Extraction Methods of Emerging Pollutants in Sewage Sludge: A Comprehensive Review
Highlights
- Non-anti-inflammatory drugs are the EPs more studied in sewage sludge.
- Ultrasound-assisted extraction represents the most used extraction method for a wide variety of compounds.
- Extraction step is the bottleneck of the determination of EP in SS.
- Traditional techniques like UAE or newer like QuEChERs do not require complex steps and are applicable to determine a great variety of EPs in SS providing good recoveries. Therefore, these are purposed like standard methods for future legislation in SS.
Abstract
1. Introduction
2. Search Strategy Methodology
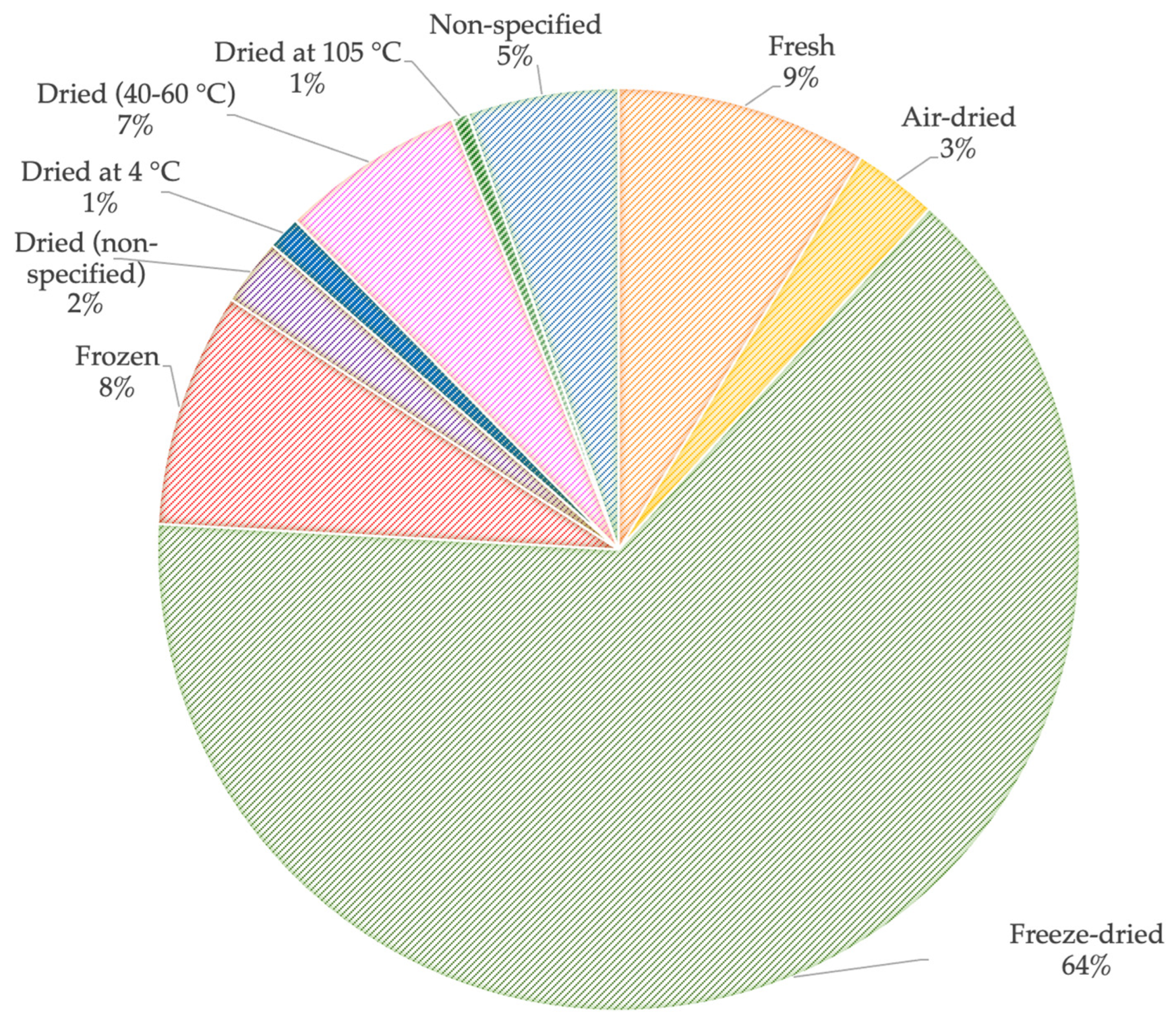
3. Pre-Treatment of Sewage Sludge Samples
4. Extraction Strategies
| Extraction Strategy | Cost | Solvent Consumption (mL) | Recoveries > 70% | Other General Information |
|---|---|---|---|---|
| Mechanical stirring | Low | 10–80 | UV filters, phthalates, PBDEs, | Filtration is not necessary [51,59]. Low-cost and basic equipment [51]. |
| Soxhlet/Soxtec | Low | 6 | Flame retardants | Soxtec was approved by EPA as standard method [48]. |
| UAE | Low | 4–35 | PFAS, PhACs, and EDCs, azoles, steroids, biocides, antibiotics, BDEs, NSAIDs, nonylphenols, PBDEs, and HBCD, | UAE using acetonitrile is valid as per EPA [59]. |
| MAE | Low | 5–30 | NSAIDs, antibiotics, EDCs, PCPs, nonylphenols, | Filtration required/clean-up required [51]. |
| PLE | High | 4–50 | β-blockers, estrogens, PFCs, BPA, carbamazepine, antibiotics, anticancer drugs, sedative-hypnotics, BDEs, TCS, | Required time: 30 min/15–45 min [36,51,60]. |
| PHWE | Low | - | - | Environmentally-friendly technique [51]. |
| QuEChERS | Low | 10–25 | Azoles, musks, UV filters, | It can be online-connected with SPE [60]. |
| MSPD | High | 5–20 | TCS, UV filters, personal care products, azoles, cardiac drugs, NSAIDs, | No clean-up is necessary after extraction or depending on the target analyte [48,51]. |
| SPME | High | 0.5–20 | Used as clean-up step [61,62]. | |
| SLE-LTP | High | 4–8 | PCBs and phthalates |
4.1. Mechanical Shaking
4.2. Soxhlet Extraction
4.3. Ultrasound-Assisted Extraction (UAE)
4.4. Microwave-Assisted Extraction (MAE)
4.5. Pressurized Liquid Extraction (PLE)
4.6. Pressurized Hot Water Extraction (PHWE)
4.7. The Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) Method
4.8. Matrix Solid-Phase Dispersion (MSPD)
4.9. Other Extraction Strategies
5. Clean-Up
6. Separation and Detection Techniques
6.1. Gas Chromatography
6.2. Liquid Chromatography
7. Validation Method
7.1. Selectivity
7.2. Accuracy and Recovery
7.3. Linearity
7.4. Precision
7.5. Sensitivity
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, personal care products and endocrine disrupting agents in the environment–a review. CLEAN–Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Singh, A.; Kumar, N.; Chauhan, G.; Singh, D.P.; Singh, A.; Rana, B.B. Emerging Pollutants in the Environment and Ecological Risks. In Management and Mitigation of Emerging Pollutants; George, N., Dwibedi, V., Rath, S.K., Chauhan, P.S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–20. ISBN 978-3-031-41005-5. [Google Scholar]
- Emerging Substances | NORMAN. 2009. Available online: https://www.norman-network.net/?q=node/19 (accessed on 5 November 2021).
- Dubey, M.; Mohapatra, S.; Tyagi, V.K.; Suthar, S.; Kazmi, A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ. Pollut. 2021, 273, 116515. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Govind, R.; Shrestha, A.; Govind, R.; Shrestha, A. Sorption of Pollutants in Wastewater Solids. In Sorption—From Fundamentals to Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Mejías, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Multiresidue Method for the Determination of Critically and Highly Important Classes of Antibiotics and Their Metabolites in Agricultural Soils and Sewage Sludge. Anal. Bioanal. Chem. 2023, 415, 7161–7173. [Google Scholar] [CrossRef]
- Zhou, T.; Li, X.; Liu, H.; Dong, S.; Zhang, Z.; Wang, Z.; Li, J.; Nghiem, L.D.; Khan, S.J.; Wang, Q. Occurrence, fate, and remediation for per-and polyfluoroalkyl substances (PFAS) in sewage sludge: A comprehensive review. J. Hazard. Mater. 2024, 466, 133637. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Decision (EU) 2018/840 Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495 (Notified Under Document C(2018) 3362). Available online: https://eur-lex.europa.eu/eli/dec_impl/2018/840/oj (accessed on 22 February 2024).
- Lindholm-Lehto, P.C.; Ahkola, H.S.J.; Knuutinen, J.S. Procedures of determining organic trace compounds in municipal sewage sludge—A review. Environ. Sci. Pollut. Res. 2017, 24, 4383–4412. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Hooda, P.S.; Swinden, J.; Barker, J.; Barton, S. Spatial distribution of organic contaminants in three rivers of Southern England bound to suspended particulate material and dissolved in water. Sci. Total Environ. 2017, 593–594, 487–497. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, J.; Wu, W.; Liu, X.; Zhang, H. A new pretreatment and improved method for determination of selected estrogens in high matrix solid sewage samples by liquid chromatography mass spectrometry. Microchem. J. 2012, 104, 49–55. [Google Scholar] [CrossRef]
- Lee, H.-B.; Lewina Svoboda, M.; Peart, T.E.; Smyth, S.A. Optimization of a microwave-assisted extraction procedure for the determination of selected alkyl, aryl, and halogenated phenols in sewage sludge and biosolids. Water Qual. Res. J. 2016, 51, 344–356. [Google Scholar] [CrossRef]
- Gabet-Giraud, V.; Miege, C.; Herbreteau, B.; Hernandez-Raquet, G.; Coquery, M. Development and validation of an analytical method by LC-MS/MS for the quantification of estrogens in sewage sludge. Anal. Bioanal. Chem. 2010, 396, 1841–1851. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Q.; Cui, J.; Zhang, K.; Tang, C.; Peng, X. Determination of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in sewage sludge by ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 399, 891–902. [Google Scholar] [CrossRef]
- Demirtepe, H.; Imamoglu, I. Levels of polybrominated diphenyl ethers and hexabromocyclododecane in treatment plant sludge: Implications on sludge management. Chemosphere 2019, 221, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ying, G.-G.; Zhao, J.-L.; Chen, F.; Yang, B.; Zhou, L.-J.; Lai, H. Trace analysis of 28 steroids in surface water, wastewater and sludge samples by rapid resolution liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Lindholm-Lehto, P.C.; Ahkola, H.S.J.; Knuutinen, J.S. Pharmaceuticals in processing of municipal sewage sludge studied by grab and passive sampling. Water Qual. Res. J. 2018, 53, 14–23. [Google Scholar] [CrossRef]
- Banihashemi, B.; Droste, R.L. Trace level determination of bisphenol-A in wastewater and sewage sludge by high-performance liquid chromatography and UV detection. Water Qual. Res. J. 2013, 48, 133–144. [Google Scholar] [CrossRef]
- Liu, S.-S.; Ying, G.-G.; Liu, S.; Lai, H.-J.; Chen, Z.-F.; Pan, C.-G.; Zhao, J.-L.; Chen, J. Analysis of 21 progestagens in various matrices by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) with diverse sample pretreatment. Anal. Bioanal. Chem. 2014, 406, 7299–7311. [Google Scholar] [CrossRef]
- Zuliani, T.; Milačič, R.; Ščančar, J. Preparation of a sewage sludge laboratory quality control material for butyltin compounds and their determination by isotope-dilution mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Llop, A.; Borrull, F.; Pocurull, E. Pressurised hot water extraction followed by simultaneous derivatization and headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry for the determination of aliphatic primary amines in sewage sludge. Anal. Chim. Acta 2010, 665, 231–236. [Google Scholar] [CrossRef]
- Vallecillos, L.; Borrull, F.; Pocurull, E. Determination of musk fragrances in sewage sludge by pressurized liquid extraction coupled to automated ionic liquid-based headspace single-drop microextraction followed by GC-MS/MS. J. Sep. Sci. 2012, 35, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Borrull, F.; Pocurull, E.; Marcé, R.M. A quick, easy, cheap, effective, rugged and safe extraction method followed by liquid chromatography-(Orbitrap) high resolution mass spectrometry to determine benzotriazole, benzothiazole and benzenesulfonamide derivates in sewage sludge. J. Chromatogr. A 2014, 1339, 34–41. [Google Scholar] [CrossRef]
- Bergé, A.; Buleté, A.; Fildier, A.; Vulliet, E. High-Resolution Mass Spectrometry as a Tool To Evaluate the Sample Preparation of Sludge. Anal. Chem. 2017, 89, 9685–9694. [Google Scholar] [CrossRef]
- Ferhi, S.; Bourdat-Deschamps, M.; Daudin, J.-J.; Houot, S.; Nélieu, S. Factors influencing the extraction of pharmaceuticals from sewage sludge and soil: An experimental design approach. Anal. Bioanal. Chem. 2016, 408, 6153–6168. [Google Scholar] [CrossRef]
- Soares, K.L.; Cerqueira, M.B.R.; Caldas, S.S.; Primel, E.G. Evaluation of alternative environmentally friendly matrix solid phase dispersion solid supports for the simultaneous extraction of 15 pesticides of different chemical classes from drinking water treatment sludge. Chemosphere 2017, 182, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.; Sanz, P.; Martínez, M.Á. Analysis of perfluorinated alkyl substances in Spanish sewage sludge by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 1277–1286. [Google Scholar] [CrossRef]
- Saleh, A.; Larsson, E.; Yamini, Y.; Jönsson, J.Å. Hollow fiber liquid phase microextraction as a preconcentration and clean-up step after pressurized hot water extraction for the determination of non-steroidal anti-inflammatory drugs in sewage sludge. J. Chromatogr. A 2011, 1218, 1331–1339. [Google Scholar] [CrossRef]
- Samaras, V.G.; Thomaidis, N.S.; Stasinakis, A.S.; Lekkas, T.D. An analytical method for the simultaneous trace determination of acidic pharmaceuticals and phenolic endocrine disrupting chemicals in wastewater and sewage sludge by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2011, 399, 2549–2561. [Google Scholar] [CrossRef]
- Dorival-García, N.; Zafra-Gómez, A.; Camino-Sánchez, F.J.; Navalón, A.; Vílchez, J.L. Analysis of quinolone antibiotic derivatives in sewage sludge samples by liquid chromatography–tandem mass spectrometry: Comparison of the efficiency of three extraction techniques. Talanta 2013, 106, 104–118. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, L. Analysis of endocrine disrupting compounds, pharmaceuticals and personal care products in sewage sludge by gas chromatography–mass spectrometry. Talanta 2012, 89, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Manso, J.; Larsson, E.; Jönsson, J.Å. Determination of 4′-isobutylacetophenone and other transformation products of anti-inflammatory drugs in water and sludge from five wastewater treatment plants in Sweden by hollow fiber liquid phase microextraction and gas chromatography–mass spectrometry. Talanta 2014, 125, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sagristà, E.; Larsson, E.; Ezoddin, M.; Hidalgo, M.; Salvadó, V.; Jönsson, J.Å. Determination of non-steroidal anti-inflammatory drugs in sewage sludge by direct hollow fiber supported liquid membrane extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2010, 1217, 6153–6158. [Google Scholar] [CrossRef]
- Martínez-Moral, M.P.; Tena, M.T. Use of microextraction by packed sorbents following selective pressurised liquid extraction for the determination of brominated diphenyl ethers in sewage sludge by gas chromatography–mass spectrometry. J. Chromatogr. A 2014, 1364, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Viglino, L.; Prévost, M.; Sauvé, S. High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. J. Environ. Monit. 2011, 13, 583–590. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, Y.; Dong, X.; Fang, B.; Wang, Z.; Chen, H.; Sun, H. Identification of emerging PFAS in industrial sludge from North China: Release risk assessment by the TOP assay. Water Res. 2025, 268, 122667. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; León-Morcillo, R.; Guzmán, S.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E. Pharmaceutical active compounds in sewage sludge: Degradation improvement and conversion into an organic amendment by bioaugmentation-composting processes. Waste Manag. 2023, 168, 167–178. [Google Scholar] [CrossRef]
- Angeles-De Paz, G.; Cubero-Cardoso, J.; Pozo, C.; Calvo, C.; Aranda, E.; Robledo-Mahón, T. Optimizing Bioaugmentation for Pharmaceutical Stabilization of Sewage Sludge: A Study on Short-Term Composting Under Real Conditions. J. Fungi 2025, 11, 67. [Google Scholar] [CrossRef]
- Wilschnack, K.; Homer, B.; Cartmell, E.; Yates, K.; Petrie, B. Targeted multi-analyte UHPLC-MS/MS methodology for emerging contaminants in septic tank wastewater, sludge and receiving surface water. Anal. Methods 2024, 16, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Miserli, K.; Kosma, C.; Konstantinou, I. Determination of pharmaceuticals and metabolites in sludge and hydrochar after hydrothermal carbonization using sonication—QuEChERS extraction method and UHPLC LTQ/Orbitrap MS. Environ. Sci. Pollut. Res. 2023, 30, 1686–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, B.; Chen, Q.; Zhu, F.; Wang, J.; Fu, X.; Zhou, T. Fate of organophosphate flame retardants (OPFRs) in the “Cambi® TH + AAD” of sludge in a WWTP in Beijing, China. Waste Manag. 2023, 169, 363–373. [Google Scholar] [CrossRef]
- Zuloaga, O.; Navarro, P.; Bizkarguenaga, E.; Iparraguirre, A.; Vallejo, A.; Olivares, M.; Prieto, A. Overview of extraction, clean-up and detection techniques for the determination of organic pollutants in sewage sludge: A review. Anal. Chim. Acta 2012, 736, 7–29. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Veenaas, C.; Haglund, P. Methodology for non-target screening of sewage sludge using comprehensive two-dimensional gas chromatography coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 4867–4883. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Q.; Deng, S.; Huang, J.; Wang, B.; Yu, G. Determination of pharmaceuticals from various therapeutic classes in dewatered sludge by pressurized liquid extraction and high performance liquid chromatography and tandem mass spectrometry (HPLC-MS/MS). Int. J. Environ. Anal. Chem. 2013, 93, 1159–1173. [Google Scholar] [CrossRef]
- Castro, G.; Carpinteiro, I.; Rodríguez, I.; Cela, R. Determination of cardiovascular drugs in sewage sludge by matrix solid-phase dispersion and ultra-performance liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 6807–6817. [Google Scholar] [CrossRef]
- Novak, P.; Zuliani, T.; Milačič, R.; Ščančar, J. Development of an analytical method for the determination of polybrominated diphenyl ethers in sewage sludge by the use of gas chromatography coupled to inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2016, 915, 27–35. [Google Scholar] [CrossRef]
- Abril, C.; Santos, J.L.; Malvar, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Determination of perfluorinated compounds, bisphenol A, anionic surfactants and personal care products in digested sludge, compost and soil by liquid-chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1576, 34–41. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Zhang, J.; Yang, Y.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, Y.; Tang, C.; Peng, X. Determination of commonly used azole antifungals in various waters and sewage sludge using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Sagristà, E.; Matamoros, V.; Fontàs, C.; Hidalgo, M.; Salvadó, V. Determination of pharmaceutical compounds in sewage sludge using a standard addition method approach. Int. J. Environ. Anal. Chem. 2014, 94, 1199–1209. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, A.O.O. Distribution and Chemical Analysis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environmental Systems: A Review. Int J Environ. Res Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; de Alarcón-Gómez, B.; Rodríguez-Gómez, R.; García-Córcoles, M.T.; Çipa, M.; Zafra-Gómez, A. Analytical methods for the determination of emerging contaminants in sewage sludge samples. A review. Talanta 2019, 192, 508–533. [Google Scholar] [CrossRef]
- López-Serna, R.; Marín-de-Jesús, D.; Irusta-Mata, R.; García-Encina, P.A.; Lebrero, R.; Fdez-Polanco, M.; Muñoz, R. Multiresidue analytical method for pharmaceuticals and personal care products in sewage and sewage sludge by online direct immersion SPME on-fiber derivatization–GCMS. Talanta 2018, 186, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Llop, A.; Borrull, F.; Pocurull, E. Pressurised hot water extraction followed by headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry for the determination of N-nitrosamines in sewage sludge. Talanta 2012, 88, 284–289. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wen, Z.; Ren, N. Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere 2014, 95, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gani, K.M.; Kazmi, A.A. Comparative assessment of phthalate removal and risk in biological wastewater treatment systems of developing countries and small communities. Sci. Total Environ. 2016, 569–570, 661–671. [Google Scholar] [CrossRef]
- Gani, K.M.; Bux, F.; Kazmi, A.A. Diethylhexyl phthalate removal in full scale activated sludge plants: Effect of operational parameters. Chemosphere 2019, 234, 885–892. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, N.; Li, Y.-F.; Kunisue, T.; Gao, D.; Kannan, K. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge. Environ. Sci. Technol. 2011, 45, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Gani, K.M.; Kazmi, A.A. Ecotoxicological risk evaluation and regulatory compliance of endocrine disruptor phthalates in a sustainable wastewater treatment scheme. Enviorn. Sci. Pollut. Res. 2020, 27, 7785–7794. [Google Scholar] [CrossRef]
- Qian, Y.; Jia, X.; Ding, T.; Yang, M.; Yang, B.; Li, J. Occurrence and removal of bisphenol analogues in wastewater treatment plants and activated sludge bioreactor. Sci. Total Environ. 2021, 758, 143606. [Google Scholar] [CrossRef]
- Eljarrat, E.; Barceló, D. Priority lists for persistent organic pollutants and emerging contaminants based on their relative toxic potency in environmental samples. TrAC Trends Anal. Chem. 2003, 22, 655–665. [Google Scholar] [CrossRef]
- Gao, S.; Tian, B.; Zeng, X.; Yu, Z. Enantiomeric analysis of polycyclic musks AHTN and HHCB and HHCB-lactone in sewage sludge by gas chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 607–612. [Google Scholar] [CrossRef]
- Vrkoslavová, J.; Demnerová, K.; Macková, M.; Zemanová, T.; Macek, T.; Hajšlová, J.; Pulkrabová, J.; Hrádková, P.; Stiborová, H. Absorption and translocation of polybrominated diphenyl ethers (PBDEs) by plants from contaminated sewage sludge. Chemosphere 2010, 81, 381–386. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T.; Misuri, L.; Lanciotti, E.; Sweetman, A.; Laschi, S.; Palchetti, I. PBDEs in Italian sewage sludge and environmental risk of using sewage sludge for land application. Environ. Pollut. 2012, 161, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Cao, S.-X.; Zhang, D.-L.; Gao, S.-T.; Yu, Z.-Q.; Li, H.-R.; Sheng, G.-Y.; Fu, J.-M. Levels and distribution of synthetic musks and polycyclic aromatic hydrocarbons in sludge collected from Guangdong Province. J. Environ. Sci Health A 2012, 47, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luque-García, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Albero, B.; García-Valcárcel, A.I. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]
- Albero, B.; Sánchez-Brunete, C.; García-Valcárcel, A.I.; Pérez, R.A.; Tadeo, J.L. Ultrasound-assisted extraction of emerging contaminants from environmental samples. TrAC Trends Anal. Chem. 2015, 71, 110–118. [Google Scholar] [CrossRef]
- Guerra, P.; Eljarrat, E.; Barceló, D. Simultaneous determination of hexabromocyclododecane, tetrabromobisphenol A, and related compounds in sewage sludge and sediment samples from Ebro River basin (Spain). Anal. Bioanal. Chem. 2010, 397, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, B.V.; Karunakar, K.K.; Anandakumar, R.; Murugathirumal, A.; Kumar, A.S. Eco-friendly extraction technologies: A comprehensive review of modern green analytical methods. Sustain. Chem. Clim. Action 2025, 6, 100054. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Borova, V.; Dasenaki, M.E.; Τhomaidis, Ν.S. Simultaneous determination of 148 pharmaceuticals and illicit drugs in sewage sludge based on ultrasound-assisted extraction and liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4287–4297. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, Q.; Feng, J.; Yuan, S.; Wang, Q.; Huang, D.; Zhang, J. Validation and application of an analytical method for the determination of selected acidic pharmaceuticals and estrogenic hormones in wastewater and sludge. J. Environ. Sci. Health Part A 2016, 51, 914–920. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Ying, G.-G.; Lai, H.-J.; Chen, F.; Su, H.-C.; Liu, Y.-S.; Peng, F.-Q.; Zhao, J.-L. Determination of biocides in different environmental matrices by use of ultra-high-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 404, 3175–3188. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, K.; Wang, Z.; Wang, C.; Peng, X. Enantiomeric determination of azole antifungals in wastewater and sludge by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moral, M.P.; Tena, M.T. Focused ultrasound solid–liquid extraction and selective pressurised liquid extraction to determine bisphenol A and alkylphenols in sewage sludge by gas chromatography–mass spectrometry. J. Sep. Sci. 2011, 34, 2513–2522. [Google Scholar] [CrossRef]
- Martínez-Moral, M.P.; Tena, M.T. Focused ultrasound solid–liquid extraction of perfluorinated compounds from sewage sludge. Talanta 2013, 109, 197–202. [Google Scholar] [CrossRef]
- Cristale, J.; Lacorte, S. Development and validation of a multiresidue method for the analysis of polybrominated diphenyl ethers, new brominated and organophosphorus flame retardants in sediment, sludge and dust. J. Chromatogr. A 2013, 1305, 267–275. [Google Scholar] [CrossRef]
- Fernández-Sanjuan, M.; Lacorte, S.; Rigol, A.; Sahuquillo, A. New quality-control materials for the determination of alkylphenols and alkylphenol ethoxylates in sewage sludge. Anal. Bioanal. Chem. 2012, 404, 2499–2505. [Google Scholar] [CrossRef]
- Koumaki, E.; Noutsopoulos, C.; Mamais, D.; Fragkiskatos, G.; Andreadakis, A. Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. Int. J. Environ. Res. Public Health 2021, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, W.; Fiedler, H.; Zhang, J.; Wei, X.; Liu, X.; Peng, M.; Zhang, T. Fate and risk assessment of emerging contaminants in reclaimed water production processes. Front. Environ. Sci. Eng. 2021, 15, 104. [Google Scholar] [CrossRef]
- Santana, J.M.; Fraga, S.V.B.; Zanatta, M.C.K.; Martins, M.R.; Pires, M.S.G. Characterization of organic compounds and drugs in sewage sludge aiming for agricultural recycling. Heliyon 2021, 7, e06771. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, R.; Andrés-Costa, M.J.; Andreu, V.; Picó, Y. Simultaneous determination of traditional and emerging illicit drugs in sediments, sludges and particulate matter. J. Chromatogr. A 2015, 1405, 103–115. [Google Scholar] [CrossRef]
- Košnář, Z.; Mercl, F.; Pierdonà, L.; Chane, A.D.; Míchal, P.; Tlustoš, P. Concentration of the main persistent organic pollutants in sewage sludge in relation to wastewater treatment plant parameters and sludge stabilisation. Environ. Pollut. 2023, 333, 122060. [Google Scholar] [CrossRef]
- Liu, R.; Ruan, T.; Wang, T.; Song, S.; Yu, M.; Gao, Y.; Shao, J.; Jiang, G. Trace analysis of mono-, di-, tri-substituted polyfluoroalkyl phosphates and perfluorinated phosphonic acids in sewage sludge by high performance liquid chromatography tandem mass spectrometry. Talanta 2013, 111, 170–177. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Okonkwo, J.O.; Sibali, L.L.; Ncube, E.J. An integrated method for the simultaneous determination of alkylphenol ethoxylates and brominated flame retardants in sewage sludge samples by ultrasonic-assisted extraction, solid phase clean-up, and GC-MS analysis. Microchem. J. 2015, 123, 230–236. [Google Scholar] [CrossRef]
- Zacs, D.; Bartkevics, V. Trace determination of perfluorooctane sulfonate and perfluorooctanoic acid in environmental samples (surface water, wastewater, biota, sediments, and sewage sludge) using liquid chromatography–Orbitrap mass spectrometry. J. Chromatogr. A 2016, 1473, 109–121. [Google Scholar] [CrossRef]
- Clara, M.; Gans, O.; Windhofer, G.; Krenn, U.; Hartl, W.; Braun, K.; Scharf, S.; Scheffknecht, C. Occurrence of polycyclic musks in wastewater and receiving water bodies and fate during wastewater treatment. Chemosphere 2011, 82, 1116–1123. [Google Scholar] [CrossRef]
- Healy, M.G.; Fenton, O.; Cormican, M.; Peyton, D.P.; Ordsmith, N.; Kimber, K.; Morrison, L. Antimicrobial compounds (triclosan and triclocarban) in sewage sludges, and their presence in runoff following land application. Ecotoxicol. Environ. Saf. 2017, 142, 448–453. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Li, X.-Y.; Leung, K.M.Y. Retinoids and oestrogenic endocrine disrupting chemicals in saline sewage treatment plants: Removal efficiencies and ecological risks to marine organisms. Environ. Int. 2019, 127, 103–113. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Lin, L.; Li, X.-Y.; Leung, K.M.Y. Removal of emerging contaminants from wastewater during chemically enhanced primary sedimentation and acidogenic sludge fermentation. Water Res. 2020, 175, 115646. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Abbott, T.; Ubay-Cokgor, E.; Eskicioglu, C. Occurrence of the Persistent Antimicrobial Triclosan in Microwave Pretreated and Anaerobically Digested Municipal Sludges under Various Process Conditions. Molecules 2020, 25, 310. [Google Scholar] [CrossRef]
- Tasselli, S.; Guzzella, L. Polycyclic musk fragrances (PMFs) in wastewater and activated sludge: Analytical protocol and application to a real case study. Environ. Sci. Pollut. Res. 2020, 27, 30977–30986. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, Y.; Wang, D.; Han, K.; Wang, H.; Kang, L. The fate of triclocarban in activated sludge and its influence on biological wastewater treatment system. J. Environ. Manag. 2020, 276, 111237. [Google Scholar] [CrossRef] [PubMed]
- Abbott, T.; Eskicioglu, C. Comparison of anaerobic, cycling aerobic/anoxic, and sequential anaerobic/aerobic/anoxic digestion to remove triclosan and triclosan metabolites from municipal biosolids. Sci. Total Environ. 2020, 745, 140953. [Google Scholar] [CrossRef] [PubMed]
- Košnář, Z.; Mercl, F.; Chane, A.D.; Pierdonà, L.; Míchal, P.; Tlustoš, P. Occurrence of synthetic polycyclic and nitro musk compounds in sewage sludge from municipal wastewater treatment plants. Sci. Total Environ. 2021, 801, 149777. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Multi-residue method for the analysis of pharmaceutical compounds in sewage sludge, compost and sediments by sonication-assisted extraction and LC determination. J. Sep. Sci. 2010, 33, 1760–1766. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Shafrir, M.; Avisar, D. Development Method for Extracting and Analyzing Antibiotic and Hormone Residues from Treated Wastewater Sludge and Composted Biosolids. Water Air Soil Pollut. 2012, 223, 2571–2587. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhao, J.-L.; Chen, F.; Zhang, R.-Q.; Peng, F.-Q.; Zhang, Q.-Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef]
- Yuan, X.; Qiang, Z.; Ben, W.; Zhu, B.; Liu, J. Rapid detection of multiple class pharmaceuticals in both municipal wastewater and sludge with ultra high performance liquid chromatography tandem mass spectrometry. J. Environ. Sci. 2014, 26, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Boix, C.; Ibáñez, M.; Fabregat-Safont, D.; Morales, E.; Pastor, L.; Sancho, J.V.; Sánchez-Ramírez, J.E.; Hernández, F. Behaviour of emerging contaminants in sewage sludge after anaerobic digestion. Chemosphere 2016, 163, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Boix, C.; Ibáñez, M.; Fabregat-Safont, D.; Morales, E.; Pastor, L.; Sancho, J.V.; Sánchez-Ramírez, J.E.; Hernández, F. Analytical methodologies based on LC–MS/MS for monitoring selected emerging compounds in liquid and solid phases of the sewage sludge. MethodsX 2016, 3, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- Abbott, T.; Kor-Bicakci, G.; Eskicioglu, C. Examination of single-stage anaerobic and anoxic/aerobic and dual-stage anaerobic-anoxic/aerobic digestion to remove pharmaceuticals from municipal biosolids. Sci. Total Environ. 2021, 791, 148237. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Lahora, A. Occurrence and fate of pharmaceuticals in a wastewater treatment plant from southeast of Spain and risk assessment. J. Environ. Manag. 2021, 279, 111565. [Google Scholar] [CrossRef]
- Mercl, F.; Košnář, Z.; Maršík, P.; Vojtíšek, M.; Dušek, J.; Száková, J.; Tlustoš, P. Pyrolysis of biosolids as an effective tool to reduce the uptake of pharmaceuticals by plants. J. Hazard. Mater. 2021, 405, 124278. [Google Scholar] [CrossRef]
- Ömeroğlu, S.; Kara Murdoch, F.; Dilek Sanin, F. Investigation of nonylphenol and nonylphenol ethoxylates in sewage sludge samples from a metropolitan wastewater treatment plant in Turkey. Talanta 2015, 131, 650–655. [Google Scholar] [CrossRef]
- Sun, X.; Peng, J.; Wang, M.; Wang, J.; Tang, C.; Yang, L.; Lei, H.; Li, F.; Wang, X.; Chen, J. Determination of nine bisphenols in sewage and sludge using dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2018, 1552, 10–16. [Google Scholar] [CrossRef] [PubMed]
- García-Valcárcel, A.I.; Tadeo, J.L. Determination of azoles in sewage sludge from Spanish wastewater treatment plants by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2011, 34, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yu, Y.; Huang, Q.; Peng, X. Simultaneous determination of fluoroquinolone and tetracycline antibacterials in sewage sludge using ultrasonic-assisted extraction and HPLC-MS/MS. Int. J. Environ. Anal. Chem. 2012, 92, 1389–1402. [Google Scholar] [CrossRef]
- Hajj-Mohamad, M.; Aboulfadl, K.; Darwano, H.; Madoux-Humery, A.-S.; Guérineau, H.; Sauvé, S.; Prévost, M.; Dorner, S. Wastewater micropollutants as tracers of sewage contamination: Analysis of combined sewer overflow and stream sediments. Environ. Sci. Process. Impacts 2014, 16, 2442–2450. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Comparison of ultrasound-assisted extraction, QuEChERS and selective pressurized liquid extraction for the determination of metabolites of parabens and pharmaceuticals in sludge. Microchem. J. 2020, 157, 104987. [Google Scholar] [CrossRef]
- Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. 2007, 77. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_1694_2007.pdf (accessed on 14 August 2024).
- US EPA. Method 3550C–Ultrasonic Extraction; Environmental Protection Agency of United States EPA: Washington, VA, USA, 2000.
- Azzouz, A.; Ballesteros, E. Determination of 13 endocrine disrupting chemicals in environmental solid samples using microwave-assisted solvent extraction and continuous solid-phase extraction followed by gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Santana-Viera, S.; Montesdeoca-Esponda, S.; Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Application of microwave-assisted extraction and ultra-high performance liquid chromatography–tandem mass spectrometry for the analysis of sex hormones and corticosteroids in sewage sludge samples. Anal. Bioanal. Chem. 2016, 408, 6833–6844. [Google Scholar] [CrossRef]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef]
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of various estradiol mimicking-compounds in sewage sludge by the combination of microwave-assisted extraction and LC–MS/MS. Talanta 2011, 85, 1825–1834. [Google Scholar] [CrossRef]
- Cantarero, S.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Vílchez, J.L.; Verge, C.; De Ferrer, J.A. Matrix effect study in the determination of linear alkylbenzene sulfonates in sewage sludge samples. Environ. Toxicol. Chem. 2011, 30, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Dobor, J.; Varga, M.; Yao, J.; Chen, H.; Palkó, G.; Záray, G. A new sample preparation method for determination of acidic drugs in sewage sludge applying microwave assisted solvent extraction followed by gas chromatography–mass spectrometry. Microchem. J. 2010, 94, 36–41. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Combination of microwave-assisted micellar extraction with liquid chromatography tandem mass spectrometry for the determination of fluoroquinolone antibiotics in coastal marine sediments and sewage sludges samples. Biomed. Chromatogr. 2012, 26, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Devault, D.A.; Amalric, L.; Bristeau, S.; Cruz, J.; Tapie, N.; Karolak, S.; Budzinski, H.; Lévi, Y. Removal efficiency of emerging micropollutants in biofilter wastewater treatment plants in tropical areas. Environ. Sci. Pollut. Res. Int. 2021, 28, 10940–10966. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef]
- Junior, I.L.C.; Machado, C.S.; Pletsch, A.L.; Torres, Y.R. Simultaneous HPLC-PDA determination of commonly prescribed antidepressants and caffeine in sludge from sewage treatment plants and river sediments in the Itaipu reservoir region, Paraná, Brazil. Int. J. Environ. Anal. Chem. 2020, 100, 1004–1020. [Google Scholar] [CrossRef]
- Junior, I.L.C.; Machado, C.S.; Ramalho, A.N.; Pletsch, A.L.; Torres, Y.R. Optimisation of caffeine and antidepressants extraction from sediments and sewage sludge using experimental designs. Int. J. Environ. Anal. Chem. 2017, 97, 935–948. [Google Scholar] [CrossRef]
- Zhou, S.; Peng, S.; Li, Z.; Zhang, D.; Zhu, Y.; Li, X.; Hong, M.; Li, W.; Lu, P. Risk assessment of pollutants in flowback and produced waters and sludge in impoundments. Sci. Total Environ. 2022, 811, 152250. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography–mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci. Total Environ. 2012, 419, 208–215. [Google Scholar] [CrossRef]
- Rhodes, L. Microwave-assisted extraction using US EPA method 3546. LC GC N. Am. 2002, 20, 23. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Arbeláez, P.; Borrull, F.; Maria Marcé, R.; Pocurull, E. Trace-level determination of sweeteners in sewage sludge using selective pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2015, 1408, 15–21. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, S.; Barceló, D. Multiresidue trace analysis of sulfonamide antibiotics and their metabolites in soils and sewage sludge by pressurized liquid extraction followed by liquid chromatography–electrospray-quadrupole linear ion trap mass spectrometry. J. Chromatogr. A 2013, 1275, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Brar, S.K.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y. Carbamazepine in municipal wastewater and wastewater sludge: Ultrafast quantification by laser diode thermal desorption-atmospheric pressure chemical ionization coupled with tandem mass spectrometry. Talanta 2012, 99, 247–255. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Radjenović, J.; Jelić, A.; Petrović, M.; Barceló, D. Determination of pharmaceuticals in sewage sludge by pressurized liquid extraction (PLE) coupled to liquid chromatography-tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2009, 393, 1685–1695. [Google Scholar] [CrossRef]
- Salvia, M.-V.; Fieu, M.; Vulliet, E. Determination of Tetracycline and Fluoroquinolone Antibiotics at Trace Levels in Sludge and Soil. Appl. Environ. Soil Sci. 2015, 2015, e435741. [Google Scholar] [CrossRef]
- Scheurer, M.; Ramil, M.; Metcalfe, C.D.; Groh, S.; Ternes, T.A. The challenge of analyzing beta-blocker drugs in sludge and wastewater. Anal. Bioanal. Chem. 2010, 396, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Seira, J.; Claparols, C.; Joannis-Cassan, C.; Albasi, C.; Montréjaud-Vignoles, M.; Sablayrolles, C. Optimization of pressurized liquid extraction using a multivariate chemometric approach for the determination of anticancer drugs in sludge by ultra high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1283, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Esparza, X.; Moyano, E.; de Boer, J.; Galceran, M.T.; van Leeuwen, S.P.J. Analysis of perfluorinated phosponic acids and perfluorooctane sulfonic acid in water, sludge and sediment by LC–MS/MS. Talanta 2011, 86, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Gorga, M.; Insa, S.; Petrovic, M.; Barceló, D. Analysis of endocrine disrupters and related compounds in sediments and sewage sludge using on-line turbulent flow chromatography–liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1352, 29–37. [Google Scholar] [CrossRef]
- Mascolo, G.; Locaputo, V.; Mininni, G. New perspective on the determination of flame retardants in sewage sludge by using ultrahigh pressure liquid chromatography-tandem mass spectrometry with different ion sources. J Chromatogr A 2010, 1217, 4601–4611. [Google Scholar] [CrossRef]
- Mastroianni, N.; Postigo, C.; de Alda, M.L.; Barcelo, D. Illicit and abused drugs in sewage sludge: Method optimization and occurrence. J. Chromatogr. A 2013, 1322, 29–37. [Google Scholar] [CrossRef]
- Tohidi, F.; Cai, Z. GC/MS analysis of triclosan and its degradation by-products in wastewater and sludge samples from different treatments. Environ. Sci. Pollut. Res. 2015, 22, 11387–11400. [Google Scholar] [CrossRef]
- Riva, F.; Zuccato, E.; Pacciani, C.; Colombo, A.; Castiglioni, S. A multi-residue analytical method for extraction and analysis of pharmaceuticals and other selected emerging contaminants in sewage sludge. Anal. Methods 2021, 13, 526–535. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, A.; Concejero, M.A.; Martínez, M.A. Concentrations and sources of an emerging pollutant, decabromodiphenylethane (DBDPE), in sewage sludge for land application. J. Environ. Sci. 2012, 24, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Farré, M.; Picó, Y.; Barceló, D. Analysis of perfluorinated compounds in sewage sludge by pressurized solvent extraction followed by liquid chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 4840–4846. [Google Scholar] [CrossRef]
- Herrero, P.; Borrull, F.; Marcé, R.M.; Pocurull, E. Pressurised liquid extraction and ultra-high performance liquid chromatography-tandem mass spectrometry to determine endogenous and synthetic glucocorticoids in sewage sludge. Talanta 2013, 103, 186–193. [Google Scholar] [CrossRef]
- Arbeláez, P.; Granados, J.; Borrull, F.; Marcé, R.M.; Pocurull, E. Determination of sedative hypnotics in sewage sludge by pressurized liquid extraction with high-performance liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3481–3488. [Google Scholar] [CrossRef]
- vom Eyser, C.; Palmu, K.; Otterpohl, R.; Schmidt, T.C.; Tuerk, J. Determination of pharmaceuticals in sewage sludge and biochar from hydrothermal carbonization using different quantification approaches and matrix effect studies. Anal. Bioanal. Chem. 2015, 407, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Diclofenac in municipal wastewater treatment plant: Quantification using laser diode thermal desorption--atmospheric pressure chemical ionization--tandem mass spectrometry approach in comparison with an established liquid chromatography-electrospray ionization-tandem mass spectrometry method. J. Chromatogr. A 2016, 1433, 106–113. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.; Thomas, K.V. Multi-residue screening of prioritised human pharmaceuticals, illicit drugs and bactericides in sediments and sludge. J. Environ. Monit. 2011, 13, 2284–2291. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Patureau, D.; Vulliet, E.; Delgenes, N.; Danel, A.; Deshayes, S.; Eudes, V.; Guerin, S.; Moilleron, R.; et al. Fate of emerging and priority micropollutants during the sewage sludge treatment: Case study of Paris conurbation. Part 1: Contamination of the different types of sewage sludge. Waste Manag. 2017, 59, 379–393. [Google Scholar] [CrossRef]
- Shukla, R.; Ahammad, S.Z. Performance assessment of a modified trickling filter and conventional activated sludge process along with tertiary treatment in removing emerging pollutants from urban sewage. Sci. Total Environ. 2023, 858, 159833. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of Organic Pollutants from Environmental Solids with Sub- and Supercritical Water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Svahn, O.; Björklund, E. Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge. Appl. Sci. 2019, 9, 1509. [Google Scholar] [CrossRef]
- Herrero, P.; Borrull, F.; Marcé, R.M.; Pocurull, E. A pressurised hot water extraction and liquid chromatography–high resolution mass spectrometry method to determine polar benzotriazole, benzothiazole and benzenesulfonamide derivates in sewage sludge. J. Chromatogr. A 2014, 1355, 53–60. [Google Scholar] [CrossRef]
- Peysson, W.; Vulliet, E. Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography–time-of-flight-mass spectrometry. J. Chromatogr. A 2013, 1290, 46–61. [Google Scholar] [CrossRef]
- Masiá, A.; Vásquez, K.; Campo, J.; Picó, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin. J. Chromatogr. A 2015, 1378, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Robles, L.; Rivas, G.; Esteban, B.; Oller, I.; Malato, S.; Agüera, A. Determination of pesticides in sewage sludge from an agro-food industry using QuEChERS extraction followed by analysis with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 6181–6193. [Google Scholar] [CrossRef]
- Cerqueira, M.B.R.; Caldas, S.S.; Primel, E.G. New sorbent in the dispersive solid phase extraction step of quick, easy, cheap, effective, rugged, and safe for the extraction of organic contaminants in drinking water treatment sludge. J. Chromatogr. A 2014, 1336, 10–22. [Google Scholar] [CrossRef]
- Cerqueira, M.B.R.; Guilherme, J.R.; Caldas, S.S.; Martins, M.L.; Zanella, R.; Primel, E.G. Evaluation of the QuEChERS method for the extraction of pharmaceuticals and personal care products from drinking-water treatment sludge with determination by UPLC-ESI-MS/MS. Chemosphere 2014, 107, 74–82. [Google Scholar] [CrossRef]
- Rossini, D.; Ciofi, L.; Ancillotti, C.; Checchini, L.; Bruzzoniti, M.C.; Rivoira, L.; Fibbi, D.; Orlandini, S.; Del Bubba, M. Innovative combination of QuEChERS extraction with on-line solid-phase extract purification and pre-concentration, followed by liquid chromatography-tandem mass spectrometry for the determination of non-steroidal anti-inflammatory drugs and their metabolites in sewage sludge. Anal. Chim. Acta 2016, 935, 269–281. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Santos, L. Development and optimization of a QuEChERS-GC–MS/MS methodology to analyse ultraviolet-filters and synthetic musks in sewage sludge. Sci. Total Environ. 2019, 651, 2606–2614. [Google Scholar] [CrossRef]
- Rede, D.; Teixeira, I.; Delerue-Matos, C.; Fernandes, V.C. Assessing emerging and priority micropollutants in sewage sludge: Environmental insights and analytical approaches. Environ. Sci. Pollut. Res. 2024, 31, 3152–3168. [Google Scholar] [CrossRef]
- Benedetti, B.; Majone, M.; Cavaliere, C.; Montone, C.M.; Fatone, F.; Frison, N.; Laganà, A.; Capriotti, A.L. Determination of multi-class emerging contaminants in sludge and recovery materials from waste water treatment plants: Development of a modified QuEChERS method coupled to LC–MS/MS. Microchem. J. 2020, 155, 104732. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; Ledezma-Villanueva, A.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E.; Purswani, J. Assembled mixed co-cultures for emerging pollutant removal using native microorganisms from sewage sludge. Chemosphere 2023, 313, 137472. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.S.; Tisler, S.; Zwiener, C. Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Methods 2020, 12, 576–586. [Google Scholar] [CrossRef]
- Montemurro, N.; Joedicke, J.; Pérez, S. Development and application of a QuEChERS method with liquid chromatography-quadrupole time of flight-mass spectrometry for the determination of 50 wastewater-borne pollutants in earthworms exposed through treated wastewater. Chemosphere 2021, 263, 128222. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef]
- Barker, S.A. Matrix solid-phase dispersion. J. Chromatogr. A 2000, 885, 115–127. [Google Scholar] [CrossRef]
- Triñanes, S.; Casais, M.C.; Mejuto, M.C.; Cela, R. Matrix solid-phase dispersion followed by liquid chromatography tandem mass spectrometry for the determination of selective ciclooxygenase-2 inhibitors in sewage sludge samples. J. Chromatogr. A 2016, 1462, 35–43. [Google Scholar] [CrossRef]
- Sánchez-Brunete, C.; Miguel, E.; Albero, B.; Tadeo, J.L. Determination of triclosan and methyl triclosan in environmental solid samples by matrix solid-phase dispersion and gas chromatography-mass spectrometry. J. Sep. Sci. 2010, 33, 2768–2775. [Google Scholar] [CrossRef]
- González-Mariño, I.; Rodríguez, I.; Quintana, J.B.; Cela, R. Matrix solid-phase dispersion followed by gas chromatography-mass spectrometry for the determination of triclosan and methyl triclosan in sludge and sediments. Anal. Bioanal. Chem. 2010, 398, 2289–2297. [Google Scholar] [CrossRef]
- Casado, J.; Rodríguez, I.; Carpinteiro, I.; Ramil, M.; Cela, R. Gas chromatography quadrupole time-of-flight mass spectrometry determination of benzotriazole ultraviolet stabilizers in sludge samples. J. Chromatogr. A 2013, 1293, 126–132. [Google Scholar] [CrossRef]
- Casado, J.; Castro, G.; Rodríguez, I.; Ramil, M.; Cela, R. Selective extraction of antimycotic drugs from sludge samples using matrix solid-phase dispersion followed by on-line clean-up. Anal. Bioanal. Chem. 2015, 407, 907–917. [Google Scholar] [CrossRef]
- Cerqueira, M.B.R.; Soares, K.L.; Caldas, S.S.; Primel, E.G. Sample as solid support in MSPD: A new possibility for determination of pharmaceuticals, personal care and degradation products in sewage sludge. Chemosphere 2018, 211, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Q.; Li, Y.; Lv, M.; Lin, L.; Wu, Y.; Ashfaq, M.; Yu, C. Simultaneous analysis of 45 pharmaceuticals and personal care products in sludge by matrix solid-phase dispersion and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Montes, R.; Rodríguez, I.; Casado, J.; López-Sabater, M.C.; Cela, R. Determination of the cardiac drug amiodarone and its N-desethyl metabolite in sludge samples. J. Chromatogr. A 2015, 1394, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Celano, R.; Rodríguez, I.; Cela, R.; Rastrelli, L.; Piccinelli, A.L. Liquid chromatography quadrupole time-of-flight mass spectrometry quantification and screening of organophosphate compounds in sludge. Talanta 2014, 118, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Pérez, R.A.; Sánchez-Brunete, C.; Tadeo, J.L. Occurrence and analysis of parabens in municipal sewage sludge from wastewater treatment plants in Madrid (Spain). J. Hazard. Mater. 2012, 239–240, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.; Ramil, M.; Cela, R.; Rodríguez, I. Identification and determination of emerging pollutants in sewage sludge driven by UPLC-QTOF-MS data mining. Sci. Total Environ. 2021, 778, 146256. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.J.; Pawliszyn, J. Solid-Phase Microextraction. A Solvent-Free Alternative for Sample Preparation. Anal. Chem. 1994, 66, 844A–853A. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.-B.; Li, K.-Y.; Cao, Y.-Q.; Wu, S.; Wu, L. Advances in different configurations of solid-phase microextraction and their applications in food and environmental analysis. TrAC Trends Anal. Chem. 2015, 72, 141–152. [Google Scholar] [CrossRef]
- Risticevic, S.; Vuckovic, D.; Pawliszyn, J. Solid-Phase Microextraction; Handbook of Sample Preparation; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 81–101. [Google Scholar] [CrossRef]
- Wu, S.-F.; Ding, W.-H. Fast determination of synthetic polycyclic musks in sewage sludge and sediments by microwave-assisted headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 2776–2781. [Google Scholar] [CrossRef]
- Vallecillos, L.; Pocurull, E.; Borrull, F. A simple and automated method to determine macrocyclic musk fragrances in sewage sludge samples by headspace solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2013, 1314, 38–43. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Liquid-liquid-liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999, 71, 2650–2656. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of dispersive liquid–liquid microextraction method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir bar sorptive extraction: Recent applications, limitations and future trends. Talanta 2014, 130, 388–399. [Google Scholar] [CrossRef]
- David, F.; Ochiai, N.; Sandra, P. Two decades of stir bar sorptive extraction: A retrospective and future outlook. TrAC Trends Anal. Chem. 2019, 112, 102–111. [Google Scholar] [CrossRef]
- Ferreira, A.M.C.; Möder, M.; Laespada, M.E.F. Stir bar sorptive extraction of parabens, triclosan and methyl triclosan from soil, sediment and sludge with in situ derivatization and determination by gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Microextraction by packed sorbent (MEPS). TrAC Trends Anal. Chem. 2015, 67, 34–44. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by packed sorbent (MEPS): A tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef]
- Maia, M.R.; Arcanjo, A.L.P.; Pinho, G.P.; Silvério, F.O.; Maia, M.R.; Arcanjo, A.L.P.; Pinho, G.P.; Silvério, F.O. Solid-Liquid Extraction with Low Temperature Purification Coupled with Gas Chromatography and Mass Spectrometry for Determination of Polychlorinated Biphenyls in Sewage Sludge. J. Braz. Chem. Soc. 2017, 28, 179–186. [Google Scholar] [CrossRef]
- Pereira, N.G.F.; Silvério, F.O.; Pinho, G.P. Optimisation, validation and application of the solid-liquid extraction with low-temperature purification followed by gas chromatography-mass spectrometry for determination of phthalates in sewage sludge. Int. J. Environ. Anal. Chem. 2020, 100, 968–980. [Google Scholar] [CrossRef]
- Andrade, V.F.; Durães, A.F.S.; Cassimiro, D.L.; de Pinho, G.P.; Silvério, F.O. Fast extraction of polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran in sewage sludge and soil samples. J. Environ. Sci. Health Part B 2017, 52, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.A. Determination of Selected Organic Contaminants in Drinking Water by Direct Aqueous Injection—Liquid Chromatography/Tandem Mass Spectrometry (DAI-LC/MS/MS). 2009, p. 40. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-538.pdf (accessed on 25 October 2024).
- Larsson, E.; Rabayah, A. Sludge removal of nonsteroidal anti-inflammatory drugs during wastewater treatment studied by direct hollow fiber liquid phase microextraction. J. Environ. Prot. 2013, 4, 36367. [Google Scholar] [CrossRef]
- Grześkowiak, T.; Czarczyńska-Goślińska, B.; Zgoła-Grześkowiak, A. Current approaches in sample preparation for trace analysis of selected endocrine-disrupting compounds: Focus on polychlorinated biphenyls, alkylphenols, and parabens. TrAC Trends Anal. Chem. 2016, 75, 209–226. [Google Scholar] [CrossRef]
- Keçili, R.; Büyüktiryaki, S.; Dolak, İ.; Hussain, C.M. 5—The use of magnetic nanoparticles in sample preparation devices and tools. In Handbook of Nanomaterials in Analytical Chemistry; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–95. ISBN 978-0-12-816699-4. [Google Scholar]
- Luque-Muñoz, A.; Vílchez, J.L.; Zafra-Gómez, A. Multiclass method for the determination of pharmaceuticals and personal care products in compost from sewage sludge using ultrasound and salt-assisted liquid–liquid extraction followed by ultrahigh performance liquid chromatography-tandem mass spectrometry analysis. J. Chromatogr. A 2017, 1507, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Lara-Gonzalo, A.; Sánchez-Uría, J.E.; Segovia-García, E.; Sanz-Medel, A. Selected ion storage versus tandem MS/MS for organochlorine pesticides determination in drinking waters with SPME and GC-MS. Int. J. Environ. Anal. Chem. 2012, 92, 856–867. [Google Scholar] [CrossRef]
- García-Córcoles, M.T.; Rodríguez-Gómez, R.; de Alarcón-Gómez, B.; Çipa, M.; Martín-Pozo, L.; Kauffmann, J.-M.; Zafra-Gómez, A. Chromatographic Methods for the Determination of Emerging Contaminants in Natural Water and Wastewater Samples: A Review. Crit. Rev. Anal. Chem. 2019, 49, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Farré, M.; de Alda, M.L.; Perez, S.; Postigo, C.; Köck, M.; Radjenovic, J.; Gros, M.; Barcelo, D. Recent trends in the liquid chromatography–mass spectrometry analysis of organic contaminants in environmental samples. J. Chromatogr. A 2010, 1217, 4004–4017. [Google Scholar] [CrossRef]
- Farré, M.; Kantiani, L.; Petrovic, M.; Pérez, S.; Barceló, D. Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J. Chromatogr. A 2012, 1259, 86–99. [Google Scholar] [CrossRef]
- Castro, G.; Roca, M.; Rodríguez, I.; Ramil, M.; Cela, R. Identification and determination of chlorinated azoles in sludge using liquid chromatography quadrupole time-of-flight and triple quadrupole mass spectrometry platforms. J. Chromatogr. A 2016, 1476, 69–76. [Google Scholar] [CrossRef]
- Wood, R. How to validate analytical methods. TrAC Trends Anal. Chem. 1999, 18, 624–632. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Parr, M.K.; Schmidt, A.H. Life cycle management of analytical methods. J. Pharm. Biomed. Anal. 2018, 147, 506–517. [Google Scholar] [CrossRef]
- Schwesig, D.; Borchers, U.; Chancerelle, L.; Dulio, V.; Eriksson, U.; Farré, M.; Goksoyr, A.; Lamoree, M.; Leonards, P.; Wegener, J.-W.; et al. A harmonized European framework for method validation to support research on emerging pollutants. TrAC Trends Anal. Chem. 2011, 30, 1233–1242. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Magnoni, F.; Censi, R.; Di Martino, P. Compensate for or Minimize Matrix Effects? Strategies for Overcoming Matrix Effects in Liquid Chromatography-Mass Spectrometry Technique: A Tutorial Review. Molecules 2020, 25, 3047. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Method 8000C, Revision3 EPA. 2003. Available online: https://archive.epa.gov/epawaste/hazard/testmethods/web/pdf/method%208000c%2c%20revision%203%20-%202003.pdf (accessed on 3 October 2024).
- ISO 11843-6; Capability of Detection—Part 6: Methodology for the Determination of the Critical Value and the Minimum Detectable Value in Poisson Distributed Measurements by Normal Approximations. ISO: Geneva, Switzerland, 2019.

| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flame retardants (6 PBDEs) | 0.1 M HCl in MeOH, Tris-citrate buffer pH: 6 (50 mL) and iso-octane (2 mL) were used as co-extractors | GC-ICP-MS | 95–104 | <0.209–66.6 ng/g | 0.302–0.182 ng/g | 0.649–1.01 ng/g | [54] | |
| PCPs (5 benzophenones—type UV; 2 benzotriazoles) | Ethyl acetate-DCM (1:1, v/v) | SPE (Oasis HLB®) | LC-MS/MS, GC-MS | 70–116 | nd, 0.730–198 ng/g | <LOQ–5920 ng/g | 0.1–1.65 ng/g | [66] |
| Plasticizers (2 butyltin compounds) | Acetic acid | Derivatization with NaBEt4+ GC-ICP-MS | - | 534–1569 ng Sn/g | - | - | [26] a | |
| Plasticizers (6 phthalates) | n-hexane | Clean up column | GC-MS | 86–114 | nd, 126.18–9408.49 ng/g | 0.051–0.13 ng/g | - | [63] |
| Plasticizers (4 phthalates) | n-hexane | Clean up column | GC-MS | 80–95 | 0.1–38 mg/kg | 0.071–0.216 μg/L | 0.182–0.342 μg/L | [64] |
| Plasticizers (DEHP) | n-hexane-DCM (3:1, v/v) | Clean up column | GC-MS | - | 0–10,000 ng/g | - | - | [65] |
| Plasticizers (4 phthalates) | n-hexane-DCM (3:1, v/v) | 10 g of alumina | GC-MS | 80.01–95.20 | nd, 0.13–10.21 mg/kg | 0.071–0.216 μg/L | 0.182–0.342 μg/L | [67] |
| Plasticizers (BPA and analogues | Methyl tertiary butyl ether | UPLC-MS | >82.0 | <LOD. 0.1–378.5 ng/g | 10.0–6453.3 ng/g | 10.0–6453.3 ng/g | [68] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flame retardants (4 PBDEs) | DCM | GC-MS-NCI | 89–105 | 1.1–400.3 ng/g | 0.1–1.2 ng/g | [71] | ||
| Flame retardants (13 PBDEs) | Acetone-hexane (4:1, v/v) | Silica gel column | GC-MS-NCI | - | 0.2–9410 ng/g | 0.017–370 ppb | - | [72] |
| PCPs (6 musks) | DCM | Silica gel and alumina (2:1) | GC/MS | 50.90–97.19 | 270.0–8421.2 ng/g | - | - | [73] |
| PCPs (4 polycyclic musks) | DCM | Silica gel and alumina (2:1) | GC-MS | - | enantiomeric fraction provided | 0.010–0.045 μg/L | - | [70] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| EDCs (3) NSAIDs (4) | GC-MS | <LOD-6297 ng/g | [87] | |||||
| EPs (119) | ACN–water (1:1, v/v 0.1% formic acid) | UPLC-MS/MS | 5–17,000 ng/g b | 0.14–20 ng/ | [88] | |||
| EPs (43) | Extractant solvent (10.5 g of citric acid and 10.2 g of magnesium chloride in 1 L of ultrapure water (pH: 4)-ACN (1:1, v/v) | SPE (Oasis HLB®) | UPLC-TQD | 48.69–114.21 | nd, 1.9–229 ng/g | MQL: 0.94–8 ng/L | [89] | |
| EPs (178) | Hexane–DCM (1:1, v/v) | GC-MS/UPLC-ESI-MS/MS | 86–119 | >20–112.1 ng/g | 4–20 μg/kg | [90] | ||
| EPs (68) | 2% NH4OH in MeOH, 2% formic acid in MeOH and MeOH | SPE (Oasis HLB®) | UHPLC-MS/MS | 0–122 | nd-170 20 μg/kg | MDL: 0.025–7.4 mg/kg | MQL: 0.080–49 mg/kg, | [45] |
| EPs (41 illicit drugs) | McIlvain buffer–methanol (1:1, v/v) | SPE (Strata-X cartridges) | LC–MS/MS | 52–197 | 1–171 ng/g | 0.12–1.32 ng/g | 0.15–3.36 ng/g | [91] |
| Flame retardants (HBCD and TBBPA) | DCM-MeOH (1:9, v/v) | SPE (C18) | LC-QqLIT-MS | 39–120 | nd-1849 ng/g | 4.64–220 ng/g | [77] | |
| Flame retardants (13 PFAS) | MeOH | EnviCarb cartridges | HPLC-MS/MS | 69–141 | nd, <0.01–286.81 ng/g | 0.01–0.21 ng/g | 0.02–0.71 ng/g | [33] |
| Flame retardants (OPFRs, PBDEs and NBFRs) | Ethyl acetate–cyclohexane (5:2; v/v) | Florisil® cartridges | GC-EI-MS/MS | 64–140 | Not found | MDLs: 6.2–575 ng/g | - | [85] |
| Flame retardants, plasticizers, and PCPs (23 compounds) | MeOH–acetic acid (90:10 v/v) | SPE (C18) | LC-MS/MS | 69–120 | <MDL-365 ng/g | MDL: 0.01–6.17 ng/g | MQL: 0.04–20.6 ng/g | [55] |
| Flame retardants (PBDEs and HBCD) | n-hexane–DCM–acetone (7:7:1, v/v) | Alumina clean-up | GC coupled with micro-cell electro capture detector | 80.6–100.4 | <LOD-2.46–107 ng/g | 0.09–3.94 ng/g | 0.19–12.53 ng/g | [21] 2 |
| Flame retardants (7 BDEs) | can | UPLC-MS/MS | 69–104 | nd, <0.18–3.03 ng/g | 0.06–0.20 ng/g | 0.18–0.60 ng/g | [84] | |
| Flame retardants (7 PCBs) | Sodium acetate buffer (pH: 3.4) and hexane | SPE (Strata SI—silica) | GC-MS/MS QqQ | 10.5–588 ng/g | [92] | |||
| Industrial and domestic products (11 PFAS) | THF–acetic acid (1:1, v/v) or ACN-THF (1:1, v/v) | SPE (WAX cartridge and EnviCarb cartridges) | HPLC-MS/MS | 24–107 | nd, <MQLs-10.7 ng/g | MQL: 0.6–5.1 ng/g | [93] | |
| Industrial surfactants and flame retardants (APEs and BDEs) | Hexane–acetone (4:1; v/v) | Acidic silica column and Cu powder | derivatization with HFBA GC-MS | 44.93–100.88 | <LOD-664.46 ng/g | 0.12–5 ng/g | 0.72–16.40 ng/g | [94] |
| Industrial and domestic products (PFAS) | MeOH and 0.2 M NaOH solution | SPE (Strata-X-AW) clean-up with graphitized | HPLC-Orbitrap-MS | 88–95 | <RL—1.31 | RL: 0.04–0.12 ng/g | [95] | |
| Industrial and domestic products (46 PFAS) | 0.1 % (v/v) ammonia in MeOH | SPE (Oasis WAX) | LC-MS/MS | 76–102 | nd-883 ng/g | MDL: <0.01–0.12 ng/g | [42] | |
| PCPs (6 azoles) | MeOH–formic acid (100:0.1 v/v) | SPE (Oasis HLB®) | UHPLC-MS/MS | 52–110 | <MQL-1442 ng/g | 3–9 ng/g | [57] | |
| PCPs (musks) | Sodium acetate buffer (pH: 3.4) and n-hexane | Aluminum oxide column | GC-MS | 80–105 | 23–20,000 ng/g | 5–25 µg/L | 10–50 µg/L | [96] |
| PCPs and steroids (14) | ACN–ethyl acetate (5:1; v/v) | SPE (silica cartridge) | LDTD-APCI-MS/MS | 80–109 | nd, ≤LMD-106 ng/g | 2.8–16.8 ng/g (MDL) | [41] | |
| PCPs (19 biocides) | MeOH and MeOH-0.1%/v/v) formic acid in Milli-Q water (5:5, v/v) | SPE (Oasis HLB®) | UHPLC-MS/MS | 70–120 | ND-887 ng/g | - | 0.01–6.37 ng/g | [81] |
| PCPs (TCB and TCC) | ACN | SPE (HLB) | LC-MS/MS | 33.1–117.4 | ~0.04–6.5 μg/g b | 0.0024–0.006 μg/g | [97] 1 | |
| PCPs and EDCs (6 retinoids 7 EDCs) | Ethyl acetate | Silica gel column/anhydrous sodium phosphate, silica gel, and glass wool) | HPLC-MS/MS | 63–182 | nd, 9–22,900 ng/g | 0.17–4.3 ng/g | 0.56–31 ng/g | [98] |
| PCPs and EDC (5 retinoids and 7 EDCs 7) | Ethyl acetate | Silica gel column/anhydrous sodium phosphate, silica gel, and glass wool) | HPLC-MS/MS | Zhou et al. [98] | 0.34–1800 ng/g | Zhou et al. [98] | Zhou et al. [98] | [99] |
| PCPs (TCC and transformation products) | Phosphate buffer (pH 2), ACN | SPE (HLB) | UHPLC-MS/MS | 105.18–317.64 | 1700–12,790 ng/g | 0.09–1.44 ppb | 0.25–5.22 ppb | [100] |
| PCPs (5 musks) | n-hexane–acetone (3:1, v/v) | EnviCarb cartridges 120/400 | GC-MS | 63.20->100 | <LOD-21,294 ng/g | 0.1–210 ng/g | 1–526 ng/g | [101] |
| PCPs (TCB) | MeOH | Filtrate 0.22 μm organic phase membrane | HPLC | 13.5–23.4 μg/g | [102] | |||
| PCPs (TCS and metabolites) | ACN + phosphate buffer | SPE (Oasis HLB®) | UPLC | 1–19.3–145.8 | 15–4532 ng/g | 0.1–0.6 ppb | 0–1 ppb | [103] |
| PCPs (musks) | Sodium acetate buffer (pH: 3.4) and n-hexane | SPE (Strata SI—silica) | GC/MS/MS | 49.7–112.2 | <MLOD-8399 ng/g | MLOD: 0.4–2 ng/g | MLOD: 0.4–2 ng/g | [104] |
| PhACs (16) | MeOH–acetone (7:2, v/v) | SPE (Oasis HLB®) | HPLC | 41.1–115 | 1.39–360 | [105] | ||
| PhACs (18 estrogens) | Ethyl acetate | Silica gel cartridge and diluted in ethyl acetate/methanol (90:10, v/v) | RRLC-MS/MS | 62.6–138 | nd, <LOQ-372 ng/g | 0.08–2.06 ng/g | 0.34–6.86 ng/g | [25] |
| PhACs and EDCs (9) | MeOH–water (2.5:1.5, v/v) | SPE (C18) | Derivatization + GC/MS | 84.6–107 | <LOD-6560 ng/g | 15–33 ng/g | 59–108 ng/g | [35] |
| PhACs and EDCs (7) | ||||||||
| PhACs (15 antidepressants) | MeOH–0.1 acetic buffer solution pH 4.0 (1:1, v/v) | SPE (Strata-X-C) | LC-qQMS | 44–101 | nd, 3.3–3735 ng/g | 0.04–0.5 ng/g | 0.1–1.7 ng/g | [106] |
| PhACs (4 antibiotic and 2 estrogens) | 1 M citrate buffer (pH 4.7) + MeOH–water (60:40) | SPE (SAX + HLB for antibiotics; Carboprep/NAX for estrogens) | LC–MS/MS. | 17–59 | <LOD, 5600–7600. ng/g | 0.6–8.5 ng/g | 1.1–17.1 ng/g | [107] |
| PhACs (22 antibiotics) | Citric acid buffer (pH 3) | SPE (Oasis HLB®) | LC-MS/MS | 50–150 | nd, 1.45–5800 ng/g | MDLs: 0.45–8.57 ng/g | MQLs: 1.50–28.6 ng/g | [108] |
| PhACs (13 quinolones antibiotics) | MeOH–McIlvaine (50:50; v/v) pH:3 | LC–MS/MS. | 96.1–103.6 | 12–834 ng/g | 2–5 ng/g | 6–18 ng/g | [36] a | |
| PhACs (NSAIDs, lipid regulators and antibiotics) | MeOH–water (1:1; v/v) | LC-MS/MS | 76–131 | <LOD-1125 ng/g | <LOD-1125 ng/g | - | [58] | |
| PhACs (21 progestogens) | Ethyl acetate–MeOH (8:2, v/v) | Silica gel cartridge | UHPLC-MS/MS with ESI (under positive ionization mode) | 35–129 | nd, 1.2–1952 ng/g | 0.01–3.68 ng/g | 0.01–12.30 ng/g | [25] |
| PhACs (26) | MeOH–0.2 M citric acid buffer, pH: 4.4, (1:1 v/v) | SPE (Oasis HLB®) | UHPLC-MS-MS | 54–130 | <LOD-8546.21 ng/g | 0.01–0.50 ng/g | 0.02–1 ng/g | [109] |
| PhACs (13) | MeOH–water (50:50) 0.5% HCOOH | LC-MS/MS QqQ | 20–117 | 30–7500 ng/g | - | 1.2–46 ng/g | [110,111] | |
| PhACs (8) | McIlvaine buffer (0.12 M EDTA, pH 3.5) + ACN | QuEChERS | SPE–UHPLC–MS-MS | 58–118 | nd, <MLOQ-4784 ng/g | 1–180 ng/g | 9.1–1230 ng/g | [31] |
| PhACs (11 acidic drugs and estrogenic hormones) | Phosphate buffer (pH 2) solution and ACN (15:10, v/v) | SPE (ENVI-18) | GC-MS | 70.6–133 | 29.6–1796 ng/g | 0.7–5.2 ng/g | 2–15.6 ng/g | [80] |
| PhACs (5) | MeOH–acetone (7:2, v/v) | SPE (Oasis HLB®) | UPLC | 13–76 ng/g b | [112] | |||
| PhACs (5 NSAIDs) | Water–hexane–acetone | Not specified | LC-MS/MS | 0.5–250 ng/g | Linked to a previous study | [23] | ||
| PhACs (4) | ACN + phosphate buffer | SPE (C18) | UPLC | 81.8–98.1 | 252–655 ng/g | 0.1–1 ng/mL | 0–5.0 ng/mL | [113] |
| PhACs (5) | MeOH–acetone (7:2, v/v) | SPE (Oasis HLB®) | UPLC | 76.2–86.7 | 33.6–2206 ng/g | MDL: 30–710 ng/L | MQL: 70–1890 ng/L | [114] |
| PhACs (69) | MeOH–water solution (pH: 2.5, 0.5% HCOOH and 0.1% disodium-EDTA (50/50, v/v) | Filtered by RC 0.22 μm syringe filter | LC-MS/MS | 53–162 | MDL, 2.19–3808 ng/g | 0.3–9.1 ng/g | 1–28 ng/g | [115] |
| PhACs and illicit drugs (148) | MeOH–Milli-Q water (pH 2.5), FA 0.5% and 0.1% EDTA, (50:50 v/v) | Filtered through a 0.2 μm RC syringe filter | HPLC-MS/MS | 20–119 | <LOD-267 ng/g | 0.9–19.9 ng/g | 20–66.3 ng/g | [79] |
| PhACs (7 antibiotics) | MeOH–formic acid (0.5% v/v= | SPE (C18) | LC-MS/MS | 9–94 | 27–191 ng/g | MDL: 0.002–12.5 ng/g | MQL: 0.003–25 ng/g | [9] |
| Plasticizers/PCPs (3 alkylphenols) | Hexane–DCM (1:1, v/v) + DCM–acetone (1:1, v/v) | Florisil® cartridges | GC-MS | - | Study of stability | Lower warming limit: 0–50 mg/kg | Upper warming limit: 16–110 mg/kg | [86] |
| Plasticizers (2 butyltin compounds) | Acetic acid | Derivatization with NaBEt4+ GC-ICP-MS | 534–1569 ng SN/g | [26] a | ||||
| Plasticizers (7) | MeOH–acetone (50:50, v/v) | SPE (HLB and MAX cartridge) | LC-MS/MS | 57.1–101.9 | Method does not apply in SS samples | 0.03–0.86 ng/g | 0.09–0.03 ng/g | [56] |
| Plasticizers/PCPs (4 nonylphenols ethoxylates) | ACN | GC/MS | 93.5–137.8 | 5.5–19.5 ng/g | 0.03–12 ng/g | [116] | ||
| Plasticizers (9) | MeOH–water pH 12 (5:3, v/v) | THPE-DMIP column | HPLC–MS/MS | 43.6–96.7 | nd, 0.26–63.6 ng/g | - | MLOQ: 0.0004–8.28 ng/L | [117] |
| PPCPs (17 azoles) | MeOH | SPE (C18) | LC-MS/MS | 71.9–115.8 | n.d., <LOQ-4448.9 ng/g | 0.5–5 ng/g | 2.0–16.5 ng/g | [118] |
| PPCPs and industrial products (10) | ACN–water (5:3, v/v) | SPE (Oasis HLB®) | UHPLC-MS/MS | 65.3–125.3 | 1.7–5088.2 ng/g | 0.1–3 ng/g (MQLs) | [20] | |
| PPCPs (5 azoles) | MeOH–formic acid (100:0.1 v/v) | SPE (Oasis HLB®) | 1200 HPLC system coupled to an Agilent 6410 triple-quadrupole mass spectrometer with electrospray ionization used in positive mode | 71.2–94.9 | 4.9–616 ng/g | MQL: 3–29 ng/g | [82] | |
| PPCPs (7 antibiotics and antibacterial agents) | 50 % ACN in 1mM EDTA solution (pH 2.0 with HCl) | SPE (Oasis HLB®) | HPLC-MS/MS | 41–123 | nd, <LOQ, 4–17,740 ng/g | - | 10–500 ng/g | [119] |
| PPCPs (14) | MeOH–formic acid (100:1, v/v) | EnviCarb cartridges | Derivatization + GC/MS | 57.9–103.1 | <LOD-1965 ng/g | 1.6–11 ng/g | 4.7–39 ng/g | [37] |
| PPCPs (10) | MeOH–water (9: 1, v/v, pH 11) + acetone + water with 0.1% formic acid (pH: 2.65) | SPE (Oasis HLB®) | UHPLC-APCI-SRM/MS | 81.1–156 | nd, <LOD-297.04 ng/g | 0.01–14.79 ng/g | [120] | |
| PPCPs (22) | Milli-Q water (pH 9) | Online DI-SPME- | On-fiber derivatization. GC-MS | 53.98–105.15 | It was not applied in solid samples | 0.64–253.30 ng/g | 7.03–844.33 ng/g | [61] |
| PPCPs and metabolites (19) | MeOH (0.5% v/v, formic acid) | dSPE (PSA + C18) | LC-MS/MS | 22–99 | Validation method | MDL: 0.1–5.3 ng/g | MQL: 0.4–18 ng/g | [121] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| EDCs (12) | MeOH | SPE (C18) | LC-MS/MS | 71.7–103.1 | <LOD-710.2 ng/g | 0.6–3.5 ng/g | 2.0–11.6 ng/g | [127] |
| EDCs (2 butynyl compounds) | Acetic acid | Derivatization with NaBEt4+ GC-MS; GC-ICP-MS | 534–1569 ng SN/g | [26] a | ||||
| EDCs (13) | MeOH | SPE | Derivatization GC/MS | 92–102 | nd, 36–164 ng/kg | 0.5–4.5 ng/kg | 2–15 ng/kg | [124] |
| EDCs (hormones and corticoids) | MeOH | None required | UHLPC-MS/MS | 60–130 | nd, <LOQ-1440 ng/g | 2.1–192.8 ng/g | [125] | |
| EDCs (14 phenols) | 30 mL of 1:1 (v/v) acetone–hexane, and 500 μL of glacial acetic acid | Silica column + washed with DMC/hexane | GC-MS | 71–105 | <4–337,200 ng/g | MDL: 2.7–204 ng/g | [18] | |
| EDCs (BPA) | Acetone–hexane (1:1, v/v) | SPE (LC-18) | HPLC–UV | 53–90 | 125–180 ng/g | 100 ng/g | 330 ng/g | [24] 1 |
| EPs (61) | For illicit drugs: MeOH-DCM (50:50, v/v)// For PhACs: ACN–water (70:30, v/v, pH: 2) | SPE (Oasis MCX®) | LC/MS | 90–104 | < LOD-2426.9 ng/g | 0–27 ng/g | [131] | |
| PCPs (4 LAS) | MeOH | LC-FLD | 35–98 | 0.70–13.39 mg/kg | 3.3–5.4 ng/g | 11.0–18.0 ng/g | [128] | |
| PhACs (4 NSAIDs) | Water | DME + SPE (Oasis HLB®) | GC-MS(SIS) | 80–105 | 10–140 ng/g | 15–29 ng/g | [129] | |
| PhACs (5 fluoroquinolone antibiotics) | HTAB (non-ionic surfactant used as an extractant) and 5% (v/v) surfactant concentration in Milli-Q water | LC-MS/MS | 73.2–95.6 | nd, 9.57–206.1 ng/g | 0.15–0.55 ng/g | 0.49–1.85 ng/g | [130] | |
| PhACs (13 quinolones antibiotics) | MeOH–McIlvaine (50:50, v/v, pH:3) | LC-MS/MS | 96.1–103.6 | 12–834 ng/g | 2–5 ng/g | 6–18 ng/g | [36] a | |
| PhACs and Illicit drugs (18) | MeOH–water (1:1, v/v) | SPE (Oasis HLB®) | LC-MS/MS | 46.9–187.3 | 0.4–275.2 ng/g | MDL:0.02–32.73 ng/L | MQL: 0.07–109.08 ng/L | [132] |
| PhACs and stimulants (5 antidepressants and caffeine) | MeOH-ACN (43:57) pH 3 | HPLC-PDA | 60–99 | 24–1980 ng/g | 15–50 ng/g | 100–200 ng/g | [133,134] | |
| Plasticizers (phthalates) | Hexane–acetone (1:1, v/v) | Silicone–alumina column packed | GC-MS | nd | nd | nd | nd | [135] |
| PPCPs (22) | MeOH–water (3:2, v/v) | SPE (Oasis HLB®) | GC-MS | 91–100 | nd-3100 ng/kg | 0.8–5.1 ng/kg | [136] | |
| PPCPs and illicit drugs (90) | MeOH–water (50:50, v/v, pH:2) | SPE (Oasis MCX®) | UPLC-MS/MS | 40–152 | <MQL-5800 ng/g | 0.03–4.81 ng/g | 0.14–24.05 ng/g | [126] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| EDCs (estrogens and BPA) | Acetone–MeOH (1:1, v/v) | SPE (Oasis HLB®) | LC-MS/MS | 88–97 (absolute) | 0.7–92.9 ng/g | 0.05–0.20 ng/g | 0.1–0.5 ng/g | [81] |
| EDCs and caffeine (22) | Water–MeOH-acetone (1:2:1, v/v) | Column clean-up | TFC-LC-MS/MS | 53–115 | nd, 2.6–29,416 ng/g | 0.031–321 ng/g | 0.10–1071 ng/g | [148] |
| EPs (pharmaceuticals, PFOA, PFOS, 44) | Milli-Q water–MeOH (1:1, v/v) | 2 SPE (OASIS MCX and OASIS HLB®) | HPLC-MS/MS | >70 | nd-5 µg/g | 0.06–14.38 ng/g | 0.12–47.92 ng/g | [152] |
| Domestic products (8 sweeteners) | MeOH | SPE (Oasis HLB®) | LC-ESI-MS/MS | 29–87 | nd, <LOQ-481 ng/g | - | 5–10 ng/g | [139] |
| Flame retardants (15) | Toluene | Diatomaceous earth | UPC-MS/MS | 65–112 | <0.005–1208 ng/g | 0.020–6 pg/g | 0.005–1.3 ng/g | [149] |
| Flame retardants (DBDPE) | Hexane–DCM (1:1, v/v) | Purification Power Prep™ (acidic silica gel, basic alumina, and carbon columns) | HRGC-TQMS/MS | 63 | 3.25–125 ng/g | 0.3 pg/g | [153] | |
| Flame retardants (7 BDEs) | Hexane–DCM (50:50, v/v) | C18 | GC-MS/MS | 92–102 | nd, 0.21–19.6 ng/g | 0.01–0.04 ng/g | - | [40] |
| Flame retardants and PCPs (99 PCBs, musk, etc.) | 20% DCM n-hexane | GPC (for PLE), Silica (for SPLE) | GC-MS | 28–219/4–287 | Not quantified | 0.02–129.43 ng/g | 0.12–392.2 ng/g | [50] |
| Flame retardants (9) | Acetone–DCM (1:1, v/v) | Bond Elut-NH2 SPE | HPLC-MS/MS | - | 0.02–349.20 g/day | - | - | [47] |
| Industrial/domestic products (PFPAs/PFOS) | Tetrahydrofuran–water (25:75, v/v) | SPE (Oasis WAX) | LC-MS/MS | 75–85 | 0.07–48 ng/g | 0.01–0.25 ng/g | - | [147] |
| Industrial/domestic products (18 PFCs) | MeOH | SPE (Oasis WAX 3cc) | LC–QLiT-MS/MS. | 76–111 | <MLOD-121.1 ng/g | 15–837 ng/kg | 50–2772 ng/kg | [154] |
| Illicit drugs (20) | MeOH–water (9:1, v/v) | LC-ESI-(QqLIT)MS/MS) | 55–129 | nd, 0.7–579.0 ng/g | 0.1–6.4 ng/g | 0.3–22.5 ng/g | [150] | |
| PCPs (10 musks) | MeOH–water (1:1, v/v) | Florisil® cartridges | GC–MS/MS | 63–100 | nd, <LOQ-530.5 ng/g | 0.5–1.5 ng/ | 2.5–5 ng/g | [28] |
| PCPs (TCS and transformation products) | DCM | GPC (gel permeation chromatography) + multilayer silica column | GC/MS | 88–99 | nd, 51–2505.9 ng/g | 1–10 ng/g | 3–30 ng/g | [151] |
| PhACs (10 β-blockers) | MeOH–water-acetic acid (49/19/2, v/v) | SPE (Oasis MCX®) | LC-MS/MS | 76–149 | 2–95 ng/g | - | 0.5–15 ng/L | [145] |
| PhACs (5 estrogens) | Water–MeOH (80:20, v/v) | SPE (Oasis HLB®) | LC-MS/MS | 86–126 | 4.2–63 ng/g | - | 1–5 ng/g | [19] |
| PhACs (carbamazepine) | MeOH | SPE (C18) | LDTD-APCI-MS/MS | 96.9–107 | 13–94 ng/g | 3.4 ng/g | - | [141] a |
| PhACs (14) | MeOH–McIlvaine buffer (1:1, v/v) | SPE (Oasis HLB®) | HPLC-MS/MS | 66.6–118.5 | 97.6–268.0 ng/g | 0.2–5.8 ng/g | 0.6–19.4 ng/g | [52] |
| PhACs (22 sulfonamide antibiotics) | ACN–water (25:75, v/v) | SPE (Oasis HLB®) | LC-MS/MS | 19–130 | 0.22–175 ng/g | 0.03–17.40 ng/g | 0.10–58.00 ng/g | [140] |
| PhACs (13 quinolones antibiotics) | MeOH–McIlvaine (50:50; v/v) pH:3 | LC–MS/MS. | 96.1–103.6 | 12–834 ng/g | 2–5 ng/g | 6–18 ng/g | [36] b | |
| PhACs (9 glucocorticoids) | MeOH–acetone (80:20, v/v) | SPE (Bond Elut Plexa cartridges) | UHPLC-MS-MS | 8–73 | nd, <LOQ-6.1 μg/kg | 0.5–1 ng/g | 1–5 ng/g | [155] |
| PhACs (2 anticancer drugs) | MeOH–ultrapure water (65:35, v/v) | SPE (MAX/MCX cartridges) | UHPLC-MS/MS | 92–106 | <MQL-42.5 μg/kg | 2.5–74 ng/g | 6.1–186 ng/g | [146] |
| PhACs (8 sedative hypnotics) | MeOH–water (1:1, v/v) | LC-MS/MS | 88–112 | nd, <LOQ-18.9 μg/kg | 0.2–12 ng/g | [156] | ||
| PhACs (5 antibiotics) | MeOH-ACN-0.2 M citric acid (pH: 4.5) (40:40:20) | SPE (StrataX cartridges) | LC-MS | 33.5–91.4 | nd, 55–8492 ng/g | 1.5–3.8 ng/g | 5–20 ng/g | [144] |
| PhAcS (12) | MeOH | SPE | HPLC-MS/MS | 33–125 | <LOQ430 ng/g | 0.4–20 ng/g | 1.2–68 ng/g | [157] |
| PhACs (diclofenac) | MeOH | SPE (C18) | LDTD-APCI-MS/MS | 95.6 | 530–650 ng/g | 270 ng/L | 1000 ng/L | [158] |
| PPCPs and illicit drugs (14) | MeOH or MeOH–formic acid (100:0.1, v/v) | UHPLC | 56–128 | <LOD-5940 ng/g | 1–50 ng/g | - | [159] | |
| PPCPs and flame retardants (71) | Acetone, MeOH, heptane, acetate buffer, citric acid, etc., depend on the compound | SPE (StrataX, Oasis HLB, multilayer silica) or without clean-up | HPLC-MS or GC-MS | 20–103 | <LQ, 2.7–105,536 ng/g | 1–12,710 ng/g | [160] c | |
| PPCPs and metabolites (19) | MeOH (0.5% v/v, formic acid) | C18+PSA | LC-MS/MS | 42–111 | Validation method | MDL: 0.1–3.5 ng/g | MQL: 4.9–12 ng/g | [121] |
| PPCPs (17) | MeOH–water (1:1, v/v) | - | LC-QqQ-MS | - | nd-5176.6 ng/L | - | - | [161] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|
| Industrial application (aliphatic primary amines) | Water (pH 4 | Diatomaceous earth | GC–MS | 0.17–543 m/kg | nd–543 ng/kg | nd–543 ng/kg | [27] | |
| Industrial application (nitrosamines) | Milli-Q water pH 7.5 | HS-SPME | GC-CI-MS-MS | nd, <LOD-371 ng/g | 0.03–0.14 ng/g | 0.03–0.14 ng/g | [62] | |
| PhACs (NSAIDs) | NaOH (0.01 M) | HF-LPME | LC-ESI-MS | 38.9–90.3 | 7.7–588 ng/g | 0.4–3.7 ng/g | 0.4–3.7 ng/g | [34] |
| PhACs (10 azoles) | Ultrapure water | SPE (Oasis HLB®) | LC-Orbitrap-HRMS | 25–107 | <LOQ-255.4 ng/g | 0.25–25 ng/g | 0.25–25 ng/g | [29] |
| PhACs (23) | Water pH 7 | SPE (Oasis HLB®) | UPLC-MS/MS | 16–37 (absolute) | nd, 0.4–3009 ng/g | nd, 0.4–3009 ng/g | [163] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| EPs (42) | ACN | d-SPE | LC-PDA-FLD | 50–126 | <LOQ-2.05 µg/g | 0.007–0.271 µg/g | 0.0240–0.821 µg/g | [172] |
| PCPs (13 azoles and benzenesulfonamide derivates) | Cool water + ACN | d-SPE (Z-sep+) | LC-(Orbitrap)HRMS | 80–90 | nd, <LOQ-181.2 ng/g | 0.5–10 ng/g | 1–25 ng/g | [29] |
| PCPs (19 musks and UV filters) | ACN | dSPE (MgSO4 + C18 + PSA) | GC-MS/MS | 74–122 | nd, <MQL-115,486 ng/g | MDL:0.5–1394 ng/g | MDL: 2–4648 ng/g | [171] |
| PhACs (136) | S1: 0.1 M EDTA + ACN + acetic acid 1% (v/v), S2: heptane, S3: acetate buffer | LC-TOF-MS | 21–135 | nd, <MLQ-5957 ng/g | 1–2500 ng/g for the majority of the compounds | 15–6250 ng/g for the majority of the compounds | [165] | |
| PhACs (13 NSAIDs) | Water–ACN (1:2, v/v) | Online-SPE | LC-MS/MS | 36–101 | <0.39–57.1 ng/g | MDL: 0.065–6.7 ng/g | - | [170] |
| PhACs (12) | ACN-H20 (50:50) + 0.5% formic acid + 0.2% Na2EDTA | SPE (PSA) not used to ERY and CIPRO | HPLC-MS/MS | 68–104 | 0.3–21.4 ng/g | 1.7–71.4 ng/g | [173] | |
| PhACs (33) | EDTA 0.1 M | d-SPE | UHPLC-Orbitrap MS | 60–98 | <LOQ-506.5 ng/f | 0.3–8.1 ng/g | 1.1–25 ng/g | [46] |
| PhACs (72) | McIlvaine–EDTA | Oasis HLB PRiME | LC-HRMS | >80% a | [43] | |||
| PhACs (32) | McIlvaine–EDTA | Oasis HLB PRiME | LC-HRMS | >80% a | [174] | |||
| PhACs (17 antibiotics) | 0.2M Na2EDTA (in water), CAN, and MeOH | d-sSPE (MgSO4 + PSA) | LC-MS/MS | 20–147 | <MLOQ-2894 ng/g | 0.003–120.39 ng/g | 0.05–364.81 ng/g | [175] |
| PPCPs (12) | ACN acidified with acetic acid | d-SPE (chitin) | LC-ESI-MS/MS | 50–120 | nr | 0.8–15 ng/g | 5–50 ng/g | [168] |
| PPCPs (21) | ACN acidified with acetic acid | dSPE (PSA) | UPLC-MS/MS | 50–96 | nd above LOQ | 0.15–1.5 ng/g | 0.5–10 ng/g | [169] |
| PPCPs (more than 100) | ACN | dSPE (PSA) | LC-HRMS | - | Method validation | [30] | ||
| PPCPs and flame retardants (71) | Different solvents (acetone, MeOH, heptane, acetate buffer, citric acid, etc.) | dSPE | HPLC | 20–103 | <LQ, 2.7–105,536 ng/g | 1–12,710 ng/g | [160] | |
| PPCPs and metabolites (19) | MeOH (0.5% v/v, formic acid) | dSPE | LC-MS/MS | 27–86 | Validation method | MDL:0.2–16 ng/g | MQL: 0.6–54 ng/g | [121] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flame retardants (8 organophosphates compounds) | ACN | PSA sorbent | LC-QTOF-MS | 69–123 | nd, 2.2–1786 ng/g | 2–50 ng/g | [187] | |
| PCPs (TCS and MTCS) | DCM | Diatomaceous earth and silica with H2SO4 | GC-MS | 86–113 | 15–2640 ng/g | - | 6–7 ng/g | [181] |
| PCPs (TCS and MTCS) | ACN | GC-MS | 98.4–101.0 | 4–2987 ng/g | 0.10–0.12 ng/g | 0.3–0.4 ng/g | [180] | |
| PCPs (9 parabens) | Ethyl acetate-MeOH (90:10, v/v). | GC–MS/MS | 80.4–124.9 | <LOQ-44.1 ng/g | MDLs: 0.1–1.7 ng/g | 0.3–5.1 ng/g | [188] | |
| PCPs (10 UV stabilizers) | Ethyl acetate | SPE (PSA sorbent) | GC-QTOF-MS | 70–93 | nd, 6.4–292 ng/g | 2–10 ng/g | [182] | |
| PhACs (8 azoles) | MeOH to recover | SPE (SCX) | LC-ESI-QTOF-MS | 70–118 | nd, 8–800 ng/g | - | 5–8 ng/g | [183] |
| PhACs (2 cardiac drug) | MeOH to recover | SPE (SCX) | LC-ESI-QTOF-MS | 95–11 | 22–362 ng/g | - | 5–8 ng/g | [186] |
| PhACs (5 NSAIDs) | Hexane-acetone (1:2, v/v) to recover | Florisil® cartridges and Silica | LC–ESI-QTOF-MS | 84.0–104.5 | nd, 1.8–14.7 ng/g | - | 0.005–0.05 ng/g | [179] |
| PhACs (5 azoles) | MeOH-ACN-Formic acid (30:69:1) | SPE cartridges online | UPLC-MS/MS | 82–124 | nd, <LOQ-1219 ng/g | - | 2–10 ng/g | [53] |
| PPCPs (45) | MeOH and acetonitrile/5 % oxalic acid (8/2, v/v) to do the elution | SPE (C18) | LC-QqQ-MS | 50.3–107 | nd, >100–2770 μg/kg | MQL: 0.117–5.55 μg/kg | [185] | |
| PPCPs (27) | MeOH | Not specified | HILIC-MS/MS | 50–120 | nd, <1.25–5466 ng/g | MQL: 1.25–1250 μg/kg | [184] | |
| PPCPs (68) | PSA sorbent | UPLC-QTOF-MS | 49.7–112.2 | 200–8000 ng/g b | MLOD: 0.4–2 ng/g | [189] | ||
| ECs (60) | MeOH | SPE(C18) | UPLC-QTOF-MS | 79–113 | Máx. 100 ng/g | LOQs: 0.3 ng/g–45 ng/g | [189] |
| Analytes | Extraction Solvent | Clean-Up | Detection Technique | R (%) | Range of Concentration | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flame retardants (2 PCBs) by SLE-LTP | Water-extraction mixture (composed by ACN, ethyl acetate tetrahydrofuran, isopropanol) | GC-MS-SIM | 78–109 | Not quantified | 16–32 ng/kg | - | [206] | |
| Flame retardants (6 PCBs 6) by SLE-LTP | Isopropanol–ethyl acetate (13:3, v/v) | Silica–sodium sulphate cartridge | GC-MS | 66–94 | 50–70 ng/g | 3.3 ng/g | 10.0 ng/g | [204] |
| PCPs (6 musks) by MA-HS-SPME | Deionized water + 3 g NaCl + pH 1 (with HCl) | GC-MS | 68–87 | nd-2.8 ng/g (fresh weight) | 0.04–0.1 ng/g | 0.1–0.3 ng/g | [194] | |
| PCPs (8 macrocyclic musks) by HS-SPME | Ultrapure water | GC-MS | - | nd, <MQL–1.45 ng/g | 10–25 pg/g | 25–50 pg/g | [195] | |
| Plasticizers (5 phthalates) by SLE-LTP | Acetonitrile/ethyl acetate | - | GC-MS | 76–119 | nd, 0.06–10.24 mg/kg | - | 40–80 μg/L | [205] |
| PhACs (4 NSAIDs) by HF-LPME | 0.1M (NH4)2CO3 pH:9 | LC-ESI-MS | not reported | 29–138 ng/g | - | - | [39] | |
| PhACs (4 NSAIDs) by HF-LPME | Acceptor buffer solution (0.1 M (NH4)2CO3) pH:9 | C-18 column | LC-MS/MS | - | nd, 30–1480 ng/L | MDL: 0.8–14.3 ng/L | [208] | |
| PhACs (3 metabolites from NSAIDs) by HF-LPME | Reagent water (Milli-Q water) | GC-MS | - | nr-183 ng/g | 1.6–5.6 ng/g | 5.3–18.6 ng/g | [38] | |
| PPCPs (6) by SBSE | NaHCO3 and acetic acid anhydride | TD-GC-MS | 91–110 | 30–280 ng/g | 0.08–1.06 ng/g | 0.24–3.22 ng/g | [201] |
| Validation Parameter | Objective | Measures |
|---|---|---|
| Selectivity | Asses the method’s ability to accurately identify and quantify the target analyte in heterogeneous samples. | Matrix effect (ME) or interference studies |
| Accuracy | Evaluate the closeness of the measured value to the true value. | Recovery (acceptable range: 70–120%) |
| Linearity | Test the method’s ability to obtain results proportional to the analyte concentration over a specified range. | Linearity (R2) |
| Precision | Assess the repeatability (intra-day) and intermediate precision (inter-day) of the method. | %RSD (generally < 10%) |
| Sensitivity | Estimate the method’s ability to detect and quantify low concentrations of the analytes. | LODs and LOQs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo-Mahón, T.; Mercl, F.; Chary, N.S.; Száková, J.; Tlustoš, P. Extraction Methods of Emerging Pollutants in Sewage Sludge: A Comprehensive Review. Toxics 2025, 13, 661. https://doi.org/10.3390/toxics13080661
Robledo-Mahón T, Mercl F, Chary NS, Száková J, Tlustoš P. Extraction Methods of Emerging Pollutants in Sewage Sludge: A Comprehensive Review. Toxics. 2025; 13(8):661. https://doi.org/10.3390/toxics13080661
Chicago/Turabian StyleRobledo-Mahón, Tatiana, Filip Mercl, Nallanthigal Sridhara Chary, Jiřina Száková, and Pavel Tlustoš. 2025. "Extraction Methods of Emerging Pollutants in Sewage Sludge: A Comprehensive Review" Toxics 13, no. 8: 661. https://doi.org/10.3390/toxics13080661
APA StyleRobledo-Mahón, T., Mercl, F., Chary, N. S., Száková, J., & Tlustoš, P. (2025). Extraction Methods of Emerging Pollutants in Sewage Sludge: A Comprehensive Review. Toxics, 13(8), 661. https://doi.org/10.3390/toxics13080661










