Chemical Dissection of PM2.5 in Cigarette Smoke: Main and Sidestream Emission Factors and Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cigarettes Selected for the Experiment
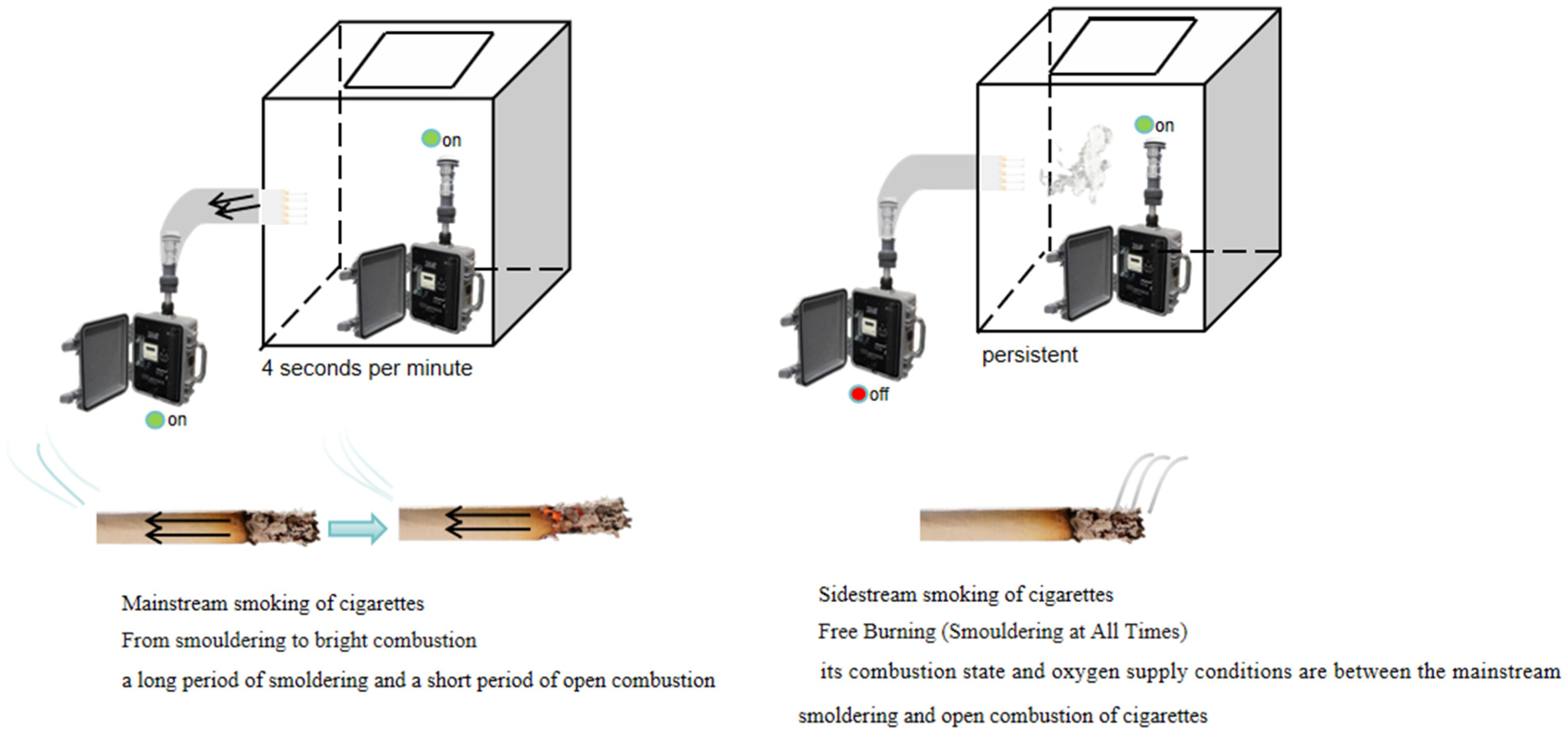
2.2. Collection of PM2.5 Sample from Main and Sidestream Smoke of Cigarettes
2.3. Determination of PM2.5 and Its Chemical Composition
2.3.1. Determination of PM2.5 Mass
2.3.2. Determination of Carbon Components
2.3.3. Determination of Water-Soluble Inorganic Ions (WSIIs)
2.3.4. Determination of Elements
2.3.5. Calculation of Emission Factors (EFs)
3. Results and Discussion
3.1. Distribution of PM2.5 and Its Chemical Composition in Smoke of Cigarettes
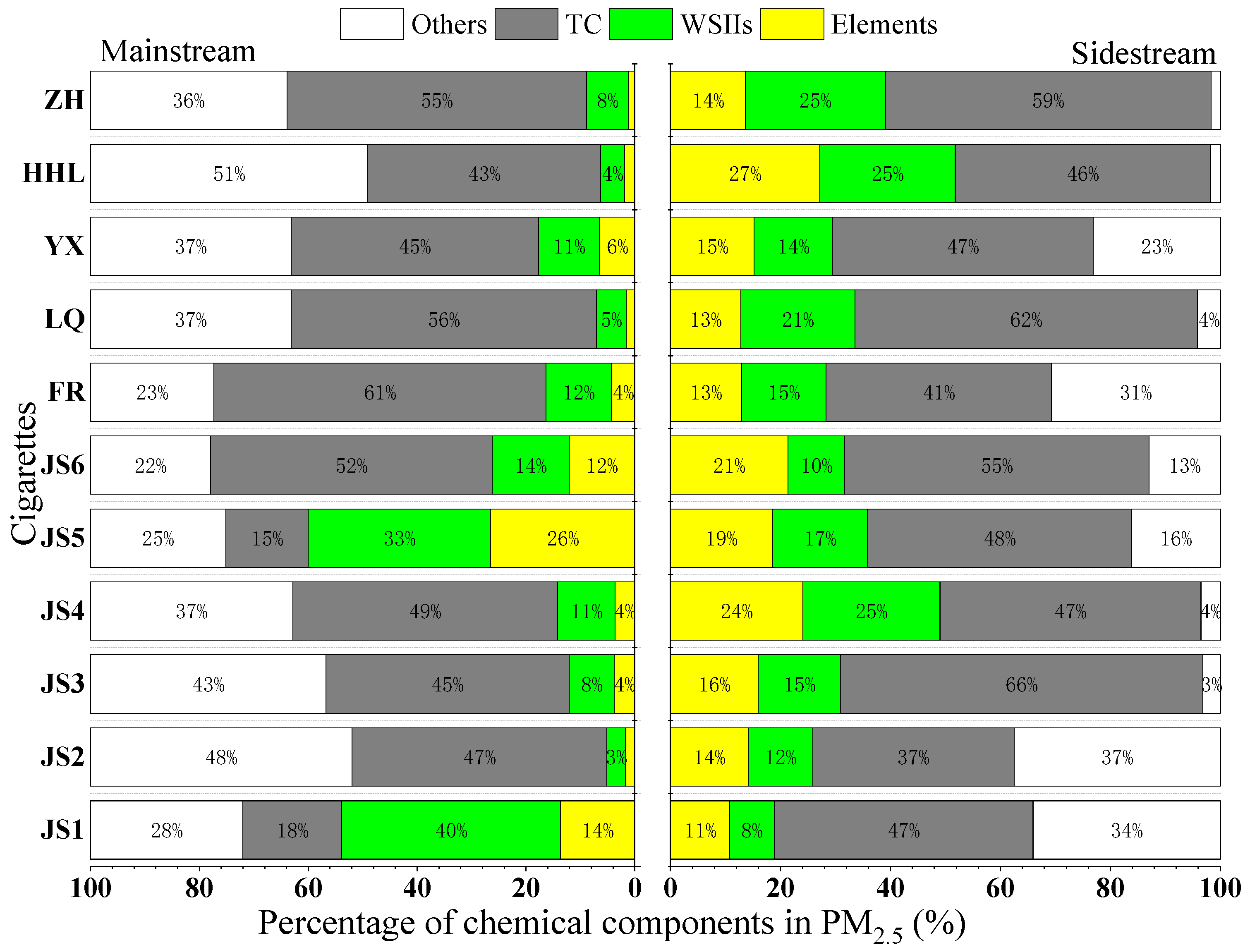
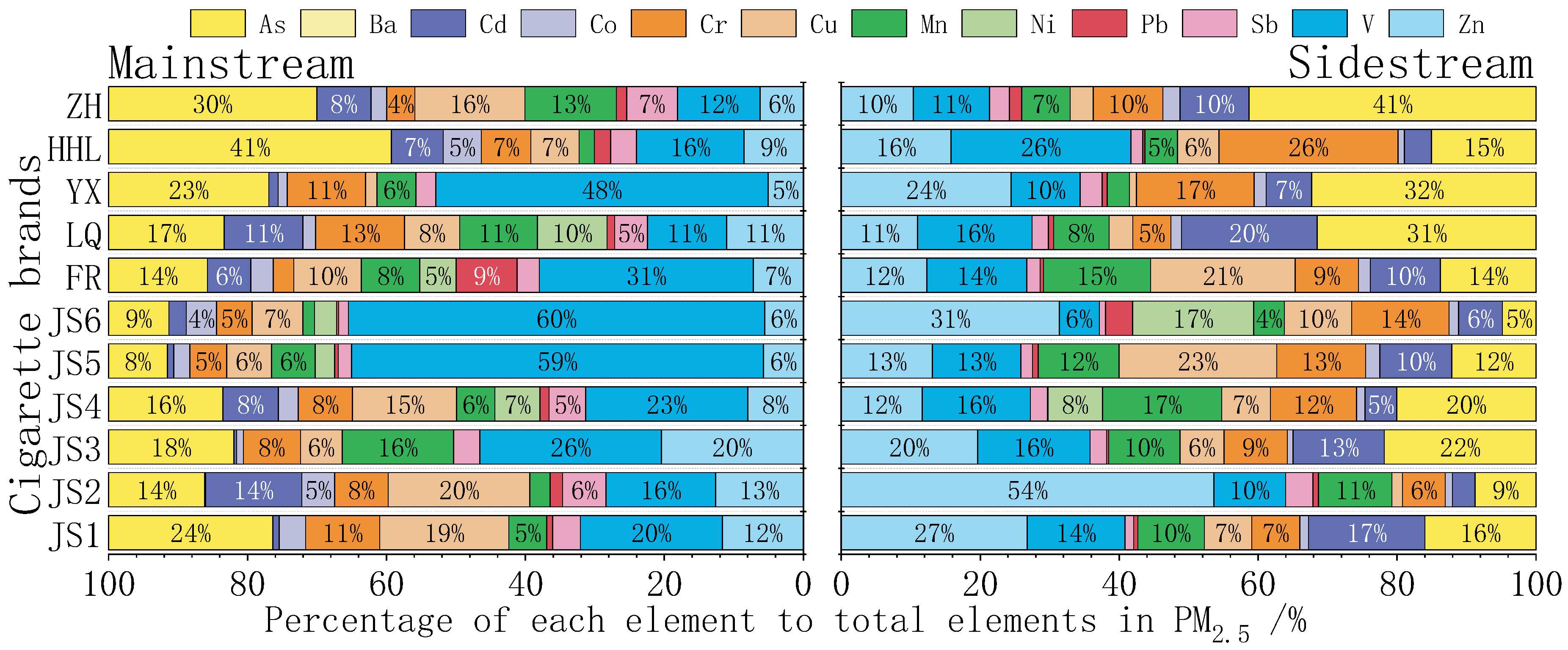
3.1.1. Percentage Composition of Chemical Components in PM2.5
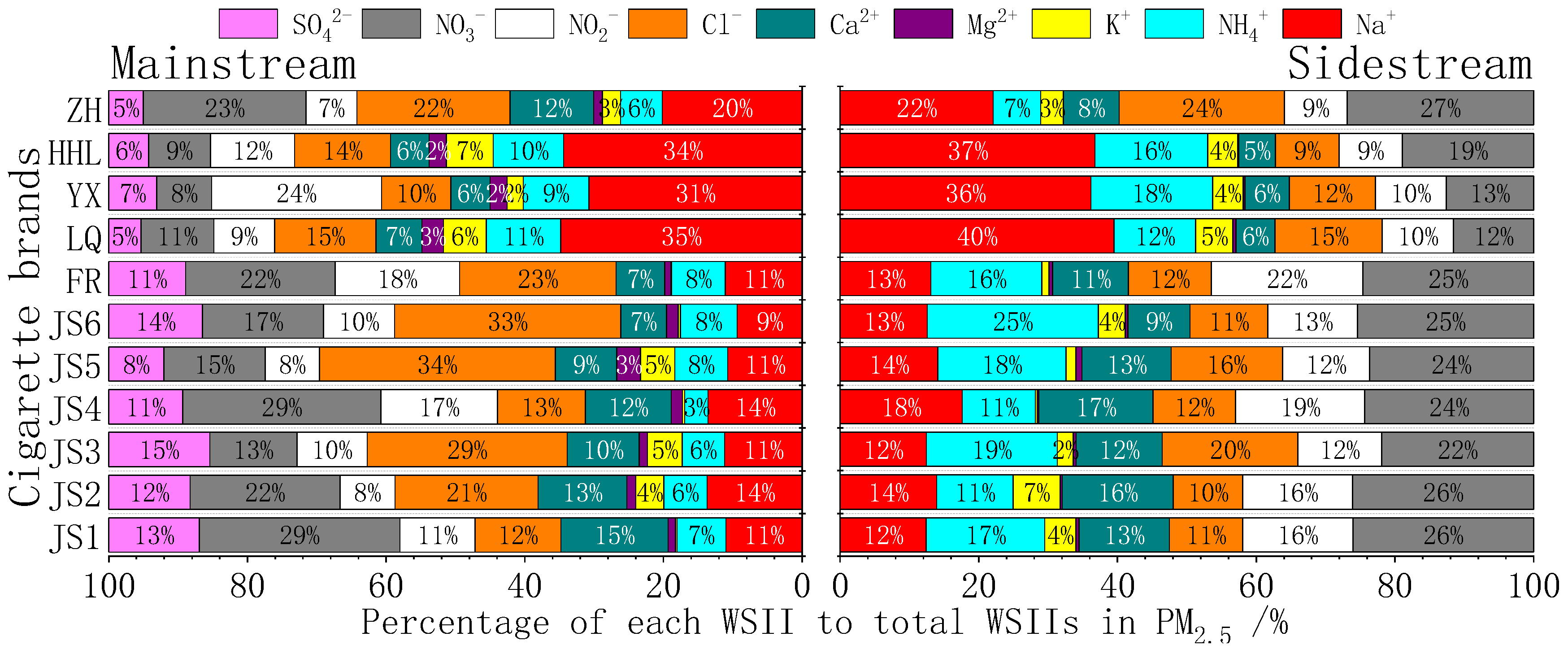
3.1.2. Distribution of Major Chemical Composition in PM2.5 (Carbon, Ions, and Metals)
3.1.3. Toxicity-Weighted Distribution of Major PM2.5 Chemical Species
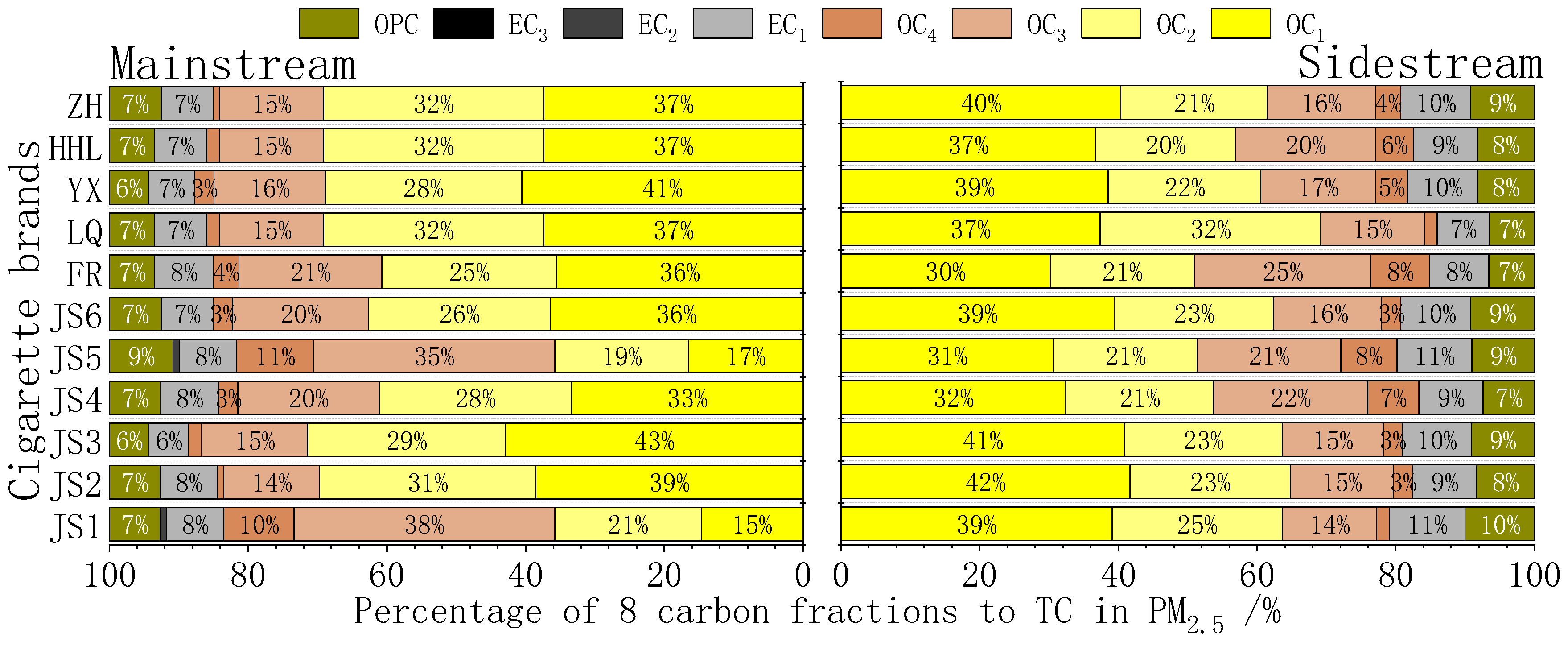
3.1.4. Distribution of Ratios of OC/EC
3.2. Emission Factors (EFs) of PM2.5 and Its Chemical Composition in Smoke of Cigarettes
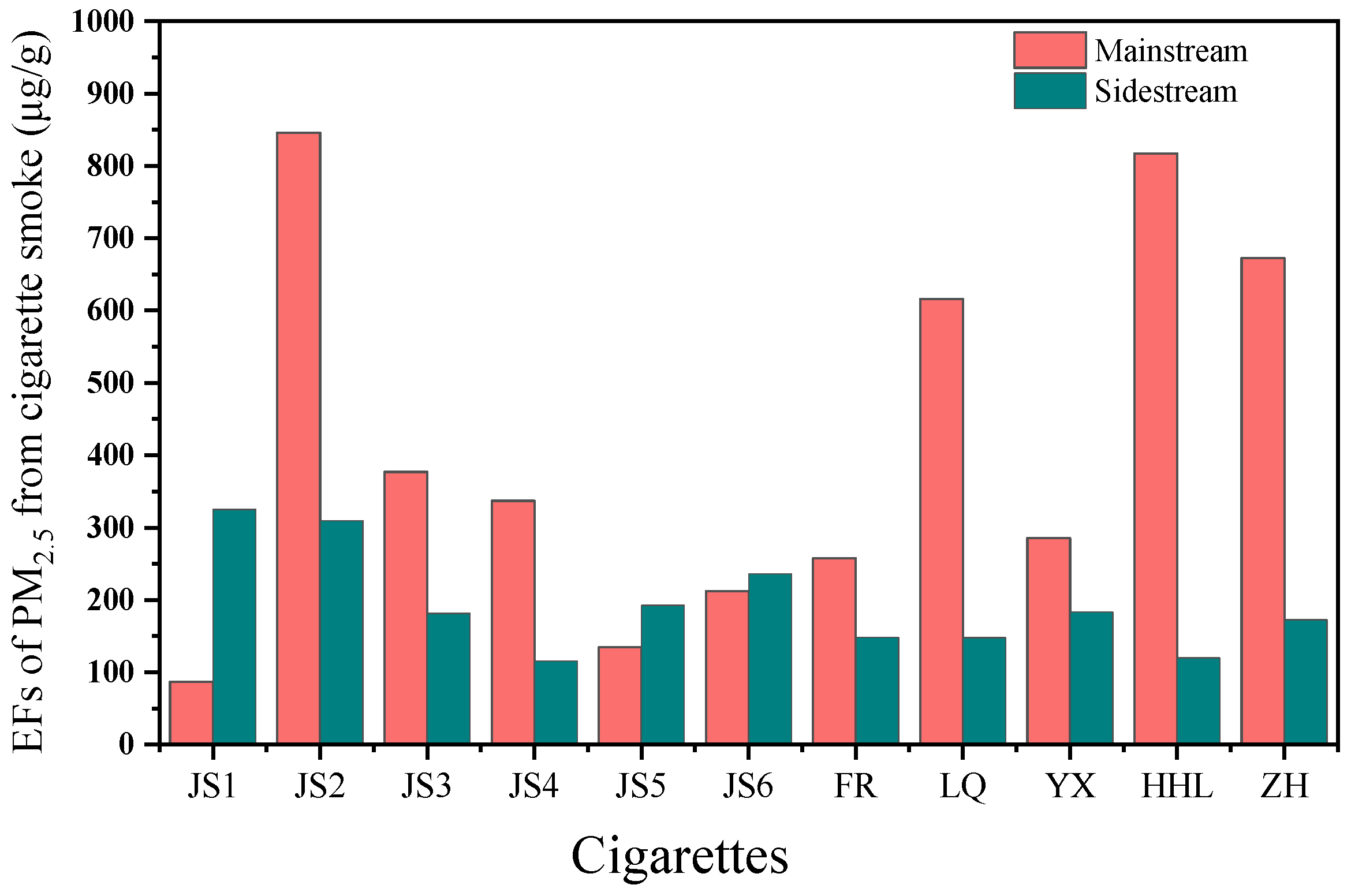
3.2.1. Emission Factors of PM2.5
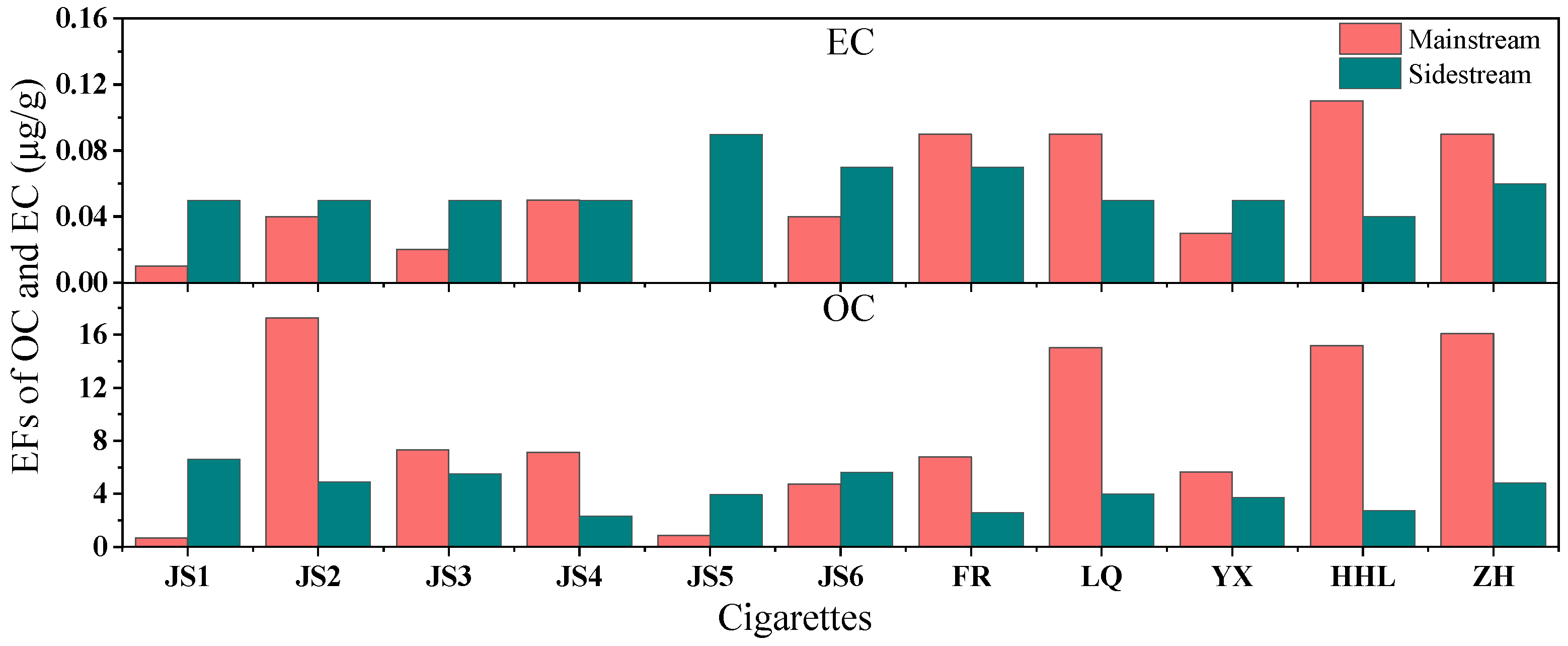
3.2.2. Emission Factors of Carbon Components
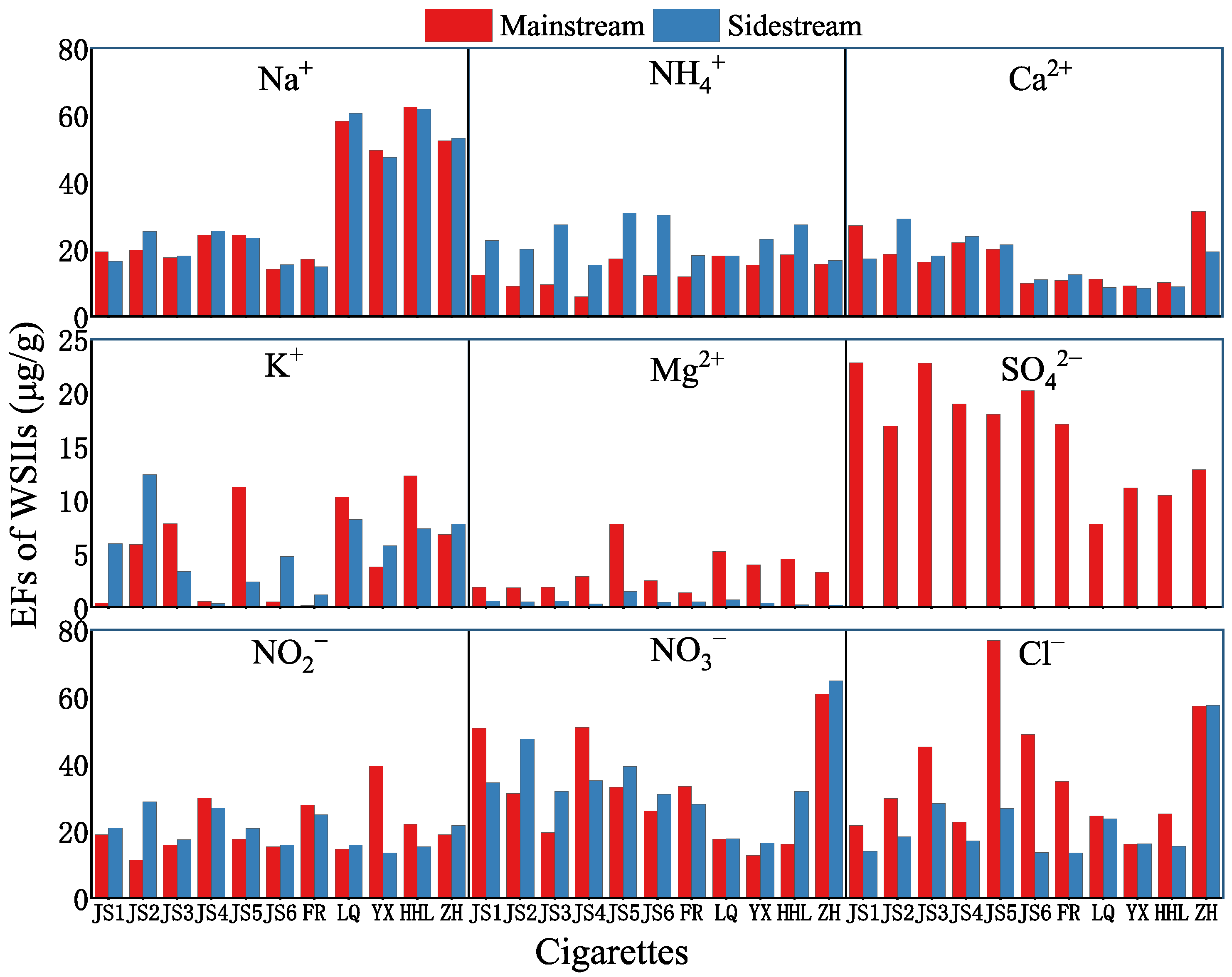
3.2.3. Emission Factors of Water-Soluble Inorganic Ions
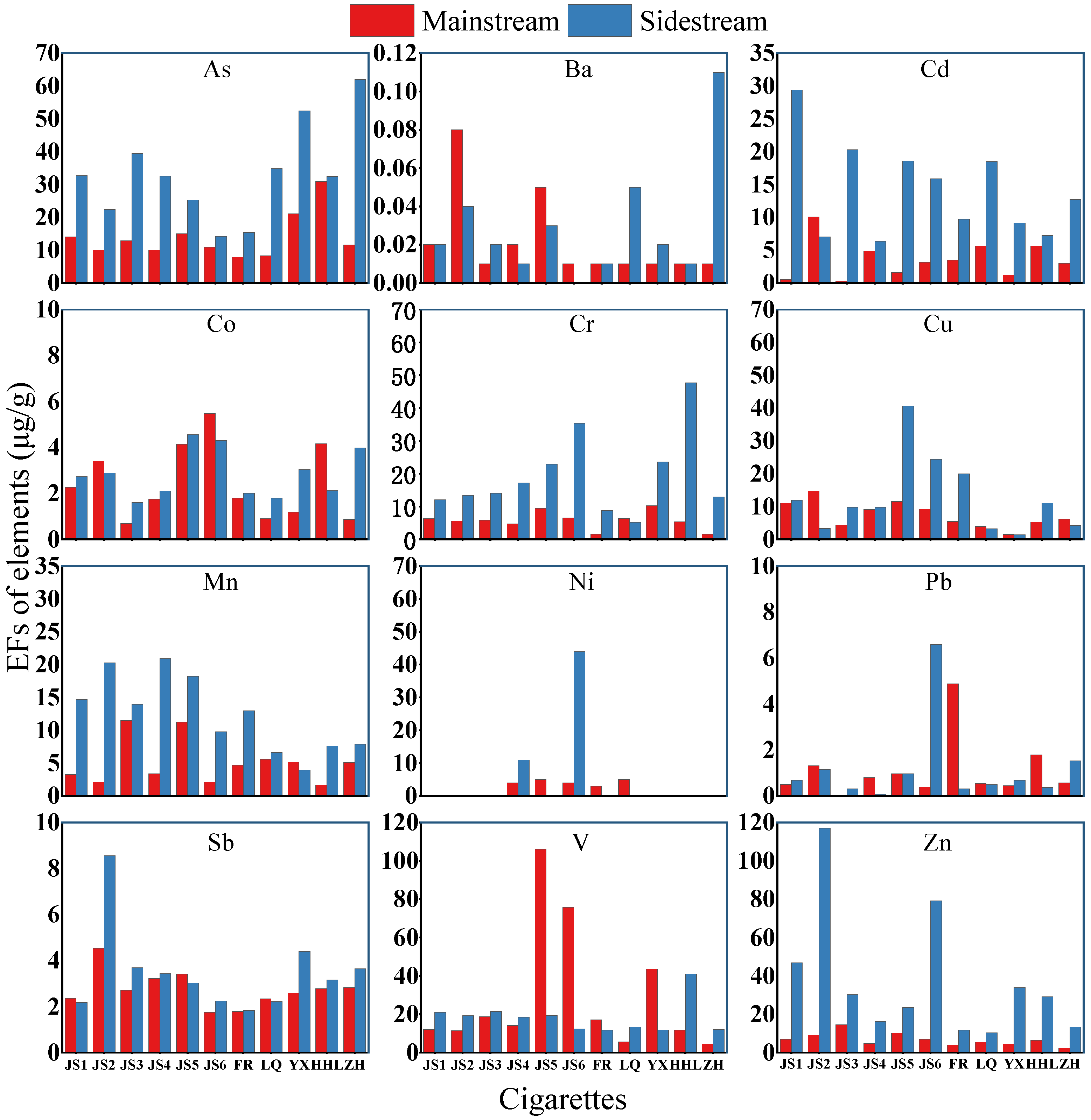
3.2.4. Emission Factors of Elements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mallah, M.A.; Soomro, T.; Ali, M.; Noreen, S.; Khatoon, N.; Kafle, A.; Feng, F.; Wang, W.; Naveed, M.; Zhang, Q. Cigarette smoking and air pollution exposure and their effects on cardiovascular diseases. Front. Public Health 2023, 11, 967047. [Google Scholar] [CrossRef]
- Hevia-Ramos, G.; Ha, E.; Hong, Y.C.; Kim, H.; Lim, Y.H.; Oh, J.A. systematic review and meta-analysis on long-term exposure to particulate matter and all-cause and cause-specific mortality in the Asia-Pacific states. J. Korean Med. Sci. 2025, 40, e156. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on the Global Tobacco Epidemic; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240077164 (accessed on 20 August 2025).
- Li, Y.; Cui, H.; Chen, L. Modeled Respiratory Tract Deposition of Smoke Aerosol from Conventional Cigarettes, Electronic Cigarettes and Heat-not-burn Products. Aerosol Air Qual. Res. 2021, 21, 200–241. [Google Scholar] [CrossRef]
- Lauren, M.P.; Mohamad, S.; Yael, D. Tobacco smoke aging in the presence of ozone: A room-sized chamber study. Atmos. Environ. 2011, 45, 4959–4965. [Google Scholar] [CrossRef]
- Barua, R.S.; Ambrose, J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1460–1467. [Google Scholar] [CrossRef]
- Hackshaw, A.; Morris, J.K.; Boniface, S. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018, 360, j5855. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.P.; Bhalla, D.K.; Whayne, T.J.; Gairola, C. Cigarette smoke and adverse health effects: An overview of research trends and future needs. Int. J. Angiol. 2007, 16, 77–83. [Google Scholar] [CrossRef]
- Dai, X.; Gil, G.F.; Reitsma, M.B. Health effects associated with smoking: A Burden of Proof study. Nat. Med. 2022, 28, 2045–2055. [Google Scholar] [CrossRef]
- Aslam, S.P.; Leslie, S.W.; Morris, J.; Bomgaars, D.L. Nicotine Addiction and Smoking: Health Effects and Interventions (Nursing). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568714/ (accessed on 3 July 2025). [PubMed]
- Alan, R.; Thomas, A.P. The Chemical Components of Tobacco and Tobacco Smoke; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429146831. [Google Scholar] [CrossRef]
- Arfaeinia, H.; Ghaemi, M.; Jahantigh, A.; Soleimani, F.; Hashemi, H. Secondhand and thirdhand smoke: A review on chemical contents, exposure routes, and protective strategies. Environ. Sci Pollut. Res. Int. 2023, 30, 78017–78029. [Google Scholar] [CrossRef]
- Behera, S.N.; Huang, X.; Rajasekhar, B. Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke. Sci. Total Environ. 2014, 472, 947–956. [Google Scholar] [CrossRef]
- Deng, B.; Chen, M.; Wu, S. The burning and pollutant formation processes during cigarette smoking under various inhalation frequencies. China Thermochim. Acta 2022, 17, 179348. [Google Scholar] [CrossRef]
- GB/T 16450-2004; Conventional Smoking Machine for Analysis—Definitions and Standard Conditions. China Standards Press: Beijing, China, 2004.
- Zou, C.; Zhang, Y.; Gao, Y.; Mao, X.; Huang, H.; Tan, Y. Characteristics, distribution, and sources of particulate carbon in rainfall collected by a sequential sampler in Nanchang. China Atmos. Environ. 2020, 235, 117619. [Google Scholar] [CrossRef]
- HJ 777-2015; Ambient Air and Waste Gas from Stationary Sources Emission—Determination of Metal Elements in Ambient Particle Matter—Inductively Coupled Plasma Optical Emission Spectrometry. China Environmental Science Press: Beijing, China, 2015.
- Marinova, D.; Karadjova, V.; Stoilova, D. Infrared spectroscopic study of SO42− ions included in M′2M″(SeO4)2·6H2O (Me′ = K, NH4+; M″ = Mg, Co, Ni, Cu, Zn) and NH4+ ions included in K2M(XO4)2·6H2O (X = S, Se; M″ = Mg, Co, Ni, Cu, Zn). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 526–534. [Google Scholar] [CrossRef]
- Gowing, L.R.; Ali, R.L.; Allsop, S.; Marsden, J.; Turf, E.E.; West, R. Global statistics on addictive behaviours: 2014 status report. Addiction 2015, 110, 904–919. [Google Scholar] [CrossRef]
- Simon, L.C.; Peter, B.; Wexler, A.S. Thermodynamic Model of the System H−NH4+−SO42−−NO3−−H2O at Tropospheric Temperatures. J. Phys. Chem. 1998, 102, 2137–2154. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010. Available online: https://stacks.cdc.gov/view/cdc/6067/cdc_6067_DS1.pdf (accessed on 6 July 2025).
- Han, Y.; Chen, Y.; Ahmad, S.; Feng, Y.; Zhang, F.; Song, W.; Cao, F.; Zhang, Y.; Yang, X.; Li, J.; et al. High time- and size-resolved measurements of PM and chemical composition from coal combustion: Implications for the EC formation process. Atmos. Chem. Phys. 2018, 52, 11. [Google Scholar] [CrossRef]
- Ray, I.; Mitra, S.; Kayee, J.; Yuan, S.; Nagendra, S.S.; Wang, X.; Das, R. Dominance of open burning signatures in PM2.5 near coal plant should redefine pollutant priorities of India. NPJ Clim. Atmos. Sci. 2024, 7, 284. [Google Scholar] [CrossRef]
- Kebe, M.; Traore, A.; Sow, M. Human health risk evaluation of particle air pollution (PM10 and PM2.5) and heavy metals in Dakar’s two urban areas. Asian J. Atmos. Environ. 2025, 19, 7. [Google Scholar] [CrossRef]
- Furuhashi, T.; Toda, K.; Weckwerth, W. Review of cancer cell volatile organic compounds: Their metabolism and evolution. Front. Mol. Biosci. 2025, 11, 1499104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakhar, R.; Styszko, K.; Samek, L. Oxidative potential of PM1, PM2.5, and PM10 collected in car and tram tunnels to analyze their impact on public health. Sci. Rep. 2025, 15, 21773. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Kim, G.-D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef]
- Cope, J.D.; Bates, K.H.; Tran, L.N.; Abellar, K.A.; Nguyen, T.B. Sulfur radical formation from the tropospheric irradiation of aqueous sulfate aerosols. Proc. Natl. Acad. Sci. USA 2022, 119, e2202857119. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution–REVIHAAP Project: Technical Report; WHO Regional Office for Europe: Geneva, Switzerland, 2013; Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2013-4101-43860-61757 (accessed on 10 July 2025).
- Pan, S.; Qiu, Y.; Li, M.; Yang, Z.; Liang, D. Recent Developments in the Determination of PM2.5 Chemical Composition. Bull. Environ. Contam. Toxicol. 2022, 108, 819–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyczyńska, M.; Gędek, M.; Brachet, A.; Stręk, W.; Flieger, J.; Teresiński, G.; Baj, J. Trace Elements in Alzheimer’s Disease and Dementia: The Current State of Knowledge. J. Clin. Med. 2024, 13, 2381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, J.; Jiang, Y.; Wang, X.; Li, X.; Zhu, Y.; Shao, Z.; Long, M. Correlating Active Sites and Oxidative Species in Single-Atom Catalyzed Fenton-like Reactions. Chem. Sci. 2024, 15, 11699–11718. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Shen, G. Characterization of Carbon Fractions in Carbonaceous Aerosols from Typical Fossil Fuel Combustion Sources. China Fuel 2019, 254, 115620. [Google Scholar] [CrossRef]
- Wang, Z.; Bi, X.; Sheng, G.; Fu, J. Characterization of Organic Compounds and Molecular Tracers from Biomass Burning Smoke in South China I: Broad-Leaf Trees and Shrubs. China Atmos. Environ. 2018, 43, 3096–3102. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.C.; Ho, K.F.; Chow, J.C. Chemically-Speciated on-Road PM2.5 Motor Vehicle Emission Factors in Hong Kong. Sci. Total Environ. 2010, 408, 1621–1627. [Google Scholar] [CrossRef]
- Shen, G.; Tao, S.; Wei, S. Field Measurement of Emission Factors of PM, EC, OC, Parent, Nitro-, and Oxy Polymerization Coal Cake, and Wood in Rural Shanxi. China Environ. Sci. Technol. 2013, 47, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Grieshop, A.P.; Jain, G.; Sethuraman, K.; Marshall, J.D. Emission Factors of Health- and Climate-relevant Pollutants. GeoHealth 2017, 1, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Guinot, B.; Wang, J.; He, K.; Ho, K.F.; Cao, J.; Shen, Z.; Sun, Z.; Lei, Y.; et al. Household heating and cooking in rural Guanzhong Plain, Northwestern China. Atmos. Environ. 2018, 185, 196–206. [Google Scholar] [CrossRef]
- Shen, G.; Chen, Y.; Xue, C. Pollutant Emissions from Improved Coal and Wood-Fuelled Cookstoves in Rural Households. China Environ. Sci. Technol. 2015, 49, 6590–6598. [Google Scholar] [CrossRef]
- Yang, G.; Kong, S.; Zheng, S.; Wu, J.; Zheng, M.; Zheng, H.; Yan, Q.; Liu, H.; Wang, W.; Wu, F.; et al. Size-resolved emission factors of carbonaceous particles from domestic coal combustion in China. Environ. Sci. 2018, 39, 3524–3534. [Google Scholar]
- Saud, T.; Gautam, R.; Mandal, T.K. Emission Estimates of Organic and Elemental Carbon from Household Biomass Fuel Used over the Indo-Gangetic Plain (IGP). India Atmos. Environ. 2012, 61, 212–220. [Google Scholar] [CrossRef]
- Ni, H.; Tian, J.; Wang, X. PM2.5 emissions and source profiles from open burning of crop residues. Atmos. Environ. 2018, 169, 229–237. [Google Scholar] [CrossRef]
- Guo, F.; Ju, Y.; Wang, G. Inorganic chemical composition of PM2.5 emissions from the combustion of six main tree species in subtropical China. Atmos. Environ. 2018, 189, 107–115. [Google Scholar] [CrossRef]
- Wang, B.; Lee, S.C.; Ho, K.F. Chemical composition of fine particles from incense burning in a large environmental chamber. Atmos. Environ. 2006, 40, 7858–7868. [Google Scholar] [CrossRef]
- Bhattu, D.; Tripathi, S.N.; Bhowmik, H.S. Local incomplete combustion emissions define the PM2.5 oxidative potential in Northern India. Nat. Commun. 2024, 15, 3517. [Google Scholar] [CrossRef]
- Yan, Z.; Ge, P.; Lu, Z.; Liu, X.; Cao, M.; Chen, W.; Chen, M. The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air-Liquid Interface Exposure on A549 Cells. Toxics 2023, 12, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mansurov, Z.A. Soot Formation in Combustion Processes (Review). Combust. Explos. Shock. Waves 2005, 41, 727–744. [Google Scholar] [CrossRef]
- Morawska, L.; Buonanno, G. The physics of particle formation and deposition during breathing. Nat. Rev. Phys. 2021, 3, 300–301. [Google Scholar] [CrossRef] [PubMed]
- AVL. Unseen but Dangerous: Understanding the Health Risks of Ultrafine Particles in Ambient Air. 2025. Available online: https://www.avl.com/en/expert-article/unseen-dangerous-understanding-health-risks-ultrafine-particles-ambient-air (accessed on 8 July 2025).
- Mastalerz, M. Validation and Application of Human in vitro Models for Investigating Bronchial Response to Cigarette Smoke. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 2020. [Google Scholar]
- Li, T.; Yu, Y.; Sun, Z. A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence. Part. Fibre Toxicol. 2022, 19, 67. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Gao, Y.; Li, C.; Zhou, L.; Qi, W.; Zhang, Y.; Ye, L. Effects of Different Components of PM2.5 on the Expression Levels of NF-κB Family Gene mRNA and Inflammatory Molecules in Human Macrophage. Int. J. Environ. Res. Public Health 2019, 16, 1408. [Google Scholar] [CrossRef] [PubMed]
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; Denier van der Gon, H.; Facchini, M.C.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U.; et al. Particulate matter, air quality and climate: Lessons learned and future needs. Atmos. Chem. Phys. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Ge, L.K.; Wang, Z.Y.; Cao, S.K.; Che, X.J.; Zhu, C.; Zhang, P.; Ma, H. Critical Review on Environmental Occurrence and Photochemical Behavior of Substituted Polycyclic Aromatic Hydrocarbons. Environ. Sci. 2023, 44, 3957–3969. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, J.W.; Zhang, L.; Yu, J.Z.; Wang, Y.H.; Li, X.D. Traffic-related PM2.5 pollution in Hong Kong: Component-specific and source-resolved health risks and cytotoxicity. Environ. Pollut. 2025, 380, 0269–7491. [Google Scholar] [CrossRef]
- Rogula-Kopiec, P.; Rogula-Kozłowska, W.; Bihałowicz, J.S. Examining Organic and Elemental Carbon Presence in Beauty Salon Service Rooms. Aerosol Air Qual. Res. 2025, 25, 23. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, K.; Qiu, P.; Xu, M. Quantifying SO2 oxidation pathways to atmospheric sulfate using stable sulfur and oxygen isotopes: Laboratory simulation and field observation. Atmos. Chem. Phys. 2024, 24, 2195–2205. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, C.; Liu, C. A review on the heterogeneous oxidation of SO2 on solid atmospheric particles: Implications for sulfate formation in haze chemistry. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1888–1911. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Fan, X.; Chen, Y.; Lin, Z.; Hou, A.; Zhang, S.; Zheng, R.; Chen, J. Measurement report: The variation properties of aerosol hygroscopic growth related to chemical composition during new particle formation days in a coastal city of Southeast China. Atmos. Chem. Phys. 2025, 25, 3669–3685. [Google Scholar] [CrossRef]
- Afridi, H.I.; Talpur, F.N.; Kazi, T.G.; Brabazon, D. Effect of trace and toxic elements of different brands of cigarettes on the essential elemental status of irish referent and diabetic mellitus consumers. Biol. Trace Elem. Res. 2015, 167, 209–224. [Google Scholar] [CrossRef]
- Afridi, H.I.; Talpur, F.N.; Kazi, T.G.; Brabazon, D. Estimation of aluminum, arsenic, lead and nickel status in the samples of different cigarettes and their effect on human health of Irish smoker hypertensive consumers. Clin. Lab. 2015, 61, 1147–1156. [Google Scholar] [CrossRef]
- Caruso, R.V.; O’Connor, R.J.; Travers, M.J.; Delnevo, C.D.; Stephens, W.E. Design char acteristics and tobacco metal concentrations in filtered cigars. Nicotine Tob. Res. 2015, 17, 1331–1336. [Google Scholar] [CrossRef]
- Caruso, R.V.; Fix, B.V.; Thrasher, J.F.; Cummings, K.M.; Fong, G.T.; Stephens, W.E. Differences in cigarette design and metal content across five countries: Results from the international tobacco control (ITC) project. Tob. Regul. Sci. 2016, 2, 166–175. [Google Scholar] [CrossRef]
- Li, W.J.; Gong, B.G.; Zhang, J.Y. Research on mineral transformation and heavy metal distribution during high-silicon coal combustion. J. Fuel Chem. Technol. 2020, 48, 1488–1497. [Google Scholar]
- Tan, Y.J.; Zhang, X.W.; Zhang, Y.H.; Luo, M.L.; Song, Z.J.; Peng, Y. Detoxification of municipal solid waste incineration fly ash and dewatered sludge based on vacuum carbothermal reduction method. J. South China Univ. (Nat. Sci. Ed.) 2024, 38, 17–24. [Google Scholar] [CrossRef]
- Alter, N.C.; Whitman, E.M.; Bellinger, D.C. Quantifying the association between PM2.5 air pollution and IQ loss in children: A systematic review and meta-analysis. Environ. Health 2024, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- McElvenny, D.; Sleeuwenhoek, A.; Copsey, H.; Clabon, K.; Mueller, W.; Dixon, K.; Fishwick, D.; Cowie, H. Chromium VI and Lung Cancer: Research into Malignant and Non-Malignant Respiratory Disease Prescriptions (Report). 2025. Health and Safety Executive. GOV.UK. 3 June 2025. Available online: https://www.gov.uk/government/publications/a-review-of-the-evidence-for-malignant-and-non-malignant-respiratory-disease-prescriptions-commissioned-by-the-industrial-injuries-advisory-council-i/chromium-vi-and-lung-cancer-report-research-into-malignant-and-non-malignant-respiratory-disease-prescriptions (accessed on 8 July 2025).
- Christian, T.J.; Kleiss, B.; Yokelson, R.J. Comprehensive laboratory measurements of biomass-burning emissions: Emissions from Indonesian, African, and other fuels. J. Geophys. Res. 2003, 108, D23. [Google Scholar] [CrossRef]
- Habib, G.; Venkataraman, C.; Bond, T.C.; Schauer, J.J. Microphysical and Optical Properties of Primary Particles from the Combustion of Biomass Fuels. Environ. Sci. Technol. 2008, 42, 8829–8834. [Google Scholar] [CrossRef]
- Reisen, F.; Meyer, C.P.; Weston, C.J.; Volkova, L. Ground-Based Field Measurements of PM2.5 Emission Factors From bright combustion and smoldering combustion in Eucalypt Forests. J. Geophys. Res. 2018, 123, 8301–8314. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Household Heating and Cooking in Rural Guanzhong Plain. Available online: https://www.epa.gov/chief (accessed on 8 July 2025).
- Zhang, Y.; Schauer, J.; Zhang, Y. Characteristics of particulate carbon emissions from real-world Chinese coal combustion. China Environ. Sci. Technol. 2008, 42, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Smith, K.R.; Ma, Y. Greenhouse gases and other airborne pollutants from household stoves in China: A database for emission factors. China Atmos. Environ. 2000, 34, 4537–4549. [Google Scholar] [CrossRef]
| Classification of Combustibles | Combustible | Combustion State | PM Emitted from Combustion | OC/EC | Source of the Data |
|---|---|---|---|---|---|
| Cigarette | Inner layer of tobacco leaves is being smothered by the burning tip | Peripheral—luminous, oxygen-rich combustion Interior—oxygen-limited smoldering pyrolysis | PM2.5 from MS smoke of cigarette | 187.3 | This study |
| Outer layer of tobacco leaves near the cigarette’s lit end | Free combustion | PM2.5 from SS smoke of cigarette | 77.5 | This study | |
| Solid fuel | Hexagonal household coal briquet | Mixed smoldering and flaming | PM2.5 | 3.9 | [38] |
| Anthracite | Mixed smoldering and flaming | TSP | 15.0 | [39] | |
| Coal cakes | Mixed smoldering and flaming | TSP | 8.4~14.7 | [36] | |
| Charcoal briquette | Mixed smoldering and flaming | TSP | 32.5~34.2 | ||
| Lumber | Mixed smoldering and flaming | TSP | 2.3~2.4 | ||
| Lump coal | Smoldering | PM1.1 | 84.9 | [40] | |
| Smoldering | PM2.1 | 81.7 | |||
| Smoldering | PM10 | 59.4 | |||
| Flaming | PM1.1 | 38.6 | |||
| Flaming | PM2.1 | 37.0 | |||
| Flaming | PM10 | 31.4 | |||
| Manure cake | Mixed smoldering and flaming | TSP | 7.9 ± 4.4 | [41] | |
| Straw | Mixed smoldering and flaming | PM2.5 | 21.3 | [42] | |
| Rice straw | Mixed smoldering and flaming | PM2.5 | 15.7 | ||
| Cornstalk | Mixed smoldering and flaming | PM2.5 | 22.5 | ||
| Foliage | Flaming | PM2.5 emitted during the late stage of leaf burning | 2.9 | [43] | |
| Smoldering | PM2.5 emitted during the initial stage of leaf burning | 5.0 | |||
| Incense | Mixed smoldering and flaming | PM2.5 | 74.3 | [44] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Huang, H.; Zou, C.; Deng, M.; Tu, X.; Deng, W.; Yu, C.; Li, J. Chemical Dissection of PM2.5 in Cigarette Smoke: Main and Sidestream Emission Factors and Compositions. Toxics 2025, 13, 711. https://doi.org/10.3390/toxics13090711
Zhou Y, Huang H, Zou C, Deng M, Tu X, Deng W, Yu C, Li J. Chemical Dissection of PM2.5 in Cigarette Smoke: Main and Sidestream Emission Factors and Compositions. Toxics. 2025; 13(9):711. https://doi.org/10.3390/toxics13090711
Chicago/Turabian StyleZhou, Yujian, Hong Huang, Changwei Zou, Mengmeng Deng, Xiang Tu, Wei Deng, Chenglong Yu, and Jianlong Li. 2025. "Chemical Dissection of PM2.5 in Cigarette Smoke: Main and Sidestream Emission Factors and Compositions" Toxics 13, no. 9: 711. https://doi.org/10.3390/toxics13090711
APA StyleZhou, Y., Huang, H., Zou, C., Deng, M., Tu, X., Deng, W., Yu, C., & Li, J. (2025). Chemical Dissection of PM2.5 in Cigarette Smoke: Main and Sidestream Emission Factors and Compositions. Toxics, 13(9), 711. https://doi.org/10.3390/toxics13090711








