; Gascon, M. Prenatal Exposure to Endocrine-Disrupting Chemicals and Asthma and Allergic Diseases. J. Investig. Allergol. Clin. Immunol. 2020, 30, 215–228. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Balmes, J.; Holland, N.; Calafat, A.M.; Harley, K.G. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environ. Int. 2018, 121 Pt 1, 538–549. [Google Scholar] [CrossRef]
- Soomro, M.H.; Baiz, N.; Philippat, C.; Vernet, C.; Siroux, V.; Maesano, C.N.; Sanyal, S.; Slama, R.; Bornehag, C.-G.; Annesi-Maesano, I. Prenatal Exposure to Phthalates and the Development of Eczema Phenotypes in Male Children: Results from the EDEN Mother-Child Cohort Study. Environ. Health Perspect. 2018, 126, 027002. [Google Scholar] [CrossRef]
- Adgent, M.A.; Carroll, K.N.; Hazlehurst, M.F.; Loftus, C.T.; Szpiro, A.A.; Karr, C.J.; Barrett, E.S.; LeWinn, K.Z.; Bush, N.R.; Tylavsky, F.A.; et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ. Int. 2020, 143, 105970. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Quirós-Alcalá, L.; Teitelbaum, S.L.; Calafat, A.M.; Wolff, M.S.; Engel, S.M. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ. Int. 2018, 115, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Smith, K.W.; Williams, P.L.; Calafat, A.M.; Berry, K.; Ehrlich, S.; Hauser, R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ. Health Perspect. 2012, 120, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, Z.; Cao, Z.; Mei, H.; Xiang, F.; Yu, L.; Hu, L.; Zhou, A.; Xiao, H. Prenatal exposure to phthalates and child growth trajectories in the first 24 months of life. Sci. Total. Environ. 2023, 898, 165518. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Davis, K.; Marro, L.; Fisher, M.; Legrand, M.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Choeurng, V.; Fraser, W.D. Phthalate and bisphenol A exposure among pregnant women in Canada—Results from the MIREC study. Environ. Int. 2014, 68, 55–65. [Google Scholar]
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; de Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; le Tertre, A.; Lefranc, A.; et al. Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Cordero, J.F.; Rivera-González, L.O.; Del Toro, L.V.A.; Ferguson, K.K.; Mukherjee, B.; Calafat, A.M.; Crespo, N.; Jiménez-Vélez, B.; Padilla, I.Y.; et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environ. Int. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Yang, T.C.; Peterson, K.E.; Meeker, J.D.; Sánchez, B.N.; Zhang, Z.; Cantoral, A.; Solano, M.; Tellez-Rojo, M.M. Exposure to Bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr. Obes. 2018, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, J.S. Lung development in the second trimester. Br. Med. Bull. 1988, 44, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Moog, N.; Entringer, S.; Heim, C.; Wadhwa, P.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R.; Alghamdi, R.; Alrushud, N.; Alnuwaysir, H.; Alnemer, M.; Aldhalaan, H.; Shoukri, M. Phthalate exposure during pregnancy and its association with thyroid hormones: A prospective cohort study. Int. J. Hyg. Environ. Health 2024, 261, 114421. [Google Scholar]
- Lottrup, G.; Andersson, A.; Leffers, H.; Mortensen, G.K.; Toppari, J.; Skakkebæk, N.E.; Main, K.M. Possible impact of phthalates on infant reproductive health. Int. J. Androl. 2006, 29, 172–180, discussion 181–185. [Google Scholar] [CrossRef]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M.A. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef]
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine Disruptor Chemicals and Children’s Health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef]
- Acharya, N.; Madi, A.; Zhang, H.; Klapholz, M.; Escobar, G.; Dulberg, S.; Christian, E.; Ferreira, M.; Dixon, K.O.; Fell, G.; et al. Endogenous Glucocorticoid Signaling Regulates CD8(+) T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity 2020, 53, 658–671.e6. [Google Scholar] [CrossRef]
- Jin, S.; Cui, S.; Mu, X.; Liu, Z.; Han, Y.; Cui, T.; Xiong, W.; Xi, W.; Zhang, X. Exposure to phthalates and their alternatives in relation to biomarkers of inflammation and oxidative stress in adults: Evidence from NHANES 2017–2018. Environ. Sci. Pollut. Res. Int. 2023, 30, 123770–123784. [Google Scholar] [CrossRef]
- Ketema, R.M.; Bamai, Y.A.; Miyashita, C.; Saito, T.; Kishi, R.; Ikeda-Araki, A. Phthalates mixture on allergies and oxidative stress biomarkers among children: The Hokkaido study. Environ. Int. 2022, 160, 107083. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Moharana, A.K.; Jena, S.R.; Samanta, L. Impact of Oxidative Stress on Embryogenesis and Fetal Development. Adv. Exp. Med. Biol. 2022, 1391, 221–241. [Google Scholar]
- Pacyga, D.C.; Gardiner, J.C.; Flaws, J.A.; Li, Z.; Calafat, A.M.; Korrick, S.A.; Schantz, S.L.; Strakovsky, R.S. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ. Int. 2021, 155, 106676. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; West, R.C. Sex differences in innate and adaptive immunity impact fetal, placental, and maternal health. Biol. Reprod. 2023, 109, 256–270. [Google Scholar] [CrossRef]
- Prokai, L.; Prokai-Tatrai, K.; Perjési, P.; Simpkins, J.W. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev. Res. 2005, 66, 118–125. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
| Characteristic | Total Sample (N = 558) | Females (N = 282) | Males (N = 276) |
|---|---|---|---|
| Maternal age at enrollment (years), Mean (SD) | 27.5 (5.54) | 27.3 (5.67) | 27.7 (5.42) |
| Maternal education at enrollment, n (%) | |||
| <High school | 225 (40.3%) | 104 (36.9%) | 121 (43.8%) |
| High school | 206 (36.9%) | 113 (40.1%) | 93 (33.7%) |
| >High school | 127 (22.8%) | 65 (23.0%) | 62 (22.5%) |
| Parity, n (%) | |||
| Primiparous | 339 (60.8%) | 165 (58.5%) | 174 (63.0%) |
| Multiparous | 219 (39.2%) | 117 (41.5%) | 102 (37.0%) |
| Child’s age, Mean (SD) | |||
| 4–6 years | 4.43 (0.567) | 4.44 (0.577) | 4.42 (0.556) |
| 6–8 years | 6.26 (0.556) | 6.26 (0.585) | 6.25 (0.527) |
| Report of a smoker in the home during pregnancy | |||
| No | 358 (64.2%) | 179 (63.5%) | 179 (64.9%) |
| Yes | 200 (35.8%) | 103 (36.5%) | 97 (35.1%) |
| Maternal pre-pregnancy BMI (kg/m2, Mean (SD) | 26.4 (4.16) | 26.1 (4.22) | 26.7 (4.08) |
| Atopic Outcomes (4–6 years), n (%) | |||
| Ever atopic dermatitis symptoms, yes | 65 (11.6%) | 34 (12.1%) | 31 (11.2%) |
| Current atopic dermatitis symptoms, yes | 58 (10.4%) | 31 (11.0%) | 27 (9.8%) |
| Ever allergic rhinitis symptoms, yes | 233 (41.8%) | 100 (35.5%) | 133 (48.2%) |
| Current allergic rhinitis symptoms, yes | 217 (38.9%) | 95 (33.7%) | 122 (44.2%) |
| Current allergic rhinitis symptoms+ itchy/watery eyes, yes | 65 (11.6%) | 31 (11.0%) | 34 (12.3%) |
| Atopic Outcomes (6–8 years), n (%) | |||
| Ever atopic dermatitis symptoms, yes | 34 (6.1%) | 16 (5.7%) | 18 (6.5%) |
| Current atopic dermatitis symptoms, yes | 26 (4.7%) | 13 (4.6%) | 13 (4.7%) |
| Ever allergic rhinitis symptoms, yes | 174 (31.2%) | 71 (25.2%) | 103 (37.3%) |
| Current allergic rhinitis symptoms, yes | 159 (28.5%) | 63 (22.3%) | 96 (34.8%) |
| Current allergic rhinitis symptoms + itchy/watery eyes, yes | 68 (12.2%) | 27 (9.6%) | 41 (14.9%) |
| Characteristic | Total Sample (N = 558) | Females (N = 282) | Males (N = 276) |
|---|---|---|---|
| Phthalate Metabolites during the 2nd Trimester, Mean (SD) (log transformed) | |||
| ∑DEHP | 6.49 (1.38) | 6.51 (1.35) | 6.47 (1.41) |
| MECPTP | 0.982 (1.52) | 1.00 (1.50) | 0.960 (1.55) |
| ∑DiNP | 2.62 (1.44) | 2.63 (1.48) | 2.60 (1.40) |
| MCNP | −0.0299 (1.20) | −0.0197 (1.23) | −0.0404 (1.17) |
| MCPP | 0.540 (1.28) | 0.604 (1.32) | 0.475 (1.24) |
| MBzP | 2.47 (1.73) | 2.49 (1.66) | 2.45 (1.80) |
| Low molecular weight | |||
| ∑DiBP | 3.64 (1.34) | 3.66 (1.30) | 3.61 (1.39) |
| ∑DBP | 6.46 (1.49) | 6.50 (1.46) | 6.42 (1.52) |
| MEP | 7.16 (1.95) | 7.12 (1.97) | 7.19 (1.94) |
| Phthalate Metabolites during the 3rd Trimester, Mean (SD) (log transformed) | |||
| High molecular weight | |||
| ∑DEHP | 6.65 (1.39) | 6.62 (1.28) | 6.68 (1.50) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
| MECPTP | 1.26 (1.61) | 1.37 (1.66) | 1.14 (1.56) |
| Missing | 69 (12.4%) | 29 (10.3%) | 40 (14.5%) |
| ∑DiNP | 2.63 (1.32) | 2.59 (1.29) | 2.66 (1.35) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
| MCNP | −0.00508 (1.14) | −0.0333 (1.16) | 0.0249 (1.12) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
| MCPP | 0.495 (1.31) | 0.512 (1.30) | 0.477 (1.31) |
| Missing | 67 (12.0%) | 28 (9.9%) | 39 (14.1%) |
| MBzP | 2.39 (1.67) | 2.40 (1.68) | 2.37 (1.67) |
| Missing | 69 (12.4%) | 31 (11.0%) | 38 (13.8%) |
| Low Molecular weight | |||
| ∑DiBP | 3.73 (1.41) | 3.76 (1.34) | 3.71 (1.49) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
| ∑DBP | 6.47 (1.48) | 6.45 (1.45) | 6.48 (1.52) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
| MEP | 7.19 (2.03) | 7.21 (2.09) | 7.17 (1.97) |
| Missing | 65 (11.6%) | 28 (9.9%) | 37 (13.4%) |
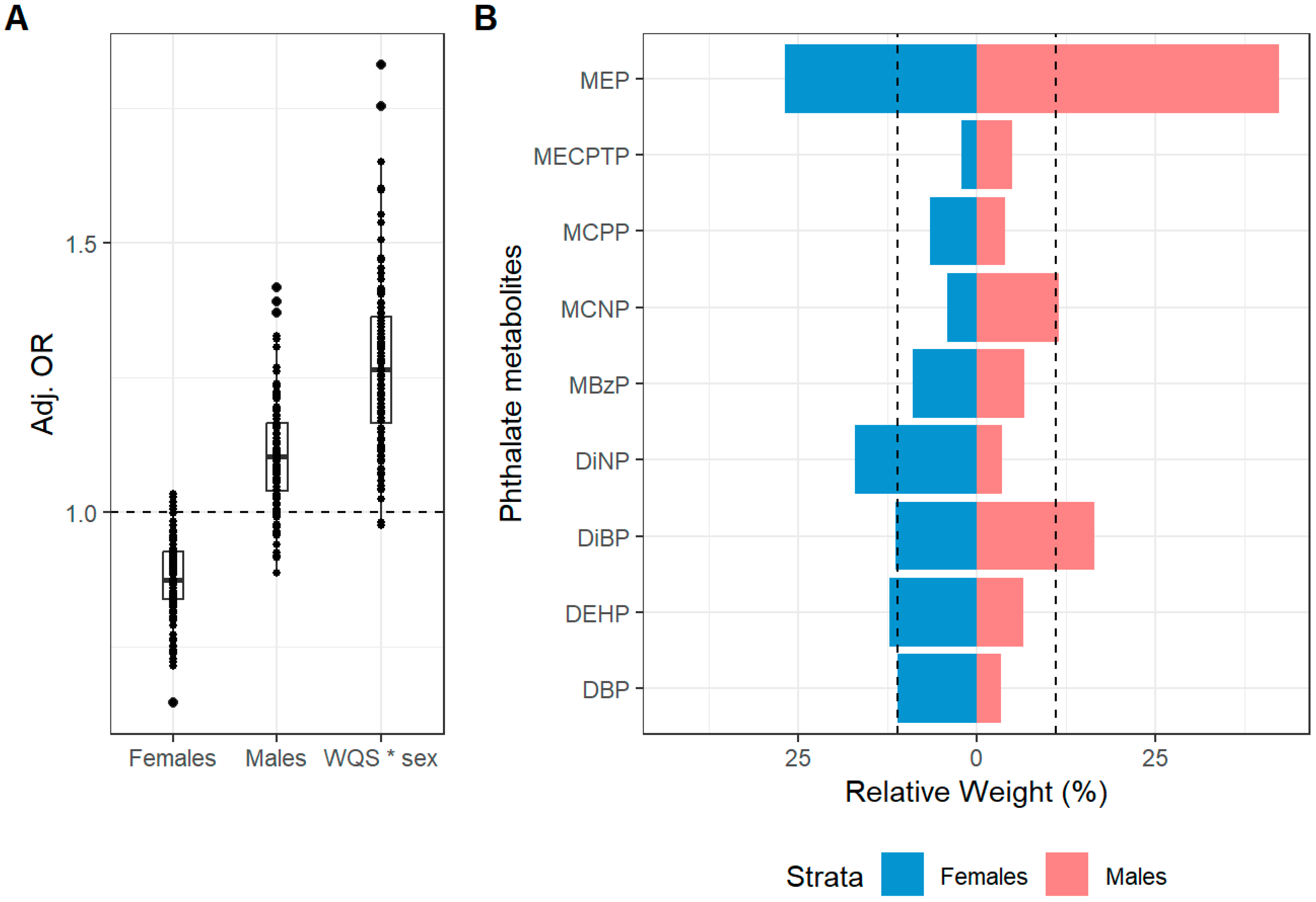
| Current Atopic Dermatitis Symptoms a | Mean OR and 95% CI b | OR > 1 c |
|---|---|---|
| WQS (b1) | 0.87 (0.73, 1.04) | 6/110 |
| WQS*sex (b12) | 1.23 (1.00, 1.60) | 106/110 |
| Betas for males and females | ||
| Females (b1) d | 0.87 (0.73, 1.04) | 6/110 |
| Males (b2) d | 1.10 (0.92, 1.32) | 93/110 |
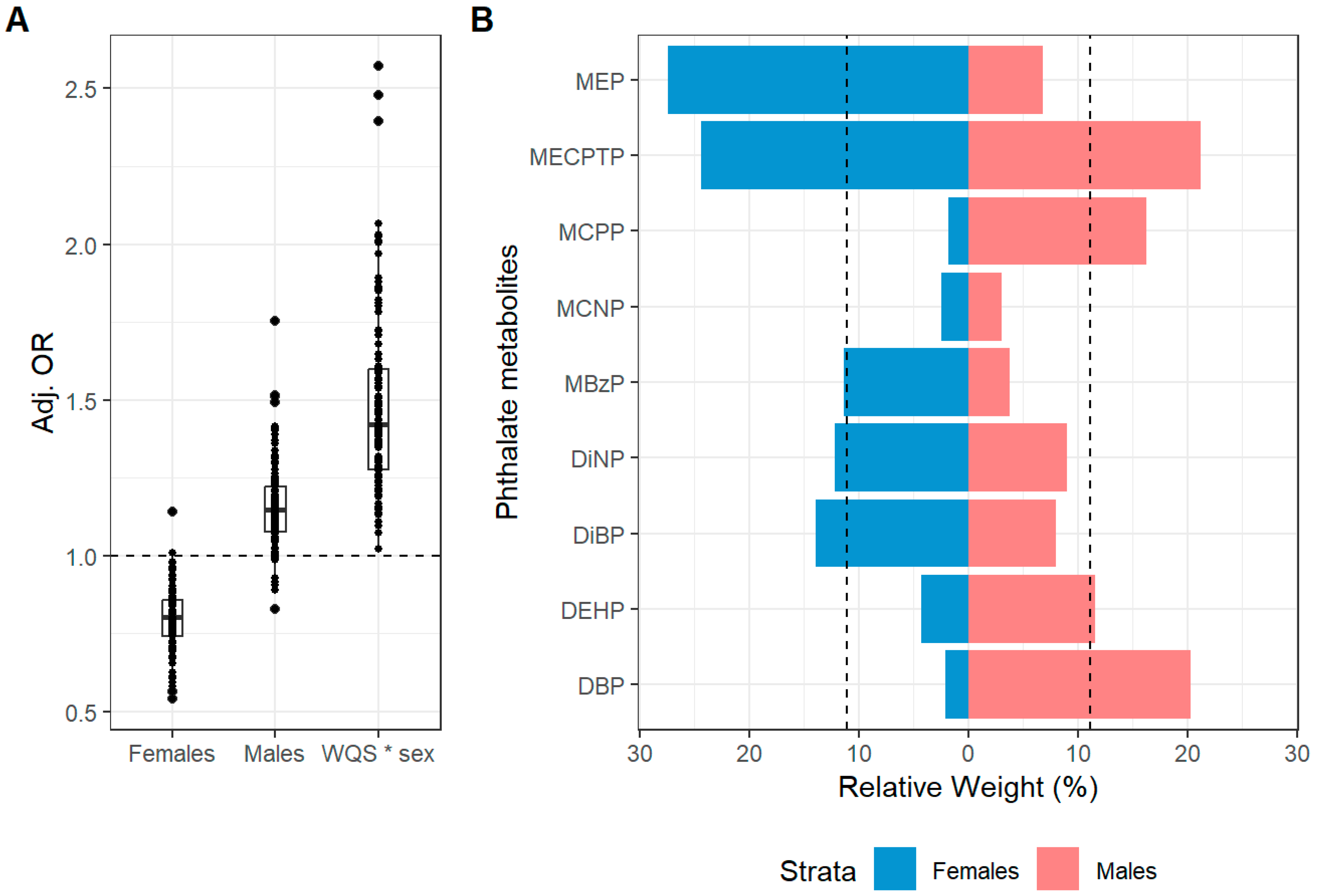
| Current Atopic Dermatitis Symptoms a | Mean OR and 95% CI b | OR > 1 c |
|---|---|---|
| WQS (b1) | 0.79 (0.62, 1.02) | 2/110 |
| WQS*sex (b12) | 1.46 (1.01, 2.1) | 109/110 |
| Betas for males and females | ||
| Females (b1) d | 0.79 (0.62, 1.02) | 2/110 |
| Males (b2) d | 1.16 (0.92, 1.46) | 99/110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, K.D.; Mistry, S.; Lamadrid-Figueroa, H.; Tamayo-Ortiz, M.; Mercado-Garcia, A.; Lane, J.M.; Téllez-Rojo, M.M.; Wright, R.O.; Wright, R.J.; Estrada-Gutierrez, G.; et al. Associations Between Prenatal Phthalate Exposure and Atopic Symptoms in Childhood: Effect Modification by Child Sex. Toxics 2025, 13, 749. https://doi.org/10.3390/toxics13090749
Bhatt KD, Mistry S, Lamadrid-Figueroa H, Tamayo-Ortiz M, Mercado-Garcia A, Lane JM, Téllez-Rojo MM, Wright RO, Wright RJ, Estrada-Gutierrez G, et al. Associations Between Prenatal Phthalate Exposure and Atopic Symptoms in Childhood: Effect Modification by Child Sex. Toxics. 2025; 13(9):749. https://doi.org/10.3390/toxics13090749
Chicago/Turabian StyleBhatt, Khushbu Dharmendra, Shachi Mistry, Héctor Lamadrid-Figueroa, Marcela Tamayo-Ortiz, Adriana Mercado-Garcia, Jamil M. Lane, Martha M. Téllez-Rojo, Robert O. Wright, Rosalind J. Wright, Guadalupe Estrada-Gutierrez, and et al. 2025. "Associations Between Prenatal Phthalate Exposure and Atopic Symptoms in Childhood: Effect Modification by Child Sex" Toxics 13, no. 9: 749. https://doi.org/10.3390/toxics13090749
APA StyleBhatt, K. D., Mistry, S., Lamadrid-Figueroa, H., Tamayo-Ortiz, M., Mercado-Garcia, A., Lane, J. M., Téllez-Rojo, M. M., Wright, R. O., Wright, R. J., Estrada-Gutierrez, G., Carroll, K. N., Alcala, C. S., & Rosa, M. J. (2025). Associations Between Prenatal Phthalate Exposure and Atopic Symptoms in Childhood: Effect Modification by Child Sex. Toxics, 13(9), 749. https://doi.org/10.3390/toxics13090749













