Exposure of Domestic Cats (Felis catus) to Rodenticidal Compounds
Abstract
1. Introduction
2. Materials and Methods
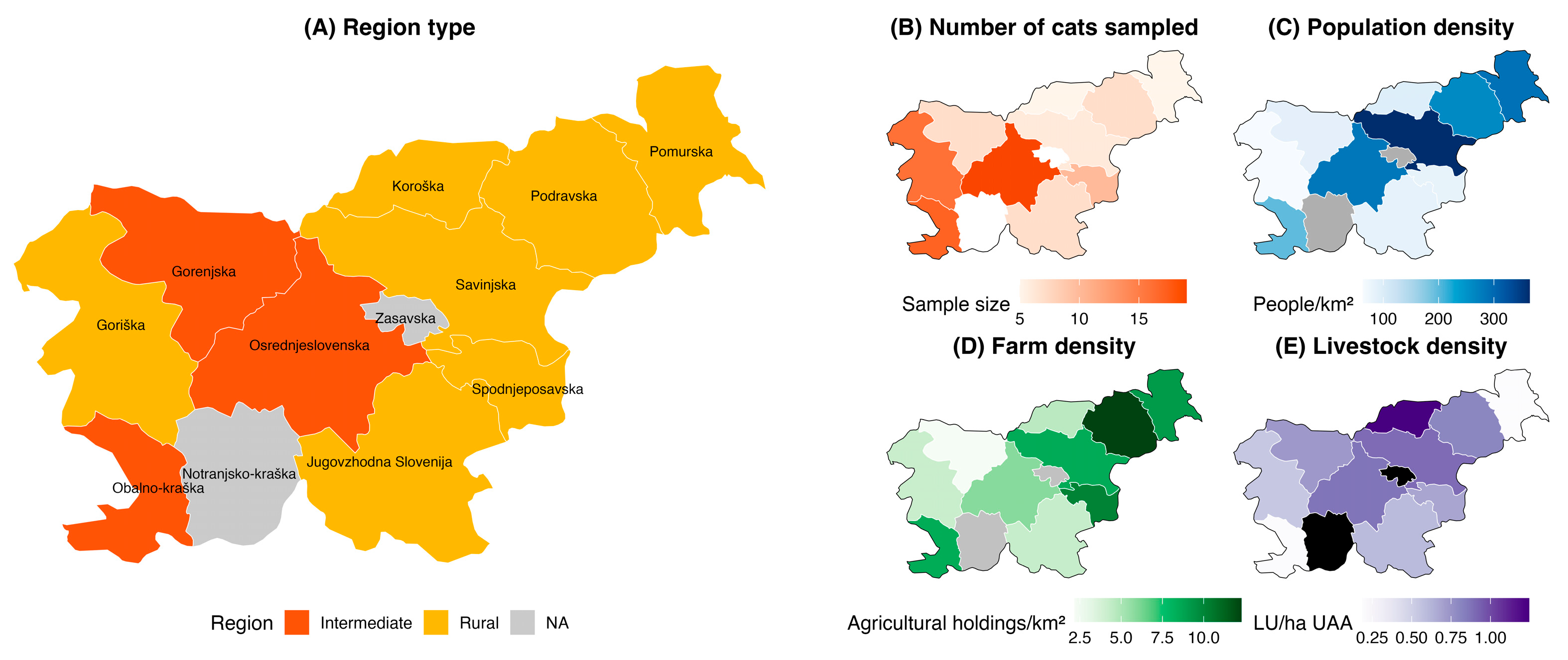
2.1. Sample Collection
2.2. Chemical Residue Analysis
2.3. Data Preparation and Methods for Statistical Analysis
2.4. Risk Assessment for Acute Toxicity Using the Example of Brodifacoum
3. Results
3.1. Necropsy
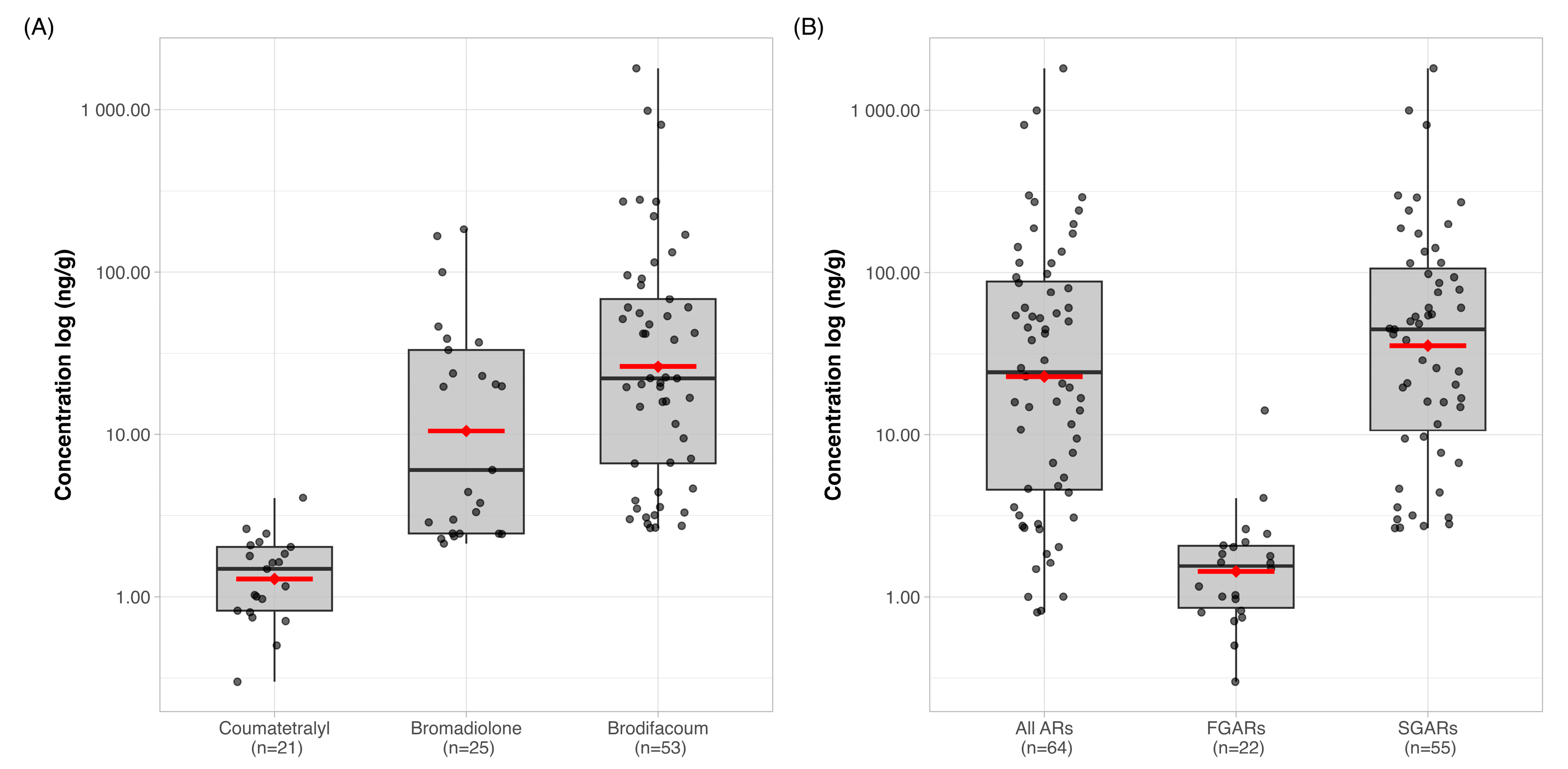
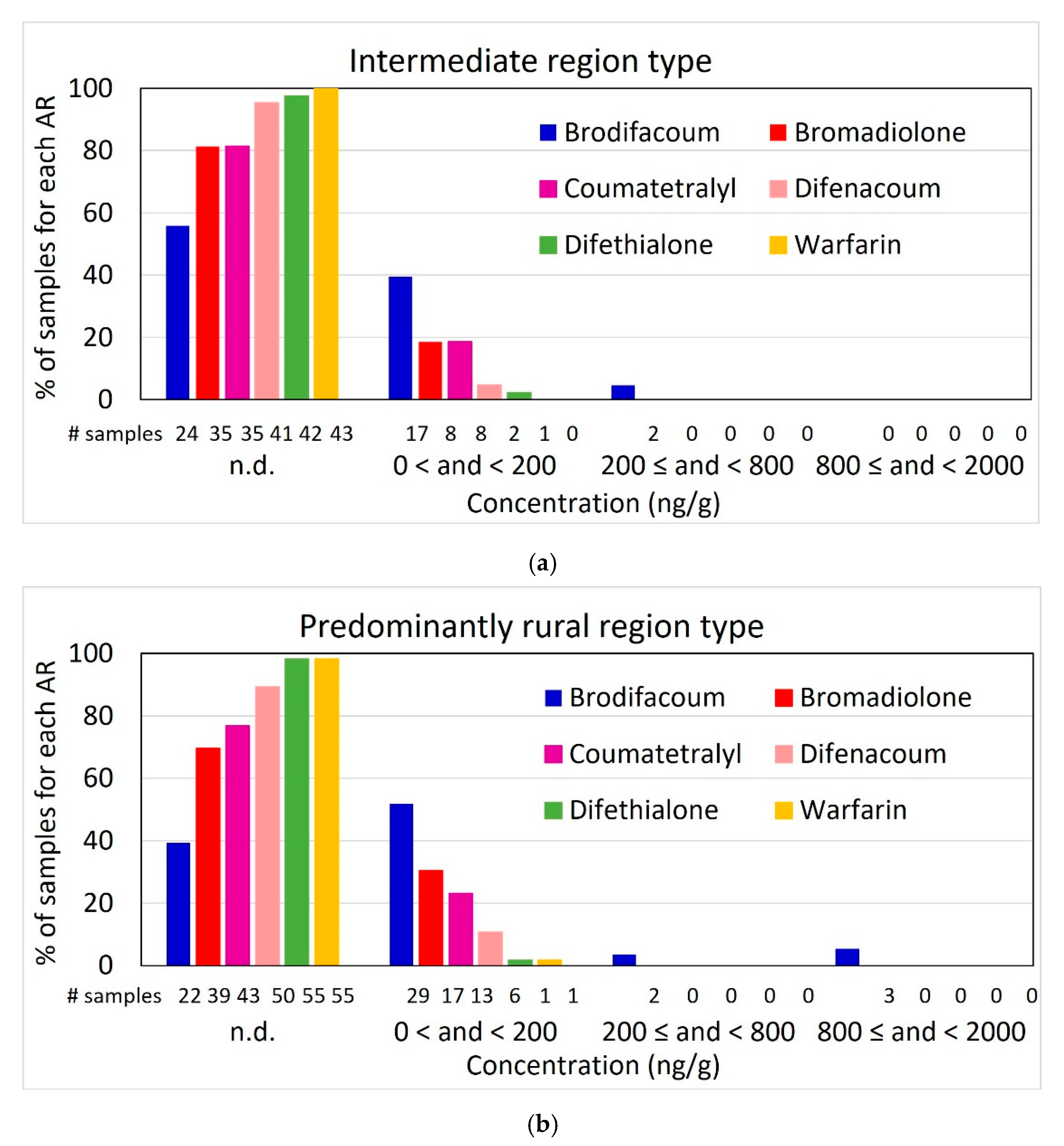
3.2. Rodenticide Residues
3.3. The Results of the Risk Assessment for Acute Toxicity Using the Example of Brodifacoum
4. Discussion
4.1. Prevalence of Rodenticides in Domestic Cats
4.2. Toxicological and Environmental Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Anticoagulant rodenticide |
| EU | European Union |
| VKORC | Enzyme vitamin K epoxide reductase complex |
| FGAR | First-generation anticoagulant rodenticide |
| SGAR | Second-generation anticoagulant rodenticide |
| CNS | Central nervous system |
| LD | Lethal dose |
| LC-ESI-MS/MS | Liquid chromatography–electrospray tandem mass spectrometry |
| ERA | Environmental risk assessment |
| PEC | Predicted environmental concentration |
| PNEC | Predicted no-effect concentration |
| RQ | Risk quotient |
| ESD | Emission Scenario Document |
| FIP | Feline infectious peritonitis |
| FIV | Feline immunodeficiency virus |
| SD | Standard deviation |
| RR | Rate ratio |
| OR | Odds ratio |
| CI | Confidence interval |
| OLS | Ordinary Least Squares |
| CKD | Chronic kidney disease |
References
- European Union. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off. J. Eur. Commun. 2012, L167, 1–123. [Google Scholar]
- Rattner, B.A.; Mastrota, F.N. Anticoagulant rodenticide toxicity to non-target wildlife under controlled exposure conditions. In Anticoagulant Rodenticides and Wildlife, Emerging Topics in Ecotoxicology 5; Van den Brink, N.W., Elliott, J.E., Shore, R.F., Rattner, B.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 45–86. [Google Scholar] [CrossRef]
- Jacob, J.; Buckle, A. Use of anticoagulant rodenticides in different applications around the world. In Anticoagulant Rodenticides and Wildlife. Emerging Topics in Ecotoxicology 5; Van den Brink, N.W., Elliott, J.E., Shore, R.F., Rattner, B.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 11–43. [Google Scholar]
- Rattner, B.A.; Lazarus, R.S.; Elliott, J.E.; Shore, R.F.; van den Brink, N. Adverse outcome pathway and risks of anticoagulant rodenticides to predatory wildlife. Environ. Sci. Technol. 2014, 48, 8433–8445. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Fourel, I.; Queffélec, S.; Vodovar, D.; Mégarbane, B.; Benoit, E.; Siguret, V.; Lattard, V. Poisoning by anticoagulant rodenticides in humans and animals: Causes and consequences. In Poisoning—From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; Hal-01668708; Version 1 (20-12-2017); InTech: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- Berny, P.; Esther, A.; Jacob, J.; Prescott, C. Risk Mitigation Measures for Anticoagulant Rodenticides as Biocidal Products; Final Report to the European Commission (contract N°07-0307/2012/638259/ETU/D3); Publications Office of the European Union: Luxembourg, 2014; 100p. [Google Scholar] [CrossRef]
- Stuart, A.M.; Jacob, J.; Awoniyi, A.M.; Costa, F.; Bosma, L.; Meheretu, Y.; Htwe, N.M.; Williamson, S.; Eddleston, M.; Dalecky, A.; et al. Alternative domestic rodent pest management approaches to address the hazardous use of metal phosphides in low- and middle-income countries. J. Pest Sci. 2024, 98, 89–111. [Google Scholar] [CrossRef]
- van den Brink, N.W.; Elliott, J.E.; Shore, R.F.; Rattner, B.A. (Eds.) Anticoagulant Rodenticides and Wildlife, Emerging Topics in Ecotoxicology 5; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Brakes, C.R.; Smith, R.H. Exposure of non-target small mammals to rodenticides: Short term effects, recovery and implications for secondary poisoning. J. Appl. Ecol. 2005, 42, 118–128. [Google Scholar] [CrossRef]
- Walther, B.; Geduhn, A.; Schenke, D.; Schlötelburg, A.; Jacob, J. Baiting location affects anticoagulant rodenticide exposure of non-target small mammals on farms. Pest Manag. Sci. 2020, 77, 611–619. [Google Scholar] [CrossRef]
- Walther, B.; Geduhn, A.; Schenke, D.; Jacob, J. Exposure of passerine birds to brodifacoum during management of Norway rats on farms. Sci. Total Environ. 2021, 762, 144160. [Google Scholar] [CrossRef]
- Alomar, H.; Chabert, A.; Coeurdassier, M.; Vey, D.; Berny, P. Accumulation of anticoagulant rodenticides (chlorophacinone, bromadiolone and brodifacoum) in a non-target invertebrate, the slug, Deroceras reticulatum. Sci. Total Environ. 2018, 610, 576–582. [Google Scholar] [CrossRef]
- Riley, S.P.D.; Bromley, C.; Poppenga, R.H.; Uzal, F.A.; Whited, L.; Sauvajot, R.M. Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban southern California. J. Wild. Manag. 2007, 71, 1874–1884. [Google Scholar] [CrossRef]
- Fraser, D.; Mouton, A.; Serieys, L.E.K.; Cole, S.; Carver, S.; Vandewoude, S.; Lappin, M.; Riley, S.P.D.; Wayne, R. Genome-wide expression reveals multiple systemic effects associated with detection of anticoagulant poisons in bobcats (Lynx rufus). Mol. Ecol. 2017, 27, 1170–1187. [Google Scholar] [CrossRef]
- Walton, K.L.; Otto, C.M. Retrospective evaluation of feline rodenticide exposure and gastrointestinal decontamination: 146 cases (2000–2010). J. Veter Emerg. Crit. Care 2018, 28, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Rached, A.; Moriceau, M.-A.; Serfaty, X.; Lefebvre, S.; Lattard, V. Biomarkers potency to monitor non-target fauna poisoning by anticoagulant rodenticides. Front. Veter Sci. 2020, 7, 616276. [Google Scholar] [CrossRef]
- Alterio, N. Secondary poisoning of stoats (Mustela erminea), feral ferrets (Mustela furo), and feral house cats (Felis catus) by the anticoagulant poison, brodifacoum. N. Z. J. Zool. 1996, 23, 331–338. [Google Scholar] [CrossRef]
- Murphy, E.C.; Clapperton, B.K.; Bradfield, P.M.F.; Speed, H.J. Brodifacoum residues in target and non-target animals following large-scale poison operations in New Zealand podocarp-hardwood forests. N. Z. J. Zool. 1998, 25, 307–314. [Google Scholar] [CrossRef]
- Spurr, E.B.; Maitland, M.J.; Taylor, G.E.; Wright, G.R.G.; Radford, C.D.; Brown, L.E. Residues of brodifacoum and other anticoagulant pesticides in target and non-target species, Nelson Lakes National Park, New Zealand. N. Z. J. Zool. 2005, 32, 237–249. [Google Scholar] [CrossRef]
- Mahjoub, T.; Krafft, E.; Garnier, L.; Mignard, A.; Hugnet, C.; Lefebvre, S.; Fourel, I.; Benoit, E.; Lattard, V. Asymptomatic anticoagulant rodenticide exposure in dogs and cats—A French and Belgian rural and urban areas study. Front. Toxicol. 2022, 4, 907892. [Google Scholar] [CrossRef] [PubMed]
- Seljetun, K.O.; Sandvik, M.; Vindenes, V.; Eliassen, E.; Øiestad, E.L.; Madslien, K.; Moe, L. Comparison of anticoagulant rodenticide concentrations in liver and feces from apparently healthy red foxes. J. Veter Diagn. Investig. 2020, 32, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, E.; Santangeli, A.; Koivisto, P.; Korkolainen, T.; Vuorisalo, T.; Hanski, I.K.; Loivamaa, I.; Koivisto, S. The prevalence and correlates of anticoagulant rodenticide exposure in non-target predators and scavengers in Finland. Sci. Total Environ. 2018, 642, 701–707. [Google Scholar] [CrossRef]
- López-Perea, J.J.; Camarero, P.R.; Sanchez-Barbudo, I.S.; Mateo, R. Urbanization and cattle density are determinants in the exposure to anticoagulant rodenticides of non-target wildlife. Environ. Pollut. 2019, 244, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Tegner, C.; Lundgren, S.; Dreimanis, K.; Tevell Åberg, A.; Windahl, U. Alpha-chloralose poisoning in cats: Clinical findings in 25 confirmed and 78 suspected cases. J. Feline Med. Surg. 2022, 24, e324–e329. [Google Scholar] [CrossRef]
- Dijkman, M.A.; Robben, J.H.; van Riel, A.J.H.P.; de Lange, D.W. Evidence of a sudden increase in α-chloralose poisoning in dogs and cats in the Netherlands between 2018 and 2021. Veter Rec. 2022, 192, e2342. [Google Scholar] [CrossRef]
- Segev, G.; Yas-Natan, E.; Shlosberg, A.; Aroch, I. Alpha-chloralose poisoning in dogs and cats: A retrospective study of 33 canine and 13 feline confirmed cases. Veter J. 2006, 172, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Windahl, U.; Tevell Åberg, A.; Kryuchkov, F.; Lundgren, S.; Tegner, C.; Dreimanis, K.; Koivisto, S.; Simola, O.; Sandvik, M.; Bernhoft, A. Alpha-chloralose poisoning in cats in three Nordic countries—The importance of secondary poisoning. BMC Veter Res. 2022, 18, 334. [Google Scholar] [CrossRef]
- Lees, P.; Pharm, B. Pharmacology and toxicology of alpha chloralose: A review. Veter Rec. 1972, 91, 330–333. [Google Scholar] [CrossRef]
- Statista. Number of Cats in the European Union in 2022, by Country (in 1000s), 2024. Cat Population in the EU by Country 2022|Statista. Available online: https://www.statista.com/statistics/515464/cat-ownership-european-union-eu-by-country/ (accessed on 30 May 2024).
- Downes, M.J.; Dean, R.S.; Stavisky, J.H.; Adams, V.J.; Grindlay, D.J.C.; Brennan, M.L. Methods used to estimate the size of the owned cat and dog population: A systematic review. BMC Veter Res. 2013, 9, 121. [Google Scholar] [CrossRef]
- Republic of Slovenia. Gospodinjstva in Družine, Slovenija, 1 January 2021. Statistical Office. 2024. Available online: https://www.stat.si/StatWeb/news/Index/9973 (accessed on 30 May 2024).
- International Companion Animal Management Coalition. Human Cat Population Management Guidance. 2011. Available online: https://www.icam-coalition.org/download/humane-cat-population-management-guidance/ (accessed on 30 May 2024).
- Castañeda, I.; Forin-Wiart, M.-A.; Pisanu, B.; de Bouillane de Lacoste, N. Spatiotemporal and individual patterns of domestic cat (Felis catus) hunting behaviour in France. Animals 2023, 13, 3507. [Google Scholar] [CrossRef]
- Krauze-Gryz, D.; Gryz, J.; Goszczyński, J. Predation by domestic cats in rural areas of central Poland: An assessment based on two methods. J. Zool. 2012, 288, 260–266. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1396. [Google Scholar] [CrossRef] [PubMed]
- Geduhn, A.; Jacob, J.; Schenke, D.; Keller, B.; Kleinschmidt, S.; Esther, A. Relation between intensity of biocide practice and residues of anticoagulant rodenticides in red foxes (Vulpes vulpes). PLoS ONE 2015, 10, e0139191. [Google Scholar] [CrossRef] [PubMed]
- Cerkvenik-Flajs, V.; Schenke, D.; Žele-Vengušt, D.; Korenjak-Černe, S.; Perpar, A.; Vengušt, G. Exposure assessment of anticoagulant rodenticides in the liver of red foxes (Vulpes vulpes) in Slovenia. Sci. Total Environ. 2024, 918, 170400. [Google Scholar] [CrossRef]
- Elmeros, M.; Lassen, P.; Bossi, R.; Topping, C.J. Exposure of stone marten (Martes foina) and polecat (Mustela putorius) to anticoagulant rodenticides: Effects of regulatory restrictions of rodenticide use. Sci. Total Environ. 2018, 612, 1358–1364. [Google Scholar] [CrossRef]
- López-Perea, J.J.; Camarero, P.R.; Molina-López, R.A.; Parpal, L.; Obón, E.; Solá, J.; Mateo, R. Interspecific and geographical differences in anticoagulant rodenticide residues of predatory wildlife from the Mediterranean region of Spain. Sci. Total Environ. 2015, 511, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Republic of Slovenia. Slovene Statistical Regions and Municipalities in Numbers. Statistical Office. 2023. Available online: https://www.stat.si/obcine (accessed on 19 May 2023).
- Perko, D.; Ciglič, R. Slovenia’s Regions. In The Geography of Slovenia; Perko, D., Ciglič, R., Zorn, M., Eds.; World Regional Geography Book Series; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- [Dataset] Republic of Slovenia. Population—Selected Indicators, Municipalities and Settlements, Slovenia, Annually. Statistical Office. 2023. Available online: https://pxweb.stat.si/SiStatData/pxweb/sl/Data/-/05C5006S.px (accessed on 15 February 2023).
- [Dataset] Republic of Slovenia. Agricultural Holdings, Total UAA, LSU and Average LSU per ha UAA, by Municipalities, Slovenia, 2000, 2010, 2020. Statistical Office. 2023. Available online: https://pxweb.stat.si/SiStatData/pxweb/sl/Data/-/15P0414S.px (accessed on 15 February 2023).
- EUROSTAT. Degree of Urbanisation. 2023. Available online: https://ec.europa.eu/eurostat/web/degree-of-urbanisation/methodology (accessed on 5 June 2023).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- European Chemicals Agency (ECHA). Guidance on the Biocidal Products Regulation. Volume IV Environment—Assessment and Evaluation (Parts B + C); Version 2.0; ECHA: Helsinki, Finland, October 2017.
- European Chemicals Agency (ECHA). Revised Emission Scenario Document for Product Type 14. Rodenticides. ECHA-18-H-23-EN; ECHA: Helsinki, Finland, 2018; ISBN 978-92-9020-837-2. [Google Scholar] [CrossRef]
- Eason, C.T.; Wickstrom, M. Vertebrate Pesticide Toxicology Manual (Poisons): Information on Poisons Used in New Zealand as Vertebrate Pesticides . Wellington, New Zealand: Department of Conservation, P.O. Box 10-420, 2001. Available online: https://www.doc.govt.nz/documents/science-and-technical/docts23.pdf (accessed on 14 March 2025).
- European Chemicals Agency (ECHA). Opinion on the Application for Renewal of the Approval of the Active Substance: Brodifacoum Product type: 14 ECHA/BPC/113/2016. 2016. Available online: https://echa.europa.eu/documents/10162/b85dfd6e-177b-43df-809c-180bc025b612 (accessed on 14 March 2025).
- Umweltbundesamt (UBA). Authorisation of Anticoagulant Rodenticides in Germany. FAQ on Environmental Risks, Risk Mitigation Measures and Best Practice. 4th edition. 2019. Available online: https://www.umweltbundesamt.de/en/publikationen/authorisation-of-anticoagulant-rodenticides-in (accessed on 14 March 2025).
- Seljetun, K.O.; Vindenes, V.; Øiestad, E.L.; Brochmann, G.-W.; Eliassen, E.; Moe, L. Determination of anticoagulant rodenticides in faeces of exposed dogs and in a healthy dog population. Acta Veter. Scand. 2020, 62, 30. [Google Scholar] [CrossRef] [PubMed]
- Republic of Slovenia. Regulation on the implementation of regulations (EU) on the availability of biocidal products on the market and their use. Off. Gaz. RS 2014, 20, 2351–2353. [Google Scholar]
- Republic of Slovenia. Regulation on the implementation of regulations (EU) on the availability of biocidal products on the market and their use. Off. Gaz. RS 2018, 81, 12995–12997. [Google Scholar]
- [Dataset] Republic of Slovenia. Register of Biocidal Products on the Market of the Republic of Slovenia, Ministry of Health Office of the Republic of Slovenia for Chemicals. 2024. Available online: https://podatki.gov.si/dataset/register-biocidnih-proizvodov-na-trgu-rs (accessed on 8 April 2024).
- Carrera, A.; Navas, I.; María-Mojica, P.; García-Fernandez, A.J. Greater predisposition to second generation anticoagulant rodenticide exposure in red foxes (Vulpes vulpes) weakened by suspected infectious disease. Sci. Total Environ. 2024, 907, 167780. [Google Scholar] [CrossRef]
- Kohn, B.; Weingart, C.; Giger, U. Haemorrhage in seven cats with suspected anticoagulant rodenticide intoxication. J. Feline Med. Surg. 2003, 5, 295–304. [Google Scholar] [CrossRef]
- Meehan, A.P. Rodenticidal activity of bromadiolone—A new anticoagulant. In Proceedings of the 8th Vertebrate Pest Conference, Sacramento, CA, USA, 7–9 March 1978. [Google Scholar]
- Bull, J.O. Laboratory and field investigations with difenacoum, a promising new rodenticide. In Proceedings of the 7th Vertebrate Pest Conference, Monterey, CA, USA, 9–11 March 1976. [Google Scholar]
- Valchev, I.; Binev, R.; Yordanova, V.; Nikolov, Y. Anticoagulant rodenticide intoxication in animals—A review. Turk. J. Veter Anim. Sci. 2008, 32, 237–243. [Google Scholar]
- Kopanke, J.H.; Horak, K.E.; Musselman, E.; Miller, C.A.; Bennett, K.; Olver, C.S.; Volker, S.F.; VandeWoude, S.; Bevins, S.N. Effects of low-level brodifacoum exposure on the feline immune response. Sci. Rep. 2018, 8, 8168. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, D.L.; Akpa, B.S.; Ayee, M.A.; Boullerne, A.I.; Braun, D.; Brodsky, S.V.; Gidalevitz, D.; Hauck, Z.; Kalinin, S.; Kowal, K.; et al. The emerging threat of superwarfarins: History, detection, mechanisms, and countermeasures. Ann. N. Y. Acad. Sci. 2016, 1374, 111–122. [Google Scholar] [CrossRef]
- Paretsis, B.F.; Mario, L.C.; Sasahara, T.H.D.C.; da Silva, L.C.G.; dos Santos, J.M.; Kfoury Júnior, J.R.; Leandro, R.M. Stereological analysis of metanephros from domestic cat (Felis catus, Linnaeus 1798) embryos and fetus. Anat. Histol. Embryol. 2021, 50, 965–973. [Google Scholar] [CrossRef]
- Alborough, R.; Grau-Roma, L.; de Brot, S.; Hantke, G.; Vazquez, S.; Gardner, D.S. Renal accumulation of prooxidant mineral elements and CKD in domestic cats. Sci. Rep. 2020, 10, 3160. [Google Scholar] [CrossRef]
- Brown, C.A.; Elliott, J.; Schmiedt, C.W.; Brown, S.A. Chronic kidney disease in aged cats: Clinical features, morphology, and proposed pathogeneses. Veter Pathol. 2016, 53, 309–326. [Google Scholar] [CrossRef]
- Chen, H.; Dunaevich, A.; Apfelbaum, N.; Kuzi, S.; Mazaki-Tovi, M.; Aroch, I.; Segev, G. Acute on chronic kidney disease in cats: Etiology, clinical and clinicopathologic findings, prognostic markers, and outcome. J. Veter Intern. Med. 2020, 34, 1496–1506. [Google Scholar] [CrossRef]
- Sánchez-Barbudo, I.S.; Camarero, P.R.; Mateo, R. Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci. Total Environ. 2012, 420, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H. Feline drug metabolism and disposition: Pharmacokinetic evidence for species differences and molecular mechanisms. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 20. [Google Scholar] [CrossRef] [PubMed]
- Rammell, C.G.; Hoogenboom, J.J.L.; Cotter, M.; Williams, J.M.; Bell, J. Brodifacoum residues in target and non-target animals following rabbit poisoning trials. N. Z. J. Exp. Agric. 1984, 12, 107–111. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). International Program on Chemical Safety. In Environmental Health Criteria 175; Anticoagulant Rodenticides; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Vandenbroucke, V.; Bousquet-Melou, A.; De Backer, P.; Croubels, S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J. Veter Pharmacol. Ther. 2008, 31, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cerkvenik-Flajs, V.; Schenke, D.; Korenjak-Černe, S.; Cvetko, M.; Švara, T.; Gombač, M. Environmental exposure to anticoagulant rodenticides and α-chloralose in the liver of domestic cats (Felis catus) in Slovenia. In Science-Based Solutions in Times of Crisis: Integrating Science and Policy for Environmental Challenges: Abstract Book, Proceedings of the SETAC Europe 34th Annual Meeting, Seville, Spain, 5–9 May 2024; SciVie—Gozdarski inštitut Slovenije: Ljubljana, Slovenia, 2024. [Google Scholar]
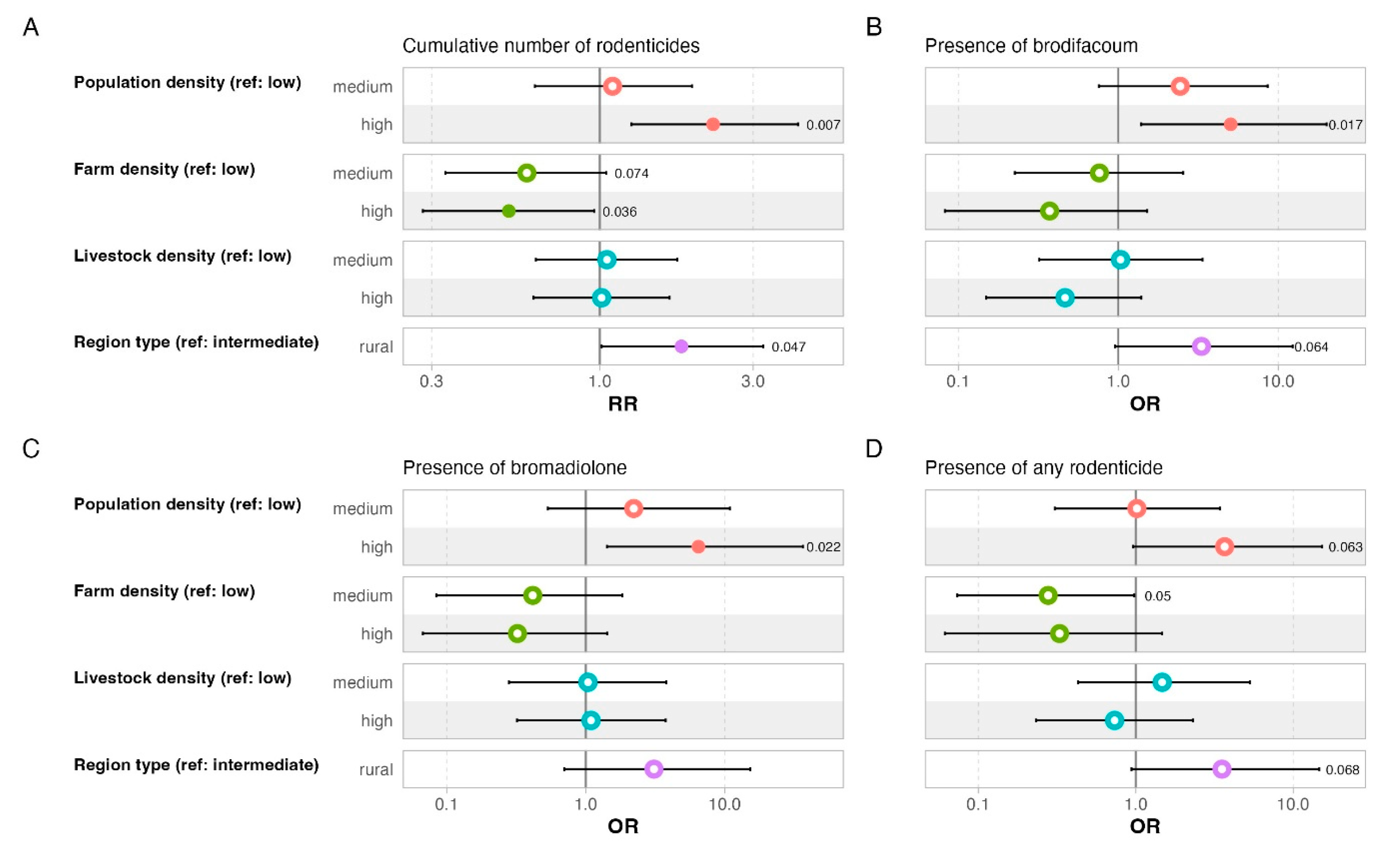
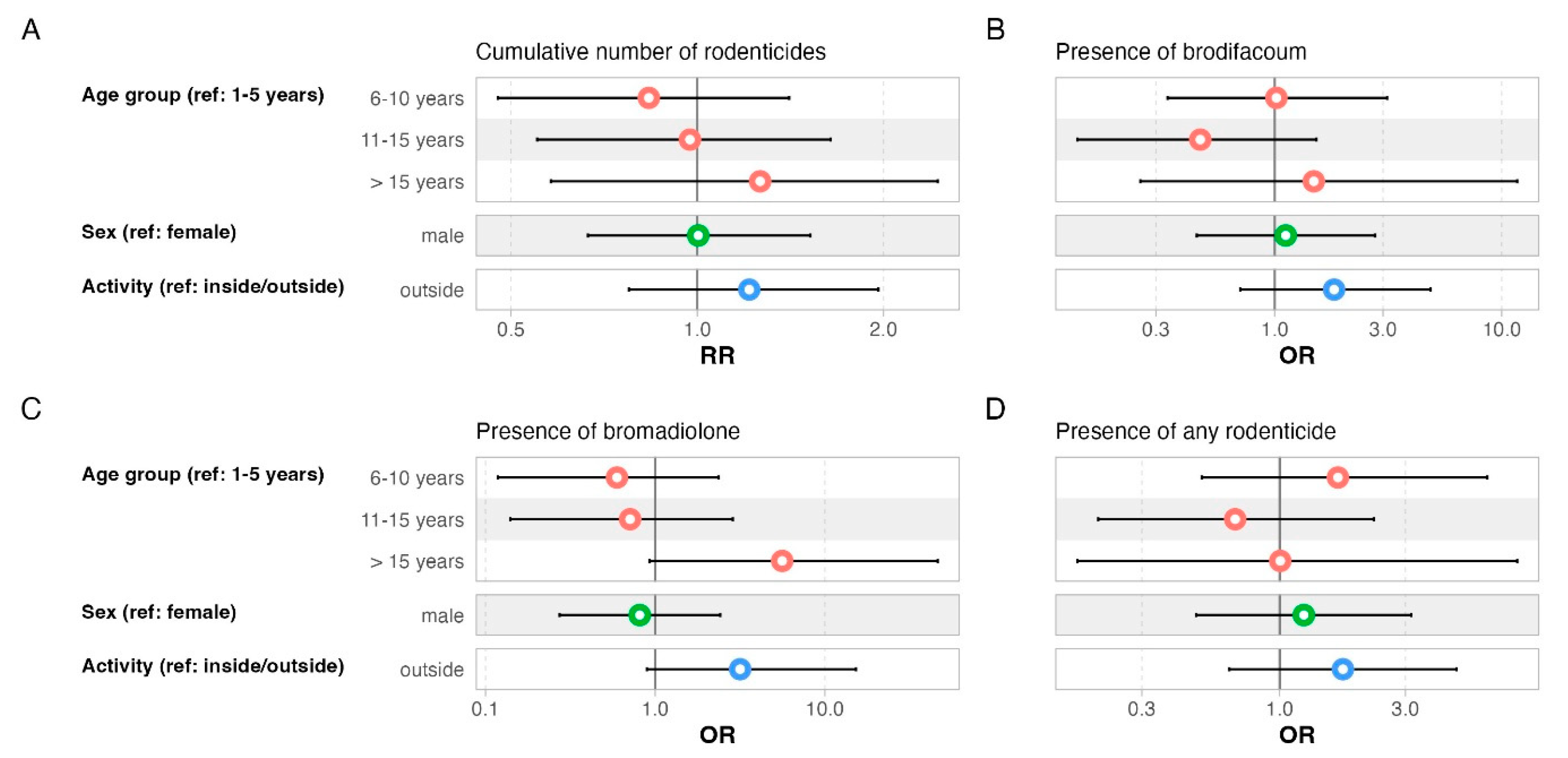
| Estimate | Bootstrap CI | p-Value | |
|---|---|---|---|
| Population density (ref: low) | |||
| medium | 0.54 | [−1.04, 2.04] | 0.48 |
| high | 1.13 | [−0.55, 2.73] | 0.16 |
| Farm density (ref: low) | |||
| medium | −0.62 | [−2.11, 0.82] | 0.37 |
| high | −1.73 | [−3.18, −0.12] | 0.03 |
| Livestock density (ref: low) | |||
| medium | 0.58 | [−0.75, 1.79] | 0.35 |
| high | 0.43 | [−0.83, 1.73] | 0.47 |
| Region type (ref: intermediate) | |||
| rural | 1.42 | [−0.18, 2.92] | 0.07 |
| Estimate | Bootstrap CI | p-Value | |
|---|---|---|---|
| Age (ref: 1–5 years) | |||
| 6–10 years | −0.58 | [−1.91, 0.7] | 0.38 |
| 11–15 years | −0.04 | [−1.57, 1.7] | 0.95 |
| >15 years | −0.06 | [−2.06, 1.25] | 0.94 |
| Sex (ref: female) | |||
| male | −0.16 | [−1.31, 1] | 0.79 |
| Activity (ref: inside/outside) | |||
| outside | 0.48 | [−0.64, 1.61] | 0.39 |
| Estimate | Bootstrap CI | p-Value | |
|---|---|---|---|
| Age (ref: 1–5 years) | |||
| 6–10 years | −1 | [−2.35, 0.49] | 0.16 |
| 11–15 years | 0.21 | [−1.82, 1.69] | 0.82 |
| >15 years | −0.74 | [−2.12, 1.14] | 0.38 |
| Sex (ref: female) | |||
| Male | −0.45 | [−1.68, 0.90] | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerkvenik-Flajs, V.; Schenke, D.; Korenjak-Černe, S.; Perpar, A.; Jacob, J.; Schwonbeck, S.; Kleine Bardenhorst, S.; Hahn, T.; Cvetko, M.; Gombač, M. Exposure of Domestic Cats (Felis catus) to Rodenticidal Compounds. Toxics 2025, 13, 663. https://doi.org/10.3390/toxics13080663
Cerkvenik-Flajs V, Schenke D, Korenjak-Černe S, Perpar A, Jacob J, Schwonbeck S, Kleine Bardenhorst S, Hahn T, Cvetko M, Gombač M. Exposure of Domestic Cats (Felis catus) to Rodenticidal Compounds. Toxics. 2025; 13(8):663. https://doi.org/10.3390/toxics13080663
Chicago/Turabian StyleCerkvenik-Flajs, Vesna, Detlef Schenke, Simona Korenjak-Černe, Anton Perpar, Jens Jacob, Susanne Schwonbeck, Sven Kleine Bardenhorst, Torsten Hahn, Marko Cvetko, and Mitja Gombač. 2025. "Exposure of Domestic Cats (Felis catus) to Rodenticidal Compounds" Toxics 13, no. 8: 663. https://doi.org/10.3390/toxics13080663
APA StyleCerkvenik-Flajs, V., Schenke, D., Korenjak-Černe, S., Perpar, A., Jacob, J., Schwonbeck, S., Kleine Bardenhorst, S., Hahn, T., Cvetko, M., & Gombač, M. (2025). Exposure of Domestic Cats (Felis catus) to Rodenticidal Compounds. Toxics, 13(8), 663. https://doi.org/10.3390/toxics13080663






