Toxic Effects of Waterborne Nitrite on LC50, Hematological Parameters, and Plasma Biochemistry in Starry Flounder (Platichthys stellatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Environment
2.2. Lethal Concentration 50% (LC50)
2.3. Hematological Parameters
2.4. Plasma Components
2.5. Statistical Analysis Method
2.6. Approval of Animal Experimental Ethics
3. Results
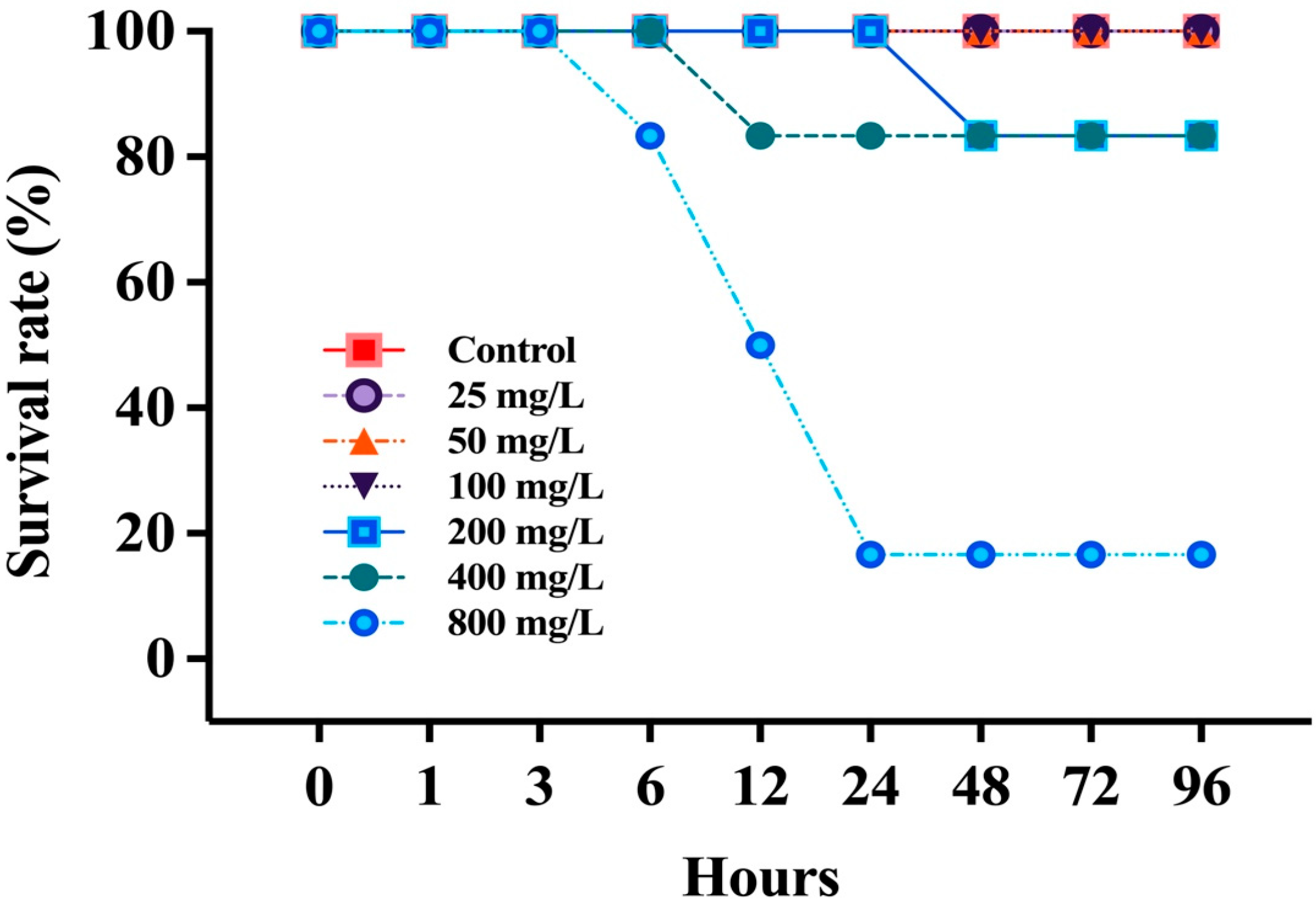
3.1. Survival Rate and Lethal Concentration 50% (LC50)
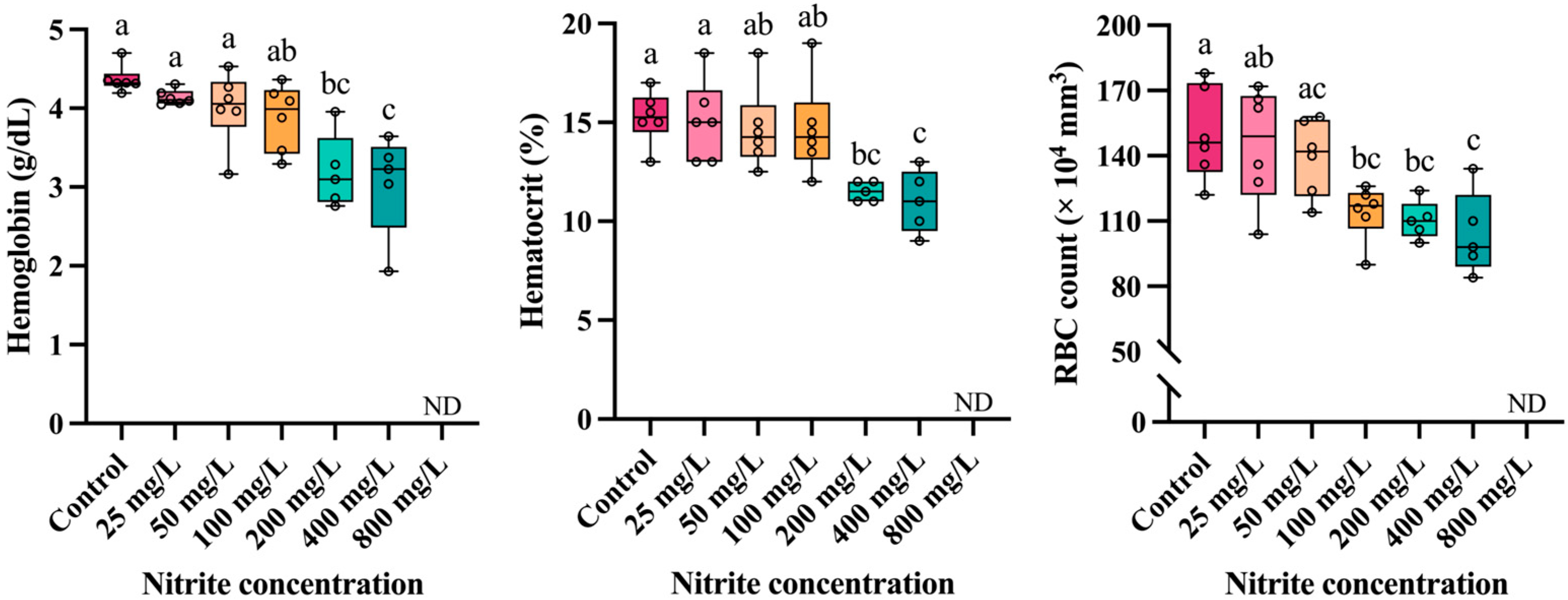
3.2. Hematological Parameters
3.3. Plasma Components
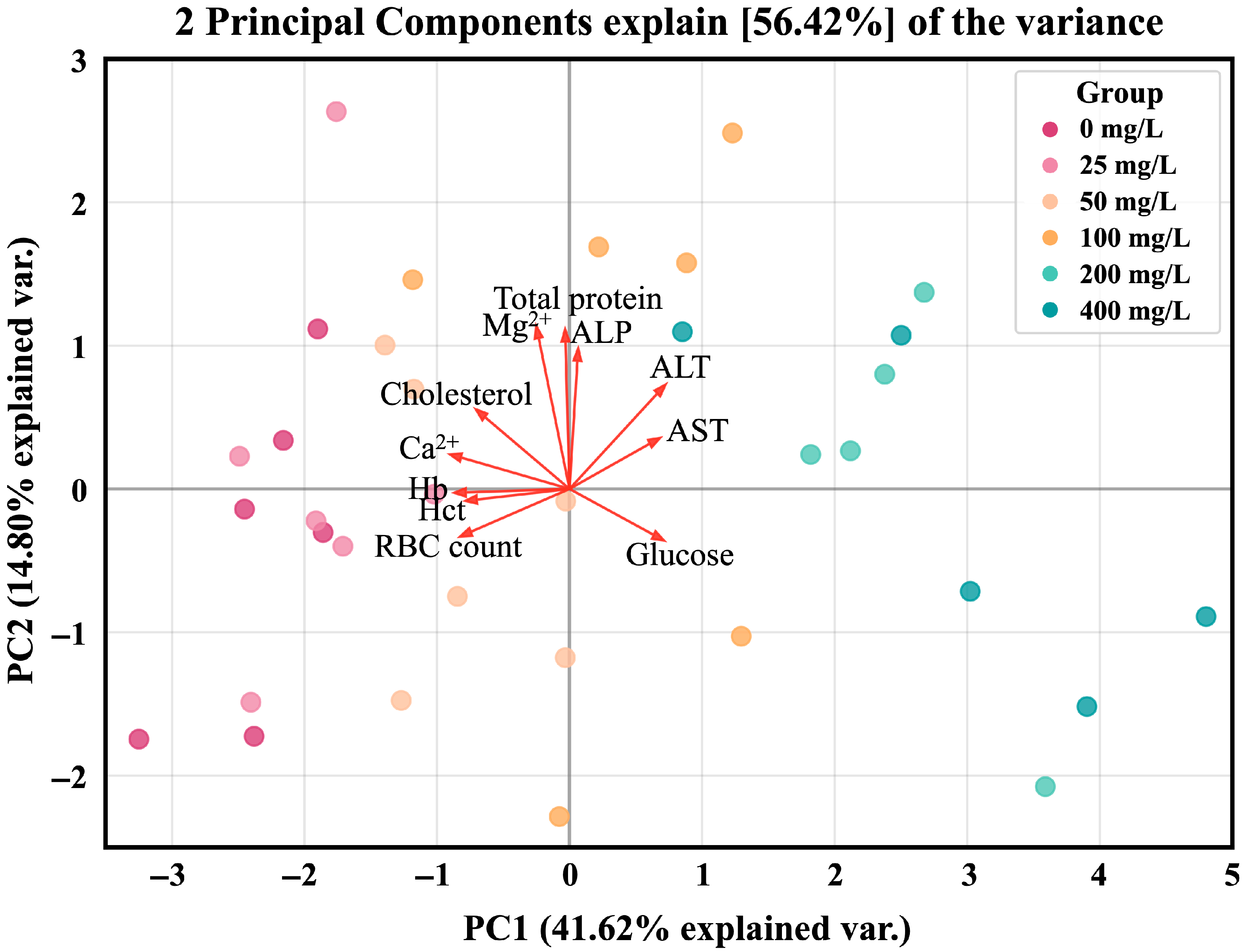
3.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, J.A.; Matheson, G.; Gilron, G.; DeForest, D.K. Evaluation of Sublethal Toxicity of Nitrite to a Suite of Aquatic Organisms in Support of the Derivation of a Chronic Environmental Water Quality Benchmark. Arch. Environ. Contam. Toxicol. 2022, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ping, J.; Ying, Y. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. Trends Analyt. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Kocour Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Máchová, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.K.; Hur, Y.B. Toxic effects of waterborne nitrite exposure on antioxidant responses, acetylcholinesterase inhibition, and immune responses in olive flounders, Paralichthys olivaceus, reared in bio-floc and seawater. Fish Shellfish Immunol. 2020, 97, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Physiological changes in Labeo rohita during nitrite exposure: Detoxification through dietary vitamin E. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 122–129. [Google Scholar] [CrossRef]
- Lin, Y.; Miao, L.H.; Pan, W.J.; Huang, X.; Dengu, J.M.; Zhang, W.X.; Ge, X.P.; Liu, B.; Ren, M.C.; Zhou, Q.L.; et al. Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish Shellfish Immunol. 2018, 76, 126–132. [Google Scholar] [CrossRef]
- Molayemraftar, T.; Peyghan, R.; Jalali, M.R.; Shahriari, A. Single and combined effects of ammonia and nitrite on common carp, Cyprinus carpio: Toxicity, hematological parameters, antioxidant defenses, acetylcholinesterase, and acid phosphatase activities. Aquaculture 2022, 548, 737676. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, X.; Li, M.; Wang, R.; Qian, Y.; Hong, M. Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 238, 108867. [Google Scholar] [CrossRef]
- Pottinger, T.G. Modulation of the stress response in wild fish is associated with variation in dissolved nitrate and nitrite. Environ. Pollut. 2017, 225, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, M.J.; da Costa, F.F.B.; Leme, F.P.; Takata, R.; Costa, D.C.; Mattioli, C.C.; Luz, R.K.; Miranda-Filho, K.C. Biological responses of Neotropical freshwater fish Lophiosilurus alexandri exposed to ammonia and nitrite. Sci. Total Environ. 2018, 616, 1566–1575. [Google Scholar] [CrossRef]
- Liang, Y.; Zhong, Y.; Xi, Y.; He, L.; Zhang, H.; Hu, X.; Gu, H. Toxic effects of combined exposure to homoyessotoxin and nitrite on the survival, antioxidative responses, and apoptosis of the abalone Haliotis discus hannai. Ecotoxicol. Environ. Saf. 2024, 272, 116058. [Google Scholar] [CrossRef]
- Lek, S.; Ut, V.N.; Phuong, N.T. Effects of nitrite at different temperatures on physiological parameters and growth in clown knifefish (Chitala ornata, Gray 1831). Aquaculture 2020, 521, 735060. [Google Scholar] [CrossRef]
- Zhang, J.M.; Fu, B.; Li, Y.C.; Sun, J.H.; Xie, J.; Wang, G.J.; Tian, J.J.; Kaneko, G.; Yu, E.M. The effect of nitrite and nitrate treatment on growth performance, nutritional composition and flavor-associated metabolites of grass carp (Ctenopharyngodon idella). Aquaculture 2023, 562, 738784. [Google Scholar] [CrossRef]
- Kroupova, H.; Stejskal, V.; Kouril, J.; Machova, J.; Piackova, V.; Zuskova, E. A wide difference in susceptibility to nitrite between Eurasian perch (Perca fluviatilis L.) and largemouth bass (Micropterus salmoides Lac.). Aquacult. Int. 2013, 21, 961–967. [Google Scholar] [CrossRef]
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef]
- Katsiadaki, I.; Ellis, T.; Andersen, L.; Antczak, P.; Blaker, E.; Burden, N.; Fisher, T.; Green, C.; Labram, B.; Pearson, A.; et al. Dying for change: A roadmap to refine the fish acute toxicity test after 40 years of applying a lethal endpoint. Ecotoxicol. Environ. Saf. 2021, 223, 112585. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Á. Post-exposure Period as a key Factor to Assess Cadmium Toxicity: Lethal vs. Behavioral Responses. Bull. Environ. Contam. Toxicol. 2023, 110, 41. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.E.; Moore, P.A. Mimicking natural systems: Changes in behavior as a result of dynamic exposure to naproxen. Ecotoxicol. Environ. Saf. 2017, 135, 347–357. [Google Scholar] [CrossRef]
- Kajimura, M.; Takimoto, K.; Takimoto, A. Acute toxicity of ammonia and nitrite to Siamese fighting fish (Betta splendens). BMC Zool. 2023, 8, 25. [Google Scholar] [CrossRef]
- Zhang, T.T.; Ma, P.; Yin, X.Y.; Yang, D.Y.; Li, D.P.; Tang, R. Acute Nitrite Exposure Induces Dysfunction and Oxidative Damage in Grass Carp Isolated Hemocytes. J. Aquat. Anim. Health 2022, 34, 58–68. [Google Scholar] [CrossRef]
- Martinez, C.B.; Souza, M.M. Acute effects of nitrite on ion regulation in two neotropical fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 151–160. [Google Scholar] [CrossRef]
- Grosell, M.; Jensen, F.B. Uptake and effects of nitrite in the marine teleost fish Platichthys flesus. Aquat. Toxicol. 2000, 50, 97–107. [Google Scholar] [CrossRef]
- Santana-Piñeros, A.M.; Cruz-Quintana, Y.; Reyes-Mero, B.M.; González-Solís, D.; Rodríguez-Canul, R. Hematological parameters of the Pacific fat sleeper, Dormitator latifrons (Pisces: Eleotridae), under natural and cultured conditions. Egypt. J. Aquat. Res. 2024, 50, 162–167. [Google Scholar] [CrossRef]
- Son, H.J.; Kang, G.; Woo, W.S.; Kim, K.H.; Sohn, M.Y.; Park, J.W.; Lee, D.; Park, C.I. Antimicrobial Activity of Identified Ubiquitin-40S Ribosomal Protein S27a (RPS27A), Ubiquitin-like Protein Fubi, and Ribosomal Protein (S30FAU) in the Starry Flounder (Platichthys stellatus). Fishes 2023, 8, 187. [Google Scholar] [CrossRef]
- Kim, Y.S.; Do, Y.H.; Min, B.H.; Lim, H.K.; Lee, B.K.; Chang, Y.J. Physiological responses of starry flounder Platichthys stellatus during freshwater acclimation with different speeds in salinity change. J. Aquacult. 2009, 22, 28–33. [Google Scholar]
- Kang, D.Y.; Kim, H.C. Functional relation of agouti signaling proteins (ASIPs) to pigmentation and color change in the starry flounder, Platichthys stellatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 291, 111524. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, J.; Liu, J.; Zhuo, H.; Zhang, Y.; Zhou, X.; Wu, G.; Guo, C.; Zhao, X. Single and combined effects of ammonia and nitrite on Litopenaeus vannamei: Histological, physiological and molecular responses. Aquac. Rep. 2024, 35, 102014. [Google Scholar] [CrossRef]
- Kim, J.H.; Sohn, S.; Kim, S.K.; Hur, Y.B. Effects on hematological parameters, antioxidant and immune responses, AChE, and stress indicators of olive flounders, Paralichthys olivaceus, raised in bio-floc and seawater challenged by Edwardsiella tarda. Fish Shellfish Immunol. 2020, 97, 194–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.F.; He, S.; Li, L. Effects of long-term low-concentration nitrite exposure and detoxification on growth performance, antioxidant capacities, and immune responses in Chinese perch (Siniperca chuatsi). Aquaculture 2021, 533, 736123. [Google Scholar] [CrossRef]
- Kir, M.; Sunar, M.C. Acute toxicity of ammonia and nitrite to Sea Bream, Sparus aurata (Linnaeus, 1758), in relation to salinity. J. World Aquac. Soc. 2018, 49, 516–522. [Google Scholar] [CrossRef]
- Pedrotti, F.; Baloi, M.; Cerqueira, V. Acute toxicity of nitrite on juveniles of common snook Centropomus undecimalis (perciformes, centropomidae). Panam. J. Aquat. Sci. 2018, 13, 162–165. [Google Scholar]
- Saoud, P.; Naamani, S.; Ghanawi, J.; Nasser, N. Effects of acute and chronic nitrite exposure on rabbitfish Siganus rivulatus growth, hematological parameters, and gill histology. J. Aquac. Res. Dev. 2014, 5, 263–271. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, D.M.; Li, B.; Zhao, Z.G.; Fang, W.; Yang, K.; Fan, Q.X. Toxicity of ammonia and nitrite to yellow catfish (Pelteobagrus fulvidraco). J. Appl. Ichthyol. 2012, 28, 82–86. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.J.; Lee, K.M. Effects of Nitrite Exposure on the Hematological Properties, Antioxidant and Stress Responses of Juvenile Hybrid Groupers, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Antioxidants 2022, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, Y.J.; Kim, K.I.; Kim, S.K.; Kim, J.H. Toxic effects of nitrogenous compounds (ammonia, nitrite, and nitrate) on acute toxicity and antioxidant responses of juvenile olive flounder, Paralichthys olivaceus. Environ. Toxicol. Pharmacol. 2019, 67, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Neves, G.C.; Presa, L.S.; Maltez, L.C.; Monserrat, J.M.; Garcia, L. Calcium carbonate addition reduces nitrite toxic effects in pacu Piaractus mesopotamicus juveniles. Aquaculture 2022, 547, 737444. [Google Scholar] [CrossRef]
- Reda, R.M.; El-Murr, A.; Abdel-Basset, N.A.; Metwally, M.M.; Ibrahim, R.E. Implications of ammonia stress for the pathogenicity of Shewanella spp. in Oreochromis niloticus: Effects on hematological, biochemical, immunological, and histopathological parameters. BMC Vet. Res. 2024, 20, 324. [Google Scholar] [CrossRef]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Akhtar, M.S. Alterations in serum electrolytes, antioxidative enzymes and haematological parameters of Labeo rohita on short-term exposure to sublethal dose of nitrite. Fish Physiol. Biochem. 2012, 38, 1355–1365. [Google Scholar] [CrossRef]
- Cogun, H.Y.; Firidin, G.; Aytekin, T.; Firat, O.; Firat, O.; Temiz, O.; Varkal, H.S.; Kargin, F. Acute toxicity of nitrite on some biochemical, hematological and antioxidant parameters in Nile Tilapia (Oreochromis niloticus L, 1758). Fresenius Environ. Bull. 2017, 26, 1712–1719. [Google Scholar]
- Jia, R.; Han, C.; Lei, J.L.; Liu, B.L.; Huang, B.; Huo, H.H.; Yin, S.T. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2015, 169, 1–9. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 135, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cao, S.; Mohsen, M.; Li, X.S.; Wang, L.; Lu, K.L.; Zhang, C.X.; Song, K. Effects of chronic nitrite exposure on hematological parameters, oxidative stress and apoptosis in spotted seabass (Lateolabrax maculatus) reared at high temperature. Aquac. Rep. 2024, 35, 102022. [Google Scholar] [CrossRef]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Physiological changes in the hemolymph of juvenile shrimp Litopenaeus vannamei to sublethal nitrite and nitrate stress in low-salinity waters. Environ. Toxicol. Pharmacol. 2020, 80, 103472. [Google Scholar] [CrossRef]
- Esposito, G.; Bergagna, S.; Colussi, S.; Shahin, K.; Rosa, R.; Volpatti, D.; Faggio, C.; Mossotto, C.; Gabetti, A.; Maganza, A.; et al. Changes in blood serum parameters in farmed rainbow trout (Oncorhynchus mykiss) during a piscine lactococcosis outbreak. J. Fish Dis. 2024, 47, e13994. [Google Scholar] [CrossRef]
- Temiz, Ö.; Kargın, D. Physiological responses of oxidative damage, genotoxicity and hematological parameters of the toxic effect of neonicotinoid-thiamethoxam in Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2024, 106, 104377. [Google Scholar] [CrossRef]
- Farris, N.W.; Willora, F.P.; Blaauw, D.H.; Gupta, S.; Santigosa, E.; Carr, I.; Zatti, K.; Bisa, S.; Kiron, V.; Haugmo, I.M.; et al. Progressive substitution of fish oil with Schizochytrium-derived algal oil in the diet of Atlantic salmon (Salmo salar) parr subjected to winter signal period. Aquac. Rep. 2024, 36, 102130. [Google Scholar] [CrossRef]
- Orlov, S.N.; Aksentsev, S.L.; Kotelevtsev, S.V. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium 2005, 38, 53–57. [Google Scholar] [CrossRef]
- Yu, Y.B.; Lee, J.W.; Jo, A.H.; Choi, Y.J.; Choi, C.Y.; Kang, J.C.; Kim, J.H. Toxic Effects of Cadmium Exposure on Hematological and Plasma Biochemical Parameters in Fish: A Review. Toxics 2024, 12, 699. [Google Scholar] [CrossRef]
- Hong, S.M.; Jo, A.H.; Kim, D.E.; Park, Y.S.; Lee, H.S.; Jeon, Y.H.; Kim, S.R.; Kim, D.H.; Kang, Y.J.; Kim, J.H. Effects of hematological parameters and plasma components of olive flounder, Paralichthys olivaceus by acute nitrite exposure according to water temperature. J. Fish Pathol. 2021, 34, 201–212. [Google Scholar] [CrossRef]
- Matsche, M.A.; Markin, E.; Donaldson, E.; Hengst, A.; Lazur, A. Effect of chloride on nitrite-induced methaemoglobinemia in Atlantic sturgeon, Acipenser oxyrinchus oxyrinchus (Mitchill). J. Fish Dis. 2012, 35, 873–885. [Google Scholar] [CrossRef]
- Griffith, M.B. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2017, 36, 576–600. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.S.; Rahmah, S.; Shirly-Lim, Y.L.; Ghaffar, M.A.; Mazelan, S.; Jalilah, M.; Lim, L.S.; Chang, Y.M.; Liang, L.Q.; Chen, Y.M.; et al. Female tilapia, Oreochromis sp. mobilised energy differently for growth and reproduction according to living environment. Sci. Rep. 2024, 14, 2903. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Souza, M.M.; Freire, C.A. Reversibility of deleterious effects of the pisciculture byproduct nitrite on cultured Nile tilapia (Oreochromis niloticus). Aquat. Living Resour. 2004, 17, 19–23. [Google Scholar] [CrossRef]
- Liu, H.J.; Dong, M.; Jiang, W.D.; Wu, P.; Liu, Y.; Jin, X.W.; Kuang, S.Y.; Tang, L.; Zhang, L.; Feng, L.; et al. Acute nitrite exposure-induced oxidative damage, endoplasmic reticulum stress, autophagy and apoptosis caused gill tissue damage of grass carp (Ctenopharyngodon idella): Relieved by dietary protein. Ecotoxicol. Environ. Saf. 2022, 243, 113994. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, E.; Xu, C.; Gan, L.; Qin, J.G.; Chen, L. Response of AMP-activated protein kinase and energy metabolism to acute nitrite exposure in the Nile tilapia Oreochromis niloticus. Aquat. Toxicol. 2016, 177, 86–97. [Google Scholar] [CrossRef]
- Sharma, G.; Chadha, P. Toxic effects of aniline in liver, gills and kidney of freshwater fish Channa punctatus after acute exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 281, 109916. [Google Scholar] [CrossRef]
- Gao, X.Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.L. Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol. Environ. Saf. 2020, 188, 109878. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Guo, M.; Fang, D.; Mei, J.; Xie, J. Analysis of acute nitrite exposure on physiological stress response, oxidative stress, gill tissue morphology and immune response of Large yellow croaker (Larimichthys crocea). Animals 2022, 12, 1791. [Google Scholar] [CrossRef]
- Alfonso, S.; Fiocchi, E.; Toomey, L.; Boscarato, M.; Manfrin, A.; Dimitroglou, A.; Papaharisis, L.; Passabi, E.; Stefani, A.; Lembo, G.; et al. Comparative analysis of blood protein fractions in two mediterranean farmed fish: Dicentrarchus labrax and Sparus aurata. BMC Vet. Res. 2024, 20, 322. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, J.; Hur, J.W.; Song, Y.C.; Na, H.C.; Noh, C.H. Acute toxicity and sublethal effects of nitrite on selected hematological parameters and tissues in dark-banded rockfish, Sebastes inermis. J. World Aquac. Soc. 2007, 38, 188–199. [Google Scholar] [CrossRef]
- Jensen, F.B.; Damsgaard, C.; Phuong, N.T.; Bayley, M. Extreme nitrite tolerance in the clown knifefish Chitala ornata is linked to up-regulation of methaemoglobin reductase activity. Aquat. Toxicol. 2017, 187, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.; Abd El-Naby, A.S.; Samir, F.; Negm, S.S. Effects of dietary Thalassodendron Ciliatum supplementation on biochemical-immunological, antioxidant and growth indices of Oreochromis niloticus exposed to ammonia toxicity. Aquaculture 2024, 585, 740702. [Google Scholar] [CrossRef]
- Gao, X.Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Han, C.; Huang, B.; Lei, J.L. The physiological performance and immune response of juvenile turbot (Scophthalmus maximus) to nitrite exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 181, 40–46. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, D.; Mei, J.; Xie, J.; Qiu, W. A preliminary study on the effects of nitrite exposure on hematological parameters, oxidative stress, and immune-related responses in Pearl gentian grouper. Fishes 2022, 7, 235. [Google Scholar] [CrossRef]

| Item | Value |
|---|---|
| Temperature (°C) | 16.8 ± 0.29 |
| pH | 8.11 ± 0.03 |
| Dissolved Oxygen (mg/L) | 6.31 ± 0.22 |
| Salinity (‰) | 30.21 ± 0.1 |
| Ammonia (µg/L) | 3.78 ± 0.45 |
| Nitrite (µg/L) | 3.59 ± 0.11 |
| Nitrate (mg/L) | 0.23 ± 0.04 |
| Phosphate (µg/L) | 8.37 ± 0.42 |
| Nitrite Concentration (mg NO2−/L) | |||||||
|---|---|---|---|---|---|---|---|
| Nitrite concentrations | Control | 25 | 50 | 100 | 200 | 400 | 800 |
| Measured nitrite concentrations | 0.01 | 26.4 | 53.2 | 108.4 | 209.4 | 416.8 | 823.6 |
| 95% Confidence Limits | |
|---|---|
| Probability | Estimate (mg/L) |
| 0.01 | 27.19 |
| 0.10 | 272.98 |
| 0.20 | 376.48 |
| 0.30 | 451.11 |
| 0.40 | 514.87 |
| 0.50 | 574.47 |
| 0.60 | 634.07 |
| 0.70 | 697.84 |
| 0.80 | 772.47 |
| 0.90 | 875.96 |
| 0.99 | 1121.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, B.; Kim, H.S.; Choi, C.Y.; Hur, S.-P.; Kim, J.-H. Toxic Effects of Waterborne Nitrite on LC50, Hematological Parameters, and Plasma Biochemistry in Starry Flounder (Platichthys stellatus). Toxics 2025, 13, 748. https://doi.org/10.3390/toxics13090748
Gong B, Kim HS, Choi CY, Hur S-P, Kim J-H. Toxic Effects of Waterborne Nitrite on LC50, Hematological Parameters, and Plasma Biochemistry in Starry Flounder (Platichthys stellatus). Toxics. 2025; 13(9):748. https://doi.org/10.3390/toxics13090748
Chicago/Turabian StyleGong, Bijae, Hyeong Su Kim, Cheol Young Choi, Sung-Pyo Hur, and Jun-Hwan Kim. 2025. "Toxic Effects of Waterborne Nitrite on LC50, Hematological Parameters, and Plasma Biochemistry in Starry Flounder (Platichthys stellatus)" Toxics 13, no. 9: 748. https://doi.org/10.3390/toxics13090748
APA StyleGong, B., Kim, H. S., Choi, C. Y., Hur, S.-P., & Kim, J.-H. (2025). Toxic Effects of Waterborne Nitrite on LC50, Hematological Parameters, and Plasma Biochemistry in Starry Flounder (Platichthys stellatus). Toxics, 13(9), 748. https://doi.org/10.3390/toxics13090748









