Gestational GenX Exposure Induces Maternal Hepatotoxicity by Disrupting the Lipid and Bile Acid Metabolism Distinguished from PFOA-Induced Pyroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
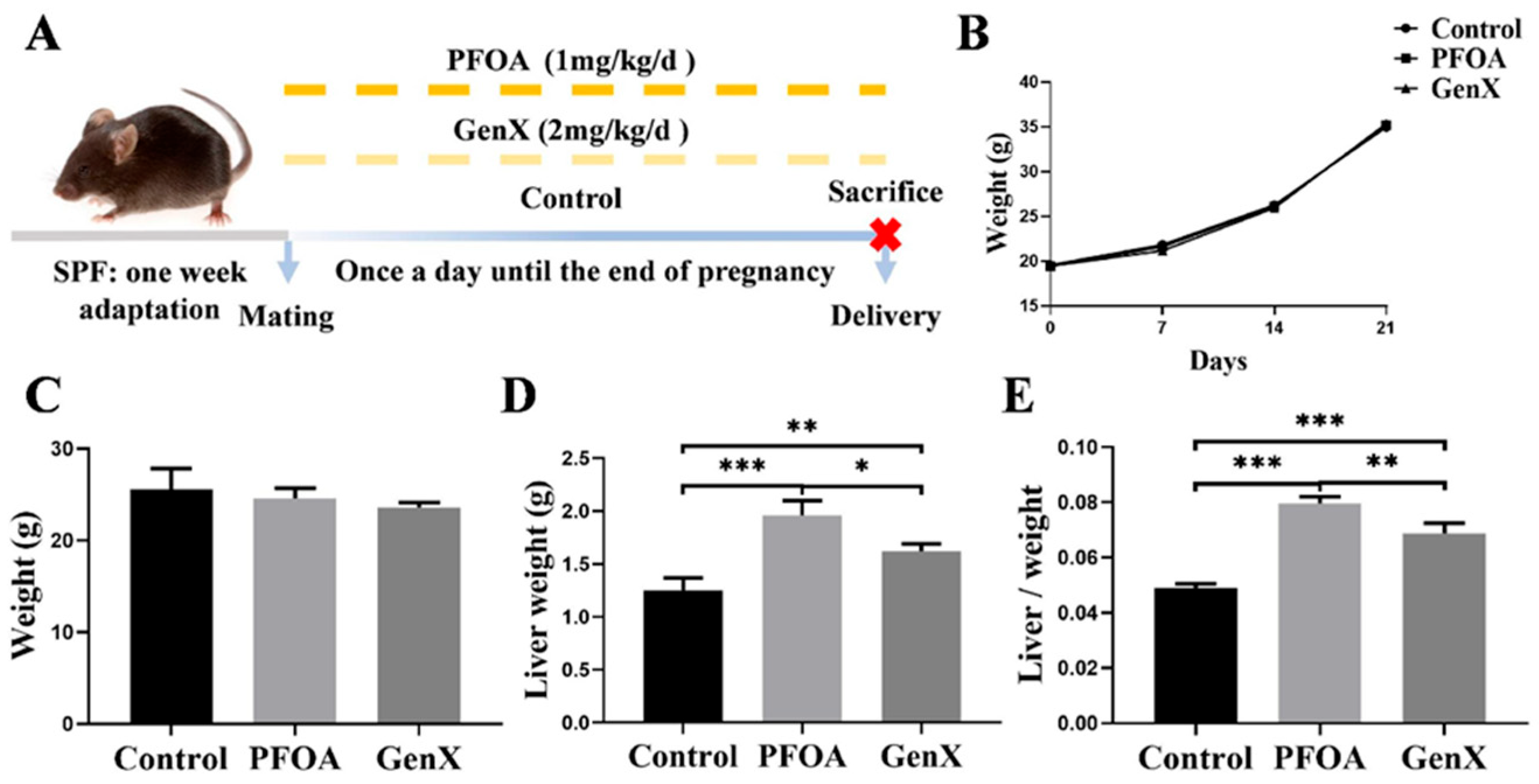
2.2. Animals and Treatments
2.3. Histopathological Analyses
2.4. Serum Biochemical Analyses
2.5. RNA Sequencing and Bioinformatics Analyses
2.6. Quantitative RT-PCR (RT-qPCR) Analysis
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Gestational Exposure to PFOA or GenX Led to Changes in the Livers of Maternal Mice
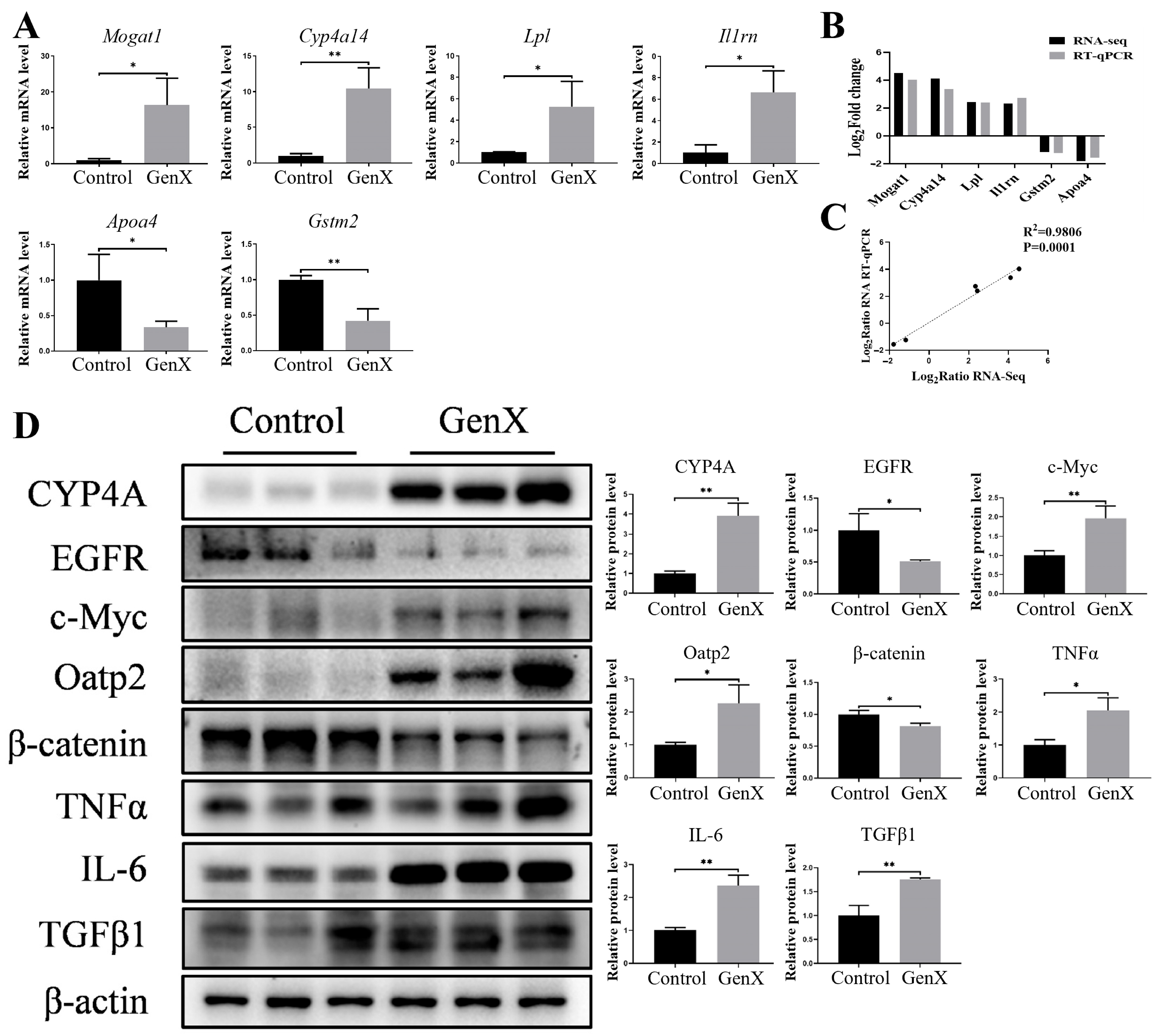
3.2. Gestational GenX Exposure Caused Maternal Hepatic Injury by Disrupting Lipid and Bile Acid Metabolism
3.3. Gestational PFOA Exposure Induced Maternal Hepatotoxicity by Activating Pyroptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muglia, L.J.; Benhalima, K.; Tong, S.; Ozanne, S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. 2022, 20, 418. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Vinnars, M.-T.; Bixo, M.; Damdimopoulou, P. Pregnancy-related maternal physiological adaptations and fetal chemical exposure. Mol. Cell. Endocrinol. 2023, 578, 112064. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, Q.; Wang, H.; Ren, Y.; Zhang, L.; Yang, L. Maternal nutrient metabolism in the liver during pregnancy. Front. Endocrinol. 2024, 15, 1295677. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Garcia, V.; Nambiar, S.M.; Jiang, H.; Dai, G. Activation of Proneuronal Transcription Factor Ascl1 in Maternal Liver Ensures a Healthy Pregnancy. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Jonas, M.M.; Taylor, S.A.; Sanchez, L.H.G.; Wolf, J.L.; Sundaram, S.S. Liver Diseases in the Perinatal Period: Interactions Between Mother and Infant. Hepatology 2020, 71, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, D.A.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’cArroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Sun, Y.; Guo, Y.; Dai, J. Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol. 2018, 52, 7621–7629. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Leeuwen, S.P. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Neth-erlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, G.; Dong, C.; Yang, R.; Pei, Z.; Li, Y.; Li, A.; Zhang, Q.; Jiang, G. Occurrence, bioaccumulation and trophodynamics of per- and polyfluoroalkyl substances (PFAS) in terrestrial and marine ecosystems of Svalbard, Arctic. Water Res. 2024, 271, 122979. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wang, Q.; Hong, P.; Ruan, Y.; Lin, H.; Xu, J.; Zhang, H.; Deng, S.; Wu, H.; Chen, L.; et al. Legacy and Emerging Per- and Polyfluoroalkyl Substances Surveillance in Bufo gargarizans from Inlet Watersheds of Chaohu Lake, China: Tissue Distribution and Bioaccumulation Potential. Environ. Sci. Technol. 2023, 57, 13148–13160. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Wang, S.; Liu, Y.; Ma, X.; Li, Y.; Li, W.; Wang, X. Decade-long historical shifts in legacy and emerging per- and polyfluoro-alkyl substances (PFAS) in surface sediments of China’s marginal seas: Ongoing production and ecological risks. Environ. Res. 2024, 263, 119978. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Usman, M.; Luo, T.; Biard, P.-F.; Lin, K.; Greenwell, H.C.; Hanna, K. Retention and transport of PFOA and its fluorinated substitute, GenX, through water-saturated soil columns. Environ. Pollut. 2023, 337, 122530. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Feng, H.; Zheng, Y.; Luo, H.; Sun, C.; Pan, Y. Determination of Perfluorooctane Sulfonyl Fluoride and Perfluoro-hexane Sulfonyl Fluoride in Soil by Chemical Derivatization and Liquid Chromatography-Tandem Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 4180–4186. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, J.; Yang, Y.; Hui, Y.; Ge, Y.; Larssen, T.; Yu, G.; Deng, S.; Wang, B.; Harman, C. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment. Environ. Sci. Technol. 2013, 47, 10163–10170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, Y.; Shi, Y.; Xu, L.; Cai, Y. Occurrence and transport of perfluoroalkyl acids (PFAAs), including short-chain PFAAs in Tangxun Lake, China. Environ. Sci. Technol. 2013, 47, 9249–9257. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Abbas, T.; Ok, Y.S.; Tsang, D.C.; Bhatnagar, A.; Khan, E. GenX is not always a better fluorinated organic compound than PFOA: A critical review on aqueous phase treatability by adsorption and its associated cost. Water Res. 2021, 205, 117683. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, Y.; Cui, S.; Tang, X.; Zhang, P.; Wang, C.; Liang, Y.; Sun, H.; Ma, C.; Xing, B. Novel Defluorination Pathways of Perfluoroether Compounds (GenX): α-Fe2O3 Nanoparticle Layer Retains Higher Concentrations of Effective Hydrated Electrons. Environ. Sci. Technol. 2024, 58, 5567–5577. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Sheppard, N.; Joseph, S.; Duckworth, O.W.; Higgins, C.P.; Knappe, D.R.U. Residential Garden Produce Harvested Near a Fluorochemical Manufacturer in North Carolina Can Be An Important Fluoroether Exposure Pathway. J. Agric. Food Chem. 2024, 72, 26874–26883. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.T.; Avula, V.; Fry, R.C. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: A Potential Mechanistic Role for Placental Peroxisome Proliferator–Activated Receptors (PPARs). Curr. Environ. Health Rep. 2020, 7, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-L.; Chen, Y.-K.; Zhang, Q.-Y.; Chen, L.-J.; Zhang, K.-K.; Li, J.-H.; Liu, J.-L.; Wang, Q.; Xie, X.-L. Gestational exposure to GenX induces hepatic alterations by the gut-liver axis in maternal mice: A similar mechanism as PFOA. Sci. Total. Environ. 2022, 820, 153281. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Z.; Li, X.; Chen, J.; Liang, X.; Li, J. GenX Disturbs the Indicators of Hepatic Lipid Metabolism Even at Envi-ronmental Concentration in Drinking Water via PPARα Signaling Pathways. Chem. Res. Toxicol. 2024, 37, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Hair, K.; Macleod, M.R.; Sena, E.S. A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus). Res. Integr. Peer Rev. 2019, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Tihanyi, D.K.; Szijarto, A.; Fülöp, A.; Denecke, B.; Lurje, G.; Neumann, U.P.; Czigany, Z.; Tolba, R. Systematic Review on Characteristics and Reporting Quality of Animal Studies in Liver Regeneration Triggered by Portal Vein Occlusion and Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Adherence to the ARRIVE Guidelines. J. Surg. Res. 2019, 235, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Olonisakin, T.F.; Pribis, J.P.; Zupetic, J.; Yoon, J.H.; Holleran, K.M.; Jeong, K.; Shaikh, N.; Rubio, D.M.; Lee, J.S. A checklist is associated with increased quality of reporting preclinical biomedical research: A systematic review. PLoS ONE 2017, 12, e0183591. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S. ARRIVE has not ARRIVEd: Support for the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, e0197882. [Google Scholar] [CrossRef] [PubMed]
- Chatzimanouil, M.K.T.; Wilkens, L.; Anders, H.J. Quantity and Reporting Quality of Kidney Research. J. Am. Soc. Nephrol. 2019, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jiang, Y.; Zhang, T.; Shang, Z.; Zhang, W.; Hu, K.; Chen, F.; Mei, F.; Gao, Q.; Zhao, L.; et al. Quality of interventional animal experiments in Chinese journals: Compliance with ARRIVE guidelines. BMC Vet. Res. 2020, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of Perfluorooctanoic Acid Exposure during Pregnancy in the Mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). 2016. Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf (accessed on 17 July 2024).
- Blake, B.E.; Cope, H.A.; Hall, S.M.; Keys, R.D.; Mahler, B.W.; McCord, J.; Scott, B.; Stapleton, H.M.; Strynar, M.J.; Elmore, S.A.; et al. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect. 2020, 128, 27006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Xu, L.-L.; Zhong, M.-T.; Chen, Y.-K.; Lai, M.-Q.; Wang, Q.; Xie, X.-L. Gestational GenX and PFOA exposures induce hepatotoxicity, metabolic pathway, and microbiome shifts in weanling mice. Sci. Total Environ. 2024, 907, 168059. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, N.; Li, C.; Lin, M.; Chen, Z.; Li, W.; Zhou, H. β-Sitosterol suppresses NLRP3 Inflammasome activation and Pyroptosis in myocardial ischemia/reperfusion injury via inhibition of PPARγ2. Int. Immunopharmacol. 2025, 154, 114543. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Mo, J.; Zuo, J.; Ye, L. Caspase-3/GSDME dependent pyroptosis contributes to offspring lung injury induced by gestational PFOS exposure via PERK/ATF4 signaling. Arch. Toxicol. 2024, 98, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Gu, T.; Ling, J.; Luo, J.; Zhao, J.; Hu, B.; Hua, L.; Wan, C.; Jiang, S. PFOS facilitates liver inflammation and steatosis: An involvement of NLRP3 inflammasome-mediated hepatocyte pyroptosis. J. Appl. Toxicol. 2022, 42, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.-Y.; Li, L.-Y.; She, Y.-C.; Zheng, Y.-Y.; Ye, X.-Y.; Bao, Q.; et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 2021, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, E.A.; Al-Abbas, N.S.; Atia, M.M.; Alzahrani, F.; Ahmed, E.-M.M.; Ali, S.S.; Al Jaouni, S.K. Can fig and olive ameliorate the toxicity induced by 2-nitropropane in some organs of mice? Role of inflammatory versus anti-inflammatory gene. J. Tradit. Chin. Med. 2021, 41, 891–899. [Google Scholar] [CrossRef]
- Li, S.; Xie, J.; Zhang, D.; Zhao, G.; Bai, Y.; Li, K.; Li, X.; Li, Q.; Tang, X.; Ge, X. Lycopene abolishes typical polyhalogenated carbazoles (PHCZs)-induced hepatic injury in yellow catfish (Pelteobagrus fulvidraco): Involvement of ROS/PI3K-AKT/NF-κB signaling. Fish Shellfish Immunol. 2023, 139, 108897. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Song, Y.; Xiao, W.; Gong, Z. Regulatory Effects and Mechanisms of L-Theanine on Neurotransmitters via Liver-Brain Axis Under a High Protein Diet. Mol. Neurobiol. 2024, 61, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, H.; Niu, J.; Chen, X.; Li, H.; Rao, Z.; Guo, Y.; Zhang, W.; Wang, Z. Curcumin alleviates zearalenone-induced liver injury in mice by scav-enging reactive oxygen species and inhibiting mitochondrial apoptosis pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116343. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Cui, X.-X.; Tan, Y.-W.; Dong, P.-X.; Ou, Y.-Q.; Li, Q.-Q.; Chu, C.; Wu, L.-Y.; Liang, L.-X.; Qin, S.-J.; et al. Per- and perfluoroalkyl substances alternatives, mixtures and liver function in adults: A community-based population study in China. Environ. Int. 2022, 163, 107179. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Pourfarzam, M.; Sharifabad, A.H.; Neisiani, A.K.; Mousavi, M.K.; Aliomrani, M. Effect of pretreatment with a synbiotic on Perfluo-rooctanoic acid-induced liver damage after sub-acute oral exposure in C57BL/6J mice. Toxicol. Appl. Pharmacol. 2023, 459, 116360. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Zhang, Z.; Chen, J.; Li, M.; Li, J. GenX caused liver injury and potential hepatocellular carcinoma of mice via drinking water even at environmental concentration. Environ. Pollut. 2024, 346, 123574. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Niu, Y.; Luan, H.; Li, M.; Zheng, L.; Pan, Y.; Liu, W. Effects of legacy and emerging per- and polyfluoroalkyl substances on PPARα/β/γ regulation and osteogenic/adipogenic differentiation. Environ. Int. 2022, 170, 107584. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, V.; Bil, W.; Vandebriel, R.; Granum, B.; Luijten, M.; Lindeman, B.; Grandjean, P.; Kaiser, A.-M.; Hauzenberger, I.; Hartmann, C.; et al. Consideration of pathways for immunotoxicity of per- and polyfluoroalkyl substances (PFAS). Environ. Health 2023, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D.; Wood, C.R.; Watkins, A.M.; Tatum-Gibbs, K.; Das, K.P.; Lau, C. Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and post-natal CD-1 mouse tissues. Reprod. Toxicol. 2012, 33, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, Z.; Guo, H.; Gou, Y.; Dai, J.; Zhou, X.; Sheng, N. GenX analogs exposure induced greater hepatotoxicity than GenX mainly via activation of PPARα pathway while caused hepatomegaly in the absence of PPARα in female mice. Environ. Pollut. 2024, 344, 123314. [Google Scholar] [CrossRef] [PubMed]
- Attema, B.; Janssen, A.W.; Rijkers, D.; van Schothorst, E.M.; Hooiveld, G.J.; Kersten, S. Exposure to low-dose perfluorooctanoic acid promotes hepatic steatosis and disrupts the hepatic transcriptome in mice. Mol. Metab. 2022, 66, 101602. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, J.; Zhang, H.; Yao, J.; Sheng, N.; Li, Q.; Guo, Y.; Wu, C.; Xie, W.; Dai, J. Exposure to GenX and Its Novel Analogs Disrupts Hepatic Bile Acid Metabolism in Male Mice. Environ. Sci. Technol. 2022, 56, 6133–6143. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sheng, N.; Guo, Y.; Wu, C.; Xie, W.; Dai, J. Exposure to GenX and its novel analogs disrupts fatty acid metabolism in male mice. Environ. Pollut. 2021, 291, 118202. [Google Scholar] [CrossRef] [PubMed]
- Hägglöf, T.; Vanz, C.; Kumagai, A.; Dudley, E.; Ortega, V.; Siller, M.; Parthasarathy, R.; Keegan, J.; Koenigs, A.; Shute, T.; et al. T-bet+ B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity. Cell Metab. 2022, 34, 1121–1136.e6. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Q.; Fang, X.; Li, L.; Yang, G.; Xu, X.; Yang, M. Nuclear factor erythroid 2-related factor 2 ameliorates disordered glucose and lipid metabolism in liver: Involvement of gasdermin D in regulating pyroptosis. Clin. Transl. Med. 2025, 15, e70233. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Per- and Polyfluoroalkyl Substances (PFAS) Final PFAS National Primary Drinking Water Regulation. 2024. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 20 July 2024).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-J.; Chen, Y.-K.; Chen, Y.-Q.; Zhang, Q.-Y.; Liu, Y.; Wang, Q.; Xie, X.-L. Gestational GenX Exposure Induces Maternal Hepatotoxicity by Disrupting the Lipid and Bile Acid Metabolism Distinguished from PFOA-Induced Pyroptosis. Toxics 2025, 13, 617. https://doi.org/10.3390/toxics13080617
Zhang J-J, Chen Y-K, Chen Y-Q, Zhang Q-Y, Liu Y, Wang Q, Xie X-L. Gestational GenX Exposure Induces Maternal Hepatotoxicity by Disrupting the Lipid and Bile Acid Metabolism Distinguished from PFOA-Induced Pyroptosis. Toxics. 2025; 13(8):617. https://doi.org/10.3390/toxics13080617
Chicago/Turabian StyleZhang, Jin-Jin, Yu-Kui Chen, Ya-Qi Chen, Qin-Yao Zhang, Yu Liu, Qi Wang, and Xiao-Li Xie. 2025. "Gestational GenX Exposure Induces Maternal Hepatotoxicity by Disrupting the Lipid and Bile Acid Metabolism Distinguished from PFOA-Induced Pyroptosis" Toxics 13, no. 8: 617. https://doi.org/10.3390/toxics13080617
APA StyleZhang, J.-J., Chen, Y.-K., Chen, Y.-Q., Zhang, Q.-Y., Liu, Y., Wang, Q., & Xie, X.-L. (2025). Gestational GenX Exposure Induces Maternal Hepatotoxicity by Disrupting the Lipid and Bile Acid Metabolism Distinguished from PFOA-Induced Pyroptosis. Toxics, 13(8), 617. https://doi.org/10.3390/toxics13080617







