Highlights
What are the main findings?
- PAHs disrupt fish reproduction by affecting endocrine signaling, gene expression, and gametogenesis.
- They alter vitellogenin levels, hormone balance, and gonadal structure via HPGL axis disruption.
What is the implication of the main finding?
- Chronic PAH exposure threatens fish populations and marine ecosystem stability.
- The findings support the use of biomarkers to inform conservation policies and strengthen marine pollution regulations.
Abstract
The biodiversity of marine and coastal ecosystems is constantly threatened by pollutants from a diversity of human activities. The polycyclic aromatic hydrocarbons (PAHs) are a class of pollutants widely released and deposited in these environments, leading to several impacts on the community of organisms that integrate these ecosystems. As lipophilic compounds, PAHs become bioavailable to organisms and can enter the trophic chain, leading to physiological changes and affecting different levels of biological organization. Several studies demonstrate that PAHs act as endocrine disruptors in marine fish, interfering with endocrine signaling through hormonal disturbances and, consequently, causing inhibition or overexpression of genes, enzymes, and proteins that are essential for reproduction success. These changes, in turn, can lead to population decline and cause immeasurable ecosystem damage. This review synthesizes studies published mainly between 2015 and 2025, aiming to critically present research that identifies different endocrine-reproductive changes in marine fish species exposed to PAHs in contaminated sites, highlighting the involved cellular mechanisms. Finally, we provide a survey of patents developed to identify PAHs in aquatic environments and how these techniques can be used in marine biomonitoring to evaluate water quality and the risk of exposure to biota and human populations.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a diverse group of organic contaminants originating from both natural and anthropogenic sources, including oil extraction, industrial activities, vehicular emissions, and fossil fuel burning [1]. They are widely distributed in marine environments and recognized as indicators of environmental contamination, particularly in areas impacted by oil spills and urban runoff [2].
Due to their hydrophobic nature, PAHs partition to suspended particles and accumulate in sediments, where their persistence depends on compound properties and environmental conditions [3]. In marine ecosystems, high salinity, low temperatures, and anoxic zones contribute to limited microbial activity and enhanced sorption, resulting in half-lives of years to decades [4]. In contrast, freshwater ecosystems, with lower ionic strength, more oxygenated sediments, and a more diverse and active microbial community, tend to promote faster PAH degradation [5].
Temporary accumulation can occur in lipid-rich tissues of vertebrates or in species with limited detoxification capacity, such as invertebrates [6,7]. In fish, exposure to PAHs has been associated with genotoxicity, neoplastic lesions, and disturbances in energy metabolism [8,9]. Notably, many PAHs exhibit endocrine-disrupting properties, interfering with hormonal regulation and reproductive processes [10]. Early developmental stages are especially vulnerable, given their high growth rates and underdeveloped detoxification systems [11,12].
This review critically examines the knowledge on the reproductive toxicity of PAHs in marine fish species through a comprehensive search of peer-reviewed literature available in databases including Web of Science, Scopus, and PubMed. Articles published between 2015 and 2025 were prioritized in order to highlight recent advances, although earlier seminal studies were also considered when relevant to contextualize mechanisms. Studies were included if they explored the effects of PAHs on reproductive endpoints in fish, with particular emphasis on marine species. Freshwater fish studies were included selectively to provide mechanistic insights when data for marine organisms were scarce.
2. PAHs in Aquatic Environments
PAHs are structurally characterized by multiple aromatic rings and are known for their toxicity, mutagenicity, genotoxicity, and carcinogenicity [13,14]. Over 100 PAHs have been identified, occurring individually or as mixtures [15], classified as high molecular weight (HMW, ≥4 rings) or low molecular weight (LMW, ≤3 rings) [16]. Their sources include natural ecological processes (wildfires, volcanic activity) or anthropogenic activities (oil extraction, industrial activities, vehicular emissions, fossil fuel burning) [17], and they can be found as components of crude oil and its derivatives (asphalt, gasoline, diesel). The widespread use of these compounds has resulted in their pervasive presence in the environment, particularly within aquatic ecosystems [18].
These compounds exhibit low to extremely low water solubility and vapor pressures ranging from low to moderately high [19,20]. These characteristics, combined with their widespread emissions, make them prevalent in water and sediments [21]. In marine environments, PAHs undergo partial volatilization and dissolution in seawater, while a significant fraction rapidly adsorbs onto suspended particles and sediments or becomes associated with petroleum-derived fractions [22]. Atmospheric deposition also contributes to PAH inputs in the oceans [23], and ocean currents facilitate their dispersion [24,25].
PAHs have been detected in different marine ecosystem stratification, from surface waters to sediments [2]. Concentrations vary according to anthropogenic activity: for example, 1 to 251 ng/g dry weight (dw) in Moroccan Mediterranean sediments [26], 3.15 to 14.35 ng/g (dw) in Qatari coastal sediments [27], and 236.27 to 644.87 ng/g (dw) in the Brazilian Suape Port sediments, with highest levels near shipyards [28]. Such findings demonstrate their widespread distribution and persistent, reinforcing the need for continuous monitoring and remediation [14].
3. Uptake and Transient Accumulation of PAHs in Marine Organisms
The uptake of PAHs in marine organisms refers to the process by which these hydrophobic organic compounds enter biological tissues through water, sediments, or diet [29]. PAH absorption mechanisms in fish include direct diffusion through gills and skin, as well as ingestion of contaminated prey or particles [30,31,32].
Once absorbed, PAHs are distributed via the bloodstream, demonstrating high affinity for lipid-rich tissues, such as the liver, where they undergo biotransformation [33]. Nevertheless, transient accumulation can occur in some tissues, where higher concentrations may be detected shortly after exposure [30]. In this context, bile is another sensitive pathway, especially for evaluating recent PAH exposure, due to its role in their metabolite excretion [34,35,36].
Due to their direct contact with water, gills also accumulate PAHs, as shown in several marine fish species [32,37]. In contrast, muscle tissue typically accumulates lower concentrations of PAHs compared to lipid-rich tissues. However, its relevance increases due to its role in human consumption [38]. Other tissues, such as the brain, kidneys, and skin, may also accumulate PAHs, though studies on these tissues focus primarily on structural, physiological, or biochemical effects [35,39,40,41].
The composition of PAH mixtures in ecosystems also plays a significant role: high molecular weight (HMW) compounds tend to accumulate more in organisms, likely due to a correlation between their trophic magnification factors (TMF) and log Kow values, as well as the generally lower metabolic capacity of organisms to biotransform larger PAHs [42,43].
The bioavailability of PAHs is influenced by their total concentrations in sediments and by the fraction that is chemically active, that is, the freely dissolved and desorbable portion of PAHs that can equilibrate between sediment particles, porewater, and organisms [44]. Furthermore, physiological traits of species, such as metabolic rates, lipid storage capacity, and diet composition, significantly modulate accumulation [45,46].
In marine trophic chains, PAH concentrations can vary significantly due to interactions between prey and predators. These variations are influenced by opposing processes: biomagnification, where contaminant concentrations increase with each trophic level, and biodilution, in which concentrations decrease due to dilution effects [42]. Some studies suggest that biomagnification is dominant, particularly in predator species with high lipid content [45,47]. However, recent research highlights biodilution as a relevant mechanism in marine trophic networks, since LMW PAHs exhibit lower log Kow values and TMFs < 1, reducing their accumulation in higher trophic levels [43,48].
In longer and more lipid-rich food webs, biomagnification tends to prevail, whereas in systems dominated by species with high metabolic capacity or shorter trophic chains, biodilution is more likely [42]. This indicates that the trophic transfer dynamics of PAHs are highly complex, being influenced by both ecological characteristics and the physicochemical properties of these compounds.
4. Impacts of PAHs on Marine Fish: Bioindicator Responses and Biological Effects
Pollution by PAHs in marine ecosystems poses a significant threat to biota, especially fish, which play crucial roles in food chains [49]. The use of fish as bioindicator organisms is widely known, due to their sensitivity to environmental changes and prolonged exposure to toxic compounds [50,51,52,53,54]. Several biomarkers have been widely used to monitor the impacts of PAHs in fish, providing early information on exposure levels and toxicity [55,56,57]. Table 1 presents a summary of different effects caused by exposure to PAHs in marine fish species, providing an overview of the consequences on different species under PAH exposure, highlighting the relevance of fish as sentinel organisms in marine environments.

Table 1.
Effects of PAHs on different species of fish.
At early developmental stages, PAHs can disrupt embryogenesis and larval survival in marine fish. Cardiac malformations (pericardial edema, arrhythmias, and dysfunction) are common. Craniofacial anomalies, fin deformities, delayed hatching, and impaired swim bladder inflation can compromise juvenile recruitment and energy efficiency [29,41,69,71,72,73,74].
In adult marine fish, PAHs also impair cellular homeostasis and organ integrity. Phenanthrene disrupts intracellular calcium cycling, impairing cardiac contractility and muscle development [69,73], while alkylated PAHs such as acenaphthene suppress osteoblastic activity, leading to skeletal deformities and structural fragility [29]. HMW PAHs (e.g., BaP) bind to the aryl hydrocarbon receptor (AhR), activating xenobiotic metabolism pathways and generating reactive intermediates that induce oxidative stress and DNA damage, often culminating in carcinogenesis [29,73]. Additional effects include metabolic disruption of lipid and glucose pathways [29], ocular degeneration [75], neurotoxicity, oxidative damage [76,77,78], and immune suppression through phagocytosis, lymphocyte proliferation [79], and cytokine imbalance [80,81].
These physiological and molecular disruptions can compromise both individual and population viability. Cardiac and swim bladder impairments reduce swimming efficiency, limiting predator evasion, foraging, and migration [69]. Ocular degeneration and metabolic disruptions could explain lethargic behaviors and anxiety-like responses [70,82]. Furthermore, delayed juvenile growth reduces competitive advantage and survival, and delays sexual maturation, ultimately affecting population replenishment [71,72]. Such disruptions can also cause intergenerational effects, as epigenetic changes may perpetuate population decline. Consequently, the decrease in forage fish leads to trophic alterations, destabilizing food webs [41] and resulting in population collapses [69].
5. Impacts of PAHs on Marine Fish Reproduction
Reproduction is one of the biological processes most sensitive to interference from environmental contaminants, especially endocrine-disrupting compounds such as PAHs. In marine fish, reproduction depends on a complex network of hormonal, molecular, and physiological interactions that coordinate everything from sexual differentiation and gamete production to behaviors associated with spawning and parental care [83]. Any dysfunction in this system can compromise not only individual reproductive performance but also population viability in the medium and long term [84].
Several studies have shown that PAHs are capable of interfering at multiple levels of fish reproductive physiology, from hormonal signaling in the central nervous system to histological changes in the gonads [85]. These interferences can occur through different mechanisms, including hormonal mimicry, inhibition of steroidogenic enzymes, modulation of nuclear receptors, oxidative stress, and epigenetic changes. Furthermore, reproductive responses to PAH exposure often do not follow linear dose-response patterns and may vary according to species, sex, developmental stage, and duration of exposure [41].
This section critically addresses the main reproductive effects of PAHs in marine fish, based on recent and relevant evidence from the scientific literature. Changes in key genes, proteins, processes, and structures involved in marine fish reproduction are discussed. This integrated approach seeks to highlight not only the isolated effects of PAHs but also their systemic and ecological implications on marine reproduction in the face of PAH contamination.
To synthesize the current scientific evidence on PAH-induced reproductive effects in marine fish, Table 2 presents a structured summary of experimental and field studies published in the last ten years. This compilation includes details of the literature references and provides an integrative overview of how various PAH compounds affect marine fish reproduction across different biological and ecological contexts.

Table 2.
Summary of studies on the reproductive effects of PAHs in marine fish.
5.1. Metabolism of PAHs, Induction of Cytochrome P450 Enzymes, and Reproduction
The biotransformation of PAHs in marine organisms is a process largely mediated by enzymes of the cytochrome P450 family, notably CYP1A, whose expression is regulated by the activation of the aryl hydrocarbon receptor (AhR) after the pollutant enters the cell [104,113]. This mechanism has been consistently identified as one of the main detoxification pathways, but it is not entirely risk-free, since the metabolites formed are often more toxic and reactive than the original compounds [29,114]. In addition, the metabolic activation of PAHs through CYP1A can generate reactive oxygen species (ROS), which contribute to oxidative stress and further exacerbate cellular and reproductive damage [29].
Aromatase, an enzyme encoded by the Cyp19 gene and responsible for the conversion of androgens into estrogens, plays a central role in the regulation of reproduction in fish. In Liza klunzingeri, cellular exposure to benzo[a]pyrene (BaP) was shown to significantly inhibit aromatase activity in both ovarian and brain cells, consequently reducing 17β-estradiol (E2) levels. This inhibition, observed in a dose-dependent manner, highlights the antiestrogenic potential of BaP, an endocrine disruptor that interferes with steroid homeostasis through mechanisms mediated by AhR [92]. Such mechanisms include direct suppression of Cyp19a1 gene transcription, in addition to conversion of estrogens into less active forms, impairing estrogen signaling. Reduced aromatase activity compromises estrogen biosynthesis and may negatively affect the hypothalamic-pituitary-gonadal (HPG) axis, impacting fertility and reproductive function of fish exposed to PAHs such as BaP [115]. These results highlight aromatase as a sensitive target of PAH contamination and a promising biomarker of environmental endocrine dysfunction, as shown in Figure 1.
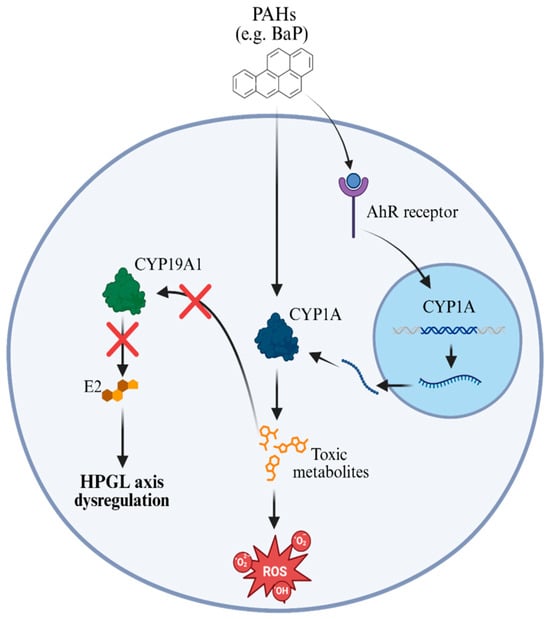
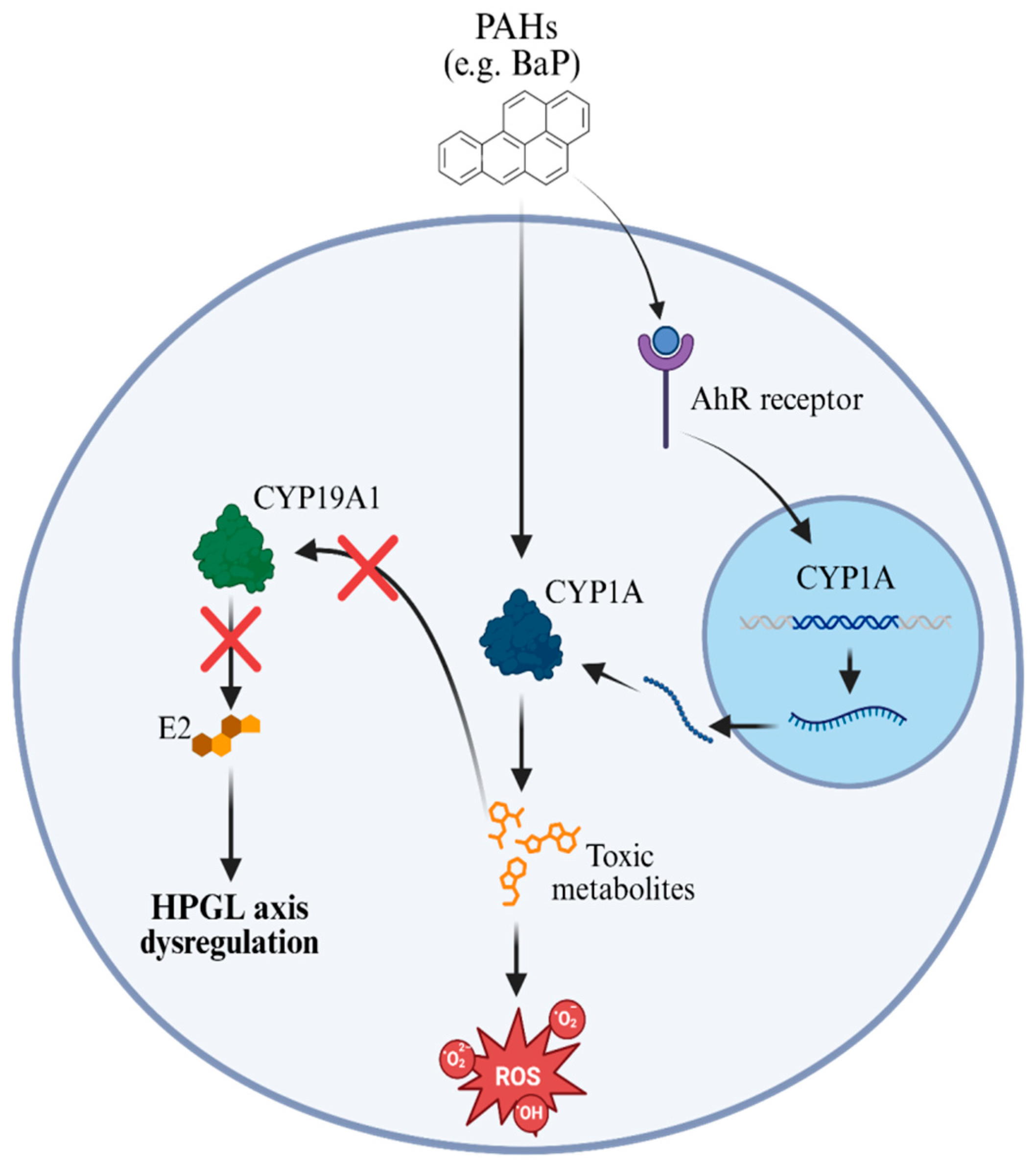
Figure 1.
AhR-CYP1A pathway induced by PAHs and interference in the HPGL axis. After crossing the cell membrane, PAHs bind to the aromatic hydrocarbon receptor (AhR), activating the transcription of the Cyp1a gene in the nucleus. The CYP1A enzyme metabolizes PAHs into reactive and toxic compounds, which promote the generation of reactive oxygen species (ROS), leading to oxidative stress and cellular damage. In parallel, these metabolites negatively interfere with the expression and function of aromatase (CYP19A1), the enzyme responsible for the conversion of androgens into 17β-estradiol (E2). The reduction in E2 synthesis compromises hormonal signaling and results in dysregulation of the hypothalamic-pituitary-gonad-lobe (HPGL) axis, negatively affecting the reproductive function of exposed fish.
Studies using precision-cut liver slices (PCLS) of cod species have further expanded the understanding of PAH antiestrogenic effects and its interaction with estrogen signaling. In Gadus morhua, exposure to BaP, EE2, and their equimolar mixtures for 48 h revealed distinct transcriptional profiles: BaP strongly induced AhR-related genes such as CYP1a and AHRr, whereas EE2 upregulated classic estrogen-responsive genes such as VTG and ZP. However, co-exposure resulted in attenuation of the estrogenic response, indicating functional antagonism by BaP [97]. A complementary study in Boreogadus saida simulated early vitellogenic conditions using physiological concentrations of EE2 (5 nM) and prolonged exposure (72 h) and demonstrated dose-dependent suppression of ESR1 and VTG1 expression by BaP. Transcriptomic analyses suggested that these antiestrogenic effects may involve multiple molecular mechanisms, including estrogen receptor degradation, interference with promoter binding, competition for coactivators, and depletion of endogenous estrogens [97]. Together, these findings confirm that AhR activation by BaP not only drives xenobiotic metabolism through CYP1 induction but also disrupts estrogen signaling pathways, posing a risk to reproductive fitness in fish chronically exposed to PAH mixtures.
Chronic in vivo exposure studies have also demonstrated the biological relevance of CYP1A induction in environmentally realistic scenarios [102,116,117,118,119]. Juvenile southern flounder (Paralichthys lethostigma) exposed for 32 days under controlled laboratory conditions to five concentrations of weathered Macondo MC252 oil mixed with field-collected sediments exhibited a dose-dependent upregulation of CYP1A expression in liver tissue. In these exposure sediments contained between 0.04 and 395 mg/kg of total PAHs (tPAH50), and fish exposed to concentrations above 8 mg/kg showed significantly reduced growth and increased mortality. These findings emphasize that PAH-induced molecular responses, such as CYP1A induction, are not only sensitive biomarkers of exposure but also correlate with significant physiological impairments in fish health [90].
The induction of the Cyp1a gene is also commonly monitored through the activity of the enzyme EROD (ethoxyresorufin-O-deethylase), which is widely considered a reliable biomarker of PAH exposure in fish [105,120]. In Sebastes schlegelii (black rockfish), exposure to benzo[a]pyrene (BaP) at concentrations of 2, 20, and 200 μg/g body weight resulted in a dose-dependent increase in both hepatic EROD activity and microsomal CYP1A protein levels. Notably, at 200 μg/g, BaP induced a four-fold increase in EROD activity and a six-fold increase in CYP1A protein expression [96]. In studies with polar cod (Boreogadus saida), prolonged exposure to crude oil also demonstrated a gradual increase in EROD activity, evidencing a cumulative effect of environmental exposure [101].
However, it is important to highlight that, although the increase in CYP1A is useful as a marker of exposure, the correlation with adverse physiological effects is not always linear [12,103,121]. As evidenced by [99,122], AhR activation can occur with different potencies depending on the composition of the PAH mixture, the species, and the sex, generating variable responses even under similar exposure conditions.
Furthermore, interference by endogenous estrogens on CYP1A activity may significantly affect detoxification and steroid regulation mechanisms, especially during critical reproductive periods, as observed by [123]. CYP1A suppression by estrogens during sexual maturation, for example, may represent an adaptive response to protect hormone levels necessary for reproduction but may also increase vulnerability to toxicity from unmetabolized PAHs [106].
The discrepancy between enzyme activation and effective toxicity suggests that biomarkers such as CYP1A and EROD, while informative, should not be interpreted in isolation. A comprehensive assessment requires their integration with physiological, reproductive, and genomic analyses to accurately elucidate the real impact of chronic exposure to PAHs on the reproductive biology of marine fish [124,125].
5.2. Disruption of the Hypothalamic-Pituitary-Gonad-Lobe (HPGL) Axis
The functional integrity of the hypothalamic-pituitary-gonad-lobe (HPGL) axis is essential for the regulation of reproduction in marine fish, coordinating hormonal synthesis from the central nervous system to the final maturation of gametes [126]. Exposure to PAHs has been shown to be a significant disruption factor in this axis, resulting in hormonal, epigenetic, and histological changes that seriously compromise the reproductive success of exposed species [127].
Studies with marine medaka (Oryzias melastigma) chronically exposed to phenanthrene (PHE) and PHE-adsorbed microplastics have demonstrated bioaccumulation of the contaminant in the brain and ovaries, accompanied by reduced expression of key genes in the HPGL axis, such as GnRH, LHβ, FSHβ, and CYP19a [91]. These changes are directly associated with decreased synthesis of gonadotropins (LH and FSH), compromising steroidogenesis and, consequently, the production of sex hormones such as estradiol (E2) and testosterone (T). This condition translates clinically into follicular atresia, inhibited ovarian maturation, and lower egg production, with potentially transgenerational effects [115].
In addition to the direct modulation of HPGL axis genes, there is evidence that PAHs interfere with hormonal feedback mechanisms that regulate hypothalamic and pituitary function. Ref. [89] demonstrated that cod exposed to mixtures of PAHs showed reduced GnRH2 expression and a concomitant increase in dopaminergic receptors (DRD2a) in the brain, indicating that the dopaminergic pathway is particularly sensitive to exposure to PAHs. Since dopamine negatively regulates GnRH secretion, these neural changes can cause significant reductions in pituitary stimulation, resulting in downstream gonadal dysfunction [115].
An additional and critical layer of disruption is mediated through epigenetic mechanisms. Studies have shown that embryonic exposure to water-soluble fractions of petroleum (WAF) increases methylation of the VTG gene, associated with decreased expression of GnRH, LHβ, FSHβ, and CYP19b, with deleterious consequences for ovarian maturation and hormonal balance [22]. This form of epigenetic modification is particularly concerning, as it may lead to latent or heritable effects, affecting populations across generations even after the end of direct exposure.
Ref. [87] observed that PHE exposure caused a U-shaped dose-response curve in the mRNA levels of GnRH, FSH, and LH in male fish, a phenomenon known as hormesis. The interpretation of this pattern is complex, but it points to non-linear neuroendocrine dysfunctions, challenging the traditional idea of dose-dependence and making it difficult to predict the population effects of PAH contamination.
The impact on HPGL is even more evident in species with sexual plasticity, such as Epinephelus marginatus, a protogynous hermaphrodite. Ref. [86] observed that exposure to PHE caused reductions in the levels of 11-ketotestosterone (11-KT) in this fish, essential for sexual inversion, in addition to dysfunctions in steroidogenesis, directly affecting reproductive development and population dynamics.
Therefore, the available evidence suggests that PAHs interfere at multiple levels of the HPGL axis, ranging from the expression of GnRH in the hypothalamus to gametogenesis. This interference does not always manifest itself in a time-, dose-, and compound mixture-dependent manner, in addition to being modulated by neuroendocrine and epigenetic interactions. These findings reveal that HPGL is not only one of the main targets of PAH toxicity but also a possible link between individual alterations and population collapses in marine fish exposed to these pollutants [128]. These multilevel interferences along the HPGL axis are illustrated in Figure 2, which summarizes the main neuroendocrine and epigenetic pathways through which PAHs disrupt fish reproduction.
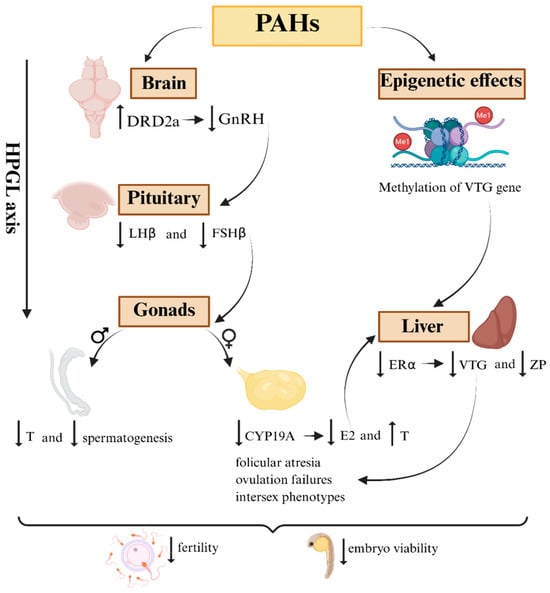
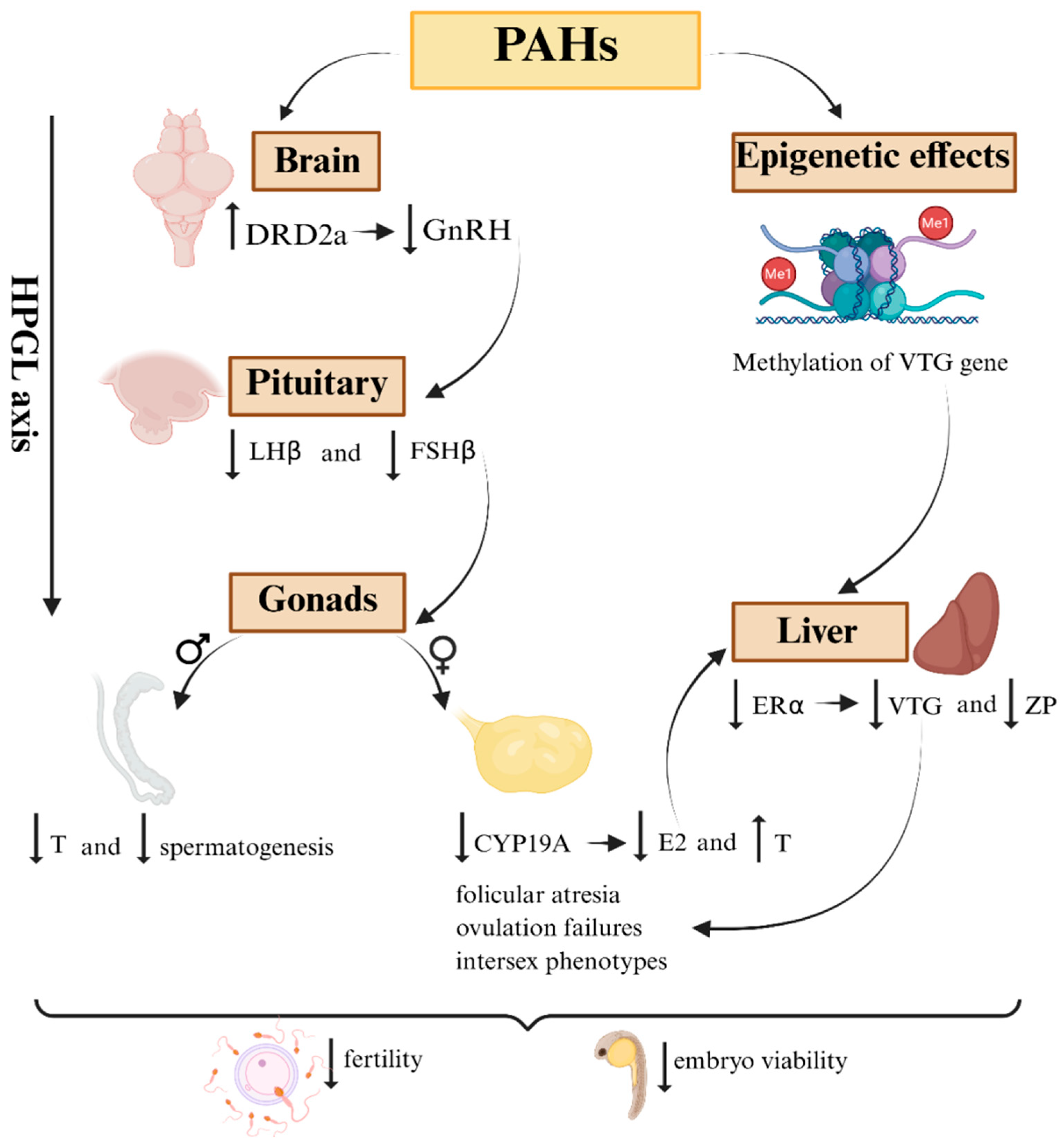
Figure 2.
Schematic representation of how PAHs disrupt the HPGL axis in marine fish. In the brain, an increase in the expression of dopaminergic receptors (DRD2) inhibits the release of gonadotropin-releasing hormone (GnRH). This dysregulation reduces the hypothalamic stimulus to the pituitary gland, resulting in a decrease in the expression and release of the gonadotropins LHβ and FSHβ. Subsequently, the effects extend to the gonads, where aromatase (CYP19a) is suppressed. As a consequence, there is an accumulation of testosterone (T) and a reduction in estradiol levels (E2). This leads to a reduction in the expression of estrogen receptors (ERα), vitellogenin (VTG), and zona pellucida proteins (ZP2 and ZP3) in the liver. Epigenetics represents an additional axis of dysregulation, with increased methylation in key genes such as VTG, resulting in persistent or transgenerational changes even after the end of exposure.
5.3. Changes in Reproductive Hormones
Exposure to PAHs has been widely associated with changes in sex hormone levels in marine fish, affecting both the synthesis and regulation of reproductive steroids such as 17β-estradiol (E2), testosterone (T), 11-ketotestosterone (11-KT), LH, FSH, and maturation-inducing hormones. These hormonal changes underlie several reproductive dysfunctions observed in fish exposed to PAHs in contaminated environments, indicating a profound interference in the reproductive neuroendocrine axis [129].
Several studies have shown that PAHs can significantly reduce circulating levels of estradiol and testosterone, with direct implications for gonadal development and fertility [10]. Disruption in the biosynthesis of these sex hormones compromises the natural reproductive cycle and may interfere with the population dynamics of several species [130]. For example, studies with marine fish exposed to PAHs indicated consistent reductions in plasma E2 levels and parallel increases in T levels, altering the balance between estrogens and androgens and resulting in follicular atresia, reduced egg production, and decreased oocyte quality [91]. Elevated testosterone levels, in addition to indicating a failure in aromatization (conversion of T to E2), may also exert inhibitory effects on ovarian maturation through negative feedback mechanisms, aggravating hormonal dysfunction [131].
Another critical aspect observed refers to dysfunction in the expression and regulation of gonadotropic hormones, such as FSH and LH. Ref. [87] reported that exposure to PAHs led to downregulation of the LHβ and FSHβ genes, with effects observed on both transcription and hormone release, which compromises ovarian follicle maturation and gamete production. These changes can occur even at environmentally relevant levels of PAHs, suggesting high sensitivity of the pituitary axis to these compounds [132].
Interestingly, Ref. [22] observed that embryonic exposure to WAF significantly altered the expression levels of GnRH, LHβ, and FSHβ in adulthood, indicating an early endocrine programming effect with late consequences on hormonal regulation. This finding reinforces the hypothesis that PAHs can generate latent reproductive effects, including transgenerational ones, through interference in the HPGL axis during embryonic development [133].
Although the most frequently reported effects involve the suppression of female hormones such as E2, there are also records of paradoxical increases in this hormone in some species and conditions. In juvenile female cod exposed to low doses of PAHs, an increase in plasma E2 levels was observed [89]. This nonlinear response is attributed to the complex interaction between PAH metabolization pathways, AhR activation, and hormone receptors, in addition to the modulation of dopaminergic pathways that participate in the control of GnRH and, consequently, the release of gonadotropins.
This diversity of responses demonstrates that the effects of PAHs on reproductive hormones do not conform to a straightforward dose-response relationship. Instead, they are often modulated by factors such as species, sex, developmental stage, route of exposure, mixture of compounds, and duration of contact. Furthermore, interference in the expression of steroidogenic enzymes, such as CYP17 and CYP19, further exacerbates hormonal imbalances, indicating that the metabolism of sex steroids is one of the main targets of PAH toxicity [130].
Finally, it is important to highlight that hormonal effects do not occur in isolation but are integrated into a cascade of physiological and behavioral events that culminate in reproductive dysfunction. The reduction of sex hormones compromises the development of gonads, gamete production, embryo viability, and even behaviors associated with copulation and spawning, rendering PAHs particularly detrimental to the reproductive success of marine fish in ecosystems impacted by oil pollution [100].
5.4. Estrogenic and Anti-Estrogenic Effects
The estrogenic and antiestrogenic effects of PAHs represent one of the most complex aspects of endocrine disruption induced by these compounds in marine fish. Several PAHs and their hydroxylated metabolites have chemical structures similar to that of 17β-estradiol (E2), enabling them to bind to estrogen receptors (ERs). Depending on the biological context, target tissue, and dose, these compounds may act as either agonists or antagonists, thereby modulating estrogenic signaling in diverse and sometimes unpredictable ways [29].
This dual nature of PAHs poses a challenge for predicting their reproductive effects and for environmental toxicological modeling. Recent literature highlights that many PAHs, especially their hydroxylated derivatives such as 9-OH-benzo[a]pyrene and 1-hydroxynaphthalene, activate ERs in vitro, promoting gene transcription and expression of proteins typical of estrogenic action, such as vitellogenin [134]. On the other hand, other PAHs act as ER antagonists, blocking the action of estradiol and reducing the expression of estrogen-sensitive genes, as observed in the decrease in VTG and ERα in the liver and ovaries of Solea solea females exposed to benzo[a]pyrene [88].
The duality in the effects of PAHs is also reflected in the different types of estrogen receptors expressed in the target tissues. Even under conditions of reduced E2, there was a compensatory increase in the expression of ERα and ERβ in the liver and ovaries of marine medaka exposed to WAF. This overexpression can be interpreted as a cellular attempt to restore estrogen signaling in the face of hormonal deficiency, but it can also aggravate tissue sensitivity to xenoestrogenic compounds, amplifying toxicity in scenarios of prolonged exposure [22].
The inconsistency between plasma E2 levels and tissue expression of ERs and target genes also points to the existence of regulatory pathways independent of endogenous estrogen. Ref. [89] demonstrated that, although exposure to low doses of PAHs increased plasma E2 levels in female cod, there was a significant reduction in the brain expression of ERα and aromatase CYP19a1b, suggesting that dopaminergic or epigenetic mechanisms could be interfering with brain estrogen sensitivity. This finding reinforces the idea that the estrogenic activity of a compound cannot be inferred solely based on circulating hormone levels [89].
Consistently, inhibition of aromatase (CYP19a) has been reported in several studies, reducing the conversion of T into E2 and promoting hormonal imbalances that impair ovulogenesis, alter reproductive behavior, and decrease fecundity [22,135]. In addition, the action of PAHs on ERs may be affected by the coactivation of alternative intracellular pathways, such as the aryl hydrocarbon receptor (AhR) [122]. Simultaneous activation of AhRs and ERs can lead to cross-talk responses, such as competition for transcription cofactors and mutual repression of gene expression, which contributes to the variability of the estrogenic effects of PAHs [122].
PAHs exert highly contextual estrogenic and antiestrogenic effects, mediated by interactions with ERs, aromatase inhibition, AhR activation, epigenetic alterations, and modulation of dopaminergic pathways. These multiple routes of action make the effects difficult to predict and indicate that PAHs can profoundly dysregulate estrogenic signaling in marine fish, with severe consequences for reproduction, especially in species with hormonal plasticity or estrogen-dependent development [136].
Production and Expression of Vitellogenin and Vitellin
Vitellogenin (VTG), a phosphoprotein precursor of vitellin, is one of the most sensitive and widely used biomarkers for monitoring endocrine disruption in fish, especially in the context of exposure to estrogenic and antiestrogenic contaminants such as PAHs [98,137]. VTG synthesis is induced by estrogens, particularly 17β-estradiol (E2), and occurs predominantly in the liver of maturing females. Following its secretion into the bloodstream, VTG is incorporated into developing oocytes, where it is proteolytically cleaved into various yolk proteins (e.g., vitellin, lipovitellin, phosvitin) that serve as key nutrient sources during embryogenesis. Any dysfunction in this process severely compromises reproduction [138,139].
Exposure to PAHs has been shown to exert bidirectional effects on VTG expression: aberrant induction of VTG in males and juveniles, evidence of exogenous estrogenic action, and/or suppression of VTG expression in females, reflecting antiestrogenic effects that impair ovarian function [130].
The study by [95] demonstrated a marked and recurrent induction of vtg gene expression in male flatfish from different regions of the Gulf of Mexico, strongly associated with the presence of PAHs in tissues and biliary metabolites. VTG expression was detected in up to 47.69% of males in some oceanographic campaigns, with levels comparable to or exceeding those observed in females, thereby indicating substantial hormonal dysregulation. PAHs were consistently correlated with this VTG expression, particularly the metabolites hydroxynaphthalene and hydroxyphenanthrene, which may substantially compromise the reproductive function of affected individuals. The persistent induction of VTG in males represents a warning sign for diffuse contamination by PAHs in the marine environment and its possible ecological repercussions [95].
In polar cod exposed to water-soluble fractions of crude oil, a significant decrease in the expression of hepatic genes associated with vitellogenesis, such as VTG α, ESR1, ZP2, and ZP3, all positively regulated by estrogens and typically expressed at high levels during the vitellogenesis phase of the reproductive cycle, was observed. The reduction of these genes suggests an antiestrogenic effect of oil exposure, potentially responsible for earlier spawning in exposed females, also observed in the study. Despite a later increase in VTG α expression after 131 days of exposure, this delayed response may reflect changes in the PAH composition of the oil mixture over time, which influence estrogen receptors differently depending on the concentration and type of bioavailable PAHs [100].
In addition to gene regulation, changes in hormonal signaling also explain the reduction in VTG. Fish exposed to PAHs showed a significant decrease in ER-α levels in the liver and ovaries, which probably contributed to the decrease in hepatic VTG production. Considering that estrogen receptor activation is a necessary step for VTG induction, the downregulation of ERs represents an important antiestrogenic mechanism [88].
Another important consideration is the distinction between VTG expression and its functional activity. Even when VTG is induced, as observed following exposure to PAH metabolites with estrogenic properties, this induction does not necessarily ensure that the resulting protein is functionally effective. Oocyte quality may be compromised, and elevated VTG levels in males or juveniles are often associated with the occurrence of intersex testes or infertility [140]. Therefore, the presence of VTG outside the expected physiological context is a marker of endocrine dysfunction. From an ecological perspective, the sustained reduction of VTG expression in females may result in reproductive failure at the population level, posing a significant threat to the long-term viability of affected fish communities. Persistently low levels of VTG are associated with reduced oocyte number and quality, lower hatching rate, and decline in the recruitment of new generations. This makes VTG not only a biomarker of exposure but also a functional indicator of reproductive success in impacted environments [141].
Also, it is important to consider the indirect effects of PAHs on VTG production, such as those mediated by alterations in CYP19a (aromatase), responsible for the conversion of testosterone (T) to estradiol (E2). The suppression of CYP19a, observed in several studies [87,91], reduces the levels of E2 available to stimulate VTG production, creating a cascade effect that compromises the entire vitellogenesis process.
5.5. Androgenic and Anti-Androgenic Effects
The action of PAHs on the androgenic pathway in marine fish has been less explored compared to their estrogenic effects, but recent studies reveal that these contaminants also exert important androgenic and anti-androgenic effects, with critical implications for sexual differentiation, gonadal maturation, and reproductive success, once PAHs can interfere with the synthesis, metabolism, signaling, and gene expression of androgens such as testosterone (T) and 11-ketotestosterone (11-KT) [142].
One of the main routes of toxicity involves the modulation of aromatase (CYP19), because of its role in converting T to estradiol (E2). When the expression of CYP19a is suppressed, androgens accumulate, with consequent hormonal imbalance. It indicates that the androgenic toxicity of PAHs can manifest itself both by androgen deficiency (antiandrogenic effect) and by secondary accumulation of T due to failure in conversion to E2 (indirect androgenic effect) [143].
At the molecular level, the expression of androgen receptors (arα and arβ) in fish exposed to PAHs may increase. This overexpression may be a compensatory attempt to the reduction of bioavailable T or an adaptive response to high levels of circulating T [142]. However, excessive activation of androgen receptors in female tissues can lead to partial masculinization, in addition to morphological and functional alterations in the gonads [22].
Previous studies also demonstrated that exposure to BaP reduced the expression of CYP19a and ERα, while increasing the expression of androgen receptors in the testes and liver of medaka, promoting a favored androgenic profile [94]. These changes were accompanied by changes in sex ratio, with a greater number of males in the offspring, suggesting that BaP may act as a masculinizing agent, including at embryonic stages. This may be particularly harmful for species with sensitive environmental or physiological sexing, as sex imbalances compromise population recruitment [144].
In addition, there is evidence that PAHs modulate androgen signaling indirectly, through activation of the AhR, which may interfere with steroidogenic pathways. This interaction was suggested by [89], who observed changes in the expression of dopaminergic and estrogenic genes after exposure to PAHs, but also changes in the expression of CYP19a1b, an enzyme that integrates the central control of estrogen production in the brain and influences the HPG axis. Thus, central aromatase dysregulation may also have an indirect impact on androgen homeostasis.
It is also important to note that the androgenic and antiandrogenic effects of PAHs, as well as estrogens, often follow non-monotonic curves and non-linear dose-dependent responses, which makes it difficult to predict population effects from laboratory studies with single or limited doses [145].
5.6. Thyroid Hormone Disruption and Its Effects on Reproduction
Although the effects of PAHs on the reproductive axis are widely recognized, recent studies have shown that these compounds also significantly interfere with the homeostasis of thyroid hormones (THs), directly and indirectly impacting the reproduction of marine fish [146]. Thyroid hormones, especially thyroxine (T4) and triiodothyronine (T3), are essential for the development, metabolism, and regulation of several physiological processes, including reproduction, metamorphosis, and sexual differentiation [112].
The synthesis and activation of THs occur through the hypothalamic-pituitary-thyroid (HPT) axis, with TSH (thyroid-stimulating hormone) being the central regulator of T4 production in the thyroid gland [147]. The peripheral conversion of T4 to T3, which is more biologically active, depends on the activity of hepatic and tissue deiodinases [148]. Dysfunction at any stage of this process can compromise gonadal development, reproductive cycling, and embryonic viability [149].
Studies conducted by [150,151] highlight that PAHs can interfere with thyroid function at multiple levels, including hormone synthesis, peripheral conversion of T4 to T3, and signaling via nuclear TH receptors. These interferences result in significant side effects for reproduction, especially during critical windows of larval and juvenile development. Exposure of some species to water-soluble fractions of petroleum containing PAHs resulted in an increase in T4, without a corresponding elevation in T3, suggesting a block in hormone bioconversion. This dysregulation of T3/T4 ratios can negatively affect metamorphosis, gonadal differentiation, and reproductive behavior [150].
Epigenetic modulation of thyroid function has also been documented as a mechanism of disruption. Exposure to BaP and other mixtures containing PAHs has been shown to cause thyroid hyperplasia and alterations in iodine metabolism, interfering with both TH availability and intracellular signaling [84]. These changes, in addition to directly impacting basal metabolism and somatic growth, may compromise cross-signaling between the HPT and HPGL axes, both of which are interdependent for gonadal maturation and sex hormone production [152].
Some PAH exposure increases free T3 levels while reducing circulating sex steroids in marine fish. This isolated elevation of T3 may represent a compensatory attempt by the organism to maintain energy or maturational metabolism under chemical stress, but it may also aggravate the dysregulation of other hormonal axes, since T3 modulates the expression of steroidogenic and gonadotropic genes [151].
Dysregulation of the thyroid cascade can compromise the timing of reproduction, gamete quality, and larval survival, affecting long-term population persistence, as evidenced by [153], who observed that chronic exposure to PAHs combined with warmer winter temperatures disrupted the reproductive phenology of Platichthys flesus, delaying gonadal maturation and potentially extending the spawning period, with consequences for recruitment timing in estuarine environments.
PAHs disrupt the thyroid physiology of marine fish through various mechanisms: alteration in hormone synthesis and conversion, interference in iodine uptake, inhibition of deiodinases, activation of nuclear receptors, and epigenetic modulation of gene expression [111]. These changes directly impact reproduction through effects on the HPT axis and its connections with other neuroendocrine axes, especially HPGL. Considering the importance of THs for the life cycle of marine fish, thyroid dysfunction caused by PAHs should be seen as a first-order reproductive risk, with the potential to compromise both individual reproduction and the ecological resilience of exposed populations [152].
5.7. Histological Changes in Gonads
Histological changes in the gonads of marine fish exposed to PAHs represent an unequivocal marker of the reproductive toxicity of these compounds. While the molecular and hormonal effects may be transient or compensated by regulatory mechanisms, histological damage is often irreversible and reflects long-term structural and functional dysfunctions, with a direct impact on fertility and population reproduction [154].
Recent studies demonstrate that exposure to PAHs is associated with follicular atresia, delayed gonadal development, changes in spermatogenesis, testicular necrosis, and disorganization of the germinal epithelium [115,155]. These changes have been consistently observed both in controlled experimental conditions and in marine environments contaminated by oil and its derivatives [107].
It is worth noting that histological damage is not homogeneous between the sexes. While females tend to present follicular atresia and ovarian immaturity, males often exhibit germinal necrosis, disorganization of the seminiferous tubules, and reduced sperm production [152]. Such alterations reflect differences in reproductive physiology and sensitivity to disruptive hormonal pathways, especially those mediated by estrogens and androgens.
The study by [94] also pointed out that exposure to BaP caused delayed sexual maturation and gonadal deformations in marine medaka, with clear implications for sex ratio and fertilization rates. This confirms that histological dysfunction of the gonads is not only a consequence of toxicity but also an important causal link between chemical exposure and reproductive collapse [156].
Finally, it is important to consider that histological alterations can be used as reliable biomarkers in environmental biomonitoring, especially when associated with other functional and molecular markers [109]. The presence of anomalous gonadal structures, such as ovotestes or the absence of maturing gametocytes, is indicative of chronic exposure and suggests potential transgenerational disruption, since the integrity of the gonads is essential to produce viable embryos [141].
5.8. Impacts on Sex Ratio and Intersex Incidence
Exposure to PAHs has been associated with significant changes in sex ratio and increased incidence of intersex in marine fish, demonstrating their ability to profoundly interfere with sex determination and differentiation [108]. These effects, although sometimes subtle, have the potential to destabilize population structure, since the balance between males and females is essential for maintaining fertility and genetic variability [129].
Disruption in sex ratio occurs, in many cases, through mechanisms related to the modulation of aromatase [94,157], which can cause a delay in the hormonal stimulus required for gonadal development, leading to a disruption in sexual maturity. This indicates that PAHs also affect the timing and speed of sexual maturation, potentially disrupting the synchrony between the sexes and reducing the reproductive efficiency of the group [94].
These changes are not only hormonal or molecular but also have clear morphological consequences. The presence of intersex individuals, fish with testes containing oocytes or ovaries with testicular structures, has been reported in areas contaminated by oil and PAHs, with the formation of oocytes in testes of exposed males [10,129]. Although intersex is more common in freshwater environments, there is growing evidence that marine species are also affected, especially in coastal regions polluted by industrial effluents and oil spills [154,158].
From an ecological perspective, these changes are alarming. Male feminization, detected by the induction of VTG in male organisms, can reduce sperm production, alter sexual behavior, and compromise fertilization. On the other hand, masculinization of females, by suppression of the estrogenic pathway, can lead to incomplete ovulation, spawning failures, or deregulation and infertility [157]. In both cases, the presence of intersex individuals and the alteration of the sex ratio can seriously compromise reproductive dynamics and population resilience [140,156,159].
Furthermore, mate choice is a critical component of sexual selection and an important determinant of individual fitness in fish. To date, no studies have directly investigated the effects of PAHs on mate choice or sexual selection, although evidence from other endocrine-disrupting chemicals indicates that altered sex ratios, feminization, or masculinization can impair courtship behavior, mate recognition, and reproductive success [83]. This gap highlights an important avenue for future research on the ecological impacts of PAHs.
The effects on the sex ratio and occurrence of intersex individuals in marine fish are worrying not only because they compromise individual reproduction but also because they can result in silent population collapses, where fertility gradually deteriorates even in apparently abundant populations [140]. Investigation and monitoring of these phenomena should be a priority in marine ecosystems contaminated by oil and PAHs.
5.9. Gonadosomatic Index (GSI)
The gonadosomatic index (GSI), defined as the ratio between the weight of the gonads and the total body weight of the fish, is widely used as a physiological indicator of sexual maturation and reproductive investment in aquatic organisms [160]. In marine fish, this index is particularly informative, as it reflects the seasonal cyclicity of reproduction, hormonal status, and the progression of gametogenesis [161]. Reductions in GSI are often associated with endocrine disruption, gonadal tissue damage, or failures in energy allocation for reproduction and are therefore an integrative marker of reproductive health in the face of environmental stressors such as PAHs [108].
Recent studies have shown that exposure to PAHs can significantly reduce GSI in marine fish, reflecting the interference of these contaminants in gonadal function, such as decreased egg production. Bioaccumulation of PAHs in the ovaries, inhibition of vitellogenesis, and increased follicular atresia are mechanisms associated with the reduction in functional gonadal mass, which together can explain the observed decrease in GSI [54,162].
Field evidence corroborates the experimental findings. In silversides (Fundulus grandis) collected in areas impacted by the Deepwater Horizon spill in the Gulf of Mexico, males had GSI approximately 50% lower than those from reference areas one year after the event [163]. Females from these same regions showed underdeveloped oocytes and gonads with lower mass, suggesting that chronic contamination by PAHs may compromise the reproductive investment and reproductive success of exposed populations in a lasting way [108].
The physiological and hormonal mechanisms underlying the reduction of GSI by PAHs are multiple and interrelated. The suppression of sex hormones, such as E2 and 11-KT, is widely documented in marine fish exposed to PAHs [86]. This hormonal decline compromises the progression of gametogenesis, leading to the inhibition of vitellogenesis in females and incomplete spermatogenesis in males, resulting in less developed gonads and, consequently, reduced GSI. Furthermore, inhibition of aromatase, as already discussed, has been consistently observed in fish exposed to PAHs, contributing to hormonal imbalance and impaired gonadal maturation [87].
Histological changes in the gonads, especially follicular atresia, are another critical factor for the decline in GSI. By promoting the degeneration of developing oocytes, PAHs contribute to the loss of functional gonadal mass. The presence of atresia in advanced vitellogenic stages indicates that the oocyte development process was interrupted late, resulting in substantial losses in reproductive biomass [88].
As a consequence of the decrease in GSI, there is not only a decrease in individual fertility but also a decrease in spawning success and population recruitment, especially in species with seasonal reproduction or low fecundity [153]. Therefore, GSI proves to be not only a useful marker for measuring reproductive status but also a sensitive indicator of the sublethal effects of contaminants such as PAHs.
6. Cell Signaling Mechanisms Involved in PAHs’ Impacts on Fish Reproduction
As previously described, extensive evidence gathered over decades shows that PAHs can disrupt fish reproductive systems [10]. This disruption primarily results from PAHs’ interference with the endocrine system, as well as their cytotoxic and mutagenic effects on germ cells [164].
PAHs can mimic endogenous estrogen molecules and disrupt estrogenic signaling through both genomic and non-genomic pathways [165,166]. The genomic pathway represents the classical mechanism of estrogen signaling and primarily involves nuclear receptor proteins known as estrogen receptors (ERs), specifically ERα, encoded by the Esr1 gene, and ERβ, encoded by the Esr2a and Esr2b genes [167]. In fish, these receptor isoforms play a crucial role in regulating gonadotropin expression and vitellogenesis [139]. Additionally, these nuclear receptors play a critical role in regulating the expression of various genes related to reproduction and hormonal balance [168]. In contrast, the non-genomic pathways engage intracellular signaling cascades and membrane proteins, such as the G protein-coupled estrogen receptor 1 (Gper1), which is crucial for regulating the non-genomic estrogen pathway in aquatic organisms [169].
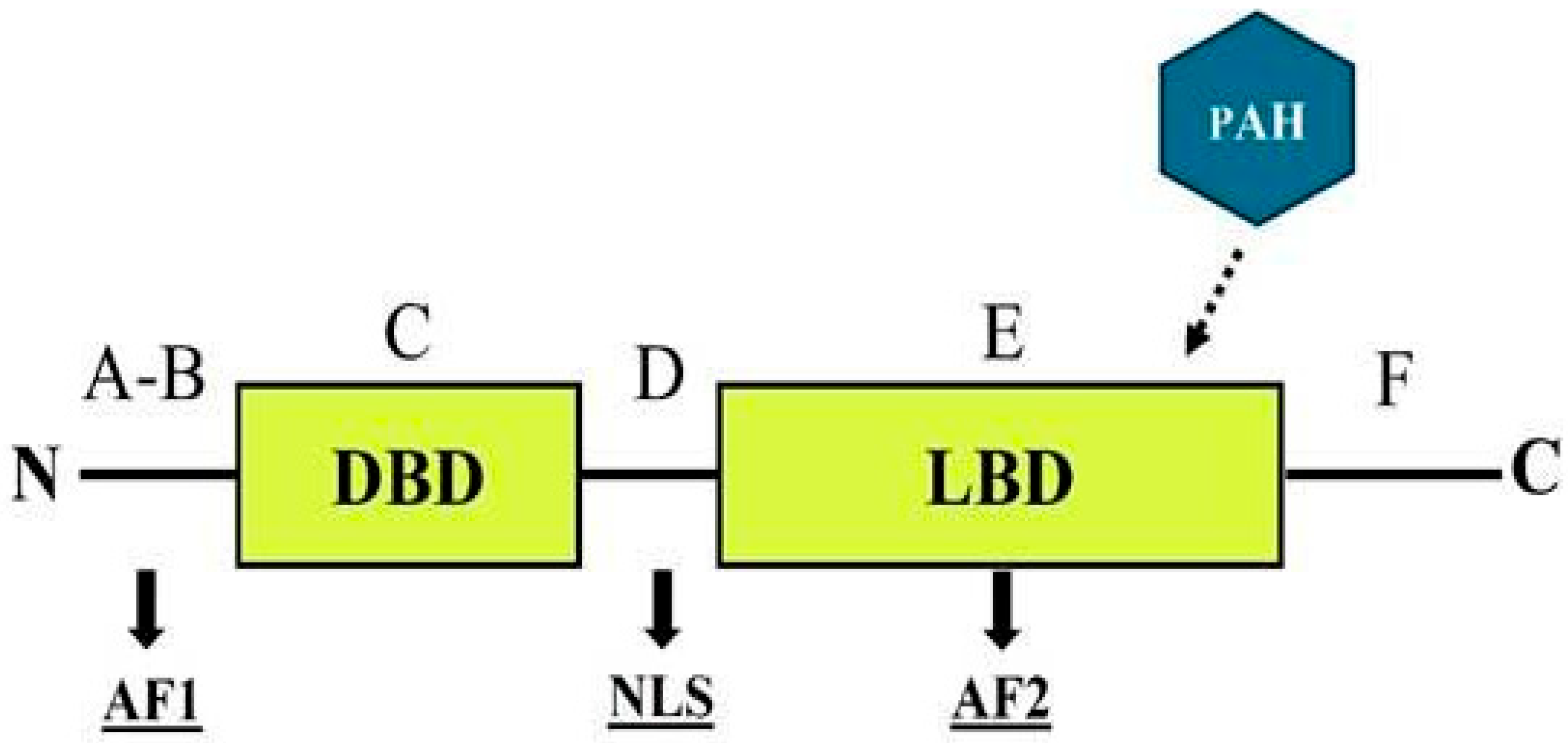
ERs are part of a superfamily of nuclear receptors that specifically bind to small molecules known as ligands, including steroids and thyroid hormones, and are characterized by a distinct domain structure [170]. The ER structure comprises six functionally distinct regions, labeled A through F (Figure 3), that can interact with environmental contaminants in various ways [171,172].
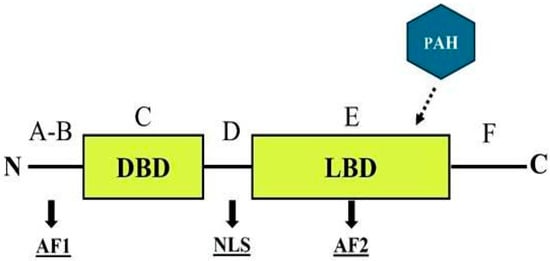
Figure 3.
General structure of a nuclear estrogen receptor, emphasizing the most probable interaction site for PAHs. DBD = DNA binding domain; LBD = ligand binding domain; AF1-2: = transcriptional activation function 1 and 2; NLS = nuclear localization signal.
The A–B regions contain a transcriptional activation function (AF-1) located in the N-terminal domain, which exhibits significant variability across species. The C region encompasses the DNA-binding domain, which is highly conserved among species, with a similarity level of 97%. The D region acts as a flexible hinge and contains a nuclear localization signal (NLS). The E region includes the ligand-binding domain (LBD), which harbors a ligand-dependent transcriptional activation function (AF-2). This domain is crucial for most receptor functions, including hormone binding, dimerization, and interactions with coregulators. Finally, the F region extends from AF-2 to the C-terminus of the receptor [170,172]. Although its specific function remains poorly understood, studies suggest that the ER F region may play a role in modulating LBD activity [173,174].
In the absence of ligands, ERs are primarily located in the cytoplasm, where they are associated with heat shock inhibitory proteins [170]. When ligands such as 17β-estradiol are present, the inhibitory complexes disassemble, enabling the ligand to attach to the ER. This binding triggers conformational changes that lead to dimerization, followed by the translocation of the receptor to the nucleus, where it interacts with specific DNA regions in the promoters of target genes, known as estrogen response elements (EREs) [175].
PAHs can act as endocrine disruptors by interacting with estrogen receptors, primarily within the ligand-binding domain (LBD), thereby activating estrogen response mechanisms [176]. Although research has long established that PAHs and their metabolites can interact with estrogen receptors [177], their specific effects on estrogen signaling vary depending on the PAH type, the estrogen receptor isoforms involved, and whether exposure occurs as a single compound or a mixture of PAHs [10].
A recent study conducted a virtual screening of 72 PAHs and identified a positive correlation between the number of aromatic rings in these compounds and their binding affinity to key proteins involved in endocrine regulation in zebrafish (Danio rerio) [139]. The findings specifically indicated that as the number of aromatic rings in each PAH increased, its binding affinity to the targeted proteins also strengthened. In this context, while E2 (estradiol) and EE2 (17α-ethynylestradiol) were used as true ligands for Erα, comparisons with the 72 PAHs revealed that benzo(g)chrysene, benzo(k)fluoranthene, benzo(e)pyrene, benzo(a)anthracene, and benzo(c)phenanthrene exhibit high binding affinity scores ranging from −11.5 to −9.6 kcal/mol. Notably, all of these PAHs demonstrated 2D and 3D similarity scores exceeding 0.5 and 0.7, respectively. Among them, benzo(g)chrysene displayed the highest 3D similarity to EE2, with an almost perfect structural overlap [139].
Estrogenic responses can also be mediated through non-genomic pathways, wherein estrogen binds to membrane or cytoplasmic receptors, such as G-protein-coupled estrogen receptor 1 (Gper1), initiating a rapid intracellular signaling cascade [178]. However, beyond endogenous estrogens, various environmental pollutants have been shown to interact with Gper1, potentially functioning as endocrine disruptors [178]. For instance, exposure to a mixture of 16 PAHs resulted in the downregulation of Esr1 and Gper1 in the human granulosa cell line (HGrC1). These genes are involved in genomic and non-genomic estrogenic pathways, respectively, and their suppression was associated with a reduction in estradiol secretion [179]. Conversely, no significant alterations in Gper1 expression were observed in primary hepatocytes of O. niloticus following exposure to a commercial EPA PAH mixture [180]. In this context, a 2018 review highlighted the lack of substantial evidence supporting the activation of non-genomic estrogenic signaling by PAHs [181]. However, compared to genomic pathways, research on the effects of PAHs on non-genomic estrogenic mechanisms, particularly in fish models, remains limited, thereby constraining a comprehensive understanding of their potential impact.
Compared to female fish, the reproductive toxicity of PAHs in male fish remains less well understood. Although some studies suggest that PAH exposure can disrupt testosterone levels, the effects reported in the literature vary depending on the specific PAH compounds and the fish species examined. Despite the scarcity of studies elucidating these mechanisms in marine species, the literature provides several findings in freshwater fish, which help illustrate how PAHs can affect male endocrine regulation. For instance, decreased testosterone levels have been observed in Danio rerio exposed to phenanthrene at concentrations of 5 and 50 nmol/L [133]. Similar effects were observed in the turbot (Scophthalmus maximus) exposed to PAHs extracted from Prestige fuel oil, as well as in the white-throated slug (Catostomus commersonii) from a river site polluted with PAHs [182]. Conversely, PAH exposure has been shown to enhance testosterone production in goldfish and rainbow trout by stimulating testicular steroidogenesis [183]. These contrasting findings highlight the complexity of PAH-induced endocrine disruption and underscore the need for further research to elucidate species-specific and compound-specific effects.
Similar to estrogen receptors, the structure of androgen receptors (AR) is composed of three main domains: an N-terminal hypervariable transcriptional activation domain (TAD), a central highly conserved DNA-binding domain (DBD), and a C-terminal ligand-binding domain (LBD) [184]. The LBD also plays a crucial role, as it is where the modulation of receptor activity takes place [139]. Due to the high conservation of these protein domains between fish and mammals, many antiandrogenic effects caused by environmental contaminants are linked to reproductive dysfunctions that can impact population size, such as decreased sperm quality in various vertebrates [142].
Androgen receptors perform their functions in a manner very similar to that previously described for estrogen receptors. In the absence of ligands, AR remains in the cytoplasm, associated with chaperone proteins, primarily heat shock proteins [185]. When an androgen binds to AR, it induces a conformational change, leading to the dissociation of these chaperone proteins and the exposure of the NLS region. The androgen/AR complex then translocates to the nucleus, dimerizing and binding to specific promoter regions known as androgen response elements (AREs) within classical target genes. Thus, it modulates gene transcription [186]. The transcriptional activity of the androgen-bound AR is also influenced by specific proteins called coregulators. These proteins bind to the activated AR in a ligand-dependent manner, either enhancing its ability to transactivate target genes as coactivators or repressing it as corepressors [187,188].
A molecular docking study was conducted to assess the binding affinity of several PAHs—cyclopenta[cd]pyrene, benzo(b)chrysene, dibenz(a,h)anthracene, benzo(k)fluoranthene, and dibenzo(a,e)pyrene—toward the androgen receptors of D. rerio, using testosterone and dihydrotestosterone (DHT) as endogenous ligands. The results revealed that these PAHs exhibited significant 2D and 3D structural similarities to the endogenous ligands, with binding affinities ranging from −9.7 to −8.8 kcal/mol [139]. Based on the information provided, it can be inferred that these PAHs may interact with and modulate the activity of the androgen receptor. These findings suggest that the tested PAHs may interact with and potentially modulate androgen receptor activity. This is particularly concerning given the androgen receptor’s role in regulating gene expression associated with reproductive development and responding to androgenic molecules such as testosterone in mammals, as well as 11-ketotestosterone and 5α-dihydrotestosterone (DHT) in fish [142].
In summary, the documented impacts of environmentally relevant PAHs on fish reproduction necessitate further investigation, particularly concerning their interference with reproductive mechanisms in male and female fish through non-genomic pathways. Additionally, considering the variability in effects across species and the diverse origins of PAHs explored in prior studies, it is imperative that future research enhance ecological relevance by evaluating regionally significant PAHs, both individually and in mixtures, in key native species within their respective ecosystems.
7. Limitations and Challenges of Current Methodologies for Biomonitoring of Marine Ecosystems Threatened by PAHs
Biomonitoring is a useful tool for assessing the impact of PAHs on marine ecosystems. Although several biomonitoring approaches have been developed to assess the environmental and biological impacts of PAHs, these methodologies face several limitations and challenges that hinder their effectiveness. The selection of the exposure method, species, and developmental stages, as well as the duration of the study and the biomarkers of exposure or effects, can directly influence the conclusions of the research [189]. PAHs can induce a range of sublethal effects, such as oxidative stress, DNA damage, and endocrine disruption [190]. Laboratory studies often report stronger or more conclusive effects than field and active biomonitoring studies, likely because they are conducted under controlled conditions, usually with higher contaminant concentrations and reduced interference from other environmental variables or chemicals [120]. Therefore, these elements must be carefully chosen based on the specific questions that the researchers intend to investigate.
One of the main challenges in biomonitoring is the selection of suitable species for the assessment of the effects of PAHs. Marine species have varied sensitivities and can develop metabolic adaptations to stressors that make it difficult to detect the adverse effects caused by PAHs [189]. Mollusks and fish are frequently used due to their capacity to accumulate this contaminant, and the responses to exposure vary significantly according to species, sex, age, and physiological conditions [191].
The use of early life stages of marine organisms for toxicity assessment is scarce, although they have advantages over adults and juveniles [191]. Early life stages are critical for the health and recruitment of marine populations, offering advantages for risk assessment in the field. Assessing biomarkers in organisms of only one sex may lead to biased results, since sex differences can increase or decrease the susceptibility of organisms, in addition to presenting physiological differences [192]. Variability in biological models can make it difficult to interpret biomonitoring data and generalize findings across ecosystems.
Biomonitoring of PAH-contaminated environments faces challenges in analytical issues. Detection and quantification in different matrices require the use of advanced analytical techniques and equipment, such as gas chromatography coupled with mass spectrometry (GC-MS) or high-performance liquid chromatography-ultraviolet (HPLC-UV) [193]. One of the main obstacles is the sensitivity of analytical instruments, especially when it comes to monitoring low concentrations in large marine areas. Furthermore, the complexity of the matrices present in biological samples can interfere with detection, resulting in possible underestimations or overestimations of contamination levels [194]. Even with advances in techniques, the sample collection and preparation process is laborious, costs are high, with prolonged analysis time and the need for specialists for the analyses [195]. In addition, PAH monitoring in marine environments is challenged by temporal and spatial variability, which can further complicate the interpretation and generalization of biomonitoring data [196].
Biomonitoring of PAHs is also challenged by the presence of other contaminants and the impacts of climate change [197]. Climate change variables can influence the fate of contaminants throughout marine ecosystems, and the interaction between toxicants and physical stressors can affect the response of biomarkers. Ocean acidification and rising temperatures can interact with PAHs and exacerbate their negative impacts [198]. Biomonitoring methodologies must consider and adapt to the synergistic effects of climate change and marine pollution [199].
Future research should prioritize improving the robustness, sensitivity, and applicability of biomonitoring approaches, aiming to more effectively protect marine ecosystems from contamination by PAHs. To overcome current limitations, it is essential to adopt a multidisciplinary approach that integrates advanced technologies, careful selection of biomarkers, and consideration of the impacts of climate change. In addition, it is essential to intensify efforts to assess the effects of contamination, considering the wide variety of contaminants present in aquatic ecosystems. In this context, the development of non-targeted methods capable of covering the great diversity of pollutants potentially present in these environments becomes an urgent need.
8. Patents and Research Trends on the Detection of PAHs and Its Impacts in Aquatic Ecosystems
Accurately estimating the total PAH load entering aquatic environments remains a significant challenge [200]. Nevertheless, assessments of PAH sources and their ecological effects have been documented since the 1980s, and the presence of these contaminants in industrial and municipal effluents, as well as in adjacent environments, has been recognized since the 1970s [201,202]. Despite this long-standing awareness, identifying the specific sources of PAHs to effectively prevent environmental contamination remains a major limitation, largely due to their typically low concentrations in aquatic ecosystems [203].
Since the United States Environmental Protection Agency (U.S. EPA) has listed the 16 PAHs for monitoring [204], various methodologies have been used to measure concentrations in water, namely, gravimetry, chromatography, fluorescence, and spectrometric analysis. Gas chromatography (GC), mass spectrometry, and fluorescence spectrometry in special have been providing reliable results in many cases, and fluorescence methods for detection of petrogenic PAHs can generate fast and cost-effective results [205]. However, conventional methods may supply the demand of analyses nowadays, and market analysis for PAHs indicates a growing demand for testing services due to increasing environmental regulations and concerns about their potential health risks [206]. As a result, an increase in knowledge and technological improvements has occurred in recent years, showing new monitoring strategies and detection of PAHs.
In this section, an overview of technologies on various patent databases will be presented. To shortlist potentially relevant patents, the search was narrowed by submitting the keywords, i.e., “polycyclic aromatic hydrocarbons, PAH, detection, aquatic”. The search was conducted on WIPO (World Intellectual Property Organization) and EspaceNet (EPO—European Patent Office) and followed by screening patents of interest. A total of 25 patents were selected as related to technologies to detect PAHs in aquatic environments.
During this search it was noticeable that most of the collections related to PAHs in terms of environmental contamination and biotechnology are related to bioremediation of hydrocarbon and food analysis technologies. At first search in those databases, almost a thousand patents were found, but only 25 were relevant (Table 3) at detection and aquatic environment monitoring. As PAHs are naturally found, present technologies here may collaborate to eventually map possible non-natural origins of these contaminants.

Table 3.
Selected patent survey about technologies to detect PAHs.
The complete analysis of the collected patent documents was conducted and showed technological advancements in the field of analysis, detection, and monitoring of PAHs in the environment. The results of patent filings showed similarities with five broad categories as follows:
- Technologies that may assist conventional methods (i.e., GC, HPLC, spectrometry) for better sensibility, accuracy, or low cost;
- Protocol or apparatus with some advantage over the conventional ones;
- Innovative new apparatus;
- Methodology to analyze, prevent, and monitor environmental risks by PAHs and even other contaminant accidents;
- Bioindicators are serving to detect and monitor PAH contamination.
8.1. Technologies That May Assist Analytical Methods
Reinforcing the challenges found in conventional analytical methods, such as low selectivity leading to the non-detection of isomers or PAHs already incorporated into the sample, the technology reported by the patent US11029303B2 [209] proposed the use of two specific antibodies targeting two PAHs. This patented technology facilitates the production of these specific biological markers, particularly for methylated phenanthrenes, which are major PAHs in petroleum. These markers enhance fluorescence analyses, improving both precision and selectivity.
Some samples, due to the low concentration of pollutants, may have their results further compromised or even remain undetected. To increase sensitivity, the patent CN115078579A [211] developed a technique utilizing naphthyl-modified magnetic ferroferric oxide to assist in PAH detection.
8.2. Protocol or Apparatus with Some Advantage over the Conventional Ones
The determination of PAHs in aquatic samples is often performed using gas chromatography-mass spectrometry (GC-MS). Conventional methods are time-consuming and prone to false positives due to matrix interferences [231]. To avoid such problems, the patent CN102854280A [208] proposes a protocol that makes use of Gas Chromatography-Triple Quadrupole Tandem Mass Spectrometry (TSQ Quantum GC), allowing the detection of 16 PAHs at a concentration of 10 μg/kg.
Common instrumental detection methods include liquid chromatography (LC), gas chromatography-mass spectrometry (GC-MS), and high-performance liquid chromatography (HPLC). The HJ 478-2009 method, a standard for PAH detection in water samples, involves liquid-liquid extraction and solid-phase extraction. However, it has significant drawbacks, including cumbersome and costly pre-treatment, and its fluorescence detector is not suitable for detecting acenaphthene. To address these limitations, a novel approach described by the patent CN112305116A [215] using CSR-LVSI technology has been developed to optimize PAH detection, significantly improving analytical sensitivity and meeting stricter environmental testing requirements.
Additionally, a molecular probe with environmentally sensitive photoluminescence has been disclosed by patent US11561175B2 [226] as a method for detecting hydrocarbon contamination in samples, representing an innovative alternative in PAH analysis. These methods refer to a technique of embodiment of PAHs in water using DNS and DNS-OH as molecular rotors.
8.3. Innovative New Apparatus for Analysis
A novel invention presented by patent WO2016207461A1 [217] is a passive ceramic sampler for measuring water pollution. This device consists of a porous ceramic casing filled with an adsorbent material, allowing for the pre-concentration of pollutants in water to determine its chemical quality. Namely, passive sampling is an integrated method of sample collection that relies on the free flow of pollutants in the environment to the receiving system. The sampling period is determined by the sampling device. The movement of analytes occurs due to differences in chemical potential between the outside and inside of the sampler until steady state or the end of the sampling period is reached.
Another advancement related to passive sampling devices designed by the patent US10551283B2 [225] is for analyzing environmental matrices such as surface water, aquatic sediments, and soil. It addresses the challenges of slow equilibrium in static sediments, and researchers developed a method to manipulate the external depletion layer of the sampler. This involves mechanically disrupting the depletion layer using periodic in situ vibrations, enhancing the accuracy of freely dissolved analyte concentration measurements in sediment porewater.
8.4. Methodology to Analyze, Prevent, and Monitor Environmental Risks of Contamination
A critical challenge in oil mining is establishing a reliable method for identifying and calculating ecological risks. The patent CN102062769A [221] proposes a methodology that integrates regional ecological risk assessment with data analysis. This approach considers the characteristics of oil mining pollutants, receptor sensitivity, and other key indicators. It is mainly based on probabilistic modeling rather than direct analytical measurements, as it uses a joint probability distribution function with a double integral method to estimate regional ecological risk status and support environmental risk prevention efforts.
8.5. Bioindicators Serving to Detect and Monitor PAH Contamination
One novel approach of patent CN111208270A [222] involves using three spiny fish CYP1 family genes as biomarkers for water pollution detection. This method provides a biological alternative to conventional chemical techniques, offering sensitive detection of PAHs through gene expression responses.
The patent US10871484B2 [223] showed that enzymatic assays have emerged as another promising tool for PAH detection. Unlike traditional methods such as GC-MS and HPLC, enzyme-based assays require less sophisticated instrumentation, making them more accessible and efficient. These assays are widely used in clinical applications for detecting DNA mutations, post-translational protein modifications, and serum content analysis. Furthermore, enzyme assays are compatible with surfactants, making them preferable for PAH extraction when hazardous organic solvents are impractical, costly, or environmentally unsafe.
However, enzyme-based methods also present methodological constraints, since their activity can be affected by temperature and other environmental variables, which may limit their robustness for routine environmental monitoring.
8.6. Other Considerations
The research covered in this section presents a diverse range of methodologies and technological innovations aimed at improving PAH detection, environmental monitoring, and pollution risk assessment. From antibody-based fluorescence techniques and molecular probes to advanced passive samplers and enzymatic assays, the field has evolved significantly to address the limitations of conventional methods. These advancements not only enhance analytical accuracy and sensitivity but also contribute to more efficient, cost-effective, and environmentally sustainable monitoring solutions. Given the ongoing need for improved pollution detection and ecological risk management, continued innovation and integration of these emerging technologies will be crucial in shaping future environmental analysis and methodologies.
9. Conclusions
PAHs remain a major class of environmental contaminants in marine ecosystems, with proven capacity to disrupt fish reproduction through endocrine interference, genotoxicity, and oxidative damage. Early life stages are particularly vulnerable, emphasizing the ecological relevance of developmental biomarkers in environmental assessments. Despite the growing use of biochemical and molecular biomarkers for monitoring PAH exposure and reproductive effects in fish, significant knowledge gaps persist regarding the long-term and population-level consequences of such exposure.
Furthermore, the bioavailability and bioaccumulative potential of PAHs in aquatic organisms raise concerns not only for ecosystem integrity but also for human health via biomagnification through the food web [46,232]. Also, a critical limitation in addressing PAH contamination lies in the regulatory framework. Current legislation varies considerably across countries and is often restricted to a limited subset of PAHs while neglecting the broader spectrum of compounds with known or suspected toxicity [233]. The detection of PAHs in local biota underscores the urgent need for more inclusive, compound-specific regulatory measures and consistent environmental monitoring [234]. Addressing these regulatory and scientific gaps will be essential for developing effective strategies to mitigate PAH-related risks in marine environments and protect both ecological and human health in the face of ongoing contamination pressures.
From an applied perspective, these findings also have important implications for fisheries and aquaculture. Reproductive impairment, altered sex ratios, and developmental toxicity in fish can directly affect stock recruitment and population sustainability, threatening wild fishery resources. In aquaculture settings, chronic PAH exposure may compromise broodstock fertility, larval survival, and growth performance, ultimately reducing productivity and economic viability. Considering the dependence of coastal communities on both fisheries and aquaculture, addressing PAH contamination is essential not only for ecosystem conservation but also for food security and sustainable resource management.
While substantial progress has been made in understanding the effects of PAHs and related compounds on fish, a few questions remain to be addressed. The findings highlighted in this paper show the exquisite sensitivity of fish in early stages to petrogenic compounds. There is substantial evidence that PAHs can cause reproductive impairment in a variety of fish through their ability to disrupt endocrine function and their cytotoxic and mutagenic effects on germ cells. Therefore, future studies are necessary to unravel the complexity of the toxic effects of PAHs.
Author Contributions
Conceptualization, R.P., W.J.M.-B., and C.A.d.O.R.; investigation, R.P., A.d.A.R., H.I., F.d.C.G., A.P.d.S., D.A.R.-V., M.d.M., F.d.O., W.J.M.-B., M.M.P., and C.A.d.O.R.; resources, W.J.M.-B., M.M.P., and C.A.d.O.R.; data curation, R.P., A.d.A.R., H.I., F.d.C.G., A.P.d.S., D.A.R.-V., M.d.M., F.d.O., W.J.M.-B., M.M.P., and C.A.d.O.R.; writing—original draft preparation, R.P., A.d.A.R., H.I., F.d.C.G., A.P.d.S., D.A.R.-V., M.d.M., and F.d.O.; writing—review and editing, R.P., A.d.A.R., W.J.M.-B., M.M.P. and C.A.d.O.R.; visualization, R.P., A.d.A.R., W.J.M.-B., M.M.P., and C.A.d.O.R.; supervision, R.P., W.J.M.-B., M.M.P., and C.A.d.O.R.; project administration, M.M.P. and C.A.d.O.R.; funding acquisition, W.J.M.-B., M.M.P., and C.A.d.O.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Araucaria Foundation (Project number JDT2022271000037) by the Postgraduate Studies Development Program in the State of Paraná, provided by the Coordination for Higher Education Staff Development—CAPES and by the Araucária Foundation (project number 88887.798741/2022-00), and by the Brazilian National Council for Scientific and Technological Development—CNPq.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Manzetti, S. Polycyclic Aromatic Hydrocarbons in the Environment: Environmental Fate and Transformation. Polycycl. Aromat. Compd. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- Teixeira, J.; Delerue-Matos, C.; Morais, S.; Oliveira, M. Environmental Contamination with Polycyclic Aromatic Hydrocarbons and Contribution from Biomonitoring Studies to the Surveillance of Global Health. Environ. Sci. Pollut. Res. 2024, 31, 54339–54362. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Queb-Suarez, J.E.; Ruiz-Marin, A.; Canedo-Lopez, Y.; Aguilar-Ucan, C.A.; Montalvo-Romero, C.; Flores-Trujillo, J.G.; Perez-Morga, N. Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico. Energies 2022, 15, 7116. [Google Scholar] [CrossRef]
- Berríos-Rolón, P.J.; Cotto, M.C.; Márquez, F. Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts. Toxics 2025, 13, 321. [Google Scholar] [CrossRef]
- Gan, N.; Martin, L.; Xu, W. Impact of Polycyclic Aromatic Hydrocarbon Accumulation on Oyster Health. Front. Physiol. 2021, 12, 734463. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Liu, W.; Kong, X.; Xu, F.; Giesy, J.P. Tissue Distribution, Bioaccumulation, and Carcinogenic Risk of Polycyclic Aromatic Hydrocarbons in Aquatic Organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef]
- Matos, B.; Bramatti, I.; Santos, C.D.; Branco, V.; Martins, M. Multi-Biomarker Analysis of Sub-Chronic PAH Mixture Effects in Fish at Environmentally Relevant Levels. Aquat. Toxicol. 2025, 283, 107350. [Google Scholar] [CrossRef]
- Yazdani, M. Comparative Toxicity of Selected PAHs in Rainbow Trout Hepatocytes: Genotoxicity, Oxidative Stress and Cytotoxicity. Drug Chem. Toxicol. 2020, 43, 71–78. [Google Scholar] [CrossRef]
- Vignet, C.; Larcher, T.; Davail, B.; Joassard, L.; Menach, K.L.; Guionnet, T.; Lyphout, L.; Ledevin, M.; Goubeau, M.; Budzinski, H.; et al. Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action. Toxics 2016, 4, 26. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Azevedo, J.; Silva, M.; Ramos, V. Effects of Marine Toxins on the Reproduction and Early Stages Development of Aquatic Organisms. Mar. Drugs 2010, 8, 59–79. [Google Scholar] [CrossRef]
- Martins, M.; Santos, J.M.; Diniz, M.S.; Ferreira, A.M.; Costa, M.H.; Costa, P.M. Effects of Carcinogenic versus Non-Carcinogenic AHR-Active PAHs and Their Mixtures: Lessons from Ecological Relevance. Environ. Res. 2015, 138, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kocak, T.K.; Kocak, G.O.; Stuart, A.L. Polycyclic Aromatic Hydrocarbons in Aquatic Media of Turkey: A Systematic Review of Cancer and Ecological Risk. Mar. Pollut. Bull. 2023, 188, 114671. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency—UEPA. Polycyclic Aromatic Hydrocarbons; Elsevier: Washington, WA, USA, 2008.
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation Approaches for Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soils: Technological Constraints, Emerging Trends and Future Directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Na, M.; Zhao, Y.; Rina, S.; Wang, R.; Liu, X.; Tong, Z.; Zhang, J. Residues, Potential Source and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Water of the East Liao River, Jilin Province, China. Sci. Total Environ. 2023, 886, 163977. [Google Scholar] [CrossRef]
- Malhat, F.; Loutfy, N.; El Menyawi, M.A.I.; Ahmed, M.T. Review of Contamination by Polycyclic Aromatic Hydrocarbons (PAHs) in Egyptian Aquatic Environment. Polycycl. Aromat. Compd. 2021, 41, 1447–1458. [Google Scholar] [CrossRef]
- Derafshi, M.; Matin, N.H.; Hassani, A. A Short Review on Polycyclic Aromatic Hydrocarbon Contamination. Acta. Hortic. Regiotect. 2022, 25, 174–180. [Google Scholar] [CrossRef]
- Montano, L.; Baldini, G.M.; Piscopo, M.; Liguori, G.; Lombardi, R.; Ricciardi, M.; Esposito, G.; Pinto, G.; Fontanarosa, C.; Spinelli, M.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Occupational Exposure, Health Risks and Fertility Implications. Toxics 2025, 13, 151. [Google Scholar] [CrossRef]
- Othman, H.B.; Pick, F.R.; Hlaili, A.S.; Leboulanger, C. Effects of Polycyclic Aromatic Hydrocarbons on Marine and Freshwater Microalgae—A Review. J. Hazard. Mater. 2023, 441, 129869. [Google Scholar] [CrossRef]
- Wang, C.; Lou, Y.; Wang, T.; Li, R.; Peng, M.; Gao, D.; Lei, W. Embryonic Exposure to Water Accommodated Fraction of Crude Oil Inhibits Reproductive Capability in Adult Female Marine Medaka (Oryzias melastigma). Chemosphere 2024, 362, 142616. [Google Scholar] [CrossRef]
- Castillo-Ilabaca, C.; Gutiérrez, M.H.; Aranda, M.; Henríquez-Aedo, K.; Pereira, A.; Salamanca, M.; Galand, P.E.; Jessen, G.L.; Pantoja-Gutiérrez, S. PAH Contamination in Coastal Surface Sediments and Associated Bacterial Communities. Sci. Rep. 2024, 14, 29053. [Google Scholar] [CrossRef]
- Anyahara, J.N. Effects of Polycyclic Aromatic Hydrocarbons (PAHs) on the Environment: A Systematic Review. Int. J. Adv. Acad. Res. 2021, 7, 2488–9849. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Liu, L.Y.; Zeng, E.Y. Polycyclic Aromatic Hydrocarbons Affiliated with Microplastics in Surface Waters of Bohai and Huanghai Seas, China. Environ. Pollut. 2018, 241, 834–840. [Google Scholar] [CrossRef]
- Bouzekry, A.; Mghili, B.; Bottari, T.; Bouadil, O.; Mancuso, M.; Benomar, M.; Aksissou, M. Polycyclic Aromatic Hydrocarbons in Sediments and Bivalves along the Moroccan Mediterranean Coast: Spatial Distribution, Sources, and Risk Assessment. Environ. Pollut. 2024, 363, 125073. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Castillo, A.B.; Yigiterhan, O.; Elobaid, E.A.; Al-Obaidly, A.; Al-Ansari, E.; Obbard, J.P. Baseline Concentrations and Distributions of Polycyclic Aromatic Hydrocarbons in Surface Sediments from the Qatar Marine Environment. Mar. Pollut. Bull. 2018, 126, 58–62. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.F.B.; Gomes, B.R.S.; França, R.S.; Moreira, T.C.S.; Moraes, A.S.; Bataglion, G.A.; Santos, J.M. A Multi-Geochemical Characterization to Evaluate Anthropogenic Contamination in Marine Sediments from Port of Suape, Northeast of Brazil. J. Braz. Chem. Soc. 2023, 35, e-20230154. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Q.; Gu, Y.; Du, F.; Wang, X.; Shi, F.; Ke, C.; Xiang, M.; Yu, Y. Bioaccumulation of Polycyclic Aromatic Hydrocarbons (PAHs)in Wild Marine Fish from the Coastal Waters of the Northern South China Sea: Risk Assessment for Human Health. Ecotoxicol. Environ. Saf. 2019, 180, 742–748. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Cai, Y.; Chen, Y. Distribution of Polycyclic Aromatic Hydrocarbon (PAH) Residues in Several Tissues of Edible Fishes from the Largest Freshwater Lake in China, Poyang Lake, and Associated Human Health Risk Assessment. Ecotoxicol. Environ. Saf. 2014, 104, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic Aromatic Compounds (PAHs and Oxygenated PAHs) and Trace Metals in Fish Species from Ghana (West Africa): Bioaccumulation and Health Risk Assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
- D’Costa, A.; Shyama, S.K.; Praveen Kumar, M.K. Bioaccumulation of Trace Metals and Total Petroleum and Genotoxicity Responses in an Edible Fish Population as Indicators of Marine Pollution. Ecotoxicol. Environ. Saf. 2017, 142, 22–28. [Google Scholar] [CrossRef]
- Baali, A.; Kammann, U.; Hanel, R.; El Qoraychy, I.; Yahyaoui, A. Bile Metabolites of Polycyclic Aromatic Hydrocarbons (PAHs) in Three Species of Fish from Morocco. Environ. Sci. Eur. 2016, 28, 25. [Google Scholar] [CrossRef]
- Collier, T.K.; Anulacion, B.F.; Arkoosh, M.R.; Dietrich, J.P.; Incardona, J.P.; Johnson, L.L.; Ylitalo, G.M.; Myers, M.S. Effects on Fish of Polycyclic Aromatic HydrocarbonS (PAHS) and Naphthenic Acid Exposures. In Organic Chemical Toxicology of Fishes; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 33, pp. 195–255. [Google Scholar]
- Vethaak, A.D.; Baggelaar, P.K.; van Lieverloo, J.H.M.; Ariese, F. Decadal Trends in Polycyclic Aromatic Hydrocarbon (PAH) Contamination Assessed by 1-Hydroxypyrene in Fish Bile Fluid in The Netherlands: Declining in Marine Waters but Still a Concern in Estuaries. Front. Mar. Sci. 2016, 3, 215. [Google Scholar] [CrossRef]
- Kono, K.; Ito, M.; Hano, T.; Ohkubo, N. Estimation of the Uptake of Polycyclic Aromatic Hydrocarbons Desorbed from Polyethylene Microplastics in the Digestive Tract of the Red Seabream (Pagrus major) and Mummichog (Fundulus heteroclitus). Mar. Pollut. Bull. 2024, 209, 117216. [Google Scholar] [CrossRef] [PubMed]
- Al-Khion, D. Distribution and Source of Polycyclic Aromatic Hydrocarbons (PAHs) in the Aquatic Species of Iraqi Marine Waters. Int. J. Sci. Res. 2018, 7, 1911–1917. [Google Scholar] [CrossRef]
- Kreitsberg, R.; Zemit, I.; Freiberg, R.; Tambets, M.; Tuvikene, A. Responses of Metabolic Pathways to Polycyclic Aromatic Compounds in Flounder Following Oil Spill in the Baltic Sea near the Estonian Coast. Aquat. Toxicol. 2010, 99, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Khursigara, A.J.; Ackerly, K.L.; Esbaugh, A.J. Pyrene Drives Reduced Brain Size during Early Life Exposure in an Estuarine Fish, the Red Drum (Sciaenops ocellatus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 259, 109397. [Google Scholar] [CrossRef] [PubMed]
- Filatova, T.S.; Abramochkin, D.V. Physiological Effects of Polycyclic Aromatic Hydrocarbons in Fish Organisms. Mosc. Univ. Biol. Sci. Bull. 2023, 78, 115–127. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Zou, S.; Li, P.; Yang, Y.; Wang, J. Pollution Status and Trophic Transfer of Polycyclic Aromatic Hydrocarbons in Coral Reef Ecosystems of the South China Sea. ICES J. Mar. Sci. 2021, 78, 2053–2064. [Google Scholar] [CrossRef]
- Jin, S.; Cao, S.; Li, R.; Gao, H.; Na, G. Trophic Transfer of Polycyclic Aromatic Hydrocarbons through the Food Web of the Fildes Peninsula, Antarctica. Environ. Sci. Pollut. Res. 2023, 30, 55057–55066. [Google Scholar] [CrossRef]
- Rojo-Nieto, E.; Perales, J.A. Estimating Baseline Toxicity of PAHs from Marine Chronically Polluted Sediments and Bioaccumulation in Target Organs of Fish Hypothetically Exposed to Them: A New Tool in Risk Assessment. Environ. Sci. Process. Impacts 2015, 17, 1331–1339. [Google Scholar] [CrossRef]
- Li, H.; Ran, Y. Distribution and Bioconcentration of Polycyclic Aromatic Hydrocarbons in Surface Water and Fishes. Sci. World J. 2012, 2012, 632910. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Q.; Sun, K.; Wen, Z. Spatiotemporal Distribution, Bioaccumulation, and Ecological and Human Health Risks of Polycyclic Aromatic Hydrocarbons in Surface Water: A Comprehensive Review. Sustainability 2024, 16, 10346. [Google Scholar] [CrossRef]
- Wang, H.; Shu, Y.; Kuang, Z.; Han, Z.; Wu, J.; Huang, X.; Song, X.; Yang, J.; Fan, Z. Bioaccumulation and Potential Human Health Risks of PAHs in Marine Food Webs: A Trophic Transfer Perspective. J. Hazard. Mater. 2025, 485, 136946. [Google Scholar] [CrossRef]
- Qadeer, A.; Liu, M.; Yang, J.; Liu, X.; Khalil, S.K.; Huang, Y.; Habibullah-Al-Mamun, M.; Gao, D.; Yang, Y. Trophodynamics and Parabolic Behaviors of Polycyclic Aromatic Hydrocarbons in an Urbanized Lake Food Web, Shanghai. Ecotoxicol. Environ. Saf. 2019, 178, 17–24. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Florek-łuszczki, M.; Wojciechowska, M.; Wójcik, T.; Bąk-Badowska, J.; Wójtowicz, B.; Zięba, E.; Gworek, B.; Chmielewski, J. Fish as Bio-Indicators of environmental pollutants and associated health risks to the consumer. J. Elem. 2022, 27, 879–896. [Google Scholar] [CrossRef]
- Dalzochio, T.; Rodrigues, G.Z.P.; Petry, I.E.; Gehlen, G.; da Silva, L.B. The Use of Biomarkers to Assess the Health of Aquatic Ecosystems in Brazil: A Review. Int. Aquat. Res. 2016, 8, 283–298. [Google Scholar] [CrossRef]
- Recabarren-Villalón, T.; Ronda, A.C.; Girones, L.; Marcovecchio, J.; Amodeo, M.; Arias, A.H. Can Environmental Factors Increase Oxidative Responses in Fish Exposed to Polycyclic Aromatic Hydrocarbons (PAHs)? Chemosphere 2024, 355, 141793. [Google Scholar] [CrossRef]
- Bencheikh, Z.; Refes, W.; Mela Prodocimo, M.; de Oliveira Ribeiro, C.A. Ultrastructural Biomarkers in Target Organs of Fish from Algeria Coastline to Access Water Quality. Water Air Soil Pollut. 2024, 235, 387. [Google Scholar] [CrossRef]
- Salgado, L.D.; Marques, A.E.M.L.; Kramer, R.D.; Garrido de Oliveira, F.; Moretto, S.L.; Alves de Lima, B.; Prodocimo, M.M.; Cestari, M.M.; de Azevedo, J.C.R.; Silva de Assis, H.C. Sediment Contamination and Toxic Effects on Violet Goby Fish (Gobioides broussonnetii—Gobiidae) from a Marine Protected Area in South Atlantic. Environ. Res. 2021, 195, 110308. [Google Scholar] [CrossRef]
- Bencheikh, Z.; Refes, W.; Brito, P.M.; Prodocimo, M.M.; Gusso-Choueri, P.K.; Choueri, R.B.; de Oliveira Ribeiro, C.A. Chemical Pollution Impairs the Health of Fish Species and Fishery Activities along the Algeria Coastline, Mediterranean Sea. Environ. Monit. Assess. 2022, 194, 497. [Google Scholar] [CrossRef]
- Beg, M.U.; Butt, S.A.; Al-Dufaileej, S.; Karam, Q.; Al-Sharrah, T.K.; Saeed, T. Biomarkers in Fish as a Measure of the State of Marine Environment of Kuwait. Environ. Monit. Assess. 2018, 190, 325. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, C.; Xu, K.; Ke, A.; Zheng, Y.; Lu, R.; Xu, J. Pollution Level and Health Risk Assessment of the Total Petroleum Hydrocarbon in Marine Environment and Aquatic Products: A Case of China. Environ. Sci. Pollut. Res. 2022, 29, 86887–86897. [Google Scholar] [CrossRef]
- Nakken, C.L.; Meier, S.; Mjøs, S.A.; Bijlsma, L.; Rowland, S.J.; Donald, C.E. Discovery of Polycyclic Aromatic Acid Metabolites in Fish Exposed to the Petroleum Compounds 1-Methylphenanthrene and 1,4-Dimethylphenanthrene. Sci. Total Environ. 2024, 918, 170496. [Google Scholar] [CrossRef]
- Wills, L.P.; Jung, D.; Koehrn, K.; Zhu, S.; Willett, K.L.; Hinton, D.E.; di Giulio, R.T. Comparative Chronic Liver Toxicity of Benzo[a]Pyrene in Two Populations of the Atlantic Killifish (Fundulus Heteroclitus) with Different Exposure Histories. Environ. Health Perspect. 2010, 118, 1376–1381. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kumaraguru, A.K.; Ramakritinan, C.M.; Anand, M. Biochemical Response of Anthracene and Benzo [a] Pyrene in Milkfish Chanos chanos. Ecotoxicol. Environ. Saf. 2012, 75, 187–197. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and Combined Effects of Microplastics and Pyrene on Juveniles (0+ Group) of the Common Goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Ali, A.O.; Hohn, C.; Allen, P.J.; Ford, L.; Dail, M.B.; Pruett, S.; Petrie-Hanson, L. The Effects of Oil Exposure on Peripheral Blood Leukocytes and Splenic Melano-Macrophage Centers of Gulf of Mexico Fishes. Mar. Pollut. Bull. 2014, 79, 87–93. [Google Scholar] [CrossRef]
- Bo, J.; Gopalakrishnan, S.; Chen, F.Y.; Wang, K.J. Benzo[a]Pyrene Modulates the Biotransformation, DNA Damage and Cortisol Level of Red Sea Bream Challenged with Lipopolysaccharide. Mar. Pollut. Bull. 2014, 85, 463–470. [Google Scholar] [CrossRef]
- Sørensen, L.; Sørhus, E.; Nordtug, T.; Incardona, J.P.; Linbo, T.L.; Giovanetti, L.; Karlsen, Ø.; Meier, S. Oil Droplet Fouling and Differential Toxicokinetics of Polycyclic Aromatic Hydrocarbons in Embryos of Atlantic Haddock and Cod. PLoS ONE 2017, 12, e0180048. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, J.; Yu, L.; Wei, Y.; Miao, Z.; Chen, M.; Huang, R. Characterization of Transcriptional Responses Mediated by Benzo[a]Pyrene Stress in a New Marine Fish Model of Goby, Mugilogobius Chulae. Genes Genom. 2019, 41, 113–123. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Li, Y.; Zheng, H.; Mi, W. Polycyclic Aromatic Hydrocarbons in Benthos of the Northern Bering Sea Shelf and Chukchi Sea Shelf. J. Environ. Sci. 2020, 97, 194–199. [Google Scholar] [CrossRef]
- Zanaty, M.I.; Sawada, N.; Kitani, Y.; Nassar, H.F.; Mahmoud, H.M.; Hayakawa, K.; Sekiguchi, T.; Ogiso, S.; Tabuchi, Y.; Urata, M.; et al. Influence of Benz[a]Anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata. Int. J. Environ. Res. Public Health 2020, 17, 1391. [Google Scholar] [CrossRef]
- Hano, T.; Ito, M.; Ito, K.; Uchida, M. Alterations of Stool Metabolome, Phenome, and Microbiome of the Marine Fish, Red Sea Bream, Pagrus Major, Following Exposure to Phenanthrene: A Non-Invasive Approach for Exposure Assessment. Sci. Total Environ. 2021, 752, 141796. [Google Scholar] [CrossRef]
- Arkoosh, M.R.; Collier, T.K. Ecological Risk Assessment Paradigm for Salmon: Analyzing Immune Function to Evaluate Risk. Hum. Ecol. Risk Assess. 2002, 8, 265–276. [Google Scholar] [CrossRef]
- Cherr, G.N.; Fairbairn, E.; Whitehead, A. Impacts of Petroleum-Derived Pollutants on Fish Development. Annu. Rev. Anim. Biosci. 2017, 5, 185–203. [Google Scholar] [CrossRef]
- Cousin, X.; Cachot, J. PAHs and Fish—Exposure Monitoring and Adverse Effects—From Molecular to Individual Level. Environ. Sci. Pollut. Res. 2014, 21, 13685–13688. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yue, Z.; Samreen; Li, Z.; Li, X.; Wang, J. Teratogenic Effects of Environmentally Relevant Concentrations of Phenanthrene on the Early Development of Marine Medaka (Oryzia melastigma). Chemosphere 2020, 254, 126900. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R.; Mager, E.M. The Effects of Exposure to Crude Oil or PAHs on Fish Swim Bladder Development and Function. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108853. [Google Scholar] [CrossRef] [PubMed]
- Hodson, P.V. The Toxicity to Fish Embryos of PAH in Crude and Refined Oils. Arch. Environ. Contam. Toxicol. 2017, 73, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.; Hecht, S.; Kuivila, K. The Challenge: “Bridging the Gap” with Fish: Advances in Assessing Exposure and Effects across Biological Scales. Environ. Toxicol. Chem. 2015, 34, 459. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Bautista, N.M.; Lucero, J.A.; Lund, A.K.; Xu, E.G.; Schlenk, D.; Burggren, W.W.; Roberts, A.P. Exposure to Crude Oil Induces Retinal Apoptosis and Impairs Visual Function in Fish. Environ. Sci. Technol. 2020, 54, 2843–2850. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Lopes, R.M.; Ziolli, R.L. Inihibition of Mullet (M. Liza) Brain Acetylcholinesterase Activity by in Vitro Polycyclic Aromatic Hydrocarbon Exposure. Mar. Pollut. Bull. 2019, 140, 30–34. [Google Scholar] [CrossRef]
- Sathikumaran, R.; Madhuvandhi, J.; Priya, K.K.; Sridevi, A.; Krishnamurthy, R.; Thilagam, H. Evaluation of Benzo[a]Pyrene-Induced Toxicity in the Estuarine Thornfish Therapon jarbua. Toxicol. Rep. 2022, 9, 720–727. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O. Neurotoxicity of Polycyclic Aromatic Hydrocarbons: A Systematic Mapping and Review of Neuropathological Mechanisms. Toxics 2022, 10, 417. [Google Scholar] [CrossRef]
- Carlson, E.A.; Li, Y.; Zelikoff, J.T. Exposure of Japanese Medaka (Oryzias latipes) to Benzo[a]Pyrene Suppresses Immune Function and Host Resistance against Bacterial Challenge. Aquat. Toxicol. 2002, 56, 289–301. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The Effects of Polycyclic Aromatic Hydrocarbons on the Immune System of Fish: A Review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef]
- Hogan, N.S.; Lee, K.S.; Köllner, B.; van den Heuvel, M.R. The Effects of the Alkyl Polycyclic Aromatic Hydrocarbon Retene on Rainbow Trout (Oncorhynchus mykiss) Immune Response. Aquat. Toxicol. 2010, 100, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Devier, M.H.; Le Menach, K.; Lyphout, L.; Potier, J.; Cachot, J.; Budzinski, H.; Bégout, M.L.; Cousin, X. Long-Term Disruption of Growth, Reproduction, and Behavior after Embryonic Exposure of Zebrafish to PAH-Spiked Sediment. Environ. Sci. Pollut. Res. 2014, 21, 13877–13887. [Google Scholar] [CrossRef] [PubMed]
- Ramasre, J.R.; Kashyap, N.; Chandravanshi, S.; Mishra, S.; Baidya, S.; Lal, J.; Dhruve, D. Endocrine Disrupting Chemicals and Their Harmful Effects in Fish: A Comprehensive Review. Int. J. Adv. Biochem. Res. 2024, 8, 5–11. [Google Scholar] [CrossRef]
- Kar, S.; Sangem, P.; Anusha, N.; Senthilkumaran, B. Endocrine Disruptors in Teleosts: Evaluating Environmental Risks and Biomarkers. Aquac. Fish. 2021, 6, 1–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological Impact of Environmental Polycyclic Aromatic Hydrocarbons (EPAHs) as Endocrine Disruptors. Environ. Pollut. 2016, 213, 809–824. [Google Scholar] [CrossRef] [PubMed]
- de Campos, M.F.; Lo Nostro, F.L.; Da Cuña, R.H.; Moreira, R.G. Endocrine Disruption of Phenanthrene in the Protogynous Dusky Grouper Epinephelus marginatus (Serranidae: Perciformes). Gen. Comp. Endocrinol. 2018, 257, 255–263. [Google Scholar] [CrossRef]
- Sun, L.; Zuo, Z.; Chen, M.; Chen, Y.; Wang, C. Reproductive and Transgenerational Toxicities of Phenanthrene on Female Marine Medaka (Oryzias melastigma). Aquat. Toxicol. 2015, 162, 109–116. [Google Scholar] [CrossRef]
- Abdelmeguid, N.E.; Ghanem, S.F.; Assem, S.S.; Abou Shabana, N.M.; Ismail, R.F.; Sultan, A.S. Ameliorative Effects of Chitosan in Water Remediation, Endocrine Disruption and Reproductive Impairment of Solea Solea after Exposure to Benzo (a) Pyrene. Int. Aquat. Res. 2024, 16, 71–90. [Google Scholar] [CrossRef]
- Khan, E.A.; Bertotto, L.B.; Dale, K.; Lille-Langøy, R.; Yadetie, F.; Karlsen, O.A.; Goksøyr, A.; Schlenk, D.; Arukwe, A. Modulation of Neuro-Dopamine Homeostasis in Juvenile Female Atlantic Cod (Gadus morhua) Exposed to Polycyclic Aromatic Hydrocarbons and Perfluoroalkyl Substances. Environ. Sci. Technol. 2019, 53, 7036–7044. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Krasnec, M.; Takeshita, R.; Ryan, C.N.; Griffitt, K.J.; Lay, C.; Mayer, G.D.; Bayha, K.M.; Hawkins, W.E.; Lipton, I.; et al. A Multiple Endpoint Analysis of the Effects of Chronic Exposure to Sediment Contaminated with Deepwater Horizon Oil on Juvenile Southern Flounder and Their Associated Microbiomes. Aquat. Toxicol. 2015, 165, 197–209. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Wang, J.; Lu, L.; Li, X.; Zheng, Y.; Zhang, Z.; Ru, S. Microplastics Increase the Accumulation of Phenanthrene in the Ovaries of Marine Medaka (Oryzias melastigma) and Its Transgenerational Toxicity. J. Hazard. Mater. 2022, 424, 127754. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, Z.; Salamat, N.; Movahedinia, A.A.; Hashemitabar, M.; Bayati, V. Using Ovarian and Brain Cell Culture from the Mullet, Liza Klunzingeri, to Assess the Inhibitory Effects of Benzo[a]Pyrene on Aromatase Activity. J. Appl. Toxicol. 2020, 40, 991–1003. [Google Scholar] [CrossRef]
- Yadetie, F.; Zhang, X.; Hanna, E.M.; Aranguren-Abadía, L.; Eide, M.; Blaser, N.; Brun, M.; Jonassen, I.; Goksøyr, A.; Karlsen, O.A. RNA-Seq Analysis of Transcriptome Responses in Atlantic Cod (Gadus morhua) Precision-Cut Liver Slices Exposed to Benzo[a]Pyrene and 17A-Ethynylestradiol. Aquat. Toxicol. 2018, 201, 174–186. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Q.; Zhu, B.; Lan, Y.; Duan, S. Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias melastigma). Int. J. Environ. Res. Public Health 2020, 17, 970. [Google Scholar] [CrossRef]
- Cañizares-Martínez, M.A.; Quintanilla-Mena, M.A.; Árcega-Cabrera, F.; Ceja-Moreno, V.; Del Río-García, M.; Reyes-Solian, S.G.; Rivas-Reyes, I.; Rivera-Bustamante, R.F.; Puch-Hau, C.A. Transcriptional Response of Vitellogenin Gene in Flatfish to Environmental Pollutants from Two Regions of the Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2024, 112, 11. [Google Scholar] [CrossRef]
- Woo, S.J. Effects of Benzo[a]Pyrene Exposure on Black Rockfish (Sebastes schlegelii): EROD Activity, CYP1A Protein, and Immunohistochemical and Histopathological Alterations. Environ. Sci. Pollut. Res. 2022, 29, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Yadetie, F.; Brun, N.R.; Vieweg, I.; Nahrgang, J.; Karlsen, O.A.; Goksøyr, A. Transcriptome Responses in Polar Cod (Boreogadus saida) Liver Slice Culture Exposed to Benzo[a]Pyrene and Ethynylestradiol: Insights into Anti-Estrogenic Effects. Toxicol. Vitr. 2021, 75, 105193. [Google Scholar] [CrossRef]
- Marsili, L.; Coppola, D.; Giannetti, M.; Casini, S.; Fossi, M.C.; van Wyk, J.H.; Sperone, E.; Tripepi, S.; Micarelli, P.; Rizzuto, S. Skin Biopsies as a Sensitive Non-Lethal Technique for the Ecotoxicological Studies of Great White Shark (Carcharodon carcharias) Sampled in South Africa. Expert Opin. Environ. Biol. 2016, 4, 2. [Google Scholar] [CrossRef]
- Imbery, J.J.; Buday, C.; Miliano, R.C.; Shang, D.; Round, J.M.; Kwok, H.; Van Aggelen, G.; Helbing, C.C. Evaluation of Gene Bioindicators in the Liver and Caudal Fin of Juvenile Pacific Coho Salmon in Response to Low Sulfur Marine Diesel Seawater-Accommodated Fraction Exposure. Environ. Sci. Technol. 2019, 53, 1627–1638. [Google Scholar] [CrossRef]
- Strople, L.C.; Vieweg, I.; Yadetie, F.; Odei, D.K.; Thorsen, A.; Karlsen, O.A.; Goksøyr, A.; Sørensen, L.; Sarno, A.; Hansen, B.H.; et al. Spawning Time in Adult Polar Cod (Boreogadus saida) Altered by Crude Oil Exposure, Independent of Food Availability. J. Toxicol. Environ. Health Part A Curr. Issues 2025, 88, 43–66. [Google Scholar] [CrossRef]
- Bender, M.L.; Frantzen, M.; Vieweg, I.; Falk-Petersen, I.B.; Johnsen, H.K.; Rudolfsen, G.; Tollefsen, K.E.; Dubourg, P.; Nahrgang, J. Effects of Chronic Dietary Petroleum Exposure on Reproductive Development in Polar Cod (Boreogadus saida). Aquat. Toxicol. 2016, 180, 196–208. [Google Scholar] [CrossRef]
- Meier, S.; Karlsen, Ø.; Le Goff, J.; Sørensen, L.; Sørhus, E.; Pampanin, D.M.; Donald, C.E.; Fjelldal, P.G.; Dunaevskaya, E.; Romano, M.; et al. DNA Damage and Health Effects in Juvenile Haddock (Melanogrammus aeglefinus) Exposed to PAHs Associated with Oil-Polluted Sediment or Produced Water. PLoS ONE 2020, 15, e0240307. [Google Scholar] [CrossRef] [PubMed]
- Bramatti, I.; Matos, B.; Figueiredo, N.; Pousão-Ferreira, P.; Branco, V.; Martins, M. Interaction of Polycyclic Aromatic Hydrocarbon Compounds in Fish Primary Hepatocytes: From Molecular Mechanisms to Genotoxic Effects. Sci. Total Environ. 2023, 855, 158783. [Google Scholar] [CrossRef]
- Strobel, A.; Burkhardt-Holm, P.; Schmid, P.; Segner, H. Benzo(a)Pyrene Metabolism and EROD and GST Biotransformation Activity in the Liver of Red- and White-Blooded Antarctic Fish. Environ. Sci. Technol. 2015, 49, 8022–8032. [Google Scholar] [CrossRef] [PubMed]
- Bozcaarmutlu, A.; Sapmaz, C.; Kaleli, G.; Turna, S.; Yenisoy-Karakaş, S. Combined Use of PAH Levels and EROD Activities in the Determination of PAH Pollution in Flathead Mullet (Mugil Cephalus) Caught from the West Black Sea Coast of Turkey. Environ. Sci. Pollut. Res. 2015, 22, 2515–2525. [Google Scholar] [CrossRef]
- Smeltz, M.; Rowland-Faux, L.; Ghiran, C.; Patterson, W.F.; Garner, S.B.; Beers, A.; Mièvre, Q.; Kane, A.S.; James, M.O. A Multi-Year Study of Hepatic Biomarkers in Coastal Fishes from the Gulf of Mexico after the Deepwater Horizon Oil Spill. Mar. Environ. Res. 2017, 129, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Noleto, K.S.; Mendes, D.C.S.; Carvalho, I.F.S.; Ribeiro, D.L.S.; Santos, D.M.S.; Ferreira, A.P.M.; Marques, A.L.B.; Carvalho-Neta, R.N.F.; Tchaicka, L.; Torres-Júnior, J.R.S. Aquatic Pollutants Are Associated with Reproductive Alterations and Genotoxicity in Estuarine Fish (Sciades herzbergii-Bloch, 1794) from the Amazon Equatorial Coast. Braz. J. Biol. 2022, 82, e267996. [Google Scholar] [CrossRef]
- Carr, D.L.; Smith, E.E.; Thiyagarajah, A.; Cromie, M.; Crumly, C.; Davis, A.; Dong, M.; Garcia, C.; Heintzman, L.; Hopper, T.; et al. Assessment of Gonadal and Thyroid Histology in Gulf Killifish (Fundulus grandis) from Barataria Bay Louisiana One Year after the Deepwater Horizon Oil Spill. Ecotoxicol. Environ. Saf. 2018, 154, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, F.; Su, X.; Li, M.; Yan, H.; Zheng, S.; Ma, Y.; Dong, J.; Liu, Y.; Lin, Q.; et al. Reproductive Toxicity and Molecular Mechanisms of Benzo[a]Pyrene Exposure on Ovary, Testis, and Brood Pouch of Sex-Role-Reversed Seahorses (Hippocampus erectus). Environ. Pollut. 2025, 367, 125569. [Google Scholar] [CrossRef]
- Mousavi, A.; Salamat, N.; Safahieh, A. Phenanthrene Disrupting Effects on the Thyroid System of Arabian Seabream, Acanthopagrus Arabicus: In Situ and In Vivo Study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 252, 109226. [Google Scholar] [CrossRef]
- Movahedinia, A.; Salamat, N.; Kheradmand, P. Effects of the Environmental Endocrine Disrupting Compound Benzo[a]Pyrene on Thyroidal Status of Abu Mullet (Liza abu) during Short-Term Exposure. Toxicol. Rep. 2018, 5, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, Z.; Ali Movahedinia, A.; Rastgar, S.; Alijani Ardeshir, R. Effects of Naphthalene on Plasma Cortisol and Thyroid Levels in Immature and Mature Female Klunzingeri Mulet, Liza Klunzingeri. Iran. J. Toxicol. 2016, 10, 45–49. [Google Scholar] [CrossRef]
- de Pinho, J.V.; Rodrigues, P.d.A.; Guimarães, I.D.L.; Monteiro, F.C.; Ferrari, R.G.; Hauser-Davis, R.A.; Conte-Junior, C.A. The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 1211. [Google Scholar] [CrossRef]
- Dearnley, J.M.; Killeen, C.; Davis, R.L.; Palace, V.P.; Tomy, G.T. Monitoring Polycyclic Aromatic Compounds Exposure in Fish Using Biliary Metabolites. Crit. Rev. Environ. Sci. Technol. 2022, 52, 475–519. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Endocrine-Disrupting Air Pollutants and Their Effects on the Hypothalamus-Pituitary-Gonadal Axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef]
- Bak, S.M.; Nakata, H.; Koh, D.H.; Yoo, J.; Iwata, H.; Kim, E.Y. In Vitro and in Silico AHR Assays for Assessing the Risk of Heavy Oil-Derived Polycyclic Aromatic Hydrocarbons in Fish. Ecotoxicol. Environ. Saf. 2019, 181, 214–223. [Google Scholar] [CrossRef]
- Woo, S.J.; Chung, J.K. Cytochrome P450 1 Enzymes in Black Rockfish, Sebastes Schlegelii: Molecular Characterization and Expression Patterns after Exposure to Benzo[a]Pyrene. Aquat. Toxicol. 2020, 226, 105566. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, I.; Bilbao, E.; Meador, J.P.; Cancio, I.; Bender, M.L.; Cajaraville, M.P.; Nahrgang, J. Effects of Dietary Crude Oil Exposure on Molecular and Physiological Parameters Related to Lipid Homeostasis in Polar Cod (Boreogadus saida). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 206, 54–64. [Google Scholar] [CrossRef]
- Sturve, J.; Hultman, M.T.; Wassmur, B.; Almroth, B.C. Determining Oxidative Stress and EROD Activity in Dab (Limanda limanda) in the North and Baltic Seas. Mar. Environ. Res. 2017, 124, 46–53. [Google Scholar] [CrossRef]
- Santana, M.S.; Sandrini-Neto, L.; Filipak Neto, F.; Oliveira Ribeiro, C.A.; Di Domenico, M.; Prodocimo, M.M. Biomarker Responses in Fish Exposed to Polycyclic Aromatic Hydrocarbons (PAHs): Systematic Review and Meta-Analysis. Environ. Pollut. 2018, 242, 449–461. [Google Scholar] [CrossRef]
- Duarte, R.M.; Sadauskas-Henrique, H.; de Almeida-Val, V.M.F.; Val, A.L.; Nice, H.E.; Gagnon, M.M. Biomarker Responses and PAH Ratios in Fish Inhabiting an Estuarine Urban Waterway. Environ. Toxicol. 2017, 32, 2305–2315. [Google Scholar] [CrossRef]
- Grosell, M.; Pasparakis, C. Physiological Responses of Fish to Oil Spills. Annu. Rev. Mar. Sci. 2021, 13, 137–160. [Google Scholar] [CrossRef]
- Berenguer, J.N.; Moraes, J.d.C.; Oliveira, M.M.; Raimundo, J.M.; Molisani, M.M. Effects of Diesel Oil and Environmental Quality on the Enzymatic Activities of a Tropical Estuarine Catfish and Implications for Contamination Assessment. Ecotoxicol. Environ. Contam. 2017, 12, 103–111. [Google Scholar] [CrossRef]
- Borcier, E.; Charrier, G.; Couteau, J.; Maillet, G.; Le Grand, F.; Bideau, A.; Waeles, M.; Le Floch, S.; Amara, R.; Pichereau, V.; et al. An Integrated Biomarker Approach Using Flounder to Improve Chemical Risk Assessments in the Heavily Polluted Seine Estuary. J. Xenobiot. 2020, 10, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, M.; Di Napoli, E.; Paciello, O.; d’Aquino, I.; Iaccarino, D.; D’amore, M.; Guida, M.; Cozzolino, L.; Serpe, F.P.; Fusco, G.; et al. Pathological Changes and CYP1A1 Expression as Biomarkers of Pollution in Sarpa Salpa and Diplodus Sargus. Animals 2024, 14, 3160. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Freeman, J.L. Toxicogenomics to Evaluate Endocrine Disrupting Effects of Environ-Mental Chemicals Using the Zebrafish Model. Curr. Genom. 2016, 17, 515–527. [Google Scholar] [CrossRef]
- Ghanem, S.F. Effect of Endocrine Disrupting Chemicals Exposure on Reproduction and Endocrine Functions Using the Zebrafish Model. Egypt. J. Aquat. Biol. Fish. 2021, 25, 951–981. [Google Scholar] [CrossRef]
- Hachfi, L.; Couvray, S.; Simide, R.; Tarnowska, K.; Pierre, S.; Gaillard, S.; Richard, S.; Coupé, S.; Grillasca, J.; D’Alvise, N.P. Impact of Endocrine Disrupting Chemicals [EDCs] on Hypothalamic-Pituitary-Gonad-Liver [HPGL] Axis in Fish. World J. Fish Mar. Sci. 2012, 4, 14–30. [Google Scholar]
- Adeogun, A.O.; Ibor, O.R.; Adeduntan, S.D.; Arukwe, A. Intersex and Alterations in Reproductive Development of a Cichlid, Tilapia Guineensis, from a Municipal Domestic Water Supply Lake (Eleyele) in Southwestern Nigeria. Sci. Total Environ. 2016, 541, 372–382. [Google Scholar] [CrossRef]
- Dupuis, A.; Ucan-Marin, F. A Literature Review on the Aquatic Toxicology of Petroleum Oil: An Overview of Oil Properties and Effects to Aquatic Biota; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2015. [Google Scholar]
- Mokhtar, D.M. Diversity and Dynamics of Fish Ovaries: Insights into Reproductive Strategies, Hormonal Regulation, and Ovarian Development. Histol. Histopathol. 2025, 40, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, D.; Lin, J.; Dai, Q.; Zhou, Q.; Chen, Y.; Wang, C. Benzo(a)Pyrene Exposure in Early Life Suppresses Spermatogenesis in Adult Male Zebrafish and Association with the Methylation of Germ Cell-Specific Genes. Aquat. Toxicol. 2023, 258, 106504. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Yu, Z.; Guan, Y.; Chen, R.; Wang, C. Early-Life Phenanthrene Exposure Inhibits Reproductive Ability in Adult Zebrafish and the Mechanism of Action. Chemosphere 2021, 272, 129635. [Google Scholar] [CrossRef]
- Bolden, A.L.; Rochester, J.R.; Schultz, K.; Kwiatkowski, C.F. Polycyclic Aromatic Hydrocarbons and Female Reproductive Health: A Scoping Review. Reprod. Toxicol. 2017, 73, 61–74. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian Aromatase and Estrogens: A Pivotal Role for Gonadal Sex Differentiation and Sex Change in Fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- De Coster, S.; Van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Souza, T.L.; de Morais, T.P.; Neto, F.F.; Opuskevitch, I.; Ferreira, F.C.A.S.; Randi, M.A.F.; de Oliveira Ribeiro, C.A.; de Souza, C.; Prodocimo, M.M. Physicochemical and Bioinformatic Characterization of Oreochromis Niloticus Vitellogenin as an Endocrine Disruption Biomarker. Ecotoxicology 2023, 32, 12–24. [Google Scholar] [CrossRef]
- Tramunt, B.; Montagner, A.; Tan, N.S.; Gourdy, P.; Rémignon, H.; Wahli, W. Roles of Estrogens in the Healthy and Diseased Oviparous Vertebrate Liver. Metabolites 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.L.; da Luz, J.Z.; Barreto, L.d.S.; de Oliveira Ribeiro, C.A.; Neto, F.F. Structure-Based Modeling to Assess Binding and Endocrine Disrupting Potential of Polycyclic Aromatic Hydrocarbons in Danio Rerio. Chem. Biol. Interact. 2024, 398, 111109. [Google Scholar] [CrossRef] [PubMed]
- Badamasi, I.; Odong, R.; Masembe, C. Threats Posed by Xenoestrogenic Chemicals to the Aquatic Ecosystem, Fish Reproduction and Humans: A Review. Afr. J. Aquat. Sci. 2020, 45, 243–258. [Google Scholar] [CrossRef]
- Delbes, G.; Blázquez, M.; Fernandino, J.I.; Grigorova, P.; Hales, B.F.; Metcalfe, C.; Navarro-Martín, L.; Parent, L.; Robaire, B.; Rwigemera, A.; et al. Effects of Endocrine Disrupting Chemicals on Gonad Development: Mechanistic Insights from Fish and Mammals. Environ. Res. 2022, 204, 112040. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Alavi, S.M.H. Androgen Signaling in Male Fishes: Examples of Anti-Androgenic Chemicals That Cause Reproductive Disorders. Theriogenology 2019, 139, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Chen, L.R.; Chen, K.H. In Vitro and Vivo Identification, Metabolism and Action of Xenoestrogens: An Overview. Int. J. Mol. Sci. 2021, 22, 4013. [Google Scholar] [CrossRef]
- Celino-Brady, F.T.; Lerner, D.T.; Seale, A.P. Experimental Approaches for Characterizing the Endocrine-Disrupting Effects of Environmental Chemicals in Fish. Front. Endocrinol. 2021, 11, 619361. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, J.; Wu, X.; Huang, B.; Pan, X. Nonmonotonic Responses to Low Doses of Xenoestrogens: A Review. Environ. Res. 2017, 155, 199–207. [Google Scholar] [CrossRef]
- Fu, D.; Bridle, A.; Leef, M.; Gagnon, M.M.; Hassell, K.L.; Nowak, B.F. Using a Multi-Biomarker Approach to Assess the Effects of Pollution on Sand Flathead (Platycephalus bassensis) from Port Phillip Bay, Victoria, Australia. Mar. Pollut. Bull. 2017, 119, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.R.; Ila, K.; Sahu, U.B.; Sehgal, N. Temporal Expression of Thyroid Hormone Regulating Genes (Tsh-b, Tsh-r, Dio2 and Dio3) and Their Correlation with Annual Reproductive Cycle of the Indian Freshwater Catfish, Heteropneustes Fossilis (Bloch). J. Appl. Nat. Sci. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Deal, C.K.; Volkoff, H. The Role of the Thyroid Axis in Fish. Front. Endocrinol. 2020, 11, 596585. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Liu, Z.; Chen, Q. Environmentally Relevant Concentrations of Mercury Exposure Alter Thyroid Hormone Levels and Gene Expression in the Hypothalamic–Pituitary–Thyroid Axis of Zebrafish Larvae. Fish Physiol. Biochem. 2018, 44, 1175–1183. [Google Scholar] [CrossRef]
- Gairin, E.; Dussenne, M.; Mercader, M.; Berthe, C.; Reynaud, M.; Metian, M.; Mills, S.C.; Lenfant, P.; Besseau, L.; Bertucci, F.; et al. Harbours as Unique Environmental Sites of Multiple Anthropogenic Stressors on Fish Hormonal Systems. Mol. Cell. Endocrinol. 2022, 555, 111727. [Google Scholar] [CrossRef]
- Nugegoda, D.; Kibria, G. Effects of Environmental Chemicals on Fish Thyroid Function: Implications for Fisheries and Aquaculture in Australia. Gen. Comp. Endocrinol. 2017, 244, 40–53. [Google Scholar] [CrossRef]
- Rurale, G.; Gentile, I.; Carbonero, C.; Persani, L.; Marelli, F. Short-Term Exposure Effects of the Environmental Endocrine Disruptor Benzo(a)Pyrene on Thyroid Axis Function in Zebrafish. Int. J. Mol. Sci. 2022, 23, 5833. [Google Scholar] [CrossRef] [PubMed]
- Laurent, J.; Lavergne, E.; Couteau, J.; Le Floch, S.; Ouddane, B.; Cachot, J.; Davail, B.; Clérandeau, C.; Devin, S.; Fisson, C.; et al. Impacts of Chemical Stress, Season, and Climate Change on the Flounder Population of the Highly Anthropised Seine Estuary (France). Environ. Sci. Pollut. Res. 2022, 29, 59751–59769. [Google Scholar] [CrossRef] [PubMed]
- García-Gasca, A.; Ríos-Sicairos, J.; Hernández-Cornejo, R.; Cunha, I.; Gutiérrez, J.N.; Plascencia-González, H.; de la Parra, L.M.G.; Abad-Rosales, S.; Betancourt-Lozano, M. The White Mullet (Mugil curema) as Biological Indicator to Assess Environmental Stress in Tropical Coastal Lagoons. Environ. Monit. Assess. 2016, 188, 688. [Google Scholar] [CrossRef]
- Verga, J.U.; Huff, M.; Owens, D.; Wolf, B.J.; Hardiman, G. Integrated Genomic and Bioinformatics Approaches to Identify Molecular Links between Endocrine Disruptors and Adverse Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 574. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Cowx, I.G.; Oleksiak, M.F.; Griffiths, A.M.; Grahn, M.; Stevens, J.R.; Carvalho, G.R.; Nicol, E.; Tyler, C.R. Population-Level Consequences for Wild Fish Exposed to Sublethal Concentrations of Chemicals—A Critical Review. Fish Fish. 2016, 17, 545–566. [Google Scholar] [CrossRef]
- Bautista, N.M.; Crespel, A.M.; Bautista, G.; Burggren, W.W. Dietary Crude Oil Exposure during Sex Differentiation Skewed Adult Sex Ratio towards Males in the Zebrafish. Sci. Total Environ. 2023, 892, 164449. [Google Scholar] [CrossRef]
- Ortiz-Zarragoitia, M.; Bizarro, C.; Rojo-Bartolomé, I.; De Cerio, O.D.; Cajaraville, M.P.; Cancio, I. Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments. Mar. Drugs 2014, 12, 4756–4782. [Google Scholar] [CrossRef] [PubMed]
- Bozinovic, G.; Shea, D.; Feng, Z.; Hinton, D.; Sit, T.; Oleksiak, M.F. PAH-Pollution Effects on Sensitive and Resistant Embryos: Integrating Structure and Function with Gene Expression. PLoS ONE 2021, 16, e0249432. [Google Scholar] [CrossRef]
- Pham, H.Q.; Le, H.M. Seasonal Changes in Three Indices of Gonadal Maturation in Male Golden Rabbitfish (Siganus guttatus): Implications for Artificial Propagation. Fish Physiol. Biochem. 2020, 46, 1111–1120. [Google Scholar] [CrossRef]
- Higuchi, K.; Suzuki, A.; Eba, T.; Hashimoto, H.; Kumon, K.; Morioka, T.; Shiozawa, S.; Soma, S.; Okita, K.; Takashi, T.; et al. Seasonal Changes and Endocrine Regulation of Gonadal Development in Hatchery-Produced Pacific Bluefin Tuna Thunnus Orientalis Broodstock in Sea Cages. Aquaculture 2021, 545, 737199. [Google Scholar] [CrossRef]
- Sueiro, M.C.; Palacios, M.G.; Trudeau, V.L.; Somoza, G.M.; Awruch, C.A. Anthropogenic Impact on the Reproductive Health of Two Wild Patagonian Fish Species with Differing Reproductive Strategies. Sci. Total Environ. 2022, 838, 155862. [Google Scholar] [CrossRef] [PubMed]
- Dubansky, B.; Whitehead, A.; Miller, J.T.; Rice, C.D.; Galvez, F. Multitissue Molecular, Genomic, and Developmental Effects of the Deepwater Horizon Oil Spill on Resident Gulf Killifish (Fundulus grandis). Environ. Sci. Technol. 2013, 47, 5074–5082. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, J.; Song, A.; Zhou, Y.; Miao, J.; Li, Z.; Pan, L. Study on Reproductive Endocrine Disturbance and DNA Damage Mechanism of Female Ruditapes Philippinarum under Benzo[a]Pyrene Stress. Environ. Pollut. 2024, 340, 122844. [Google Scholar] [CrossRef] [PubMed]
- Kummer, V.; Mašková, J.; Zralý, Z.; Neča, J.; Šimečková, P.; Vondráček, J.; Machala, M. Estrogenic Activity of Environmental Polycyclic Aromatic Hydrocarbons in Uterus of Immature Wistar Rats. Toxicol. Lett. 2008, 180, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Casanova-Nakayama, A.; Kase, R.; Tyler, C.R. Impact of Environmental Estrogens on Yfish Considering the Diversity of Estrogen Signaling. Gen. Comp. Endocrinol. 2013, 191, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of Estrogen Receptors in Health and Disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Toporova, L.; Balaguer, P. Nuclear Receptors Are the Major Targets of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Zhang, Y.; Cai, L.; Qiu, W.; Yang, M. G Protein-Coupled Estrogen Receptor 1 Mediates Estrogen Effect in Red Common Carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108868. [Google Scholar] [CrossRef]
- Nelson, E.R.; Habibi, H.R. Estrogen Receptor Function and Regulation in Fish and Other Vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef]
- Delfosse, V.; Grimaldi, M.; Cavaillès, V.; Balaguer, P.; Bourguet, W. Structural and Functional Profiling of Environmental Ligands for Estrogen Receptors. Environ. Health Perspect. 2015, 122, 1306–1313. [Google Scholar] [CrossRef]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular Mechanism of Estrogen–Estrogen Receptor Signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef]
- Arao, Y.; Korach, K.S. The F Domain of Estrogen Receptor Is Involved in Species-Specific, Tamoxifen-Mediated Transactivation. J. Biol. Chem. 2018, 293, 8495–8507. [Google Scholar] [CrossRef]
- Koide, A.; Zhao, C.; Naganuma, M.; Abrams, J.; Deighton-Collins, S.; Skafar, D.F.; Koide, S. Identification of Regions within the F Domain of the Human Estrogen Receptor α That Are Important for Modulating Transactivation and Protein-Protein Interactions. Mol. Endocrinol. 2007, 21, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The Many Faces of Estrogen Signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef]
- Schug, T.T.; Blawas, A.M.; Gray, K.; Heindel, J.J.; Lawler, C.P. Elucidating the Links between Endocrine Disruptors and Neurodevelopment. Endocrinology 2015, 156, 1941–1951. [Google Scholar] [CrossRef]
- Fertuck, K.C.; Kumar, S.; Sikka, H.C.; Matthews, J.B.; Zacharewski, T.R. Interaction of PAH-Related Compounds with the Alpha and Beta of the Estrogen Receptor. Toxicol. Lett. 2001, 121, 167–177. [Google Scholar] [CrossRef]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic Endocrine Disruptors: Molecular Mechanisms of Action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef] [PubMed]
- Zajda, K.; Gregoraszczuk, E.L. Environmental Polycyclic Aromatic Hydrocarbons Mixture, in Human Blood Levels, Decreased Oestradiol Secretion by Granulosa Cells via ESR1 and GPER1 but Not ESR2 Receptor. Hum. Exp. Toxicol. 2020, 39, 276–289. [Google Scholar] [CrossRef]
- de Souza, T.L.; da Luz, J.Z.; de Almeida Roque, A.; Opuskevitch, I.; da Silva Ferreira, F.C.A.; de Oliveira Ribeiro, C.A.; Neto, F.F. Exploring the Endocrine Disrupting Potential of a Complex Mixture of PAHs in the Estrogen Pathway in Oreochromis Niloticus Hepatocytes. Aquat. Toxicol. 2024, 273, 107002. [Google Scholar] [CrossRef]
- Vondráček, J.; Pivnička, J.; Machala, M. Polycyclic Aromatic Hydrocarbons and Disruption of Steroid Signaling. Curr. Opin. Toxicol. 2018, 11, 27–34. [Google Scholar] [CrossRef]
- Fernandes, D.; Porte, C. Hydroxylated PAHs Alter the Synthesis of Androgens and Estrogens in Subcellular Fractions of Carp Gonads. Sci. Total Environ. 2013, 447, 152–159. [Google Scholar] [CrossRef]
- Evanson, M.; Van Der Kraak, G.J. Stimulatory Effects of Selected PAHs on Testosterone Production in Goldfish and Rainbow Trout and Possible Mechanisms of Action. Comp. Biochem. Physiol. Part C 2001, 130, 249258. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Larsson, A.; Scherbak, N.; Olsson, P.E.; Orban, L. Zebrafish Androgen Receptor: Isolation, Molecular, and Biochemical Characterization. Biol. Reprod. 2008, 78, 361–369. [Google Scholar] [CrossRef]
- Smolinsky, A.N.; Doughman, J.M.; Kratzke, L.T.C.; Lassiter, C.S. Zebrafish (Danio rerio) Androgen Receptor: Sequence Homology and up-Regulation by the Fungicide Vinclozolin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Androg. Recept. Biol. Clin. Biochem. Rev. 2016, 37, 3. [Google Scholar]
- Heemers, H.V.; Tindall, D.J. Androgen Receptor (AR) Coregulators: A Diversity of Functions Converging on and Regulating the AR Transcriptional Complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen Receptor (AR) Coregulators: An Overview. Endocr. Rev. 2002, 23, 175–200. [Google Scholar] [CrossRef]
- Bellas, J.; Hylland, K.; Burgeot, T. Editorial: New Challenges in Marine Pollution Monitoring. Front. Mar. Sci. 2020, 6, 820. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Bancel, S.; Cachot, J.; Bon, C.; Rochard, É.; Geffard, O. A Critical Review of Pollution Active Biomonitoring Using Sentinel Fish: Challenges and Opportunities. Environ. Pollut. 2024, 360, 124661. [Google Scholar] [CrossRef]
- Guillante, T.; Fonseca, J.d.S.; Costa, P.G.; Bianchini, A.; Robaldo, R.B.; Zebral, Y.D. Sex-Biased Response of Pollution Biomarkers in Fish: Insights from the Killifish Poecilia Vivipara. Aquat. Toxicol. 2023, 261, 106613. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical Methods for Polycyclic Aromatic Hydrocarbons and Their Global Trend of Distribution in Water and Sediment: A Review. In Recent Insights in Petroleum Science and Engineering; InTech: London, UK, 2018. [Google Scholar]
- Yemele, O.M.; Zhao, Z.; Nkoh, J.N.; Ymele, E.; Usman, M. A Systematic Review of Polycyclic Aromatic Hydrocarbon Pollution: A Combined Bibliometric and Mechanistic Analysis of Research Trend toward an Environmentally Friendly Solution. Sci. Total Environ. 2024, 926, 171577. [Google Scholar] [CrossRef]
- Soursou, V.; Campo, J.; Picó, Y. Revisiting the Analytical Determination of PAHs in Environmental Samples: An Update on Recent Advances. Trends Environ. Anal. Chem. 2023, 37, e00195. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Park, M.K.; Son, J.M.; Choi, S.D. Spatial Distribution and Temporal Variation of Polycyclic Aromatic Hydrocarbons in Runoff and Surface Water. Sci. Total Environ. 2021, 793, 148339. [Google Scholar] [CrossRef]
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between Climate Change and Contaminants. Mar. Pollut. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. New Challenges of Marine Ecotoxicology in a Global Change Context. Mar. Pollut. Bull. 2021, 166, 112242. [Google Scholar] [CrossRef]
- Cabral, H.; Fonseca, V.; Sousa, T.; Leal, M.C. Synergistic Effects of Climate Change and Marine Pollution: An Overlooked Interaction in Coastal and Estuarine Areas. Int. J. Environ. Res. Public Health 2019, 16, 2737. [Google Scholar] [CrossRef]
- Zoboli, O.; Weber, N.; Braun, K.; Krampe, J.; Zessner, M. Systematic Underestimation of Polycyclic Aromatic Hydrocarbon Aqueous Concentrations in Rivers. Environ. Sci. Pollut. Res. 2024, 31, 38117–38127. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Zhang, Z.F.; Kan, Z.; Jiang, C.; Liu, L.Y.; Ma, W.L.; Song, W.W.; Nikolaev, A.; Li, Y.F. Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation. Molecules 2021, 26, 2739. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Mokoena, L.; Tshilongo, J. A Review on Recent Developments in the Extraction and Identification of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Water 2024, 16, 2520. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Bordoloi, J.; Boruah, H.P.D. Analysis of Recent Patenting Activities in the Field of Bioremediation of Petroleum Hydrocarbon Pollutants Present in the Environment. Recent. Pat. Biotechnol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- The Canadian Food Inspection Agency (CFIA). Polycyclic Aromatic Hydrocarbons in Selected Foods; Canadian Food Inspection Agency: Barrie, ON, Canada, 2020.
- Chen, D.; Hu, Y.; Guan, B.; Yuan, C.; Jiang, L.; Shi, M.; Zhou, N.; Ren, Y.; Chen, B.; Ding, H.; et al. Method for Detecting Multiple Polycyclic Aromatic in Aquatic Products and Application Thereof. Patent CN118604201A, 6 September 2024. [Google Scholar]
- Chen, Z.; Li, H.; Huo, L.; Ding, R.; Du, H.; Wang, W.; Li, R.; Wang, F.; Lv, X. Secondary Mass Spectrometry Method for Detecting 16 Kinds of Polycyclic Aromatic in Aquatic Products. Patent CN102854280A, 2 January 2013. [Google Scholar]
- Blake, D.A.; Sun, Y. Antibody Reagents to Detect Polycyclic Aromatic Hydrocarbons Found in Oil Spills. Patent US11029303B2, 30 November 2017. [Google Scholar]
- Nuhu, A.A.; Basheer, C.; Shaikh, A.A.; Al-Arfaj, A.R. Determination of Polycyclic Aromatic Hydrocarbons in Water Using Nanoporous Material Prepared from Waste Avian Egg Shell. Patent US2015377752A1, 31 December 2015. [Google Scholar]
- Peng, X.; Zheng, D.; Liu, L.; Yan, W.; Hu, X.; Li, J.; Xia, Z.; Xia, H.; Peng, L. Application of Naphthyl-Modified Magnetic Ferroferric Oxide Nano Extraction Material in Enrichment and Detection of Polycyclic Aromatic Hydrocarbon. Patent CN115078579A, 20 September 2022. [Google Scholar]
- Chernyaev, A.P.; Zyk, E.N.; Butorin, D.A. Method of Determining General and Polycyclic Aromatic Hydrocarbons in Components of Ecosystem. Patent RU2589897C1, 10 July 2016. [Google Scholar]
- Yum, S.S.; Woo, S.O.; Lee, T.K.; Jeon, H.Y.; Lee, J.R.; Song, J.I. Polycyclic Aromatic Hydrocarbons Exposure Responsive Genes in Scleronephthya Gracillimum and the Method for Diagnosing the Coastal Environment Pollution Using the Same. Patent KR101199440B1, 9 November 2012. [Google Scholar]
- Zheng, D.; Lu, A.; Jiao, H.; Wang, J.; Yang, J.; Wu, B.; Jiang, H. Method for Determining Polycyclic Aromatic Hydrocarbon Pollution in Shellfish by Adopting Gas Chromatography-Mass Spectrometry. Patent CN115561338A, 3 January 2023. [Google Scholar]
- Wei, H.; Li, Z.; Duan, L.; Lin, J.; Li, E.; Wu, Y. Method for Determining Ultra-Trace Aromatic Hydrocarbon in Water. Patent CN112305116A, 2 February 2021. [Google Scholar]
- Wang, Z.; Zhao, H.; Liu, S.; Song, J.; Chen, G.; Qin, P.; Shen, H.; Huang, S.; Wang, C.; Chen, H. Preparation Method and Application of Magnetic Metal Organic Framework Composite Material MUiO-BDA. Patent CN118063840A, 24 May 2024. [Google Scholar]
- Bruguera, S.L.; Griell, H.F.; Treviño, J.S.; Clemente, V.M.O. Passive Ceramic Sampler for Measuring Water Contamination. Patent WO2016207461A1, 29 December 2016. [Google Scholar]
- Yuan, M.; Feng, Y.; Hong, J.; Li, J.; Ding, Y.; Wang, X.; Zhu, H.; Wu, L. Chemical Analyzer for High-Selectivity Detection of Polycyclic Aromatic Hydrocarbon in Environment. Patent CN118913795A, 8 November 2024. [Google Scholar]
- Li, Y.; Feng, J.; Yang, Z.; Shen, Z. A Method for Source Apportionment of PAHs in Roadway Sediments Coupled with Transport and Transformation Process. Patent AU2020101615A4, 10 September 2020. [Google Scholar]
- Rong, Q.; Zhang, H.; Li, Y.; Cheng, H. DGT Device for Polycyclic Aromatic Monitoring and Application Thereof. Patent CN116159342A, 26 May 2023. [Google Scholar]
- Deng, X.; Zhan, J.; Wu, F.; Lin, Y.; Ma, E.; Huang, W.; Yue, N. Method for recognizing Ecological Risks and Calculating Value at Risk for Land Oil Exploitation. Patent CN102062769A, 18 May 2011. [Google Scholar]
- Gao, K.; Wang, Q.; Li, Z.; Zhao, X.; Wu, Y.; Zhao, X.; Mei, P.; Zhang, Z. Application of Three-Spined Stickleback CYP1 Family Gene in Preparation of Water Body Pollution Detection Biomarker and Detection Method Thereof. Patent CN111208270A, 29 May 2020. [Google Scholar]
- Kosa, N.M.; Krebs, J.F.; Kasal, D.N. Enzymatic Method for Detecting Hydrocarbons. Patent US10871484B2, 22 December 2020. [Google Scholar]
- Vamvounis, G. Identification of Polycyclic Aromatic Hydrocarbons. Patent AU2017200771A1, 23 August 2018. [Google Scholar]
- Ghosh, U.; Jalalizadeh, M. Actively Shaken In-Situ Passive Sampling Device. Patent US10551283B2, 4 February 2020. [Google Scholar]
- Gotor, R.; Bell, J.; Rurack, K. Detection of Hydrocarbon Contamination in Soil and Water. Patent US11561175B2, 24 January 2023. [Google Scholar]
- Usova, S.V.; Vershinin, V.I.; Mamontova, A.V. Method for Determining the Total Content of Monocyclic Aromatic Hydrocarbons in Water. Patent RU2017109544A, 21 September 2018. [Google Scholar]
- Fan, L.; Shafie, S.R. Extraction of Harmful Compounds from Materials Containing Such Harmful Compounds. Patent US20140088209, 19 September 2013. [Google Scholar]
- Krebs, J.F.; Duvall-Jisha, J.; Resnick, S. Method of Detecting the Presence of Polycyclic Aromatic Hydrocarbons. Patent US20140154723, 5 June 2015. [Google Scholar]
- Blake, D.; Sun, Y. PAH Antibodies and Uses Thereof. Patent WO2016196638, 8 June 2016. [Google Scholar]
- Zhang, Q.; Liu, P.; Li, S.; Zhang, X.; Chen, M. Progress in the Analytical Research Methods of Polycyclic Aromatic Hydrocarbons (PAHs). J. Liq. Chromatogr. Relat. Technol. 2020, 43, 425–444. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; Liu, R.; Wang, Z.; Zhai, Y.; Lin, H.; Wen, W.; Li, Y.; Wang, D.; Yang, Z.; et al. Dietary Uptake Patterns Affect Bioaccumulation and Biomagnification of Hydrophobic Organic Compounds in Fish. Environ. Sci. Technol. 2019, 53, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Ivatt, P.D.; Evans, M.J.; Kroll, J.H.; Hrdina, A.I.H.; Kohale, I.N.; White, F.M.; Engelward, B.P.; Selin, N.E. Global Cancer Risk From Unregulated Polycyclic Aromatic Hydrocarbons. Geohealth 2021, 5, e2021GH000401. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, R.A.; de Oliveira, F.F.; de Souza, J.M.; Nudi, A.H.; de Luca Rebello Wagener, Â.; de Fátima Guadalupe Meniconi, M.; Francioni, E. Monitoring of Polycyclic Aromatic Hydrocarbons in a Produced Water Disposal Area in the Potiguar Basin, Brazilian Equatorial Margin. Environ. Sci. Pollut. Res. 2016, 23, 17113–17122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).