Distinct Effects of PFOS and OBS on Neurotoxicity via PMK-1 Mediated Pathway in Caenorhabditis elegans
Highlights
- OBS exposure exhibits stronger neurotoxicity than PFOS in C. elegans, causing neurobehavioral deficits.
- OBS induces significant neurotoxicity via the PMK-1 mediated pathway in C. elegans, and its genetic mechanism is different to that of PFOS.
- The main findings highlight the distinct effects and mechanisms of PFOS and OBS on neurotoxicity in C. elegans.
- This study conducted an environmental and health risk assessment of OBS, emphasizing the need for further investigation into the safety of OBS.
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Culture
2.2. Exposure to PFOS and OBS
2.3. Neurobehavioral Activity Measurement
2.4. Neuronal Damage Observation
2.5. Reverse Transcription Quantitative PCR (RT-qPCR)
2.6. Molecular Docking
2.7. Correlation Analysis
2.8. Statistical Analysis
3. Results
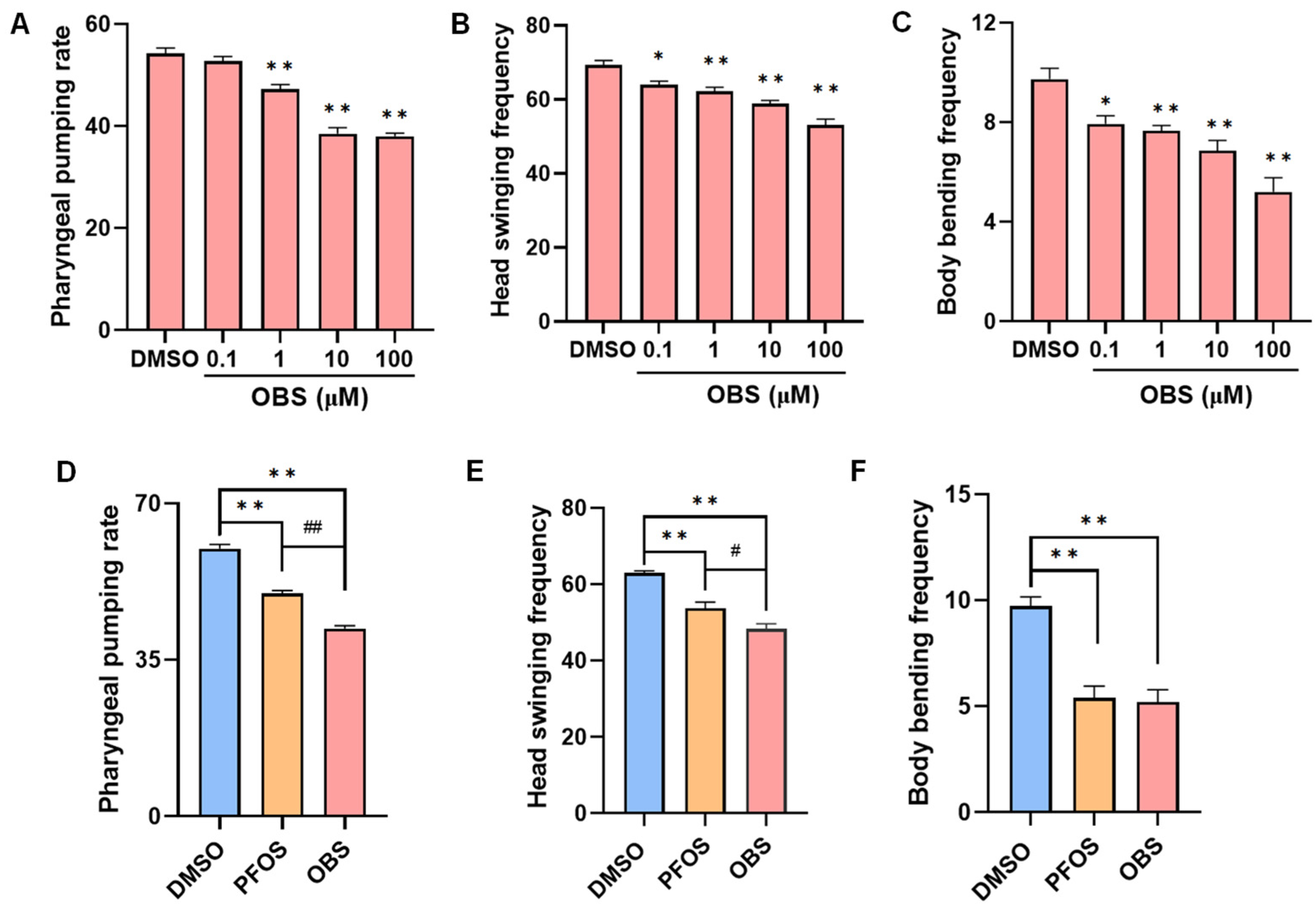
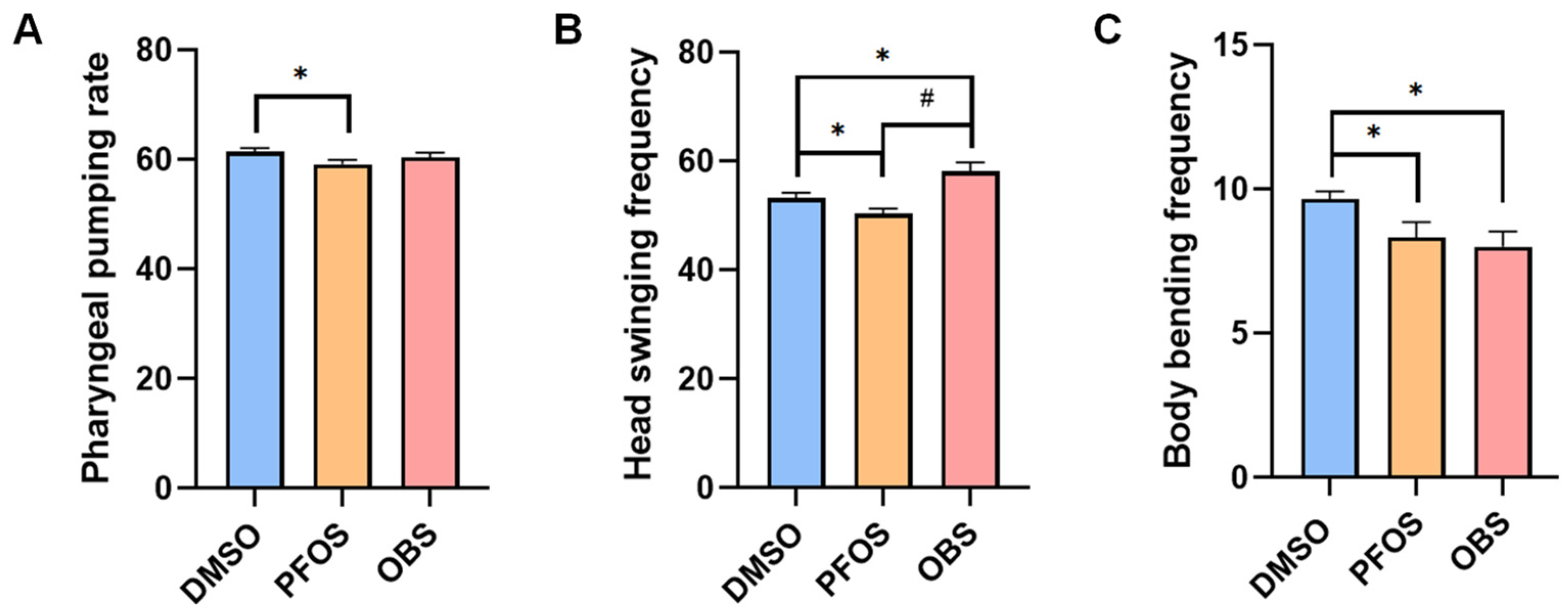
3.1. Distinct Effects of PFOS/OBS Exposure on Neurobehavioral Activity in C. elegans
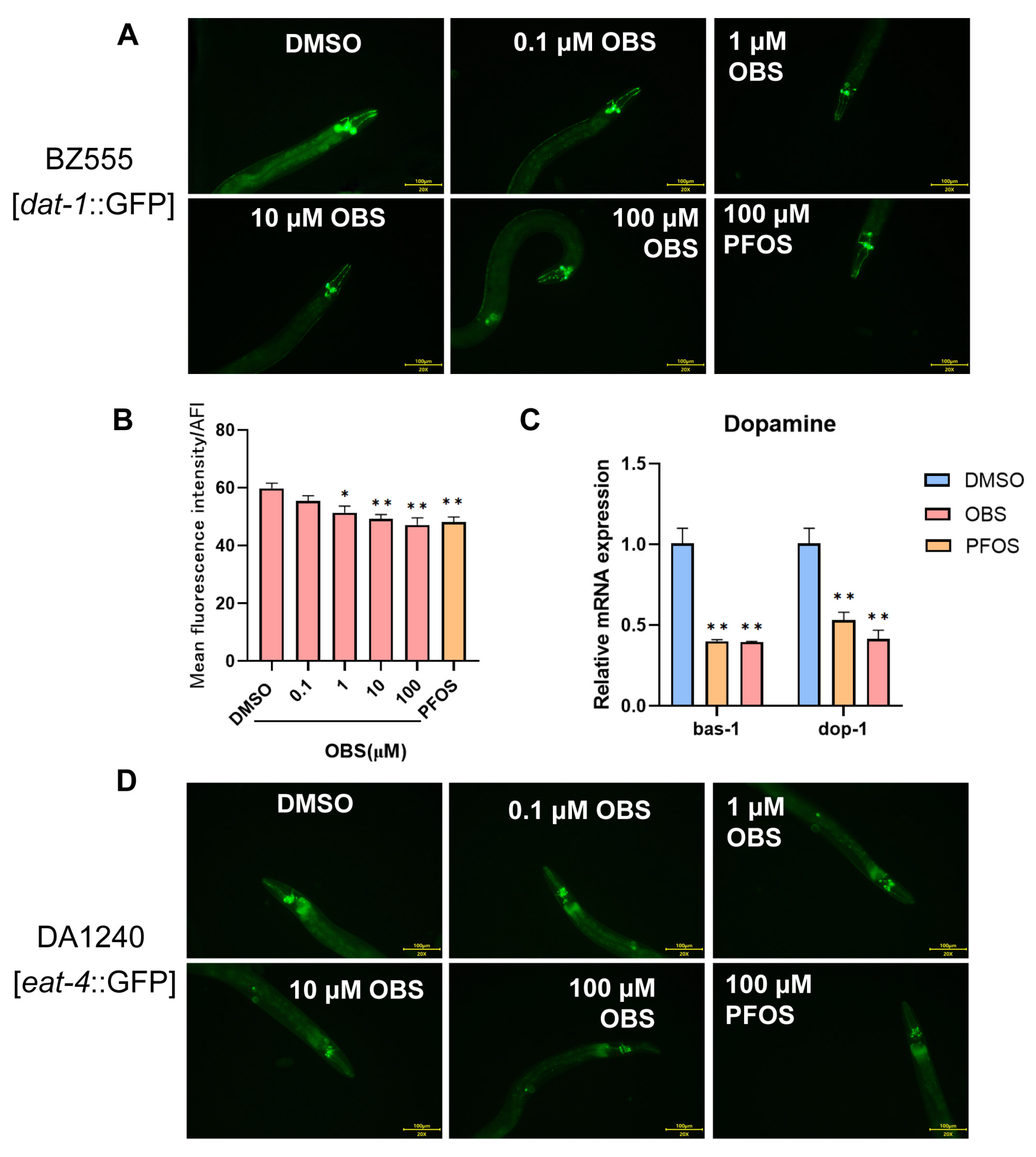
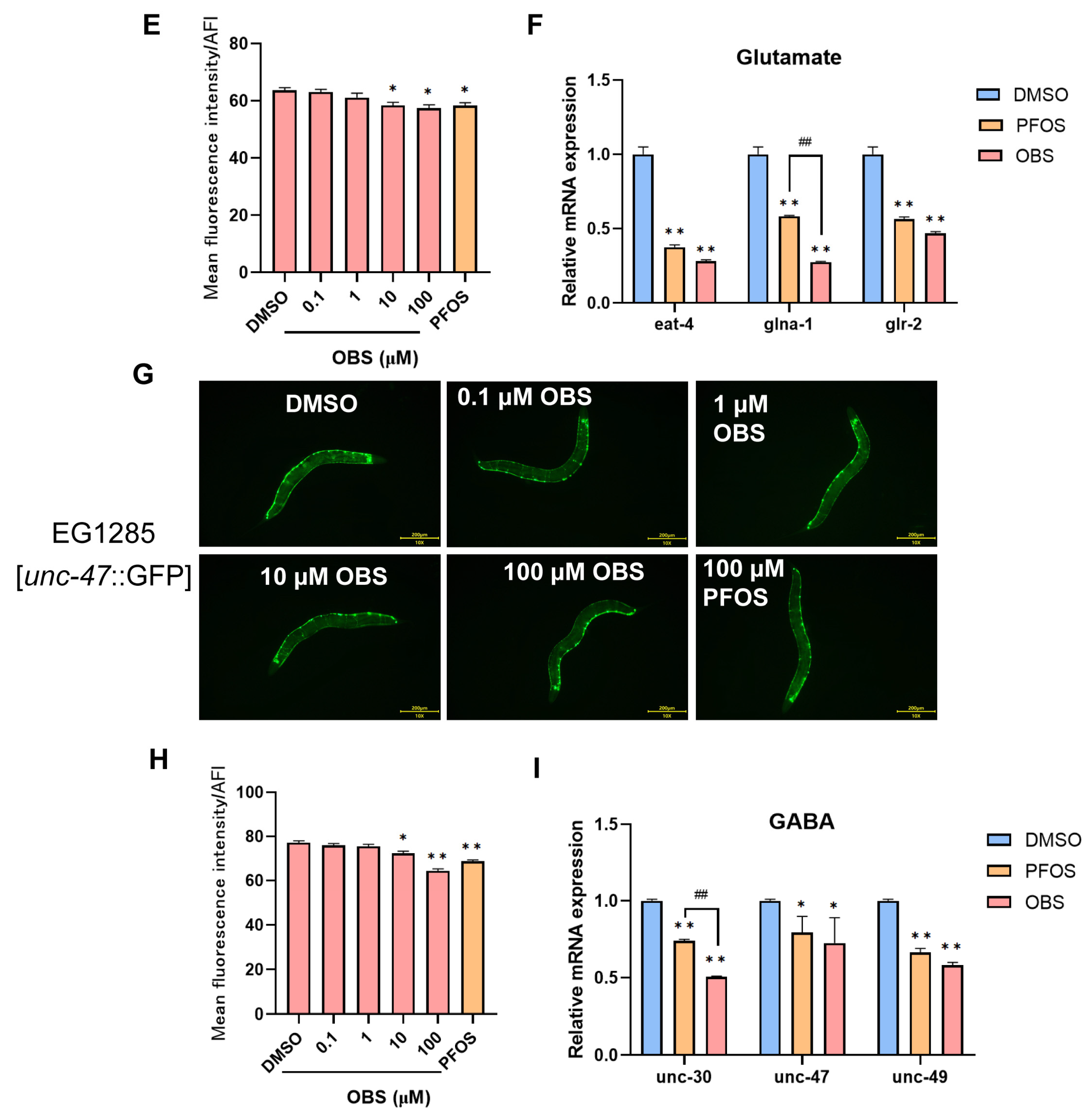
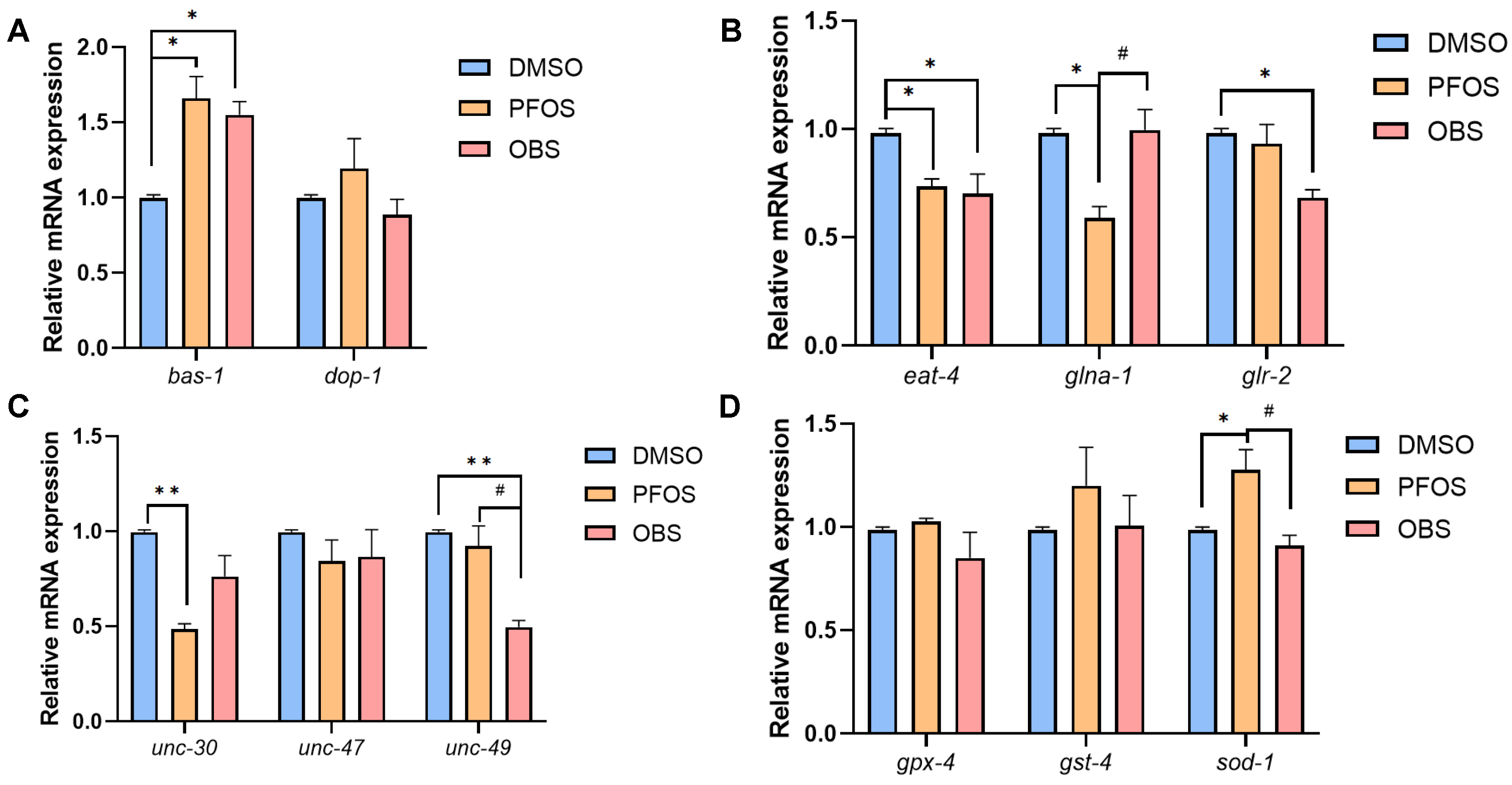
3.2. Distinct Effects of PFOS/OBS Exposure on Specific Neurotransmitter Systems in C. elegans
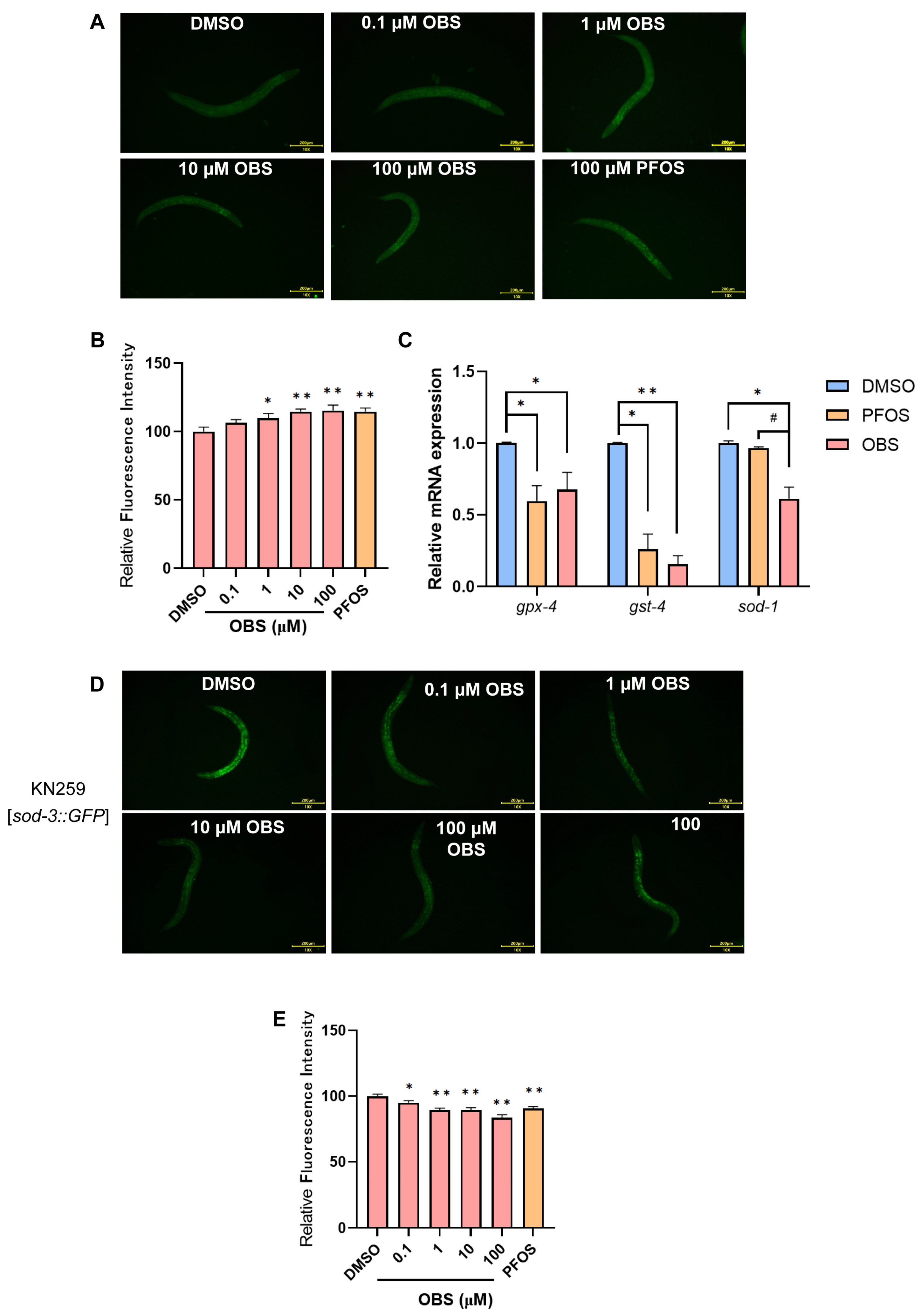
3.3. Distinct Effects of PFOS/OBS Exposure on Oxidative Stress in C. elegans
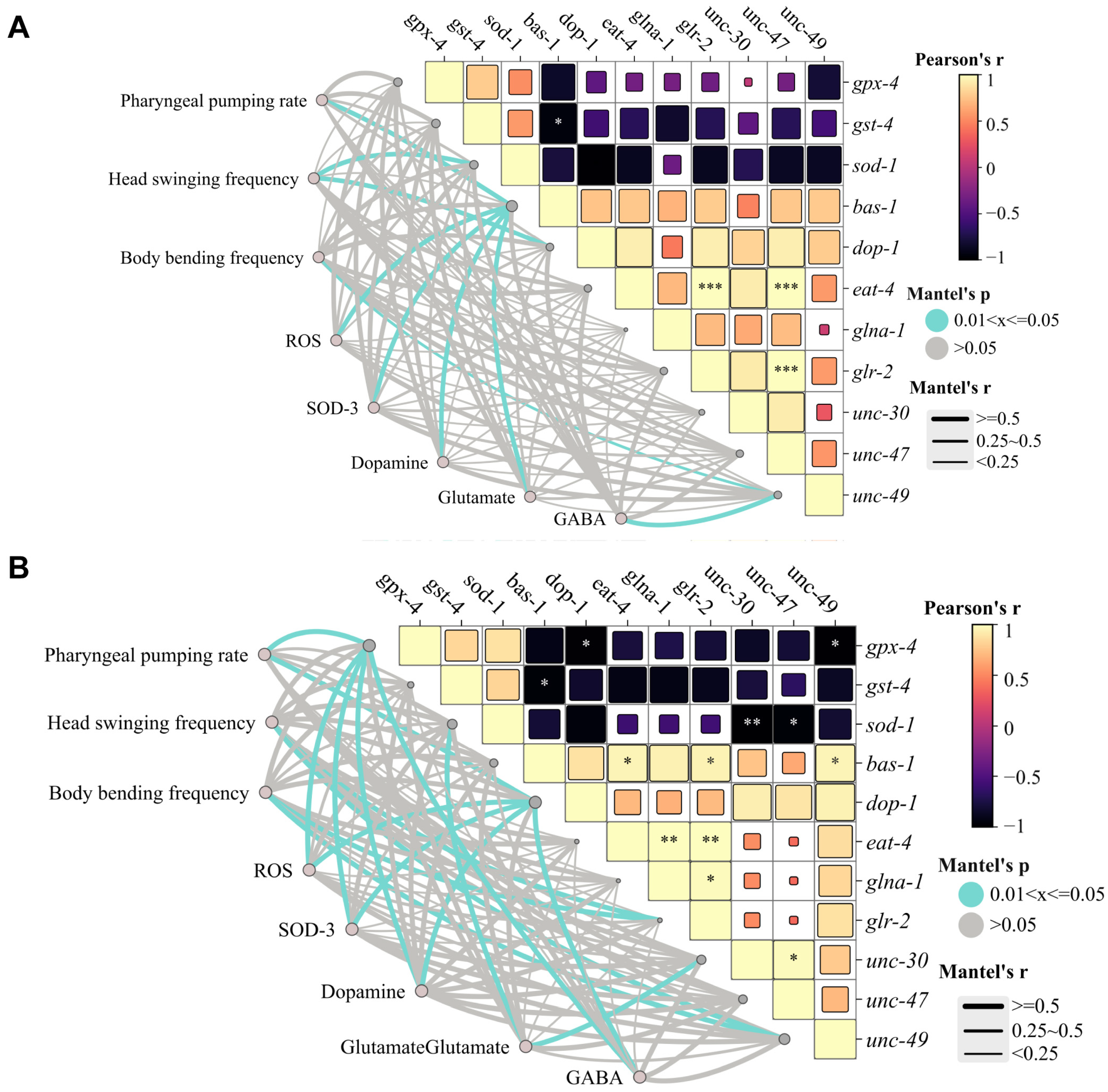
3.4. Distinct Effects of PFOS/OBS on Oxidative Stress-Neurotransmitter Regulatory Network
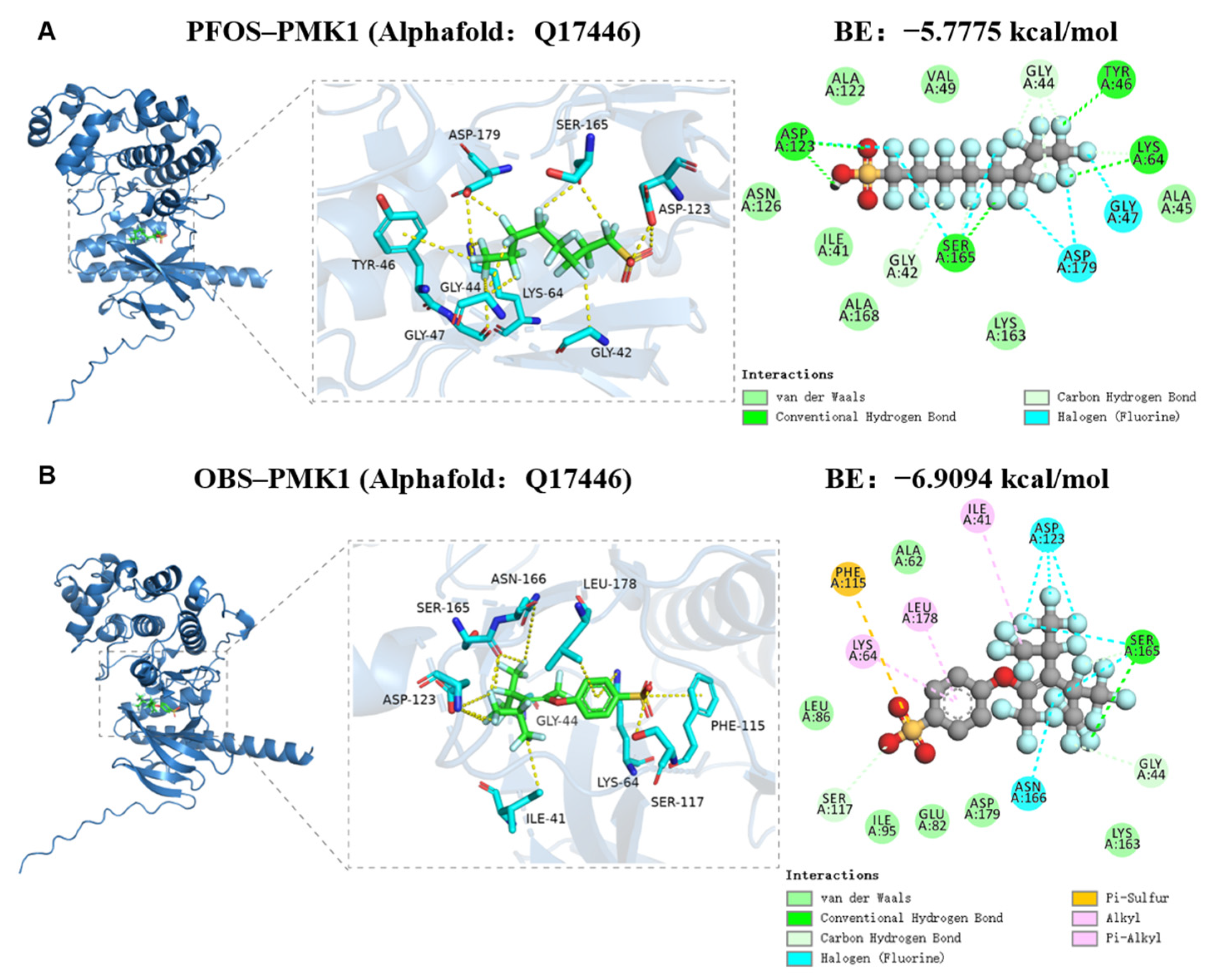
3.5. Binding Potential of PFOS and OBS with PMK-1 Protein
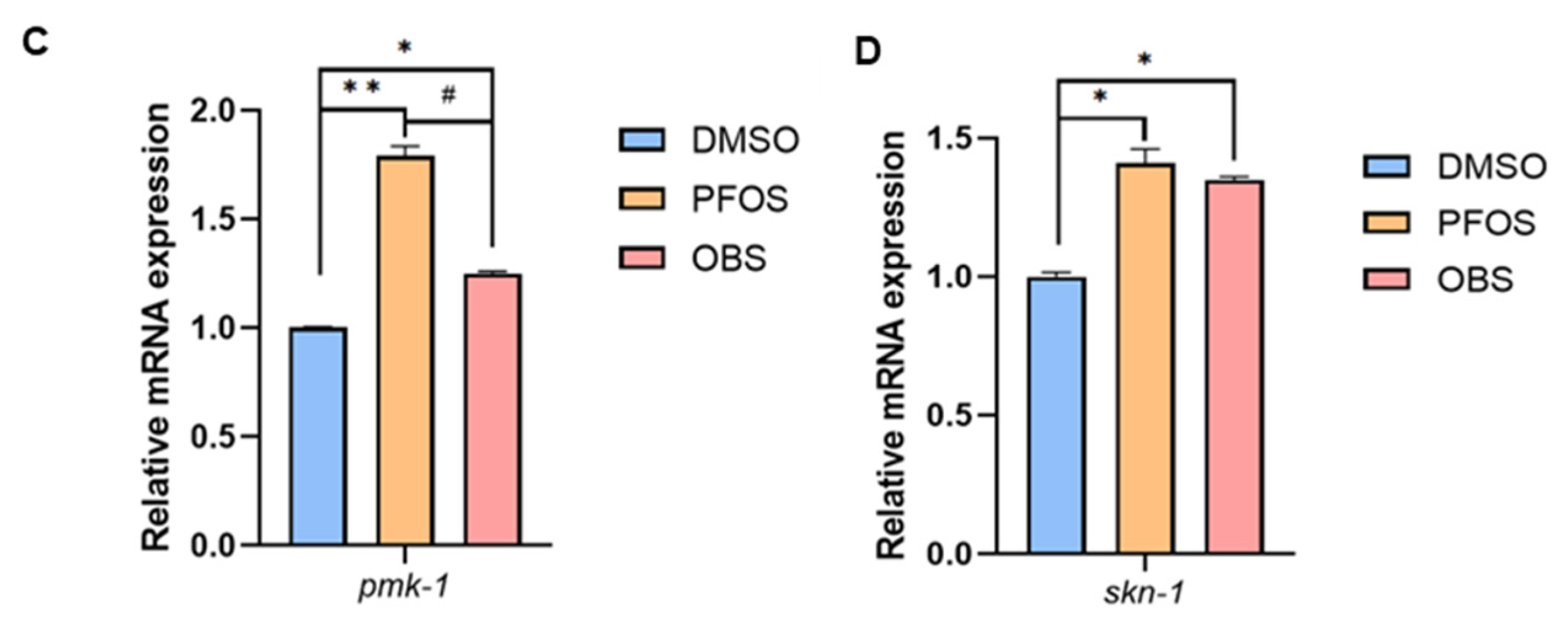
3.6. Distinct Effects of PFOS/OBS Exposure on Neurotoxicity in pmk-1 Mutant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OBS | Sodium p-perfluorous nonenoxybenzenesulfonate |
| PFOS | Perfluorooctanesulfonic acid |
| PFASs | Perfluoroalkyl and polyfluoroalkyl substances |
| C. elegans | Caenorhabditis elegans |
| GFP | Green fluorescent protein |
| DA | Dopamine |
| Glu | Glutamate |
| GABA | Gamma-aminobutyric acid |
| Ach | Acetylcholine |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MAPK | Mitogen-activated protein kinase |
| ROS | Reactive oxygen species |
| E. coli | Escherichia coli |
| NGM | Nematode growth medium |
| DMSO | Dimethyl sulfoxide |
| SOD | Superoxide dismutase |
| GST | Glutathione S-transferase |
References
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic Degradation of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Water: A Critical Review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Gaines, L.G.T. Historical and Current Usage of Per-and Polyfluoroalkyl Substances (PFAS): A Literature Review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic Acid (PFOA)—Main Concerns and Regulatory Developments in Europe from an Environmental Point of View. Environ. Sci. Eur. 2012, 24, 16. [Google Scholar] [CrossRef]
- Tang, Z.W.; Shahul Hamid, F.; Yusoff, I.; Chan, V. A Review of PFAS Research in Asia and Occurrence of PFOA and PFOS in Groundwater, Surface Water and Coastal Water in Asia. Groundw. Sustain. Dev. 2023, 22, 100947. [Google Scholar] [CrossRef]
- Xiao, F.; Simcik, M.F.; Halbach, T.R.; Gulliver, J.S. Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Soils and Groundwater of a U.S. Metropolitan Area: Migration and Implications for Human Exposure. Water Res. 2015, 72, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.; Yan, S.; Cui, J.; Liang, Y.; Ren, S. Adverse Effects of Perfluorooctane Sulfonate on the Liver and Relevant Mechanisms. Toxics 2022, 10, 265. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Chen, S.; Li, R.; Wang, M.; Wang, X.; Ding, N.; Sun, Y. Mitigation of PFOA/PFOS Toxicity in Zebrafish (Danio rerio) by Oxidative Stress Modulation and Gut Microbial Metabolism through the Use of Aquatic Probiotic Lactobacillus Rhamnosus. Water Cycle 2025, 6, 71–81. [Google Scholar] [CrossRef]
- Liu, L.; Deng, S.; Bao, Y.; Huang, J.; Yu, G. Degradation of OBS (Sodium p-Perfluorous Nonenoxybenzenesulfonate) as a Novel Per-and Polyfluoroalkyl Substance by UV/Persulfate and UV/Sulfite: Fluorinated Intermediates and Treatability in Fluoroprotein Foam. Environ. Sci. Technol. 2022, 56, 6201–6211. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, X.; Tu, W.; Wang, Q.; Liu, S.; Zhang, M.; Wu, Y.; Mai, B. Bioaccumulation, Tissue Distribution, and Maternal Transfer of Novel PFOS Alternatives (6:2 Cl-PFESA and OBS) in Wild Freshwater Fish from Poyang Lake, China. Chemosphere 2023, 336, 139253. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Li, C.; Song, X.; Qin, Z.; Cao, D.; Cai, Y. Discovery of a Novel Polyfluoroalkyl Benzenesulfonic Acid around Oilfields in Northern China. Environ. Sci. Technol. 2017, 51, 14173–14181. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Liu, S.; Zhang, M.; Liu, Y.; Sun, L.; Wu, Y.; Tu, W. Crosstalk between Histological Alterations, Oxidative Stress and Immune Aberrations of the Emerging PFOS Alternative OBS in Developing Zebrafish. Sci. Total Environ. 2021, 774, 145443. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Dong, S.; Zhao, X.; Li, X.; Zhang, M.; Shi, Y.; Ding, G. Developmental Toxicity and Cardiotoxicity Induced by PFOS and Its Novel Alternative OBS in Early Life Stage of Zebrafish (Danio rerio). Water Air Soil Pollut. 2023, 234, 481. [Google Scholar] [CrossRef]
- Wang, C.; Weng, Y.; Tu, W.; Jin, C.; Jin, Y. Maternal Exposure to Sodium ρ-Perfluorous Nonenoxybenzene Sulfonate during Pregnancy and Lactation Disrupts Intestinal Barrier and May Cause Obstacles to the Nutrient Transport and Metabolism in F0 and F1 Generations of Mice. Sci. Total Environ. 2021, 794, 148775. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, B.; Wang, Z.; Liu, L.; Zhang, Q.; Zhang, Q.; Jiang, W. Sodium P-Perfluorous Nonenoxybenzene Sulfonate Induces ROS-Mediated Necroptosis by Directly Targeting Catalase in HepG2 Cells. Sci. Total Environ. 2024, 910, 168446. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, J.; Lu, F.; Zhang, J.; Guo, L. Application of Zebrafish in the Study of the Gut Microbiome. Anim. Models Exp. Med. 2022, 5, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Deng, M.; Wang, X.; Tu, W.; Fu, Z.; Jin, Y. Bioaccumulation in the Gut and Liver Causes Gut Barrier Dysfunction and Hepatic Metabolism Disorder in Mice after Exposure to Low Doses of OBS. Environ. Int. 2019, 129, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, K.; Kucharíková, S.; Bárdyová, Z.; Botek, N.; Kaiglová, A. Applications of a Powerful Model Organism Caenorhabditis elegans to Study the Neurotoxicity Induced by Heavy Metals and Pesticides. Physiol. Res. 2023, 72, 149–166. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Luo, Y.; Xu, X.; Han, Y.; Chen, H.; Sun, H.; Xue, Y.; Ji, G. Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A Bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure. Toxics 2025, 13, 76. [Google Scholar] [CrossRef]
- Bargmann, C.I. Neurobiology of the Caenorhabditis elegans Genome. Science 1998, 282, 2028–2033. [Google Scholar] [CrossRef]
- Long, N.P.; Kang, J.S.; Kim, H.M. Caenorhabditis elegans: A Model Organism in the Toxicity Assessment of Environmental Pollutants. Environ. Sci. Pollut. Res. 2023, 30, 39273–39287. [Google Scholar] [CrossRef]
- Wang, C.; Xia, C.; Zhu, Y.; Zhang, H. Innovative Fluorescent Probes for in Vivo Visualization of Biomolecules in Living Caenorhabditis elegans. Cytom. A 2021, 99, 560–574. [Google Scholar] [CrossRef]
- Keshet, A.; Mertenskötter, A.; Winter, S.A.; Brinkmann, V.; Dölling, R.; Paul, R.J. PMK-1 P38 MAPK Promotes Cadmium Stress Resistance, the Expression of SKN-1/Nrf and DAF-16 Target Genes, and Protein Biosynthesis in Caenorhabditis elegans. Mol. Genet. Genom. 2017, 292, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of Mitochondrial Reactive Oxygen Species in Homeostasis Regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Sugawara, T.; Saraprug, D.; Sakamoto, K. Soy Sauce Increased the Oxidative Stress Tolerance of Nematode via P38 MAPK Pathway. Biosci. Biotechnol. Biochem. 2019, 83, 709–716. [Google Scholar] [CrossRef]
- Tsai, Y.; Lin, Y.-C.; Lee, Y.-H. Octopamine-MAPK-SKN-1 Signaling Suppresses Mating-Induced Oxidative Stress in Caenorhabditis elegans Gonads to Protect Fertility. iScience 2023, 26, 106162. [Google Scholar] [CrossRef]
- Lim, D.; Roh, J.; Eom, H.; Choi, J.; Hyun, J.; Choi, J. Oxidative Stress-related PMK-1 P38 MAPK Activation as a Mechanism for Toxicity of Silver Nanoparticles to Reproduction in the Nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef]
- Mertenskötter, A.; Keshet, A.; Gerke, P.; Paul, R.J. The P38 MAPK PMK-1 Shows Heat-Induced Nuclear Translocation, Supports Chaperone Expression, and Affects the Heat Tolerance of Caenorhabditis elegans. Cell Stress Chaperones 2013, 18, 293–306. [Google Scholar] [CrossRef]
- Soltanmohammadi, N.; Wang, S.; Schumacher, B. Somatic PMK-1/P38 Signaling Links Environmental Stress to Germ Cell Apoptosis and Heritable Euploidy. Nat. Commun. 2022, 13, 701. [Google Scholar] [CrossRef]
- Hou, M.; Jin, Q.; Na, G.; Cai, Y.; Shi, Y. Emissions, Isomer-Specific Environmental Behavior, and Transformation of OBS from One Major Fluorochemical Manufacturing Facility in China. Environ. Sci. Technol. 2022, 56, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Sammi, S.R.; Foguth, R.M.; Nieves, C.S.; De Perre, C.; Wipf, P.; McMurray, C.T.; Lee, L.S.; Cannon, J.R. Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology in Caenorhabditis elegans. Toxicol. Sci. 2019, 172, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Albertson, D.G.; Thompson, J. The Pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 275, 299–325. [Google Scholar] [PubMed]
- Kalyn, M.; Lee, H.; Curry, J.; Tu, W.; Ekker, M.; Mennigen, J.A. Effects of PFOS, F-53B and OBS on Locomotor Behaviour, the Dopaminergic System and Mitochondrial Function in Developing Zebrafish (Danio Rerio). Environ. Pollut. 2023, 326, 121479. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Chen, J.; He, X.-R.; Yang, S.-L.; Ma, B.-J.; Yu, J.; Qiu, J.; Qian, Y.-Z.; Xu, Y.-Y. Perfluorooctane Sulfonate (PFOS) and Benzo[a]Pyrene (BaP) Synergistically Induce Neurotoxicity in C6 Rat Glioma Cells via the Activation of Neurotransmitter and Cyp1a1-Mediated Steroid Hormone Synthesis Pathways. Food Chem. Toxicol. 2024, 193, 115058. [Google Scholar] [CrossRef]
- Sato, I.; Kawamoto, K.; Nishikawa, Y.; Tsuda, S.; Yoshida, M.; Yaegashi, K.; Saito, N.; Liu, W.; Jin, Y. Neurotoxicity of Perfluorooctane Sulfonate (PFOS) in Rats and Mice after Single Oral Exposure. J. Toxicol. Sci. 2009, 34, 569–574. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Ji, G.; Yan, L.; Hu, F.; Gu, A. Neurotoxicity of Perfluorooctane Sulfonate to Hippocampal Cells in Adult Mice. PLoS ONE 2013, 8, e54176. [Google Scholar] [CrossRef]
- Ninomiya, A.; Mshaty, A.; Haijima, A.; Yajima, H.; Kokubo, M.; Khairinisa, M.A.; Ariyani, W.; Fujiwara, Y.; Ishii, S.; Hosoi, N.; et al. The Neurotoxic Effect of Lactational PFOS Exposure on Cerebellar Functional Development in Male Mice. Food Chem. Toxicol. 2022, 159, 112751. [Google Scholar] [CrossRef]
- Pu, P.; Le, W. Dopamine Neuron Degeneration Induced by MPP+ Is Independent of CED-4 Pathway in Caenorhabditis elegans. Cell Res. 2008, 18, 978–981. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Razia, D.M.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Shariati, M.A. Dopamine in Parkinson’s Disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Yu, Y.; Hua, X.; Chen, H.; Wang, Z.; Han, Y.; Chen, X.; Yang, Y.; Xiang, M. Glutamatergic Transmission Associated with Locomotion-Related Neurotoxicity to Lindane over Generations in Caenorhabditis elegans. Chemosphere 2022, 290, 133360. [Google Scholar] [CrossRef]
- Ke, T.; Santamaria, A.; Barbosa, F.; Rocha, J.B.T.; Skalny, A.V.; Tinkov, A.A.; Bowman, A.B.; Aschner, M. Developmental Methylmercury Exposure Induced and Age-Dependent Glutamatergic Neurotoxicity in Caenorhabditis elegans. Neurochem. Res. 2023, 48, 920–928. [Google Scholar] [CrossRef]
- Wu, Q.; Zhi, L.; Qu, Y.; Wang, D. Quantum Dots Increased Fat Storage in Intestine of Caenorhabditis elegans by Influencing Molecular Basis for Fatty Acid Metabolism. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Y.; Sun, J.; Wang, J.-S.; Tang, L.; Xu, Y.; Ji, J.; Sun, X. Abnormal Neurotransmission of GABA and Serotonin in Caenorhabditis elegans Induced by Fumonisin B1. Environ. Pollut. 2022, 304, 119141. [Google Scholar] [CrossRef]
- Chen, N.; Li, J.; Li, D.; Yang, Y.; He, D. Chronic Exposure to Perfluorooctane Sulfonate Induces Behavior Defects and Neurotoxicity through Oxidative Damages, In Vivo and In Vitro. PLoS ONE 2014, 9, e113453. [Google Scholar] [CrossRef]
- Wang, K.; He, L.; Liu, X.; Wu, M. Sodium P-Perfluorinated Noneoxybenzen Sulfonate (OBS) Induced Neurotoxicity in Zebrafish through Mitochondrial Dysfunction. Chemosphere 2024, 362, 142651. [Google Scholar] [CrossRef]
- Pimentel, C.; Batista-Nascimento, L.; Rodrigues-Pousada, C.; Menezes, R.A. Oxidative Stress in Alzheimer’ s and Parkinson’ s Diseases: Insights from the Yeast Saccharomyces Cerevisiae. Oxid. Med. Cell. Longev. 2012, 2012, 132146. [Google Scholar] [CrossRef]
- Nass, R.; Blakely, R.D. The Caenorhabditis elegans Dopaminergic System: Opportunities for Insights into Dopamine Transport and Neurodegeneration. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 521–544. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in Cell Death, Autophagy, and Disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Horspool, A.M.; Chang, H.C. Superoxide Dismutase SOD-1 Modulates Caenorhabditis elegans Pathogen Avoidance Behavior. Sci. Rep. 2017, 7, 45128. [Google Scholar] [CrossRef]
- Wong, C.-H.; Haque, A.; Chang, H.C. Superoxide Dismutase SOD-3 Regulates Redox Homeostasis in the Intestine. MicroPubl. Biol. 2023, 2023, 17912. [Google Scholar]
- Urbizo-Reyes, U.; Kim, K.-H.; Reddivari, L.; Anderson, J.M.; Liceaga, A.M. Oxidative Stress Protection by Canary Seed (Phalaris canariensis L.) Peptides in Caco-2 Cells and Caenorhabditis elegans. Nutrients 2022, 14, 2415. [Google Scholar] [CrossRef]
- Wang, C.; Vidal, B.; Sural, S.; Loer, C.; Aguilar, G.R.; Merritt, D.M.; Toker, I.A.; Vogt, M.C.; Cros, C.C.; Hobert, O. A Neurotransmitter Atlas of Caenorhabditis elegans Males and Hermaphrodites. Elife 2024, 13, RP95402. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.T.; Maher, K.N.; Wani, K.A.; Betts, K.E.; Chase, D.L. Coexpressed D1-and D2-Like Dopamine Receptors Antagonistically Modulate Acetylcholine Release in Caenorhabditis elegans. Genetics 2011, 188, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, S.; Nkambeu, B.; Beaudry, F. Impaired EAT-4 Vesicular Glutamate Transporter Leads to Defective Nocifensive Response of Caenorhabditis elegans to Noxious Heat. Neurochem. Res. 2020, 45, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yang, H.; Zhang, Z.; Zheng, J.C.; Qin, Z. Loss of Mammalian Glutaminase Orthologs Impairs Sperm Function in Caenorhabditis elegans. iScience 2023, 26, 106206. [Google Scholar] [CrossRef]
- Choi, S.; Taylor, K.P.; Chatzigeorgiou, M.; Hu, Z.; Schafer, W.R.; Kaplan, J.M. Sensory Neurons Arouse Caenorhabditis elegans Locomotion via Both Glutamate and Neuropeptide Release. PLoS Genet. 2015, 11, e1005359. [Google Scholar] [CrossRef]
- Liu, S.; Alexander, K.D.; Francis, M.M. Neural Circuit Remodeling: Mechanistic Insights from Invertebrates. J. Dev. Biol. 2024, 12, 27. [Google Scholar] [CrossRef]
- Pohl, F.; Germann, A.L.; Mao, J.; Hou, S.; Bakare, B.; Kong Thoo Lin, P.; Yates, K.; Nonet, M.L.; Akk, G.; Kornfeld, K.; et al. UNC-49 Is a Redox-Sensitive GABAA Receptor That Regulates the Mitochondrial Unfolded Protein Response Cell Nonautonomously. Sci. Adv. 2023, 9, eadh2584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Liu, Q.; Zhang, B.; Zhao, L.; Xu, D. Distinct Effects of PFOS and OBS on Neurotoxicity via PMK-1 Mediated Pathway in Caenorhabditis elegans. Toxics 2025, 13, 662. https://doi.org/10.3390/toxics13080662
Jiang J, Liu Q, Zhang B, Zhao L, Xu D. Distinct Effects of PFOS and OBS on Neurotoxicity via PMK-1 Mediated Pathway in Caenorhabditis elegans. Toxics. 2025; 13(8):662. https://doi.org/10.3390/toxics13080662
Chicago/Turabian StyleJiang, Jiahong, Qi Liu, Boxiang Zhang, Lei Zhao, and Dan Xu. 2025. "Distinct Effects of PFOS and OBS on Neurotoxicity via PMK-1 Mediated Pathway in Caenorhabditis elegans" Toxics 13, no. 8: 662. https://doi.org/10.3390/toxics13080662
APA StyleJiang, J., Liu, Q., Zhang, B., Zhao, L., & Xu, D. (2025). Distinct Effects of PFOS and OBS on Neurotoxicity via PMK-1 Mediated Pathway in Caenorhabditis elegans. Toxics, 13(8), 662. https://doi.org/10.3390/toxics13080662






