Structural and Kinetic Properties of Liver Rhodanese from Coptodon zillii: Implications for Cyanide Detoxification in Gold Mining-Impacted Aquatic Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Sample Collection, and Authentication
2.2. Enzyme Extraction and Purification
2.3. Enzyme Assay and Protein Determination
2.4. Molecular Weight Determination
2.5. Kinetic Studies
2.6. pH and Temperature Studies
2.7. The Impact of Salts on Rhodanese Activity
2.8. Studies on Substrate Specificity
2.9. Studies on Heat Stability
2.10. Measures for Quality Control
2.11. Computational Study
2.12. Data Analysis
3. Results
3.1. Enzyme Purification and Molecular Features
3.2. Kinetic Properties and Substrate Interactions
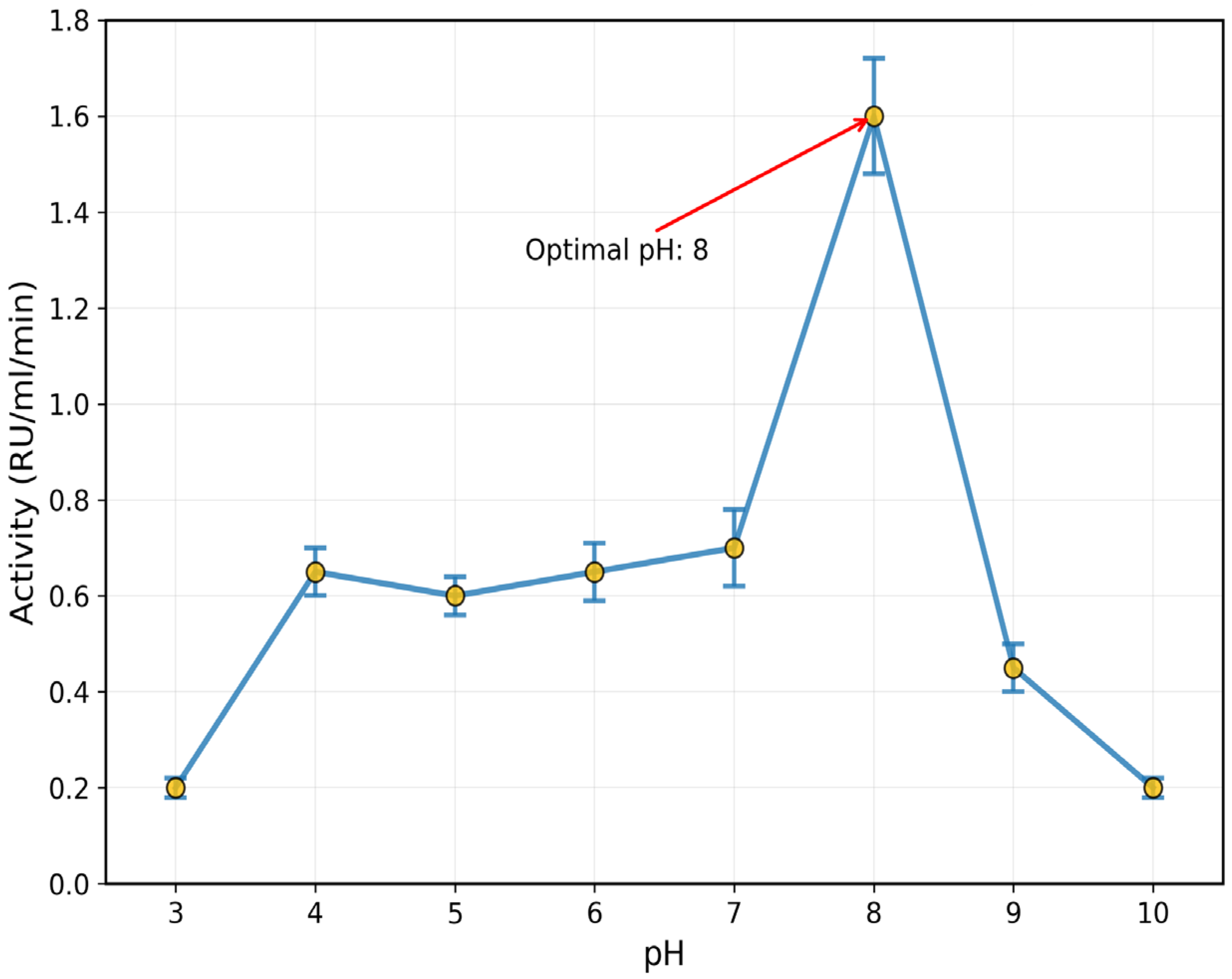
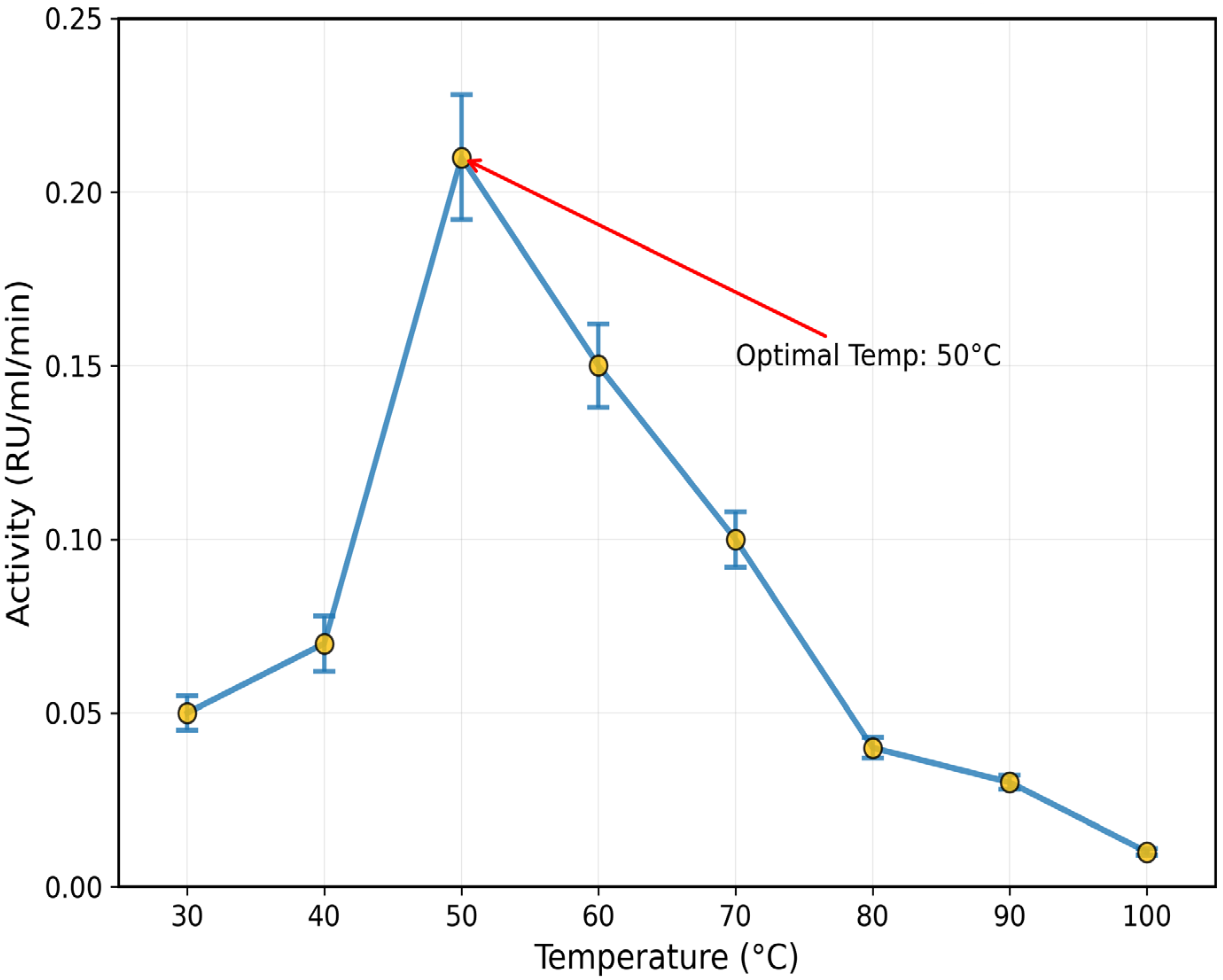
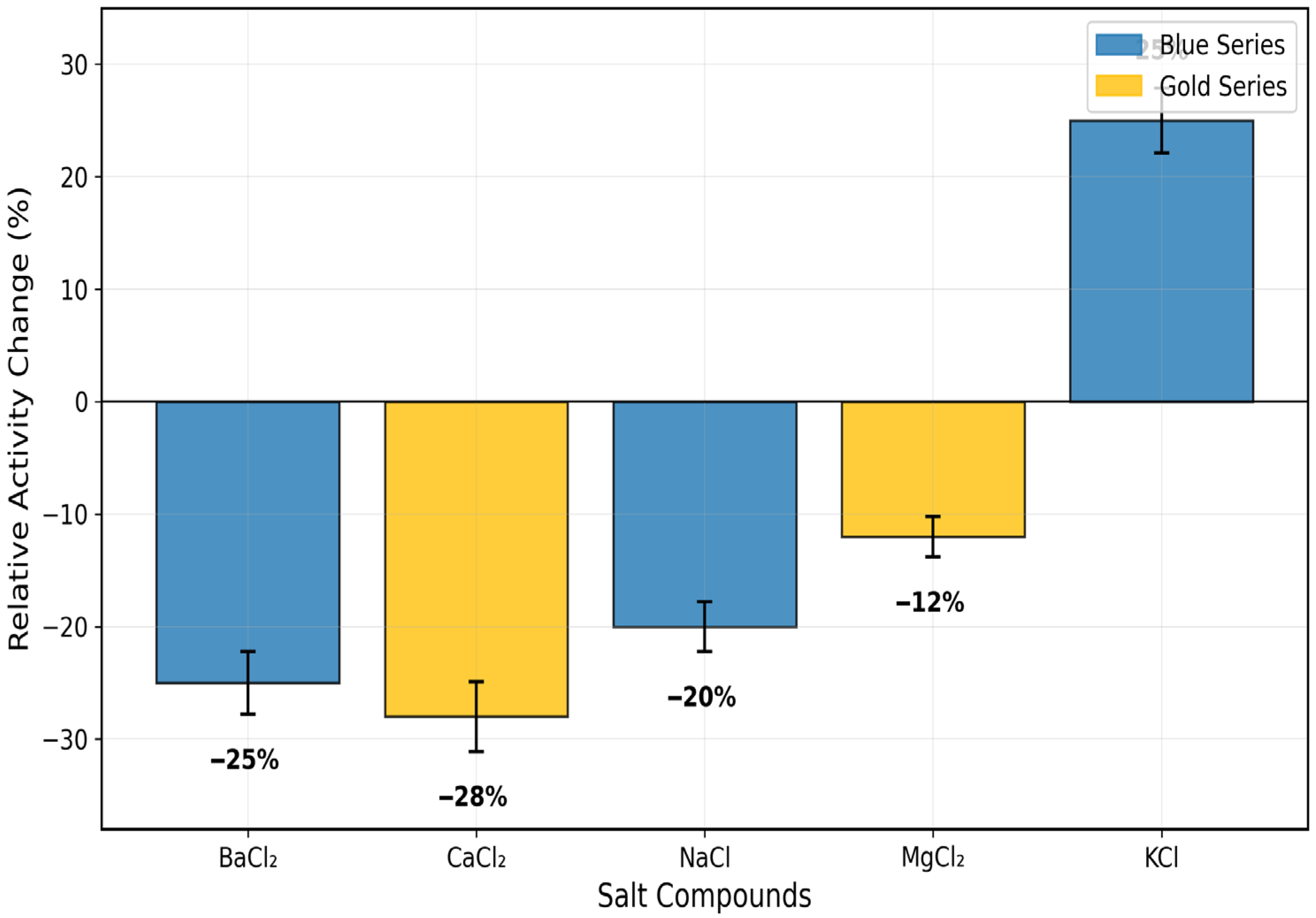
3.3. Effect of pH, Temperature, and Salts
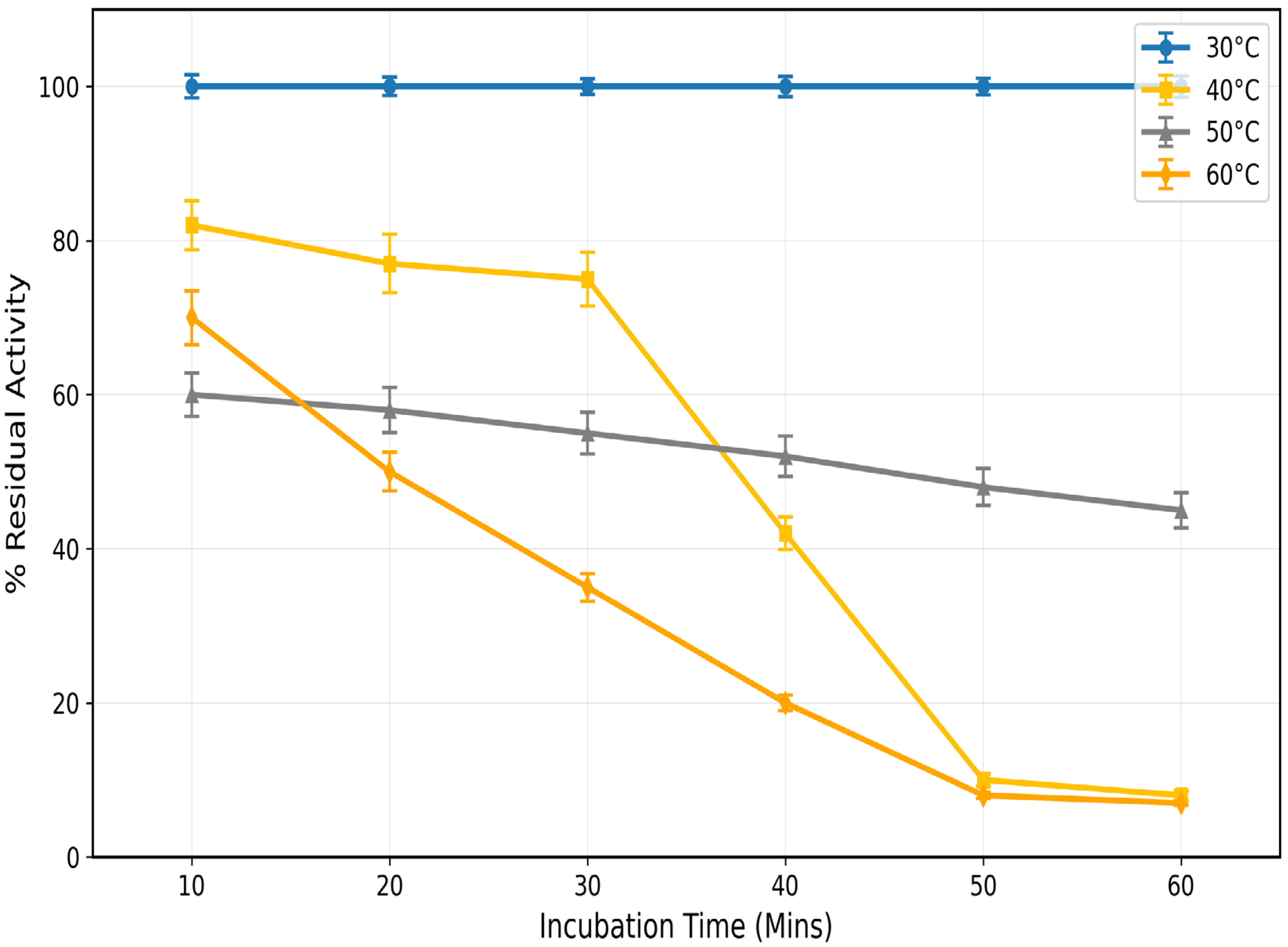
3.4. Thermal Stability Profile
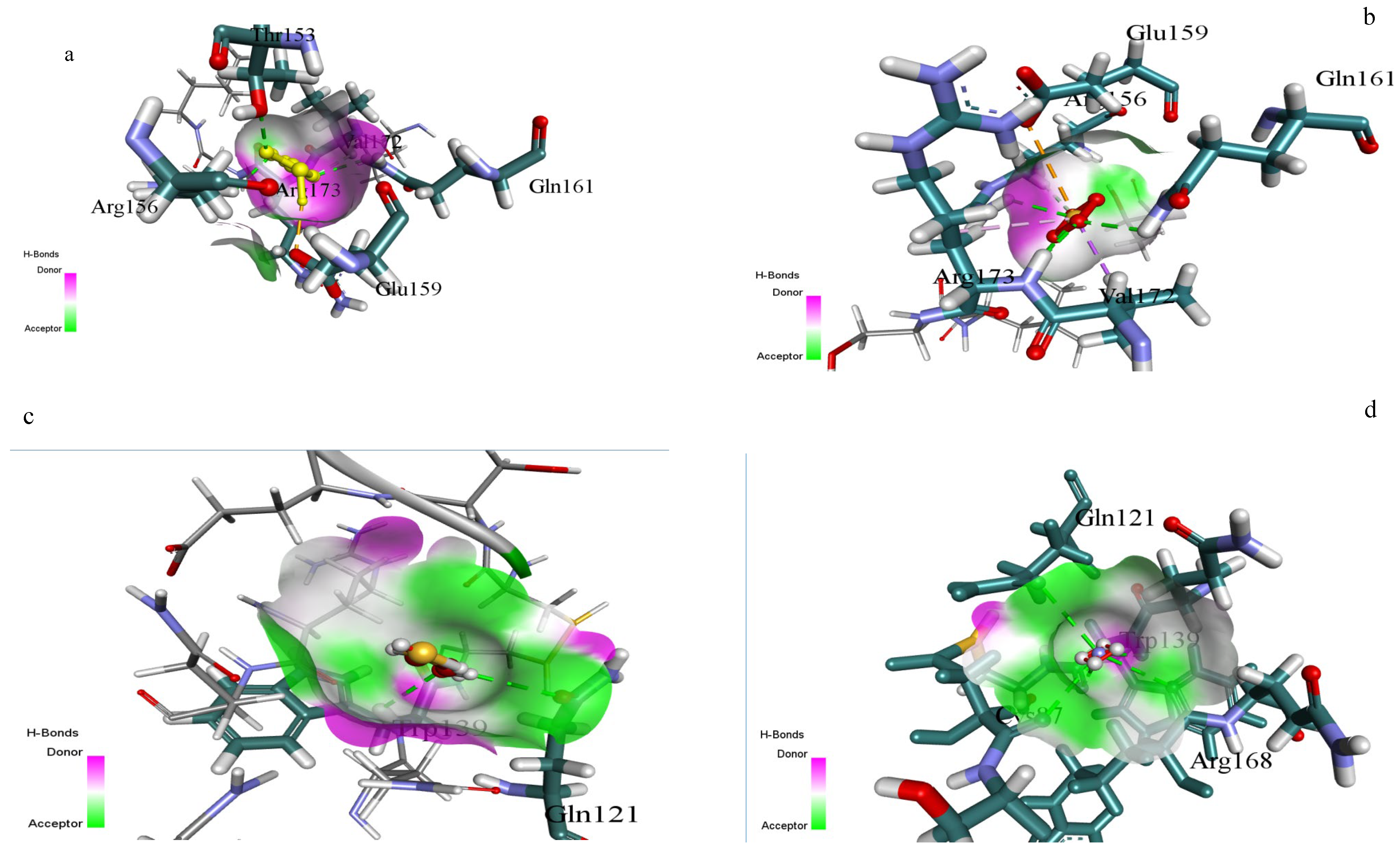
3.5. Structural Analysis
3.6. Outcomes of Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatto, A.; Chepeliev, M. Global food loss and waste estimates show increasing nutritional and environmental pressures. Nat. Food 2024, 5, 136–147. [Google Scholar] [CrossRef]
- Tacon, A.G.; Lemos, D.; Metian, M. Fish for health: Improved nutritional quality of cultured fish for human consumption. Rev. Fish. Sci. Aquac. 2020, 28, 449–458. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.B.; Ahilan, B.; Jeevagan, I.J.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Arumugam, M.; Jayaraman, S.; Sridhar, A.; Venkatasamy, V.; Brown, P.B.; Abdul Kari, Z.; Tellez-Isaias, G.; Ramasamy, T. Recent advances in tilapia production for sustainable developments in Indian aquaculture and its economic benefits. Fishes 2023, 8, 176. [Google Scholar] [CrossRef]
- Verbrugge, B.; Lanzano, C.; Libassi, M. The cyanide revolution: Efficiency gains and exclusion in artisanal- and small-scale gold mining. Geoforum 2021, 126, 267–276. [Google Scholar] [CrossRef]
- Buonvino, S.; Arciero, I.; Melino, S. Thiosulfate-cyanide sulfurtransferase, a mitochondrial essential enzyme: From cell metabolism to the biotechnological applications. Int. J. Mol. Sci. 2022, 23, 8452. [Google Scholar] [CrossRef]
- Adesulu, E.A.; Syndenham, D.H.J. The Freshwater Fishes and Fisheries of Nigeria; Macmillan Publishers Limited: Basingstoke, UK, 2007; pp. 10–234. [Google Scholar]
- Pandey, M.; Hajela, K. Isolation, purification and characterization of a protease from the seeds of Artocarpus heterophyllus. Indian J. Biochem. Biophys. (IJBB) 2023, 60, 690–702. [Google Scholar]
- Sörbo, B.H.; Lagerkvist, U.; Pesola, R.; Virtanen, A.I.; Sörensen, N.A. Crystalline Rhodanese. I. Purification and physicochemical examination. Acta Chem. Scand. 1953, 7, 1129–1136. [Google Scholar] [CrossRef]
- Lee, C.H.; Hwang, J.H.; Lee, Y.S.; Cho, K.S. Purification and Characterization of mouse liver rhodanese. J. Biochem. Mol. Biol. 1995, 28, 170–176. [Google Scholar]
- Bradford, K.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Weber, K.; Osborn, M. Protein and Sodium Dodecyl Sulphate: Molecular Weight Determination on Polyacrylamide Gels and Related Procedures; The Proteins; Academic Press: New York, NY, USA, 1975; pp. 179–223. [Google Scholar]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Sriramulu, D.K.; Lee, S.G. Effect of molecular properties of the protein-ligand complex on the prediction accuracy of AutoDock. J. Mol. Graph. Model. 2021, 106, 107921. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.E.; Ayinla, Z.A.; Agboola, S.S.; Adegbuyi, T.A.; Akinseye, J.F.; Sijuade, A.; Egbebi, A.H.; Ilesanmi, O.S.; Agboola, A.A.; Ibrahim, O.K. Molecular mechanisms underlying the erectogenic effects of nutraceutical lunamarine, a novel PDE5 inhibitor derived from watermelon (Citrullus lanatus). Discov. Food 2024, 4, 153. [Google Scholar] [CrossRef]
- Zayed, A.O. Optimizing protein-ligand docking through machine learning: Algorithm selection with AutoDock Vina. Discov. Chem. 2025, 2, 164. [Google Scholar] [CrossRef]
- Agboola, O.E.; Agboola, S.S.; Oyinloye, O.M.; Fadugba, A.E.; Omolayo, E.Y.; Ayinla, Z.A.; Osunsanmi, F.O.; Olaiya, O.E.; Olojo, F.O.; Ajiboye, B.O.; et al. Integrative genomic and in silico analysis reveals mitochondrially encoded cytochrome C oxidase III (MT-CO3) overexpression and potential neem-derived inhibitors in breast cancer. Genes 2025, 16, 546. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Agboola, O.E.; Ayeni, A.M.; Oyinloye, O.M.; Agboola, S.S.; Idowu, O.T.; Mathenjwa-Goqo, M.S.; Owolabi, O.V.; Ogunyinka, B.I.; Osunsanmi, F.O.; et al. Computational analysis of Annona muricata phytochemicals for targeted modulation of endocrine networks in polycystic ovary syndrome. Discov. Food 2025, 5, 145. [Google Scholar] [CrossRef]
- Agboola, O.E.; Ayinla, Z.A.; Apalowo, O.; Agboola, S.S.; Fajana, O.M.; Ekundayo, B.E.; Itakorode, B.O.; Oyinloye, B.E. Profilage chimio-informatique des composés phytochimiques d’Azardirachta indica en tant qu’inhibiteurs oncogènes doubles SHP2/HSP90: Identification du nimbocinol, de la nimbidinine et de la margolone. Ann. Pharm. Françaises 2025, 83, 907–920. [Google Scholar] [CrossRef]
- Agboola, O.E.; Agboola, S.S.; Agboinghale, P.E.; Ayinla, Z.A.; Oyebamiji, A.K.; Olaiya, O.E.; Fajana, O.M.; Oyinloye, O.M.; Adewale, A.I.; Idowu, O.T.; et al. Selective modulation of orexinergic receptors by neem-derived phytochemicals: Computational analysis of structure-activity relationships. Toxicol. Rep. 2025, 15, 102104. [Google Scholar] [CrossRef]
- Agboola, O.E.; Ayinla, Z.A.; Agboola, S.S.; Omolayo, E.Y.; Fadugba, A.E.; Odeghe, O.B.; Olaiya, O.E.; Oyinloye, B.E. In silico profiling of neem limonoids and gut microbiome metabolites for Alzheimer’s therapeutics: Targeted inhibition of BACE1 and elucidation of intricate molecular crosstalk with tau oligomers, and bacterial gingipains. Discov. Appl. Sci. 2025, 7, 312. [Google Scholar] [CrossRef]
- Marrufo, T.J.; Capece, B.P.; Silva, M.R. Influence of Artisanal Mining and Environmental Pollutants on Food Security in Southern Africa: A Systematic Review. Food Energy Secur. 2025, 14, e70080. [Google Scholar] [CrossRef]
- Marshall, B.G.; Veiga, M.M.; da Silva, H.A.; Guimarães, J.R. Cyanide contamination of the Puyango-Tumbes River caused by artisanal gold mining in Portovelo-Zaruma, Ecuador. Curr. Environ. Health Rep. 2020, 7, 303–310. [Google Scholar] [CrossRef]
- Geletu, T.T.; Tang, S.; Xing, Y.; Zhao, J. Ecological niche and life-history traits of redbelly tilapia (Coptodon zillii, Gervais 1848) in its native and introduced ranges. Aquat. Living Resour. 2024, 37, 2. [Google Scholar] [CrossRef]
- Akinsiku, O.T.; Agboola, F.K.; Kuku, A.; Afolayan, A. Physicochemical and kinetic characteristics of rhodanese from the liver of African catfish Clarias gariepinus Burchell in Asejire lake. Fish Physiol. Biochem. 2010, 36, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Comte, C.; Mager-Heckel, A.M.; Addis, V.; Krasheninnikov, I.A.; Martin, R.P.; Entelis, N.; Tarassov, I. Mitochondrial enzyme rhodanese is essential for 5S ribosomal RNA import into human mitochondria. J. Biol. Chem. 2010, 285, 30792–30803. [Google Scholar] [CrossRef] [PubMed]
- Ogudugu, B.E.; Ademakinwa, N.A.; Ezinma, E.N.; Agboola, F.K. Purification and physicochemical properties of rhodanese from the liver of a goat, Capra aegagrus hircus. J. Biochem. Mol. Biol. Res. 2015, 1, 105–111. [Google Scholar]
- Horowitz, P.M.; Xu, R. Acid pH-induced conformational changes in bovine liver rhodanese. J. Biol. Chem. 1992, 267, 19464–19469. [Google Scholar] [CrossRef]
- Melino, S.; Cicero, D.O.; Orsale, M.; Forlani, F.; Pagani, S.; Paci, M. Azotobacter vinelandii rhodanese: Selenium loading and ion interaction studies. Eur. J. Biochem. 2003, 270, 4208–4215. [Google Scholar] [CrossRef]
- Slobodkin, A.I.; Slobodkina, G.B. Diversity of sulfur-disproportionating microorganisms. Microbiology 2019, 88, 509–522. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Napoli, G.D.; Fissore, A.; Salladini, E.; Raccuia, E.; Oliaro-Bosso, S.; Ruggiero, A.; Berisio, R.; Medina, M.; Velazquez-Campoy, A.; Adinolfi, S.; et al. Characterization of Mycobacterium tuberculosis rhodanese-like sulfurtransferase SseA and its newly identified activator Rv3284: A new pathway to exploit as a drug target? bioRxiv 2025. [Google Scholar] [CrossRef]
- Papenbrock, J.; Guretzki, S.; Henne, M. Latest news about the sulfurtransferase protein family of higher plants. Amino Acids 2011, 41, 43–57. [Google Scholar] [CrossRef]
- Babamotemi, O.I.; Raphael, E.O.; Odunayo, A.; Nkem, T.; Chinwe, O.; Ademakinwa, O. Studies on some physicochemical properties of rhodanese synthesized by Bacillus cereus isolated from the effluents of the iron and steel smelting industry. Afr. J. Biochem. Res. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Dildar, T.; Cui, W.; Ikhwanuddin, M.; Ma, H. Aquatic Organisms in Response to Salinity Stress: Ecological Impacts, Adaptive Mechanisms, and Resilience Strategies. Biology 2025, 14, 667. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hausinger, R.P. Sulfur incorporation into biomolecules: Recent advances. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, A.; Forlani, F.; Carpen, A.; Armirotti, A.; Pagani, S.; Bolognesi, M.; Bordo, D. The “rhodanese” fold and catalytic mechanism of 3-mercaptopyruvate sulfurtransferases: Crystal structure of SseA from Escherichia coli. J. Mol. Biol. 2004, 335, 583–593. [Google Scholar] [CrossRef]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide-containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef]
- Bebarta, V.S.; Nath, A.K. Redirecting Intermediary Metabolism to Counteract Cyanide Poisoning. FASEB J. 2025, 39, e70709. [Google Scholar] [CrossRef]
- Francioso, A.; Baseggio Conrado, A.; Mosca, L.; Fontana, M. Chemistry and biochemistry of natural sulfur compounds: Key intermediates of metabolism and redox biology. Oxid. Med. Cell. Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef]
- Mesbah, N.M. Industrial biotechnology based on enzymes from extreme environments. Front. Bioeng. Biotechnol. 2022, 10, 870083. [Google Scholar] [CrossRef]
- Davis, S.; Murray, J.; Katsiadaki, I. Cyanide in the Aquatic Environment and Its Metabolism by Fish; A Report to Ornamental Aquatic Trade Association; Centre for Environment Fisheries & Aquaculture Science: Lowestoft, UK, 2017. [Google Scholar]
 Pooled Fractions.
Pooled Fractions.
 Pooled Fractions.
Pooled Fractions.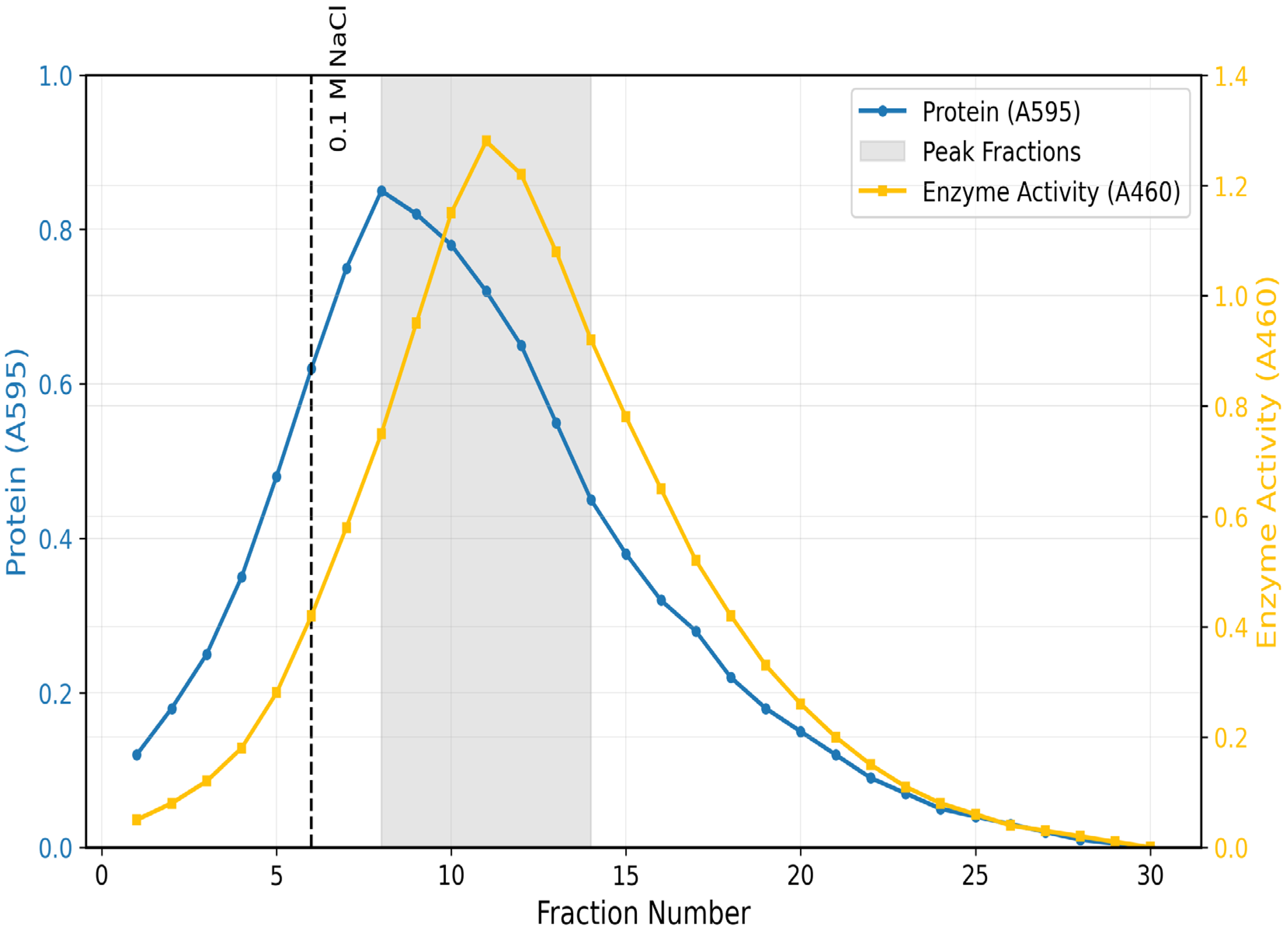
 Pooled Fractions.
Pooled Fractions.
 Pooled Fractions.
Pooled Fractions.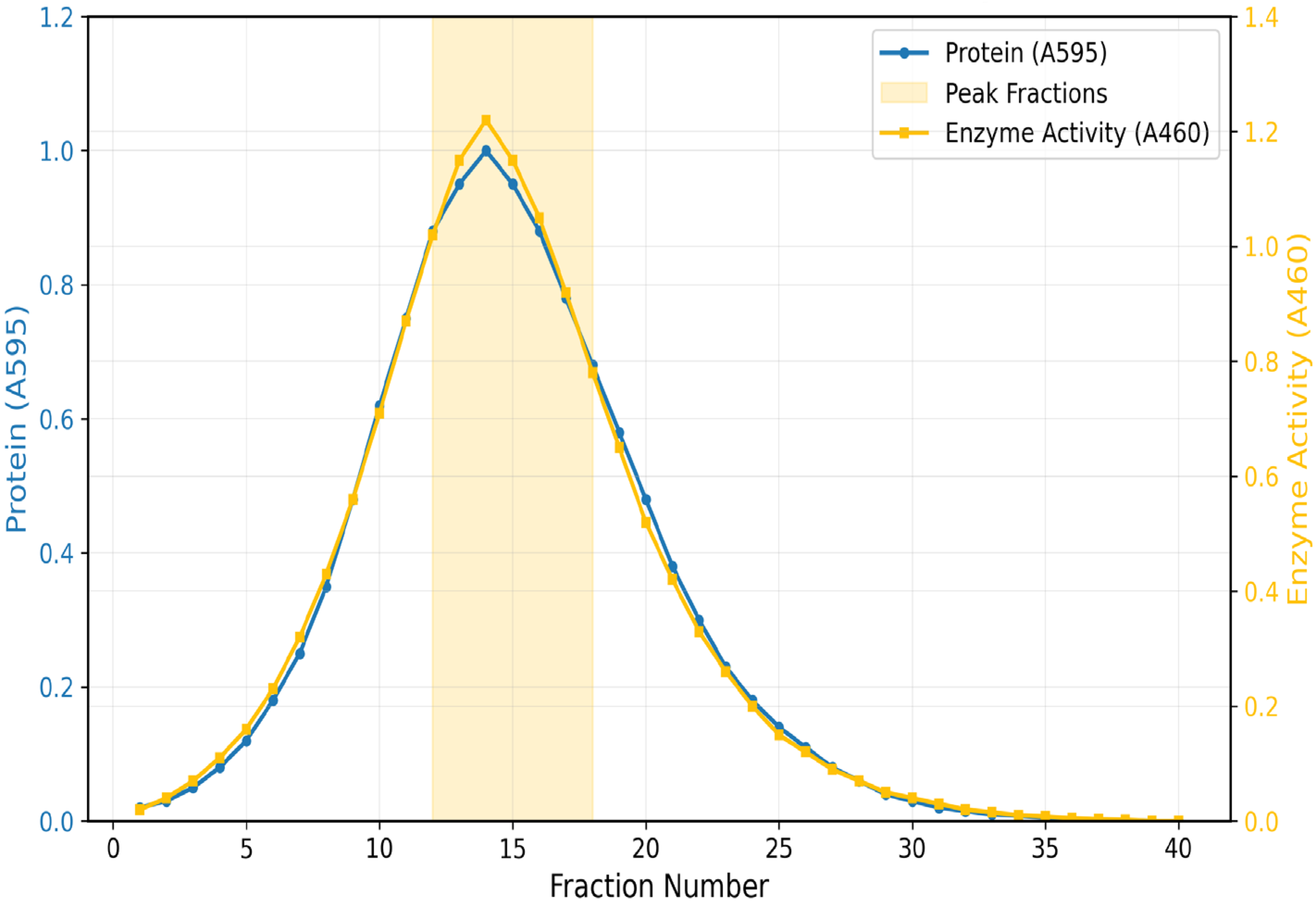
 ) and Varying Concentrations of Potassium Cyanide (KCN) (
) and Varying Concentrations of Potassium Cyanide (KCN) ( ) for Coptodon zillii Liver Rhodanese.
) for Coptodon zillii Liver Rhodanese.
 ) and Varying Concentrations of Potassium Cyanide (KCN) (
) and Varying Concentrations of Potassium Cyanide (KCN) ( ) for Coptodon zillii Liver Rhodanese.
) for Coptodon zillii Liver Rhodanese.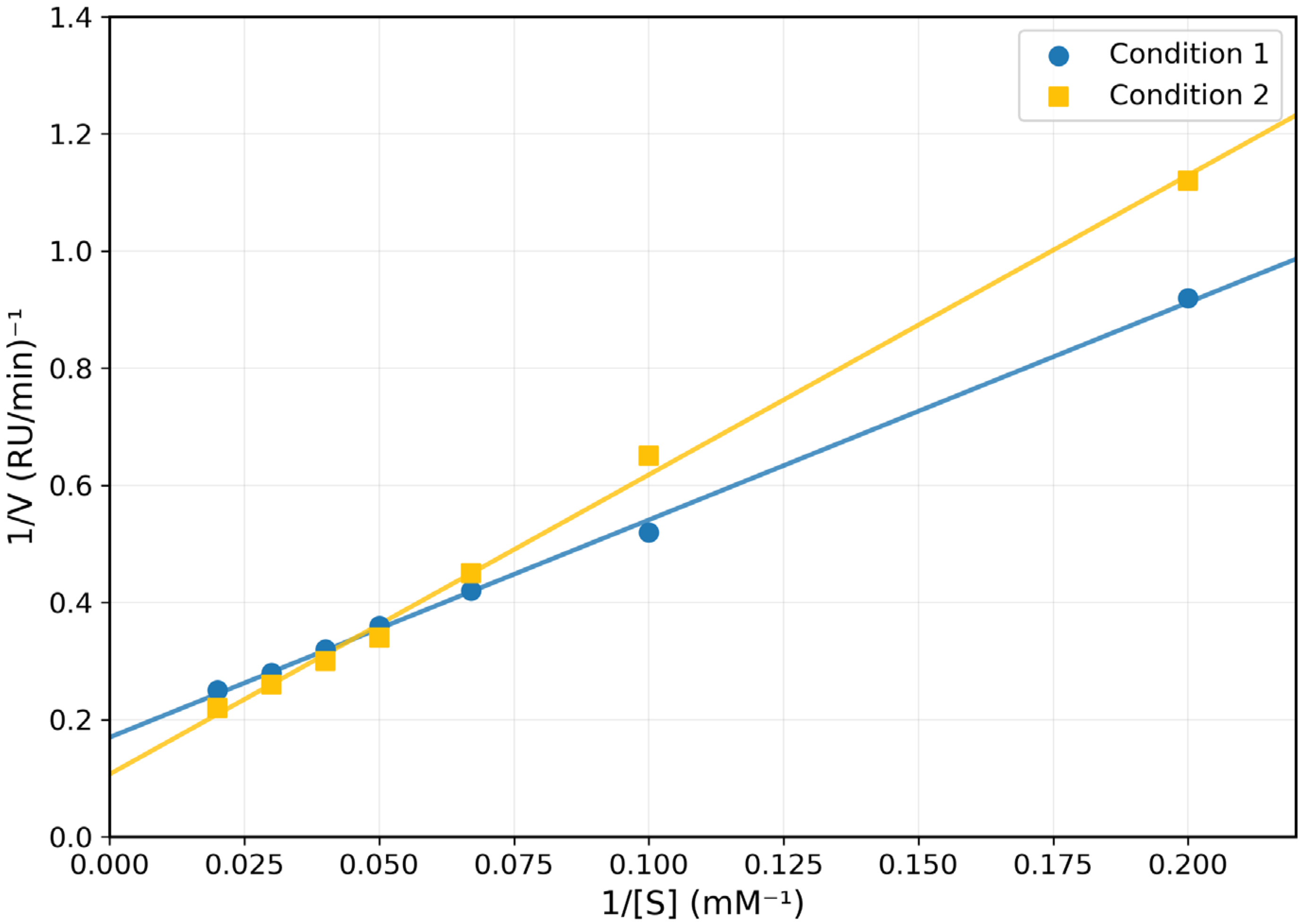
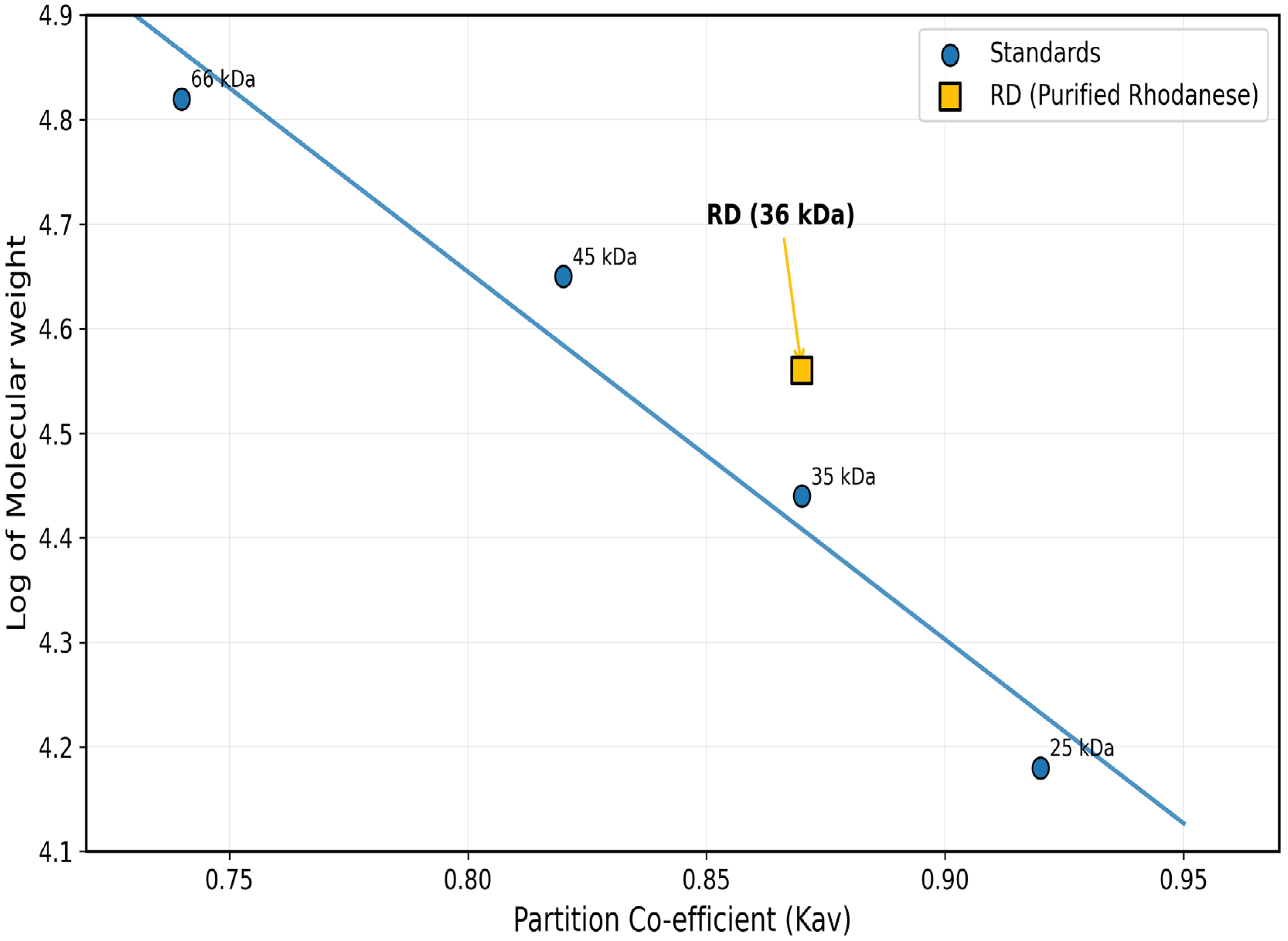
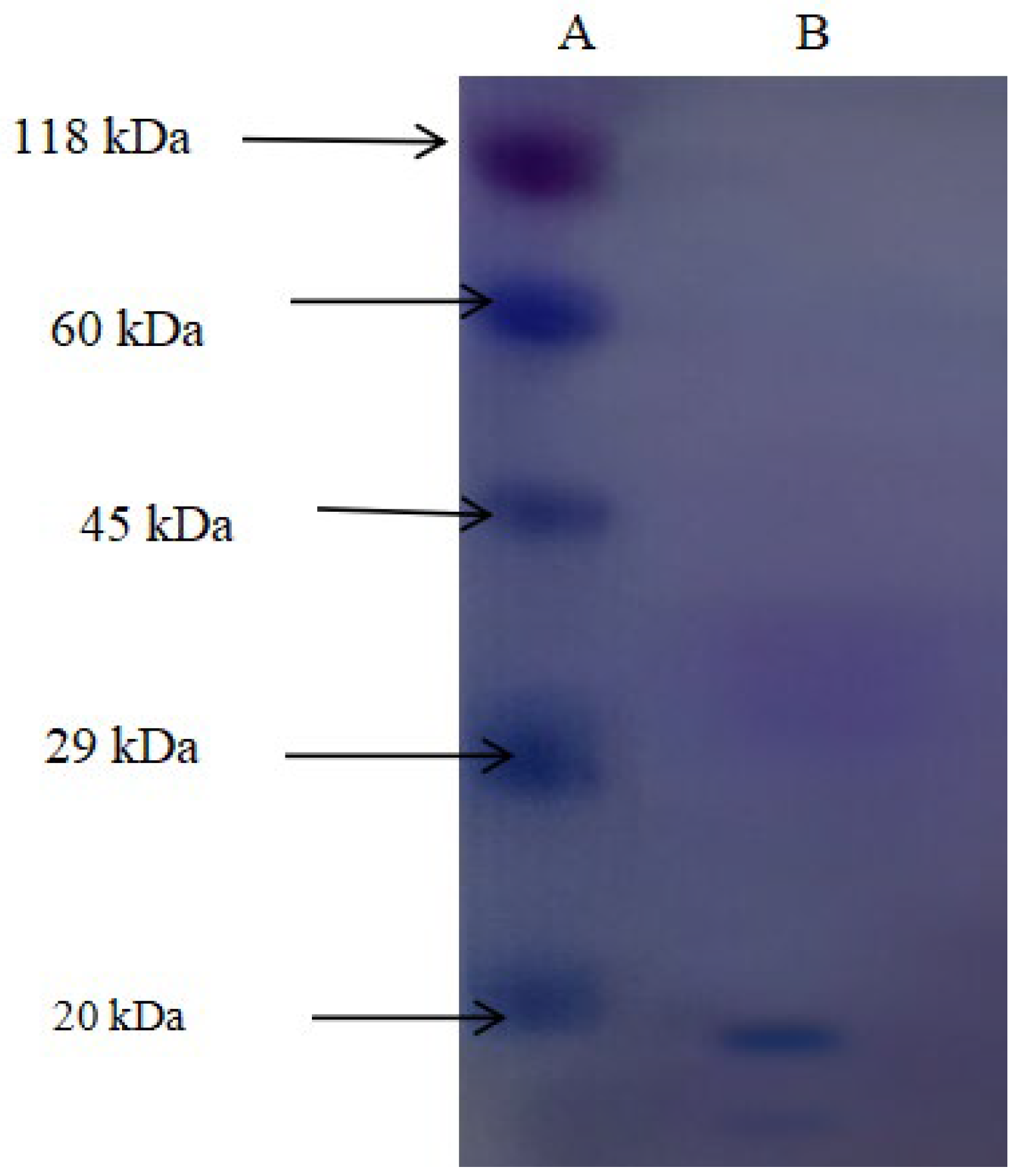
| Purification Step | Volume (mL) | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Yield (%) | Purification Fold |
|---|---|---|---|---|---|---|
| Crude Extract | 75 | 401 | 690.00 | 1.72 | 100 | 1.00 |
| 70% Ammonium Sulphate | 30 | 90 | 252.00 | 2.80 | 37 | 1.63 |
| CM-Sephadex C-50 | 17 | 43 | 140.08 | 3.30 | 20 | 1.92 |
| Biogel P-100 | 14 | 21 | 113.68 | 5.40 | 16 | 3.10 |
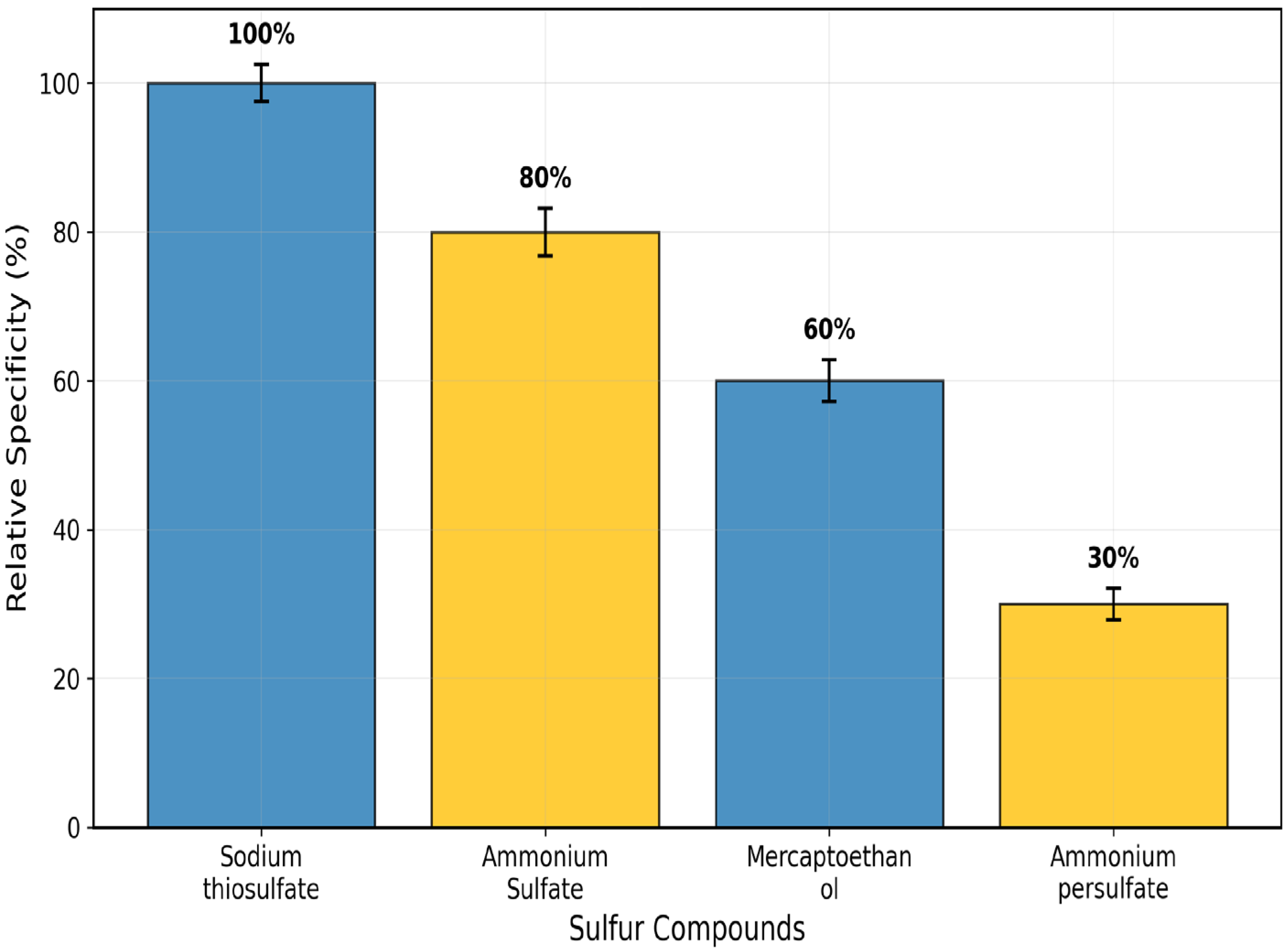
| Substrate | Docking Score (kcal/mol) | H-Bond Donors | H-Bond Acceptors | Surface Interactions | Interacting Residues |
|---|---|---|---|---|---|
| Sodium thiosulfate | −4.372 | Arg173-NH, Gln161-NH2 | Glu159-COO, SO4 group | Strong donor-acceptor (magenta-green gradient) | Glu159, Gln161, Arg173, Arg156, Val172 |
| Ammonium sulphate | −3.832 | Arg156-NH, Gln121-NH2 | SO4 group, Trp139-CO | Moderate donor-acceptor interface | Gln121, Trp139, Arg156 |
| 2-Mercaptoethanol | −2.853 | Trp139-NH, Val172-NH | OH group, Val172-CO | Weak donor-acceptor surface contact | Gln121, Trp139, Val172 |
| Ammonium persulphate | −1.488 | Gln121-NH2 | SO4 group | Limited surface complementarity | Gln121, Trp139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agboola, O.E.; Ayinla, Z.A.; Itakorode, B.O.; Akinsanya, P.O.; Okonji, R.E.; Odeghe, O.B.; Agboola, S.S.; Oluranti, O.E.; Olojo, F.O.; Oyinloye, B.E. Structural and Kinetic Properties of Liver Rhodanese from Coptodon zillii: Implications for Cyanide Detoxification in Gold Mining-Impacted Aquatic Ecosystems. Toxics 2025, 13, 750. https://doi.org/10.3390/toxics13090750
Agboola OE, Ayinla ZA, Itakorode BO, Akinsanya PO, Okonji RE, Odeghe OB, Agboola SS, Oluranti OE, Olojo FO, Oyinloye BE. Structural and Kinetic Properties of Liver Rhodanese from Coptodon zillii: Implications for Cyanide Detoxification in Gold Mining-Impacted Aquatic Ecosystems. Toxics. 2025; 13(9):750. https://doi.org/10.3390/toxics13090750
Chicago/Turabian StyleAgboola, Oluwaseun E., Zainab A. Ayinla, Babamotemi O. Itakorode, Priscilla O. Akinsanya, Raphael E. Okonji, Othuke B. Odeghe, Samuel S. Agboola, Olaiya E. Oluranti, Folake O. Olojo, and Babatunji E. Oyinloye. 2025. "Structural and Kinetic Properties of Liver Rhodanese from Coptodon zillii: Implications for Cyanide Detoxification in Gold Mining-Impacted Aquatic Ecosystems" Toxics 13, no. 9: 750. https://doi.org/10.3390/toxics13090750
APA StyleAgboola, O. E., Ayinla, Z. A., Itakorode, B. O., Akinsanya, P. O., Okonji, R. E., Odeghe, O. B., Agboola, S. S., Oluranti, O. E., Olojo, F. O., & Oyinloye, B. E. (2025). Structural and Kinetic Properties of Liver Rhodanese from Coptodon zillii: Implications for Cyanide Detoxification in Gold Mining-Impacted Aquatic Ecosystems. Toxics, 13(9), 750. https://doi.org/10.3390/toxics13090750









