Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Protocol and Data Collection
2.2.1. Anthropometric Measurements
2.2.2. Assessment of Dietary Aluminum Exposure
2.2.3. Assessment of Dietary Antioxidant Capacity
2.2.4. Biological Sample Collection
2.2.5. Aluminum Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BW | Body weight |
| FFQ | Food frequency questionnaire |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| IQR | Interquartile range |
| LOD | Limit of detection |
| M | Median |
| Max | Maximum |
| Min | Minimum |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
References
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the obesity epidemic: After the sudden rise, is the upward trajectory beginning to flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2025).
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Ricart, W.; Crujeiras, A.B.; Mateos, A.; Castells-Nobau, A.; Fernández-Real, J.M. Is obesity the next step in evolution through brain changes? Neurosci. Appl. 2024, 3, 103927. [Google Scholar] [CrossRef]
- Verde, L.; Barrea, L.; Bowman-Busato, J.; Yumuk, V.D.; Colao, A.; Muscogiuri, G. Obesogenic environments as major determinants of a disease: It is time to re-shape our cities. Diabetes Metab. Res. Rev. 2024, 40, e3748. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Chang, L.; Shen, S.; Zhang, Z.; Song, X.; Jiang, Q. Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann. Transl. Med. 2018, 6, 320. [Google Scholar] [CrossRef]
- Hellström, H.O.; Mjöberg, B.; Mallmin, H.; Michaëlsson, K. The aluminum content of bone increases with age, but is not higher in hip fracture cases with and without dementia compared to controls. Osteoporos. Int. 2005, 16, 1982–1988. [Google Scholar] [CrossRef]
- Hellström, H.O.; Michaëlsson, K.; Mallmin, H.; Mjöberg, B. The aluminium content of bone, and mortality risk. Age Ageing 2008, 37, 217–220. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 25, 794–798. [Google Scholar] [CrossRef]
- Bignucolo, A.; Lemire, J.; Auger, C.; Castonguay, Z.; Appanna, V.; Appanna, V.D. The Molecular Connection Between Aluminum Toxicity, Anemia, Inflammation and Obesity: Therapeutic Cues. In Anemia; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Skalnaya, M.G.; Aaseth, J.; Ajsuvakova, O.P.; Aschner, M.; Skalny, A.V. Aluminium levels in hair and urine are associated with overweight and obesity in a non-occupationally exposed population. J. Trace Elem. Med. Biol. 2019, 56, 139–145. [Google Scholar] [CrossRef]
- Knazicka, Z.; Bihari, M.; Janco, I.; Harangozo, L.; Arvay, J.; Kovacik, A.; Massanyi, P.; Galik, B.; Saraiva, J.M.; Habanova, M. Blood concentration of macro-and microelements in women who are overweight/obesity and their associations with serum biochemistry. Life 2024, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- San Martín, S.P.; Bauçà, J.M.; Martinez-Morillo, E. Determination of aluminum concentrations in biological specimens: Application in the clinical laboratory. Adv. Lab. Med. 2022, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Álzate, K.; Acevedo-Correa, D.; Urzola Ortega, J.J.; Fuentes-Berrio, L.; Beltrán-Cotta, L.A. Alzheimer’s disease and its association with dietary aluminum: A review. Tecnura 2023, 27, 91–120. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Scimeca, M.; Mauriello, A. The impact of aluminum exposure on human health. Arch. Toxicol. 2023, 97, 2997–2998. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.; Lemire, J.; Appanna, V. Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: A link to obesity. Cell Physiol. Biochem. 2007, 20, 627–638. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Lemire, J.; Appanna, V.D. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp. Cell Res. 2011, 317, 2231–2238. [Google Scholar] [CrossRef]
- Korchazhkina, O.V.; Ashcroft, A.E.; Croom, J.; Exley, C. Does either the gastrointestinal peptide PYY or the neuropeptide NPY bind aluminium? J. Inorg. Biochem. 2003, 94, 372–380. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V.; Grabeklis, A.R.; Serebryansky, E.P.; Demidov, V.A.; Tinkov, A.A. Hair trace elements in overweight and obese adults in association with metabolic parameters. Biol. Trace Elem. Res. 2018, 186, 12–20. [Google Scholar] [CrossRef]
- Skalny, A.V.; Kopylov, P.Y.; Paoliello, M.M.; Chang, J.-S.; Aschner, M.; Bobrovnitsky, I.P.; Chao, J.C.-J.; Aaseth, J.; Chebotarev, S.N.; Tinkov, A.A. Hair lead, aluminum, and other toxic metals in normal-weight and obese patients with coronary heart disease. Int. J. Environ. Res. Public Health 2021, 18, 8195. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Amiri, R.J.; Pirzadeh, M.; Moghadamnia, A.A. Aluminum poisoning with emphasis on its mechanism and treatment of intoxication. Emerg. Med. Int. 2022, 2022, 1480553. [Google Scholar] [CrossRef]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rakıcıoglu, N.; Acar-Tek, N.; Ayaz, A.; Pekcan, G. Yemek ve Besin Fotoğraf Kataloğu Ölçü ve Miktarlar; Ankara Nobel Tıp Kitabevleri: Ankara, Turkey, 2025. [Google Scholar]
- Merdol, T. Kurum Için Standart Yemek Tarifeleri; Hatipoglu Yayınevi: Ankara, Turkey, 2011. [Google Scholar]
- Bratakos, S.M.; Lazou, A.E.; Bratakos, M.S.; Lazos, E.S. Aluminium in food and daily dietary intake estimate in Greece. Food Addit. Contam. Part B Surveill. 2012, 5, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kolbaum, A.E.; Berg, K.; Mueller, F.; Kappenstein, O.; Lindtner, O. Dietary exposure to elements from the German pilot total diet study (TDS). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Safety of aluminium from dietary intake-scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef]
- López, F.F.; Cabrera, C.; Lorenzo, M.L.; López, M.C. Aluminium levels in spices and aromatic herbs. Sci. Total Environ. 2000, 257, 191–197. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Z.-P.; Yang, D.-J.; Liang, J.; Zhu, J.-H.; Xu, H.-B.; Li, F.-Q.; Li, N. Risk assessment of dietary exposure to aluminium in the Chinese population. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1557–1562. [Google Scholar] [CrossRef]
- Kongta, N.; Judprasong, K.; Chunhabundit, R.; Sirivarasai, J.; Karnpanit, W. Assessment of exposure to aluminum through consumption of noodle products. Foods 2023, 12, 3960. [Google Scholar] [CrossRef]
- Satia, J.A.; Watters, J.L.; Galanko, J.A. Validation of an antioxidant nutrient questionnaire in whites and African Americans. J. Am. Diet. Assoc. 2009, 109, 502–508. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Qiu, F.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The role of iron metabolism in chronic diseases related to obesity. Mol. Med. 2022, 28, 130. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron metabolism in obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- VanDuyn, N.; Settivari, R.; LeVora, J.; Zhou, S.; Unrine, J.; Nass, R. The metal transporter SMF-3/DMT-1 mediates aluminum-induced dopamine neuron degeneration. J. Neurochem. 2013, 124, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cannata, J.B.; Fernández-Menéndez, M.J.; Fernández-Martín, J.L.; Menéndez, R.; Santos, F. Gastrointestinal aluminium absorption: Is it modulated by the iron-absorptive mechanism? Proc. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1985, 21, 105–111. [Google Scholar]
- Zhang, Y.; Ding, R.; Zhang, Y.; Qi, J.; Cao, W.; Deng, L.; Zhou, L.; Ye, Y.; Xue, Y.; Liu, E. Dysfunction of DMT1 and miR-135b in the gut-testis axis in high-fat diet male mice. Genes Nutr. 2024, 19, 1. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additive and Contaminants. Available online: https://www.who.int/docs/default-source/food-safety/food-genetically-modified/9789240695405-eng.pdf (accessed on 1 April 2025).
- European Food Safety Authority. Flavourings; Aids, P. Scientific Opinion on the safety evaluation of the substance, silver zeolite A (silver zinc sodium ammonium alumino silicate), silver content 2–5%, for use in food contact materials. EFSA J. 2011, 9, 1999. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, L.; Huang, H.; Zeng, S.; Qiu, F.; Yu, M.; Li, X.; Wei, S. Dietary exposure to aluminium and health risk assessment in the residents of Shenzhen, China. PLoS ONE 2014, 9, e89715. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, G.; Zheng, W.; Zhang, M. A longitudinal assessment of aluminum contents in foodstuffs and aluminum intake of residents in Tianjin metropolis. Food Sci. Nutr. 2019, 7, 997–1003. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.; Bargellini, A.; Vinceti, M. Aluminum and tin: Food contamination and dietary intake in an Italian population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gürtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M. Aggregated aluminium exposure: Risk assessment for the general population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef]
- Fekete, V.; Vandevijvere, S.; Bolle, F.; Van Loco, J. Estimation of dietary aluminum exposure of the Belgian adult population: Evaluation of contribution of food and kitchenware. Food Chem. Toxicol. 2013, 55, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, M.I.; Gomes, M.T.S. Aluminium migration into beverages: Are dented cans safe? Sci. Total Environ. 2008, 405, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Greger, J. Dietary and other sources of aluminium intake. In Ciba Foundation Symposium 169-Aluminium in Biology and Medicine: Aluminium in Biology and Medicine: Ciba Foundation Symposium 169; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Wang, L.; Su, D.; Wang, Y. Studies on the aluminium content in Chinese foods and the maximum permitted levels of aluminum in wheat flour products. Biomed. Environ. Sci. 1994, 7, 91–99. [Google Scholar] [PubMed]
- Müller, M.; Anke, M.; Illing-Günther, H. Aluminium in foodstuffs. Food Chem. 1998, 61, 419–428. [Google Scholar] [CrossRef]
- Greger, J.; Powers, C.F. Assessment of exposure to parenteral and oral aluminum with and without citrate using a desferrioxamine test in rats. Toxicology 1992, 76, 119–132. [Google Scholar] [CrossRef]
- Cabrera-Vique, C.; Mesías, M. Content and bioaccessibility of aluminium in duplicate diets from Southern Spain. J. Food Sci. 2013, 78, 1307–1312. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 1–269. [Google Scholar] [CrossRef]
- Hardisson, A.; Revert, C.; Gonzales-Weler, D.; Rubio, C. Aluminium exposure through the diet. Food Sci. Nutr. 2017, 3, 19. [Google Scholar] [CrossRef]
- Brizio, P.; Benedetto, A.; Squadrone, S.; Curcio, A.; Pellegrino, M.; Ferrero, M.; Abete, M. Heavy metals and essential elements in Italian cereals. Food Addit. Contam. Part B Surveill. 2016, 9, 261–267. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. 2007. Available online: https://iris.who.int/bitstream/handle/10665/44788/WHO_TRS_966_eng.pdf (accessed on 1 April 2025).
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C.; Cui, L.; Wang, H.; Liu, Y.; Cui, W. Dietary aluminium intake disrupts the overall structure of gut microbiota in Wistar rats. Food Sci. Nutr. 2022, 10, 3574–3584. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut bacteria in obesity: An overview of the metabolic mechanisms and therapeutic perspectives of next-generation probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: A systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.F.; Cabrera, C.; Lorenzo, M.L.; Lopez, M.C. Aluminum content of drinking waters, fruit juices and soft drinks: Contribution on dietary intake. Sci. Total Environ. 2002, 292, 205–213. [Google Scholar] [CrossRef]
- Aonso-Diego, G.; Krotter, A.; García-Pérez, Á. Prevalence of energy drink consumption world-wide: A systematic review and meta-analysis. Addiction 2024, 119, 438–463. [Google Scholar] [CrossRef]
- Yokel, R.A. Aluminum in beverages and foods: A comprehensive compilation of regulations; concentrations in raw, prepared, and stored beverages and foods; and intake. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70175. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
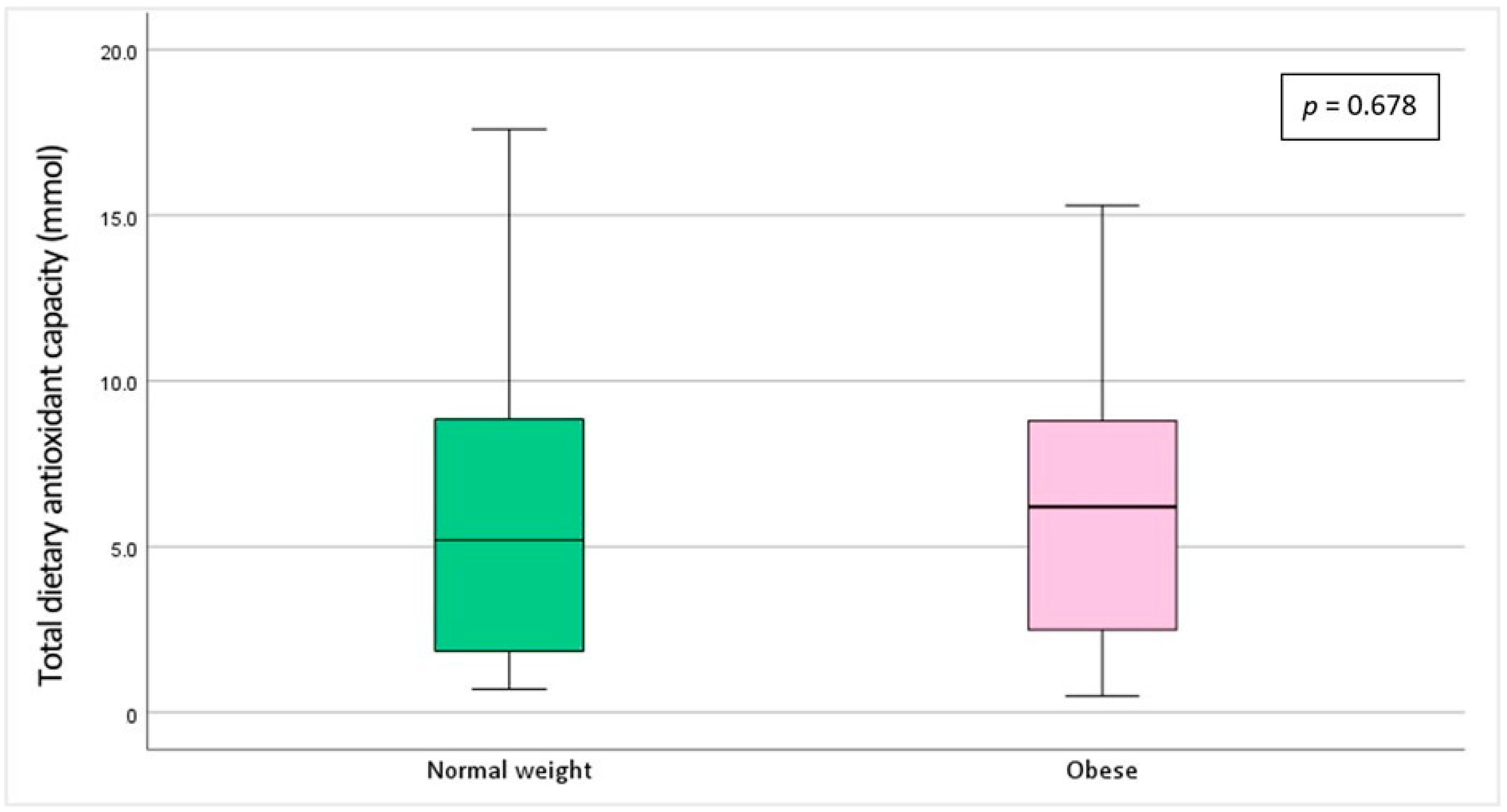
| Variables | Total (n = 54) | Normal Weight (n = 28) | Obese (n = 26) | p |
|---|---|---|---|---|
| Age (years), M (IQR) | 41.0 (25.75) | 31.5 (27.75) | 45.0 (13.62) | 0.004 |
| Education level, n (%) | ||||
| Primary school | 5 (9.3) | 0 (0) | 5 (9.3) | 0.840 |
| High school | 9 (16.7) | 4 (14.3) | 9 (16.7) | |
| University | 34 (63.0) | 20 (71.4) | 34 (63.0) | |
| Post-graduate | 6 (11.0) | 4 (14.3) | 6 (11.0) | |
| Marital status, n (%) | ||||
| Married | 30 (55.6) | 10 (35.7) | 20 (76.9) | 0.003 |
| Single | 24 (44.4) | 18 (64.3) | 6 (23.1) | |
| Income level, n (%) | ||||
| Low | 6 (11.1) | 2 (7.1) | 4 (15.4) | 0.613 |
| Medium | 33 (61.1) | 17 (60.7) | 16 (61.5) | |
| High | 15 (27.8) | 9 (32.2) | 6 (23.1) | |
| Disease status, n (%) | ||||
| With chronic disease | 18 (33.3) | 7 (25.0) | 11 (42.3) | 0.145 |
| Without chronic disease | 36 (66.7) | 21 (75.0) | 15 (57.7) | |
| Body weight (kg), mean ± SD | 74.3 ± 15.97 | 61.8 ± 7.77 | 87.8 ± 10.51 | <0.001 |
| Body mass index (kg/m2), mean ± SD | 27.8 ± 6.17 | 22.6 ± 1.94 | 33.3 ± 3.76 | <0.001 |
| Variables | Normal Weight (n = 28) | Obese (n = 26) | p | ||
|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||
| Serum aluminum (µg/L) | 127.7 ± 102.42 | 10.9–346.4 | 122.9 ± 88.37 | 9.9–299.6 | 0.449 |
| Urinary aluminum (µg/L) | 28.1 ± 12.73 | <LOD-367.1 | 14.1 ± 10.77 | 1.98–41.6 | 0.165 |
| Dietary aluminum exposure (mg/kg bw/week) | 0.61 ± 0.45 | 0.16–1.65 | 0.45 ± 0.24 | 0.04–0.95 | 0.563 |
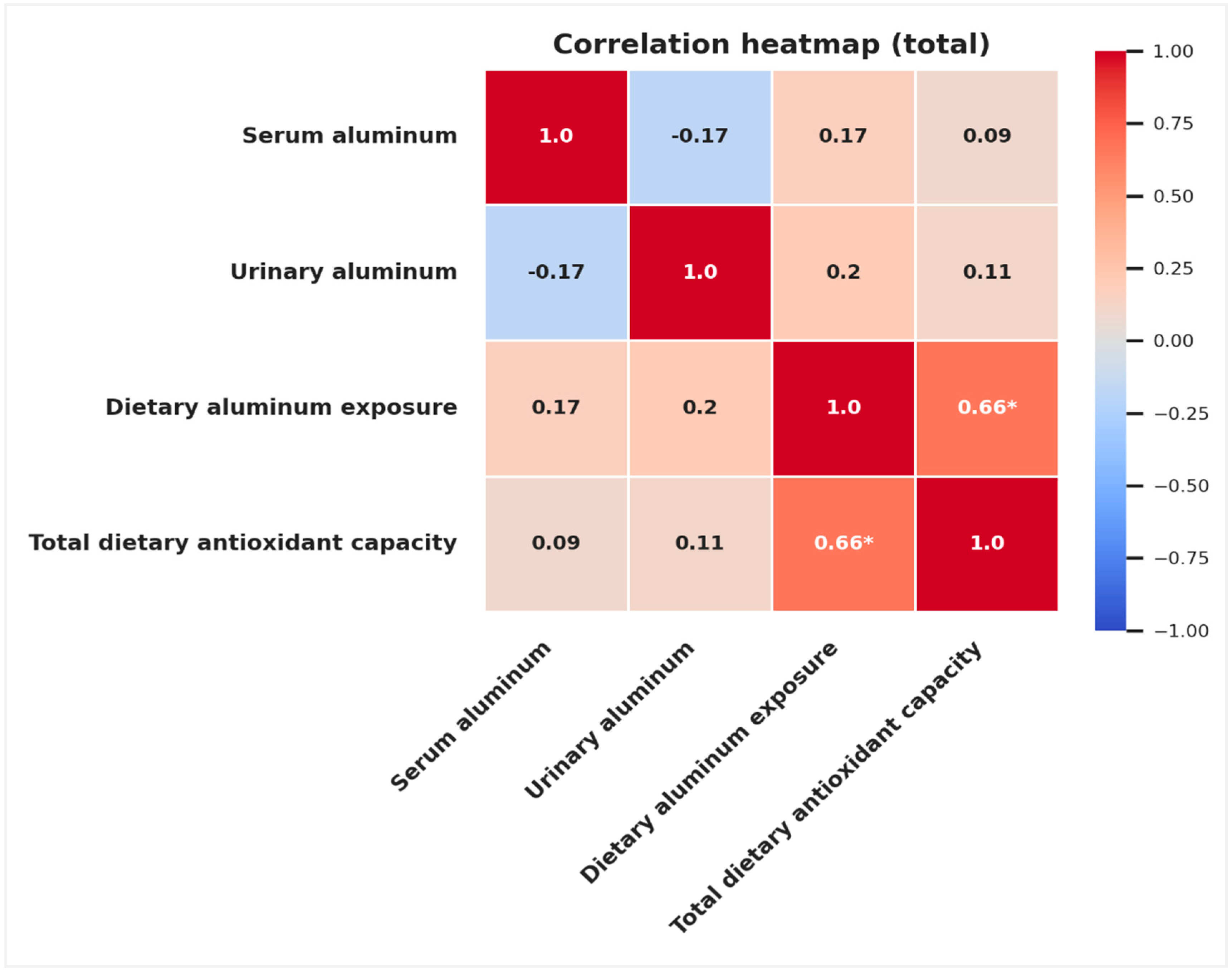
| Total Dietary Antioxidant Capacity | ||||||
|---|---|---|---|---|---|---|
| Total (n = 54) | Normal Weight (n = 28) | Obese (n = 26) | ||||
| r | p | r | p | r | p | |
| Serum aluminum (µg/L) | 0.086 | 0.539 | 0.222 | 0.256 | −0.101 | 0.624 |
| Urinary aluminum (µg/L) | 0.114 | 0.413 | 0.130 | 0.509 | 0.210 | 0.303 |
| Dietary aluminum exposure (mg/kg bw/week) | 0.665 | <0.001 | 0.798 | <0.001 | 0.500 | 0.009 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | se | p | β | se | p | β | se | p | β | se | p | β | se | p | |
| Age | 0.49 | 0.048 | <0.001 | 0.473 | 0.049 | <0.001 | 0.514 | 0.048 | <0.001 | 0.511 | 0.049 | <0.001 | 0.504 | 0.053 | <0.001 |
| Education level | −0.190 | 0.981 | 0.130 | −0.170 | 0.995 | 0.181 | −0.154 | 0.958 | 0.208 | −0.151 | 0.990 | 0.232 | −0.147 | 1.016 | 0.255 |
| Income level | −0.096 | 1.218 | 0.426 | −0.107 | 1.228 | 0.382 | −0.070 | 1.192 | 0.556 | −0.070 | 1.208 | 0.556 | −0.073 | 1.234 | 0.549 |
| Serum aluminum | −0.011 | 0.008 | 0.925 | 0.045 | 0.008 | 0.707 | 0.045 | 0.008 | 0.708 | 0.046 | 0.008 | 0.704 | |||
| Urinary aluminum | −0.152 | 0.015 | 0.220 | −0.091 | 0.015 | 0.455 | −0.090 | 0.015 | 0.463 | −0.091 | 0.015 | 0.465 | |||
| Dietary aluminum exposure | −0.271 | 2.018 | 0.031 | −0.284 | 2.704 | 0.089 | −0.242 | 5.146 | 0.440 | ||||||
| Total dietary antioxidant capacity | 0.019 | 0.213 | 0.906 | 0.054 | 0.356 | 0.845 | |||||||||
| Interaction: Dietary Aluminum × Antioxidant Capacity | −0.070 | 0.485 | 0.875 | ||||||||||||
| Adjusted R2 | 0.264 | 0.258 | 0.314 | 0.299 | 0.284 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yesildemir, O.; Filiz Ozsoz, C.; Celik, M.N.; Guclu, O.A.; Ozgur, A.; Ağagündüz, D.; Budán, F. Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index. Toxics 2025, 13, 578. https://doi.org/10.3390/toxics13070578
Yesildemir O, Filiz Ozsoz C, Celik MN, Guclu OA, Ozgur A, Ağagündüz D, Budán F. Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index. Toxics. 2025; 13(7):578. https://doi.org/10.3390/toxics13070578
Chicago/Turabian StyleYesildemir, Ozge, Ceren Filiz Ozsoz, Mensure Nur Celik, Ozge Aydin Guclu, Anil Ozgur, Duygu Ağagündüz, and Ferenc Budán. 2025. "Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index" Toxics 13, no. 7: 578. https://doi.org/10.3390/toxics13070578
APA StyleYesildemir, O., Filiz Ozsoz, C., Celik, M. N., Guclu, O. A., Ozgur, A., Ağagündüz, D., & Budán, F. (2025). Dietary Aluminum Exposure Is More Closely Linked to Antioxidant Dynamics than to Body Mass Index. Toxics, 13(7), 578. https://doi.org/10.3390/toxics13070578









