Accumulation of Mixed Heavy Metals in Maternal Hair and Risk of Pre-Eclampsia: A Prospective Nested Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data and Sample Collection
2.3. Measurement of Heavy Metals and Analysis
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Levels of Heavy Metals in Maternal Hair
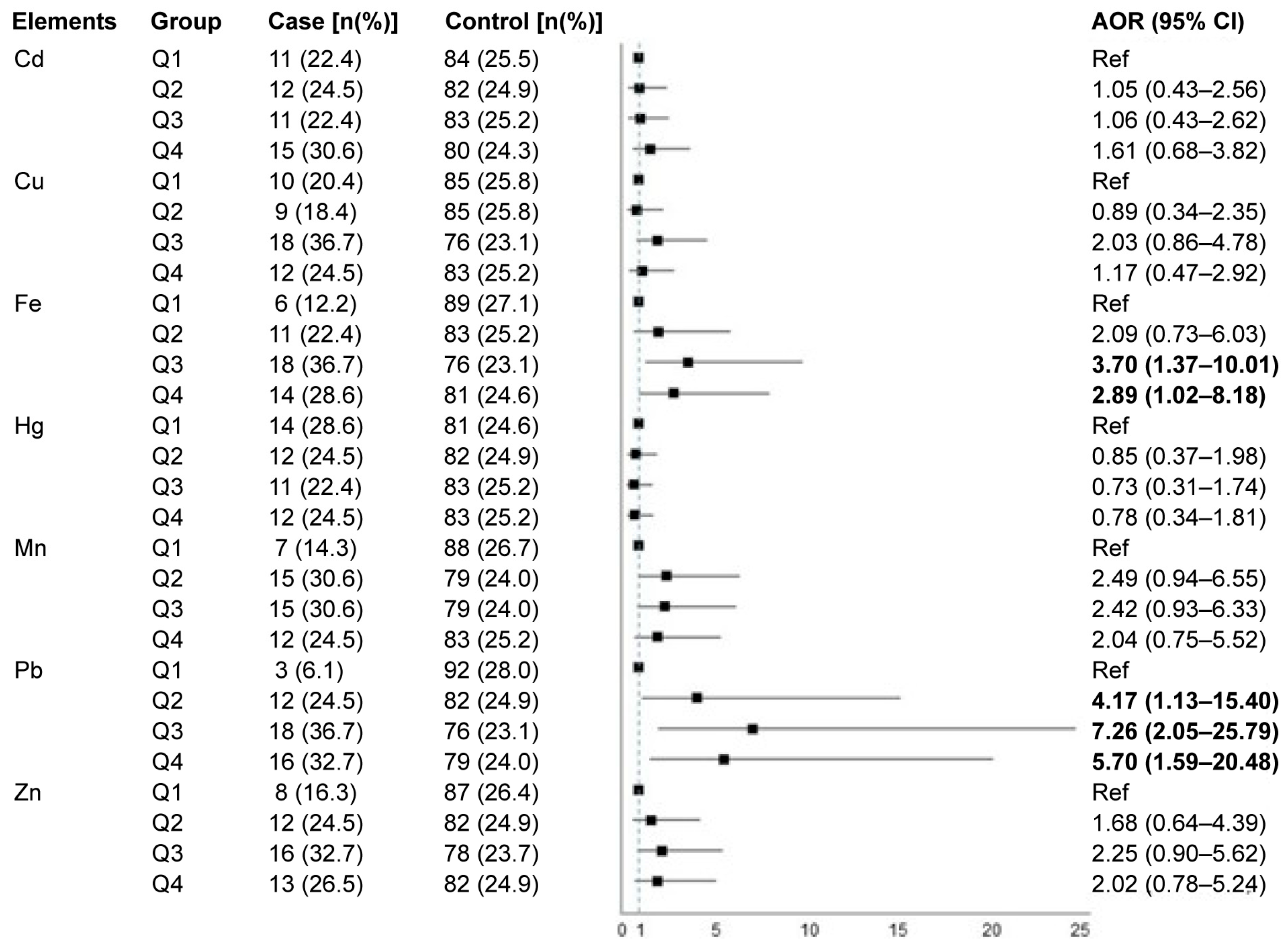
3.3. Associations of Individual Heavy Metals in Maternal Hair and Pre-Eclampsia Risk
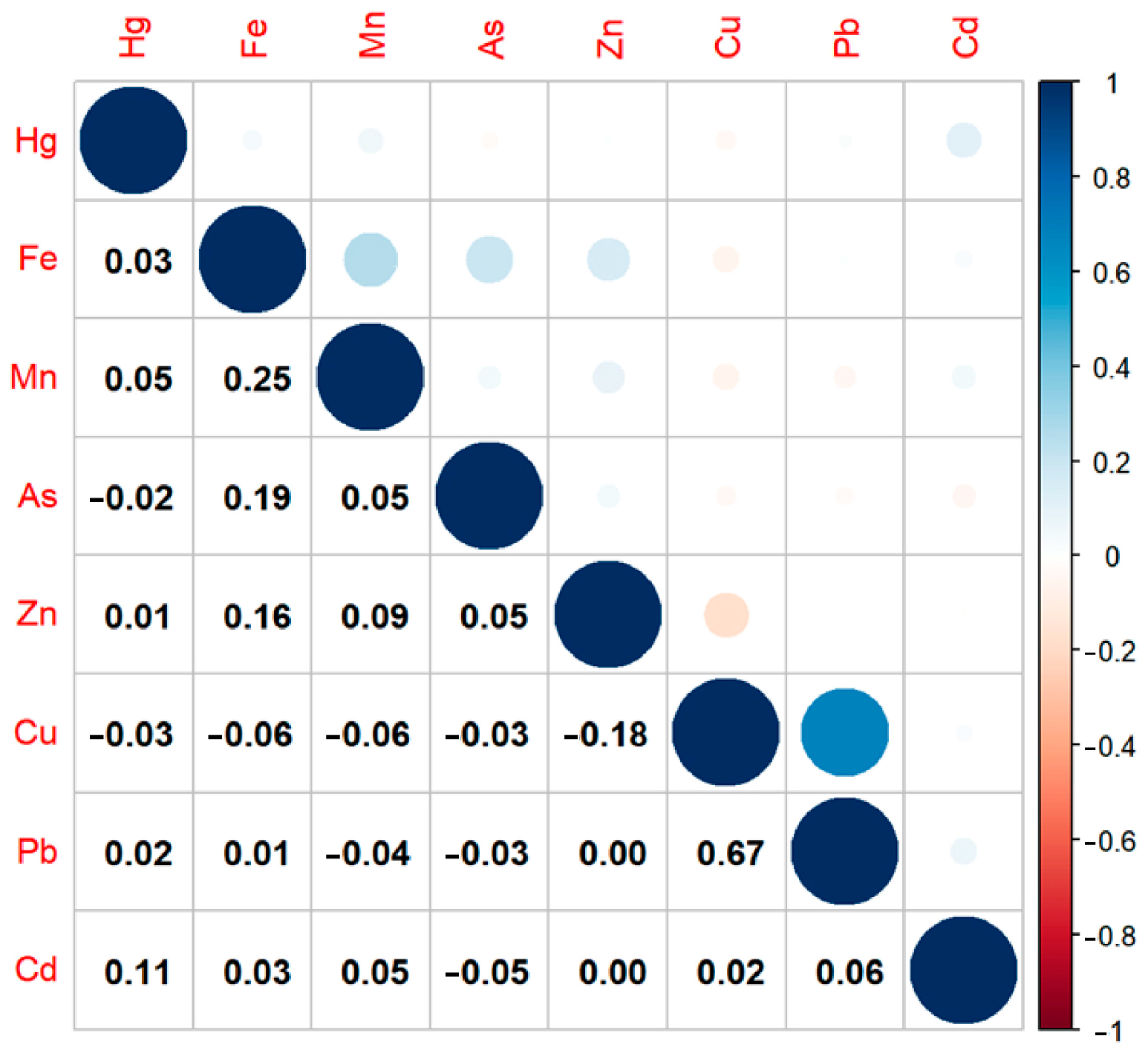
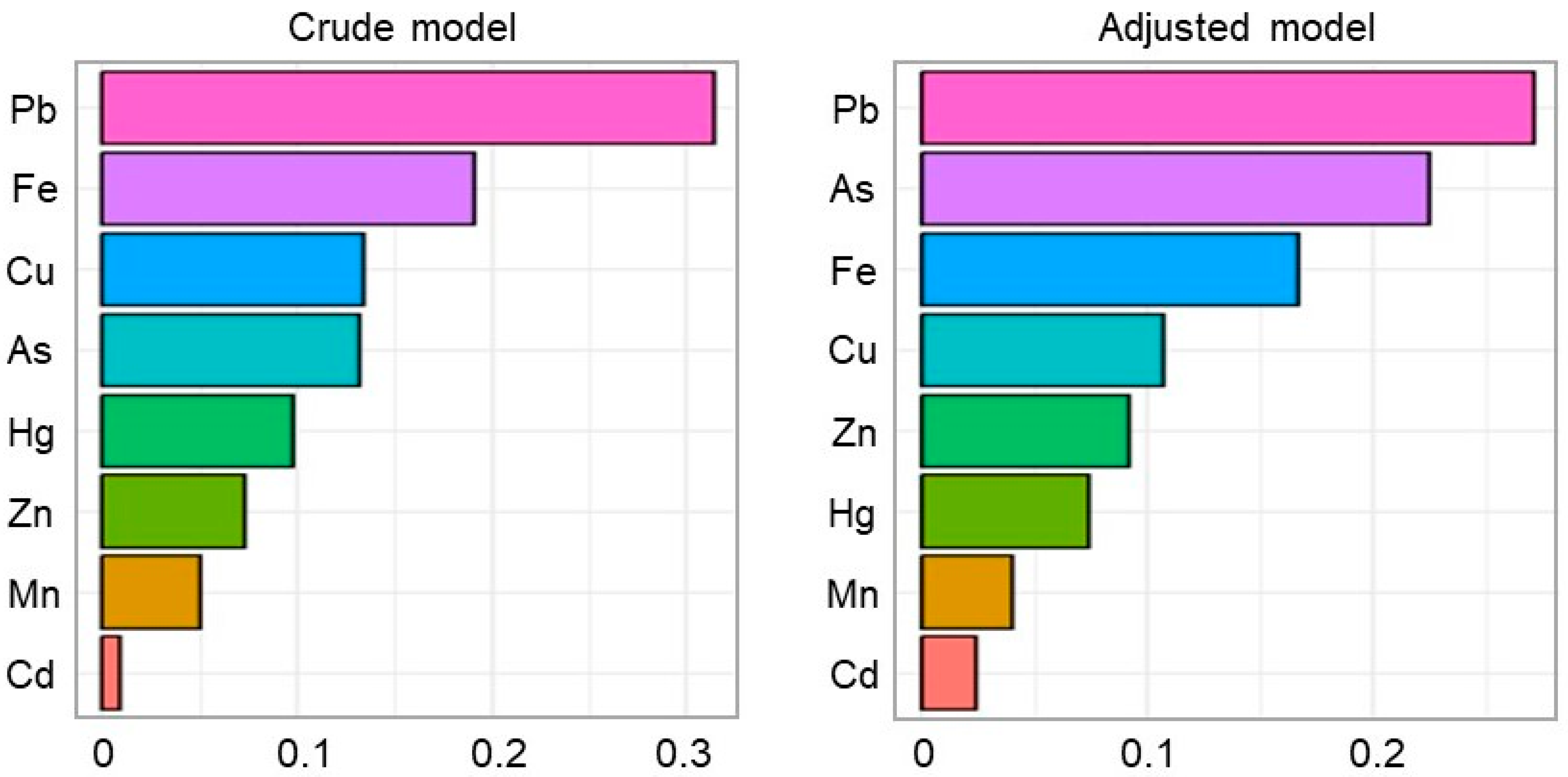
3.4. Mixed Effects of Heavy Metal Co-Exposure in Maternal Hair on Pre-Eclampsia Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Muglia, L.J.; Benhalima, K.; Tong, S.; Ozanne, S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. 2022, 20, 418. [Google Scholar] [CrossRef]
- Miao, J.; Dou, S.; Shi, T.; Wang, X.; Wei, X.; Yan, L.; Ma, B.; Huang, W.; Zhang, Y.; Li, S.; et al. Young adults’ blood selenium and lung function in Shandong province, China: A prospective cohort study. Innov. Med. 2023, 1, 100013. [Google Scholar] [CrossRef]
- Kahn, L.G.; Trasande, L. Environmental toxicant exposure and hypertensive disorders of pregnancy: Recent findings. Curr. Hypertens. Rep. 2018, 20, 87. [Google Scholar] [CrossRef]
- Poropat, A.E.; Laidlaw, M.A.S.; Lanphear, B.; Ball, A.; Mielke, H.W. Blood lead and preeclampsia: A meta-analysis and review of implications. Environ. Res. 2018, 160, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Borghese, M.M.; Fisher, M.; Ashley-Martin, J.; Fraser, W.D.; Trottier, H.; Lanphear, B.; Johnson, M.; Helewa, M.; Foster, W.; Walker, M.; et al. Individual, independent, and joint associations of toxic metals and manganese on hypertensive disorders of pregnancy: Results from the mirec Canadian pregnancy cohort. Environ. Health Perspect. 2023, 131, 47014. [Google Scholar] [CrossRef]
- Maduray, K.; Moodley, J.; Soobramoney, C.; Moodley, R.; Naicker, T. Elemental analysis of serum and hair from pre-eclamptic south African women. J. Trace Elem. Med. Biol. 2017, 43, 180–186. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, T.; Zhang, P.; Chen, X.; Wu, W.; Feng, Y.; Yang, H.; Li, M.; Xie, B.; et al. Exposure to multiple metals and prevalence for preeclampsia in Taiyuan, China. Environ. Int. 2020, 145, 106098. [Google Scholar] [CrossRef]
- El-Badry, A.; Rezk, M.; El-Sayed, H. Mercury-induced oxidative stress may adversely affect pregnancy outcome among dental staff: A cohort study. Int. J. Occup. Environ. Med. 2018, 9, 113–119. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Guallar, E.; Wang, G.; Hong, X.; Wang, X.; Mueller, N.T. Trace minerals, heavy metals, and preeclampsia: Findings from the Boston birth cohort. J. Am. Hear. Assoc. 2019, 8, e012436. [Google Scholar] [CrossRef]
- Rabinowitz, M.; Bellinger, D.; Leviton, A.; Needleman, H.; Schoenbaum, S. Pregnancy hypertension, blood pressure during labor, and blood lead levels. Hypertension 1987, 10, 447–451. [Google Scholar] [CrossRef]
- Sandoval-Carrillo, A.; Méndez-Hernández, E.M.; Antuna-Salcido, E.I.; Salas-Pacheco, S.M.; Vázquez-Alaniz, F.; Téllez-Valencia, A.; Aguilar-Durán, M.; Barraza-Salas, M.; Castellanos-Juárez, F.X.; La Llave-León, O.; et al. Arsenic exposure and risk of preeclampsia in a Mexican mestizo population. BMC Pregnancy Childbirth 2016, 16, 153. [Google Scholar] [CrossRef]
- Sowers, M.; Jannausch, M.; Scholl, T.; Li, W.; Kemp, F.W.; Bogden, J.D. Blood lead concentrations and pregnancy outcomes. Arch. Environ. Health 2002, 57, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sajdak, S.; Marciniak, W.; Lubiński, J. First trimester serum copper or zinc levels, and risk of pregnancy-induced hypertension. Nutrients 2019, 11, 2479. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Rahman, M.L.; Wang, X.; Hinkle, S.N.; Zhang, C.; Mueller, N.T. Exposure to heavy metals and trace minerals in first trimester and maternal blood pressure change over gestation. Environ. Int. 2021, 153, 106508. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, S.G.; Norwitz, E.R. The impact of iron overload and ferroptosis on reproductive disorders in humans: Implications for preeclampsia. Int. J. Mol. Sci. 2019, 20, 3283. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Yeung, E.; Sundaram, R.; Laughon, S.K.; Zhang, C. The exposome--exciting opportunities for discoveries in reproductive and perinatal epidemiology. Paediatr. Perinat. Epidemiol. 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York state. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef]
- Jin, M.; Chen, H.; Na, J.; An, H.; Li, Z.; Li, N. Passive smoking and insomnia in rural Chinese nonsmoking housewives: An environmental and genetic perspective. Environ. Int. 2022, 170, 107569. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhao, J.; Wang, B.; An, H.; Li, Y.; Jia, X.; Wang, J.; Wang, S.; Yan, L.; Liu, X.; et al. Associations between hair levels of trace elements and the risk of preterm birth among pregnant women: A prospective nested case-control study in Beijing birth cohort (bbc), China. Environ. Int. 2022, 158, 106965. [Google Scholar] [CrossRef]
- Li, Q.G.; Liu, G.H.; Qi, L.; Wang, H.C.; Ye, Z.F.; Zhao, Q.L. Heavy metal-contained wastewater in China: Discharge, management and treatment. Sci. Total Environ. 2022, 808, 152091. [Google Scholar] [CrossRef] [PubMed]
- Shahab, A.; Hui, Z.; Rad, S.; Xiao, H.; Siddique, J.; Huang, L.L.; Ullah, H.; Rashid, A.; Taha, M.R.; Zada, N. A comprehensive review on pollution status and associated health risk assessment of human exposure to selected heavy metals in road dust across different cities of the world. Environ. Geochem. Health 2023, 45, 585–606. [Google Scholar] [CrossRef]
- Manduca, P.; Diab, S.Y.; Qouta, S.R.; Albarqouni, N.M.; Punamaki, R.L. A cross sectional study of the relationship between the exposure of pregnant women to military attacks in 2014 in Gaza and the load of heavy metal contaminants in the hair of mothers and newborns. BMJ Open 2017, 7, e014035. [Google Scholar] [CrossRef]
- Muniroh, M.; Bakri, S.; Gumay, A.R.; Dewantiningrum, J.; Mulyono, M.; Hardian, H.; Yamamoto, M.; Koriyama, C. The first exposure assessment of mercury levels in hair among pregnant women and its effects on birth weight and length in semarang, central java, indonesia. Int. J. Environ. Res. Public Health 2022, 19, 10684. [Google Scholar] [CrossRef] [PubMed]
- Trdin, A.; Snoj Tratnik, J.; Stajnko, A.; Marc, J.; Mazej, D.; Sešek Briški, A.; Kastelec, D.; Prpić, I.; Petrović, O.; Špirić, Z.; et al. Trace elements and apoe polymorphisms in pregnant women and their new-Borns. Environ. Int. 2020, 143, 105626. [Google Scholar] [CrossRef] [PubMed]
- Palir, N.; Stajnko, A.; Snoj Tratnik, J.; Mazej, D.; Briški, A.S.; France-Štiglic, A.; Rosolen, V.; Mariuz, M.; Giordani, E.; Barbone, F.; et al. Alad and apoe polymorphisms are associated with lead and mercury levels in Italian pregnant women and their newborns with adequate nutritional status of zinc and selenium. Environ. Res. 2023, 220, 115226. [Google Scholar] [CrossRef]
- Black, A.P.; Knight, R.; Batty, J.; Haswell, S.J.; Lindow, S.W. An analysis of maternal and fetal hair lead levels. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 1295–1297. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, Y.; Zhao, F.; Lv, Y.; Huang, D.; Wei, J.; Ruan, C.; Huang, M.; Deng, J.; Huang, D.; et al. The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: A case control study in Chinese women. Medicine 2017, 96, e9388. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Pang, Y.; Huo, W.; Li, N.; Li, Z.; Zhang, J.; Ye, R.; Wang, B. Association between chronic exposure to tobacco smoke and accumulation of toxic metals in hair among pregnant women. Biol. Trace Elem. Res. 2018, 185, 302–310. [Google Scholar] [CrossRef]
- Kippler, M.; Gyllenhammar, I.; Glynn, A.; Levi, M.; Lignell, S.; Berglund, M. Total mercury in hair as biomarker for methylmercury exposure among women in central sweden—A 23 year long temporal trend study. Environ. Pollut. 2021, 268, 115712. [Google Scholar] [CrossRef]
- Kocyłowski, R.; Lewicka, I.; Grzesiak, M.; Gaj, Z.; Sobańska, A.; Poznaniak, J.; von Kaisenberg, C.; Suliburska, J. Assessment of dietary intake and mineral status in pregnant women. Arch. Gynecol. Obstet. 2018, 297, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Ripley, S.; Robinson, E.; Johnson-Down, L.; Andermann, A.; Ayotte, P.; Lucas, M.; Nieboer, E. Blood and hair mercury concentrations among Cree first nations of Eeyou Istchee (Quebec, Canada): Time trends, prenatal exposure and links to local fish consumption. Int. J. Circumpolar Health 2018, 77, 1474706. [Google Scholar] [CrossRef]
- Sikorski, R.; Juszkiewicz, T.; Paszkowski, T.; Radomański, T.; Szkoda, J.; Milart, P. Hair trace metal concentration of pregnant women at term in comparison with blood and milk levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 1986, 23, 349–357. [Google Scholar] [CrossRef]
- Dawson, E.B.; Evans, D.R.; Nosovitch, J. Third-trimester amniotic fluid metal levels associated with preeclampsia. Arch. Environ. Health 1999, 54, 412–415. [Google Scholar] [CrossRef]
- Ikechukwu, I.C.; Ojareva, O.I.; Ibhagbemien, A.J.; Okhoaretor, O.F.; Oluwatomi, O.B.; Akhalufo, O.S.; Oluwagbenga, A.T.; Chigaekwu, M.N. Blood lead, calcium, and phosphorus in women with preeclampsia in Edo state, Nigeria. Arch. Environ. Occup. Health 2012, 67, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Sakai, T.; Morita, Y.; Kitamura, F.; Sato, H.; Kobayashi, Y. Lead and other trace metals in preeclampsia: A case-control study in Tehran, Iran. Environ. Res. 2006, 100, 268–275. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pu, Y.; Du, Y.; Liu, H.; Wang, X.; He, S.; Ai, S.; Dang, Y. An exploratory study on the association of multiple metals in serum with preeclampsia. Front. Public Health 2024, 12, 1336188. [Google Scholar] [CrossRef]
- Huang, C.; Lai, C.; Xu, P.; Zeng, G.M.; Huang, D.L.; Zhang, J.C.; Zhang, C.; Cheng, M.; Wan, J.; Wang, R.Z. Lead-induced oxidative stress and antioxidant response provide insight into the tolerance of Phanerochaete chrysosporium to lead exposure. Chemosphere 2017, 187, 70–77. [Google Scholar] [CrossRef]
- Laine, J.E.; Ray, P.; Bodnar, W.; Cable, P.H.; Boggess, K.; Offenbacher, S.; Fry, R.C. Placental cadmium levels are associated with increased preeclampsia risk. PLoS ONE 2015, 10, e0139341. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Jaleel, A.; Kadri, H.M.; Saeed, W.A.; Tamimi, W. Iron status parameters in preeclamptic women. Arch. Gynecol. Obstet. 2011, 284, 587–591. [Google Scholar] [CrossRef]
- Gul, A.Z.; Atakul, N.; Selek, S.; Atamer, Y.; Sarıkaya, U.; Yıldız, T.; Demirel, M. Maternal serum levels of zinc, copper, and thiols in preeclampsia patients: A case-control study. Biol. Trace Elem. Res. 2022, 200, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Rafeeinia, A.; Tabandeh, A.; Khajeniazi, S.; Marjani, A.J. Serum copper, zinc and lipid peroxidation in pregnant women with preeclampsia in Gorgan. Open Biochem. J. 2014, 8, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pu, Y.; Liu, H.; Cao, A.; Du, Y.; He, S.; Ai, S.; Dang, Y. A study on the mediating role of serum hormones in the effects of heavy metals on preeclampsia. Environ. Pollut. 2024, 360, 124721. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Ahmed, S.; Ullah, M.S.; Kabir, H.; Rahman, G.K.; Hasnat, A.; Islam, M.S. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013, 154, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.K.; Yu, S.; Jeong, Y.J.; Seo, J.; Choi, S.G.; Yoon, B.Y. Source identification of arsenic contamination in agricultural soils surrounding a closed cu smelter, south Korea. Chemosphere 2019, 217, 183–194. [Google Scholar] [CrossRef]
- Mielcarek, K.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Karpińska, E.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Moskwa, J.; Nowakowski, P.; Borawska, M.H.; et al. Comparison of zinc, copper and selenium content in raw, smoked and pickled freshwater fish. Molecules 2020, 25, 3771. [Google Scholar] [CrossRef]
- Stratakis, N.; Conti, D.V.; Borras, E.; Sabido, E.; Roumeliotaki, T.; Papadopoulou, E.; Agier, L.; Basagana, X.; Bustamante, M.; Casas, M.; et al. Association of fish consumption and mercury exposure during pregnancy with metabolic health and inflammatory biomarkers in children. JAMA Netw Open 2020, 3, e201007. [Google Scholar] [CrossRef]
- White, C.M. Lead in mineral or multivitamin-multimineral products. Ann. Pharmacother. 2022, 56, 339–345. [Google Scholar] [CrossRef]
- Golding, J.; Steer, C.D.; Gregory, S.; Lowery, T.; Hibbeln, J.R.; Taylor, C.M. Dental associations with blood mercury in pregnant women. Community Dent. Oral Epidemiol. 2016, 44, 216–222. [Google Scholar] [CrossRef]
- Menezes-Filho, J.A.; Carvalho, C.F.; Rodrigues, J.L.G.; Araújo, C.F.S.; Dos Santos, N.R.; Lima, C.S.; Bandeira, M.J.; Marques, B.L.S.; Anjos, A.L.S.; Bah, H.A.F.; et al. Environmental co-exposure to lead and manganese and intellectual deficit in school-aged children. Int. J. Environ. Res. Public Health 2018, 15, 2418. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, H. Joint association of heavy metals and polycyclic aromatic hydrocarbons exposure with depression in adults. Environ. Res. 2024, 242, 117807. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kuang, H.; Li, L.; Wu, M.; Liao, Z.; Zeng, K.; Ye, Y.; Fan, R. What adverse health effects will environmental heavy metal co-exposure bring us: Based on a biological monitoring study of sanitation workers. Environ. Sci. Pollut. Res. Int. 2023, 30, 35769–35780. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xu, X.; Cao, L.; Xiang, Q.; Gao, Q.; Duan, H.; Wang, S.; Zhou, L.; Yang, X. Single and joint exposure of Pb, Cd, Hg, Se, Cu, and Zn were associated with cognitive function of older adults. Sci. Rep. 2024, 14, 28567. [Google Scholar] [CrossRef]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Hibbeln, J.R.; Halden, R.U.; Witter, F.R.; Goldman, L.R. Methyl mercury, but not inorganic mercury, associated with higher blood pressure during pregnancy. Environ. Res. 2017, 154, 247–252. [Google Scholar] [CrossRef]
| Characteristics | Control Group (N = 329) | Case Group (N = 49) | p |
|---|---|---|---|
| Age (years, mean [SD]) | 31.2 [3.78] | 31.8 [3.99] | 0.255 |
| Pre-pregnancy BMI (kg/m2, mean [SD]) | 22.3 [3.40] | 22.2 [3.33] | 0.863 |
| Parity | 0.091 | ||
| Primiparous | 262 (79.6%) | 44 (89.8%) | |
| Multiparous | 67 (20.4%) | 5 (10.2%) | |
| Ethnicity | 0.979 | ||
| Han | 313 (95.1%) | 46 (93.9%) | |
| Minority | 16 (4.9%) | 3 (6.1%) | |
| Education | 0.741 | ||
| Postgraduate | 87 (26.4%) | 13 (26.5%) | |
| Undergraduate | 145 (44.1%) | 24 (49.0%) | |
| Junior college or lower | 97 (29.5%) | 12 (24.5%) | |
| Occupation | 0.675 | ||
| Worker/business/services | 76 (23.1%) | 10 (20.4%) | |
| Professional and technical staff | 107 (32.5%) | 13 (26.5%) | |
| Public official | 74 (22.5%) | 12 (24.5%) | |
| Others | 72 (21.9%) | 14 (28.6%) |
| Elements | LOD | DR | Case (N = 49) | Control (N = 329) | p |
|---|---|---|---|---|---|
| (μg/g) | N (%) | Median (IQR) | Median (IQR) | ||
| As | 0.011 | 112 (29.6) | 0.613 (0.231–0.757) | 0.530 (0.310–0.915) | 0.323 |
| Cd | <0.001 | 378 (100.0) | 0.017 (0.012–0.026) | 0.015 (0.010–0.022) | 0.157 |
| Cu | 0.004 | 378 (100.0) | 11.0 (7.83–14.1) | 9.94 (6.99–13.8) | 0.329 |
| Fe | 0.076 | 378 (100.0) | 22.7 (19.8–28.2) | 21.0 (16.4–27.0) | 0.028 |
| Hg | 0.007 | 378 (100.0) | 0.433 (0.268–0.682) | 0.455 (0.290–0.680) | 0.766 |
| Mn | 0.002 | 378 (100.0) | 0.379 (0.296–0.550) | 0.345 (0.226–0.534) | 0.078 |
| Pb | 0.002 | 378 (100.0) | 0.409 (0.284–0.603) | 0.327 (0.221–0.522) | 0.016 |
| Zn | 0.006 | 378 (100.0) | 256 (204–380) | 238 (183–370) | 0.176 |
| Elements | Case Group | Control Group | Crude OR | Adjusted OR a | |
|---|---|---|---|---|---|
| n (%) | n (%) | (95% CI) | (95% CI) | ||
| As | |||||
| Low | 31 (63.3) | 235 (71.4) | 1 | 1 | |
| High | 18 (36.7) | 94 (28.6) | 1.45 (0.77–2.72) | 1.42 (0.75–2.69) | |
| Cd | |||||
| Low | 23 (46.9) | 166 (50.5) | 1 | 1 | |
| High | 26 (53.1) | 163 (49.5) | 1.15 (0.63–2.10) | 1.28 (0.69–2.38) | |
| Cu | |||||
| Low | 19 (38.8) | 170 (51.7) | 1 | 1 | |
| High | 30 (61.2) | 159 (48.3) | 1.69 (0.91–3.12) | 1.57 (0.83–2.98) | |
| Fe | |||||
| Low | 17 (34.7) | 172 (52.3) | 1 | 1 | |
| High | 32 (65.3) | 157 (47.7) | 2.06 (1.10–3.86) | 2.17 (1.14–4.12) | |
| Hg | |||||
| Low | 26 (53.1) | 163 (49.5) | 1 | 1 | |
| High | 23 (46.9) | 166 (50.5) | 0.87 (0.48–1.58) | 0.81 (0.44–1.51) | |
| Mn | |||||
| Low | 22 (44.9) | 167 (50.8) | 1 | 1 | |
| High | 27 (55.1) | 162 (49.2) | 1.27 (0.69–2.31) | 1.33 (0.72–2.46) | |
| Pb | |||||
| Low | 15 (30.6) | 174 (52.9) | 1 | 1 | |
| High | 34 (69.4) | 155 (47.1) | 2.54 (1.34–4.85) | 2.53 (1.31–4.86) | |
| Zn | |||||
| Low | 20 (40.8) | 169 (51.4) | 1 | 1 | |
| High | 29 (59.2) | 160 (48.6) | 1.53 (0.83–2.82) | 1.57 (0.83–2.97) | |
| Reference | Region | Study Time | Subjects | No. of | Sample Time | Age | Concentrations of Heavy Metals in Hair (µg/g) a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants | (gwk) a | (Years) a | As | Cd | Cu | Fe | Hg | Mn | Pb | Zn | ||||
| The present study | Beijing, China | 2024 | Control | 329 | 1st trimester | 31.2 ± 3.78 | 0.53 (0.31–0.92) | 0.02 (0.01–0.02) | 9.94 (6.99–13.8) | 21.0 (16.4–27.0) | 0.46 (0.29–0.68) | 0.35 (0.23–0.53) | 0.33 (0.22–0.52) | 238 (183–370) |
| PE | 49 | 31.8 ± 3.99 | 0.61 (0.23–0.76) | 0.02 (0.01–0.03) | 11.0 (7.83–14.1) | 22.7 (19.8–28.2) | 0.43 (0.27–0.68) | 0.38 (0.30–0.55) | 0.41 (0.28–0.60) | 256 (204–380) | ||||
| Maduray et al. [7] | South Africa | 2017 c | Control | 23 | / f | 24 ± 5 | 5.47 ± 2.79 | 3.75 ± 0.64 | 78.8 ± 28.2 | 449 ± 78.4 | / | 13.6 ± 1.13 | 58.8 ± 37.0 | 331 ± 29.7 |
| PE | 43 | 25 ± 5 | 7.63 ± 1.32 | 3.96 ± 0.87 | 58.9 ± 17.3 | 614 ± 107 | / | 13.1 ± 0.95 | 72.3 ± 19.8 | 396 ± 48.6 | ||||
| Manduca et al. [23] | Palestine | 2014–2015 | Total | 502 | 1st trimester | 26.9 ± 5.92 | 0.07 (0.01–1.04) | 0.04 (0.00–0.54) | 12.70 (1.90–22,700) | 14.6 (1.53–868) | 0.19 (0.01–2480) | 0.72 (0.04–14.2) | 1.50 (0.07–331) | 284 (34.9–2160) |
| Trdin et al. [25] | Croatia | 2007–2009 | Total | 222 | 3rd trimester | 30.1 ± 4.8 | / | / | / | / | 0.51 | / | / | / |
| Palir et al. [26] | Italy | 2006–2009 | Total | 873 | 2nd and 3rd trimesters | 32.7 | / | / | / | / | 0.77 (0.73–0.81) | / | / | / |
| Muniroh et al. [24] | Indonesia | 2018 | Total | 118 | 2nd trimester | 29.5 (19–39) | / | / | / | / | 0.43 (0.15–8.11) | / | / | / |
| Black et al. [27] | Britain | 1980s | Total | 71 | 37–42 | / | / | / | / | / | / | / | 0.55 (0–12.1) | / |
| Zhao et al. [28] | Guangxi, China | 2012 | Healthy group | 57 | <20 | 26.7 ± 4.73 | / | 0–0.04, 71.4% b | / | / | / | 1–1.90, 91.2% b | 0–0.68, 73.8% b | / |
| Kippler et al. [30] | Sweden | 1996 | Total | 655 | / | 29 ± 4.0 | / | / | / | / | 0.38 (0.17–1.5) 0.25 (0.03–1.1) | / | / | / |
| 2019 | ||||||||||||||
| Kocyłowski et al. [31] | Poland | 2016 | Total | 108 | 17.7 ± 5.3 | 31.4 ± 4.9 | / | / | 17.3 ± 9.9 | / | / | / | / | 179 ± 50.1 |
| Sikorski et al. [33] | Poland | 1986 | Total | 104 | 40 (37–42) | 26 (15–42) | / | / | 6.55 | 16.4 | / | / | 2.14 | 152 |
| Ripley et al. [32] | Canada | 2006–2011 | Pregnant women | 913 | Prenatal | 25.5 | / | / | / | / | 0.84 d (0.77–0.91) | / | / | / |
| Zhu et al. [29] | Northern China | 2018 c | No e | 172 | / | / | / | 0.02 (0.01–0.03) | / | / | 0.13 (0.10–0.17) | / | 0.63 (0.35–1.35) | / |
| Yes e | 84 | / | / | 0.01 (0.01–0.02) | / | / | 0.15 (0.11–0.19) | / | 0.72 (0.36–1.38) | / | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu, T.H.; Ma, G.; Jin, M.; Liu, X.; Ren, M.; Gao, S.; Wang, J.; Ye, R.; Liu, X.; Li, N. Accumulation of Mixed Heavy Metals in Maternal Hair and Risk of Pre-Eclampsia: A Prospective Nested Case–Control Study. Toxics 2025, 13, 575. https://doi.org/10.3390/toxics13070575
Luu TH, Ma G, Jin M, Liu X, Ren M, Gao S, Wang J, Ye R, Liu X, Li N. Accumulation of Mixed Heavy Metals in Maternal Hair and Risk of Pre-Eclampsia: A Prospective Nested Case–Control Study. Toxics. 2025; 13(7):575. https://doi.org/10.3390/toxics13070575
Chicago/Turabian StyleLuu, Thi Ha, Gege Ma, Ming Jin, Xiaojing Liu, Mengyuan Ren, Suhong Gao, Jiamei Wang, Rongwei Ye, Xiaohong Liu, and Nan Li. 2025. "Accumulation of Mixed Heavy Metals in Maternal Hair and Risk of Pre-Eclampsia: A Prospective Nested Case–Control Study" Toxics 13, no. 7: 575. https://doi.org/10.3390/toxics13070575
APA StyleLuu, T. H., Ma, G., Jin, M., Liu, X., Ren, M., Gao, S., Wang, J., Ye, R., Liu, X., & Li, N. (2025). Accumulation of Mixed Heavy Metals in Maternal Hair and Risk of Pre-Eclampsia: A Prospective Nested Case–Control Study. Toxics, 13(7), 575. https://doi.org/10.3390/toxics13070575








