Exposure to Metal Mixtures and Metabolic Syndrome in Residents Living near an Abandoned Lead–Zinc Mine: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analysis of Urinary Metals
2.3. Biochemical Measurements
2.4. Definition of MetS
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Urinary Concentrations of Metals
3.3. Single-Metal Logistic Regression Analyses
3.4. Selecting Important Metals Associated with MetS and Multiple Metals Logistic Regression Analyses
3.5. Bayesian Kernel Machine Regression Models
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Luo, C.L.; Gao, Y.; Li, F.B.; Lin, L.W.; Wu, C.A.; Li, X.D. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ. Pollut. 2010, 158, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zou, B.; Li, N.Y.; Li, Z.A. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 2009, 31, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, B.T.; Kim, J.Y.; Kim, K.W.; Lee, J.S. Human risk assessment for heavy metals and as contamination in the abandoned metal mine areas, Korea. Environ. Monit. Assess. 2006, 119, 233–244. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.W.; Ahn, J.S.; Ko, I.; Lee, C.H. Investigation and risk assessment modeling of As and other heavy metals contamination around five abandoned metal mines in Korea. Environ. Geochem. Health 2005, 27, 193–203. [Google Scholar] [CrossRef]
- Coelho, P.; Silva, S.; Roma-Torres, J.; Costa, C.; Henriques, A.; Teixeira, J.; Gomes, M.; Mayan, O. Health impact of living near an abandoned mine—Case study: Jales mines. Int. J. Hyg. Environ. Health 2007, 210, 399–402. [Google Scholar] [CrossRef]
- Ford, E.S. The metabolic syndrome and mortality from cardiovascular disease and all-causes: Findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004, 173, 309–314. [Google Scholar] [CrossRef]
- Ford, E.S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care 2005, 28, 1769–1778. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- He, Y.N.; Zhao, W.H.; Zhao, L.Y.; Yu, D.M.; Zhang, J.; Yang, X.G.; Ding, G.G. [Prevalence of metabolic syndrome in Chinese adults in 2010–2012]. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 212–215. [Google Scholar] [PubMed]
- Liu, L.; Li, X.; Wu, M.; Yu, M.; Wang, L.; Hu, L.; Li, Y.; Song, L.; Wang, Y.; Mei, S. Individual and joint effects of metal exposure on metabolic syndrome among Chinese adults. Chemosphere 2022, 287 Pt 3, 132295. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Wang, D.; Guo, Y.; Wang, B.; Xu, Y.; Chen, W. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020, 140, 105802. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef]
- Chung, J.Y.; Seo, M.S.; Shim, J.Y.; Lee, Y.J. Sex differences in the relationship between blood mercury concentration and metabolic syndrome risk. J. Endocrinol. Investig. 2015, 38, 65–71. [Google Scholar] [CrossRef]
- Gambelunghe, A.; Sallsten, G.; Borné, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engström, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef]
- Noor, N.; Zong, G.; Seely, E.W.; Weisskopf, M.; James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ. Int. 2018, 121 Pt 1, 349–356. [Google Scholar] [CrossRef]
- Seo, J.W.; Hong, Y.S. Comparative Evaluation of Heavy Metal Concentrations in Residents of Abandoned Metal Mines. Int. J. Environ. Res. Public Health 2020, 17, 6280. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arbuckle, T.E.; Janssen, P.; Lanphear, B.P.; Zhuang, L.H.; Braun, J.M.; Chen, A.; McCandless, L.C. Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: A Bayesian analysis using kernel machine regression. Environ. Res. 2021, 195, 110749. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, N.M.; Keil, A.P.; O’Brien, K.M.; Jackson, B.P.; Karagas, M.R.; Weinberg, C.R.; White, A.J. Metals and trace elements in relation to body mass index in a prospective study of US women. Environ. Res. 2020, 184, 109396. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ge, S.; Liu, J.; Iqbal, Y.; Jiang, Y.; Sun, R.; Ruan, X.; Wang, Y. Spatial Distribution and Risk Assessment of Heavy Metal(oid)s Contamination in Topsoil around a Lead and Zinc Smelter in Henan Province, Central China. Toxics 2023, 11, 427. [Google Scholar] [CrossRef]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef]
- Metabolic Syndrome Research Collaboration Group. Recommendations on metabolic syndrome from China Diabetes Federation. Chin. J. Diabetes 2004, 12, 156–161. [Google Scholar]
- Fan, M.; Lyu, J.; He, P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 961–964. [Google Scholar]
- Feng, W.; He, X.S.; Yu, Y.Q.; Li-Gu, Y.; Zhang, X.M.; Yuan, J.; Chen, W.H.; Wu, T.C. Association between 23 urinary metals and mean platelet volume among a community-dwelling population in Wuhan, China. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 689–697. [Google Scholar]
- Heitland, P.; Köster, H.D. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin. Chim. Acta 2006, 365, 310–318. [Google Scholar] [CrossRef]
- Osada, M.; Izuno, T.; Kobayashi, M.; Sugita, M. Relationship between environmental exposure to cadmium and bone metabolism in a non-polluted area of Japan. Environ. Health Prev. Med. 2011, 16, 341–349. [Google Scholar] [CrossRef]
- Yang, J.; Kim, E.C.; Shin, D.C.; Jo, S.J.; Lim, Y.W. Human exposure and risk assessment of cadmium for residents of abandoned metal mine areas in Korea. Environ. Geochem. Health 2015, 37, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Association Between Metal Levels and Overweight/Obesity of Zhuang Nationality Residents Aged 35~74 in Liangqing District, Nanning City. Master’s Thesis, Guangxi Medical University, Guangxi, China, 2021. [Google Scholar]
- Ding, C.; Pan, Y.; Zhang, A.; Wu, B.; Huang, H.; Zhu, C.; Liu, D.; Zhu, B.; Xu, G.; Shao, H.; et al. Study of distribution and influencing factors of lead and cadmium in whole blood and urine among population in 8 provinces in China. Zhonghua Yu Fang Yi Xue Za Zhi 2014, 48, 91–96. [Google Scholar]
- Ding, C.; Pan, Y.; Zhang, A.; Wu, B.; Huang, H.; Zhu, C.; Liu, D.; Zhu, B.; Xu, G.; Shao, H.; et al. Study of distribution and influencing factors of arsenic in whole blood and urine among population in 8 provinces in China. Zhonghua Yu Fang Yi Xue Za Zhi 2014, 48, 97–101. [Google Scholar] [PubMed]
- Pan, X.; Ding, C.; Pan, Y.; Zhang, A.; Wu, B.; Huang, H.; Zhu, C.; Liu, D.; Zhu, B.; Xu, G.; et al. Distribution of copper and zinc level in urine of general population in eight provinces of China. Zhonghua Yu Fang Yi Xue Za Zhi 2015, 49, 919–923. [Google Scholar]
- Ding, C.G.; Pan, Y.J.; Zhang, A.H.; Wu, B.H.; Huang, H.L.; Zhu, C.; Liu, D.Y.; Zhu, B.L.; Xu, G.; Shao, H.; et al. Distribution of chromium in whole blood and urine among general population in China between year 2009 and 2010. Zhonghua Yu Fang Yi Xue Za Zhi 2012, 46, 679–682. [Google Scholar]
- Pan, Y.; Ding, C.; Zhang, A.; Wu, B.; Huang, H.; Zhu, C.; Liu, D.; Zhu, B.; Xu, G.; Shao, H.; et al. Distribution of manganese, cobalt and molybdenum in blood and urine among general population in 8 provinces of China. Zhonghua Yu Fang Yi Xue Za Zhi 2014, 48, 784–790. [Google Scholar]
- Fang, C.; Wu, W.; Gu, X.; Dai, S.; Zhou, Q.; Deng, H.; Shen, F.; Chen, J. Association of serum copper, zinc and selenium levels with risk of metabolic syndrome: A nested case-control study of middle-aged and older Chinese adults. J. Trace Elem. Med. Biol. 2019, 52, 209–215. [Google Scholar] [CrossRef]
- Ahn, B.I.; Kim, M.J.; Koo, H.S.; Seo, N.; Joo, N.S.; Kim, Y.S. Serum zinc concentration is inversely associated with insulin resistance but not related with metabolic syndrome in nondiabetic Korean adults. Biol. Trace Elem. Res. 2014, 160, 169–175. [Google Scholar] [CrossRef]
- Ruz, M.; Carrasco, F.; Rojas, P.; Basfi-Fer, K.; Hernández, M.C.; Pérez, A. Nutritional Effects of Zinc on Metabolic Syndrome and Type 2 Diabetes: Mechanisms and Main Findings in Human Studies. Biol. Trace Elem. Res. 2019, 188, 177–188. [Google Scholar] [CrossRef]
- Dineley, K.E.; Votyakova, T.V.; Reynolds, I.J. Zinc inhibition of cellular energy production: Implications for mitochondria and neurodegeneration. J. Neurochem. 2003, 85, 563–570. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, F.; Liu, Y.; Lu, N. Zinc and its effects on oxidative stress in Alzheimer’s disease. Neurol. Sci. 2014, 35, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium toxicity in humans. Arh. Hig. Rada. Toksikol. 2010, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. Int. J. Environ. Res. Public Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Taguchi, T.; Mori, H.; Thorpe, M.; Yamori, Y.; Mori, M. Possible association of high urinary magnesium and taurine to creatinine ratios with metabolic syndrome risk reduction in Australian aboriginals. Cardiol. Res. Pract. 2011, 2011, 235653. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef]
- Yang, N.; He, L.; Li, Y.; Xu, L.; Ping, F.; Li, W.; Zhang, H. Reduced Insulin Resistance Partly Mediated the Association of High Dietary Magnesium Intake with Less Metabolic Syndrome in a Large Chinese Population. Diabetes Metab. Syndr. Obes. 2020, 13, 2541–2550. [Google Scholar] [CrossRef]
- Lu, L.; Chen, C.; Yang, K.; Zhu, J.; Xun, P.; Shikany, J.M.; He, K. Magnesium intake is inversely associated with risk of obesity in a 30-year prospective follow-up study among American young adults. Eur. J. Nutr. 2020, 59, 3745–3753. [Google Scholar] [CrossRef]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef]
- De Long, N.E.; Holloway, A.C. Early-life chemical exposures and risk of metabolic syndrome. Diabetes Metab. Syndr. Obes. 2017, 10, 101–109. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A. Factors moderating cadmium bioavailability: Key considerations for comparing blood cadmium levels between groups. Food Chem. Toxicol. 2024, 191, 114865. [Google Scholar] [CrossRef]

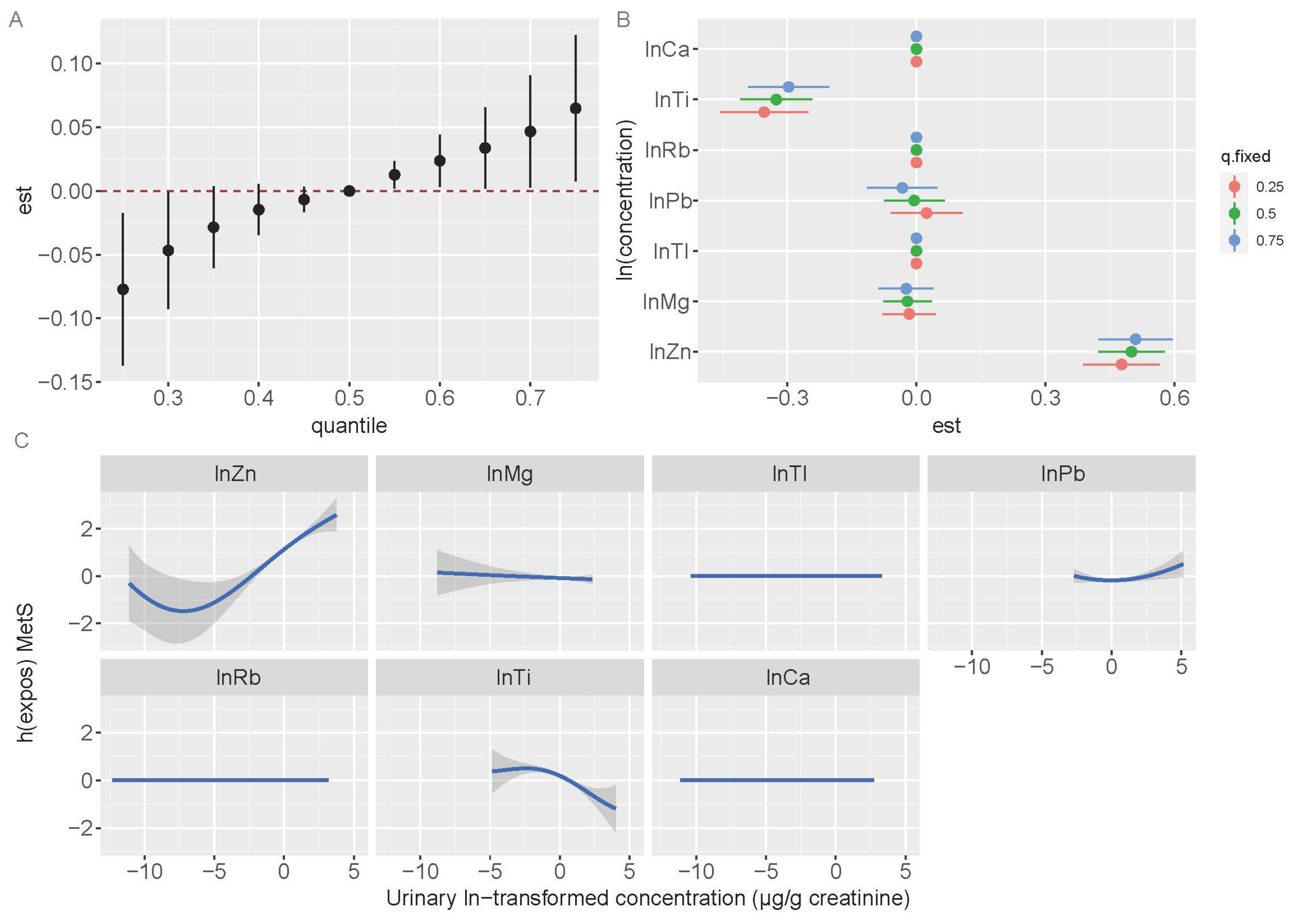
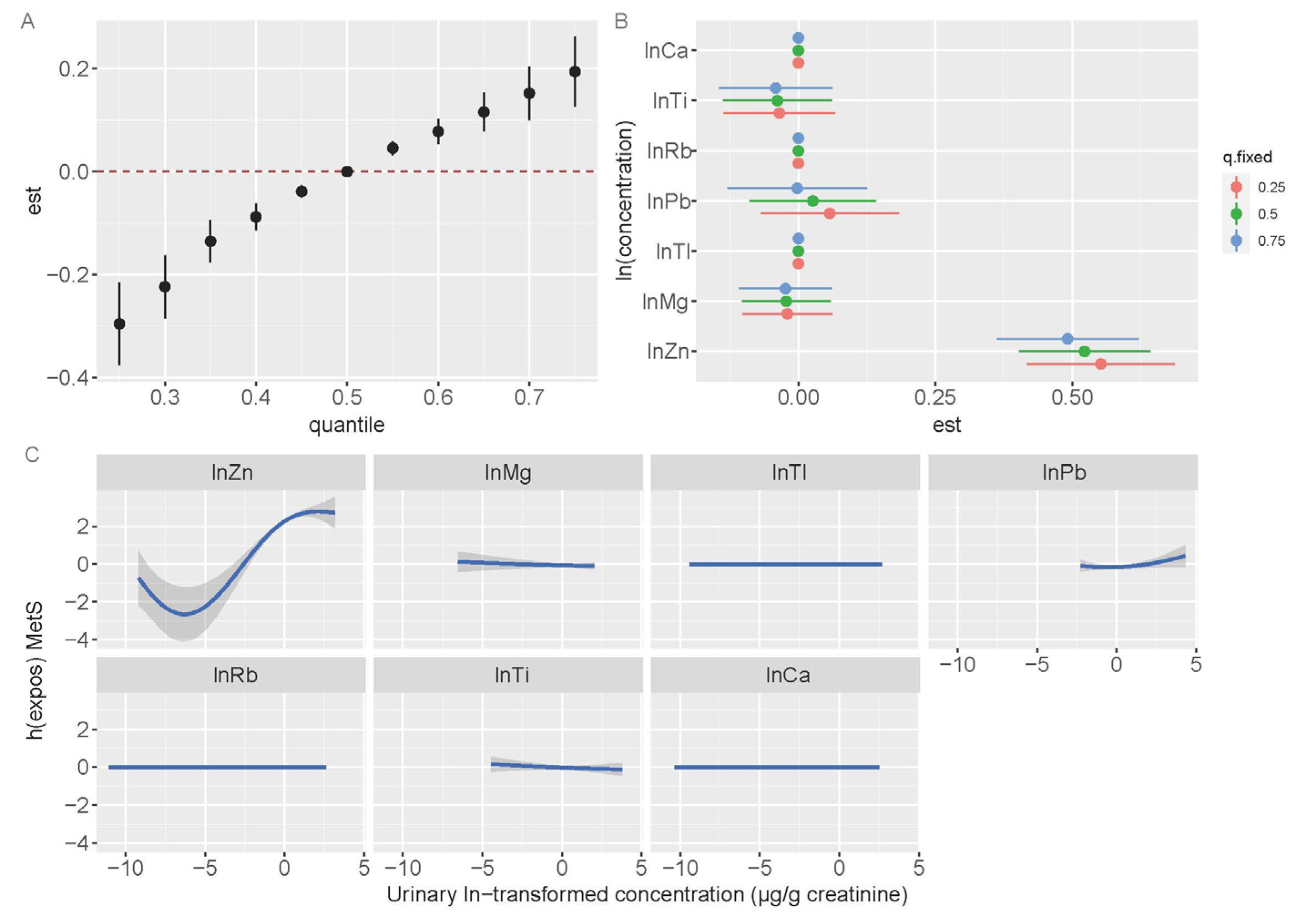
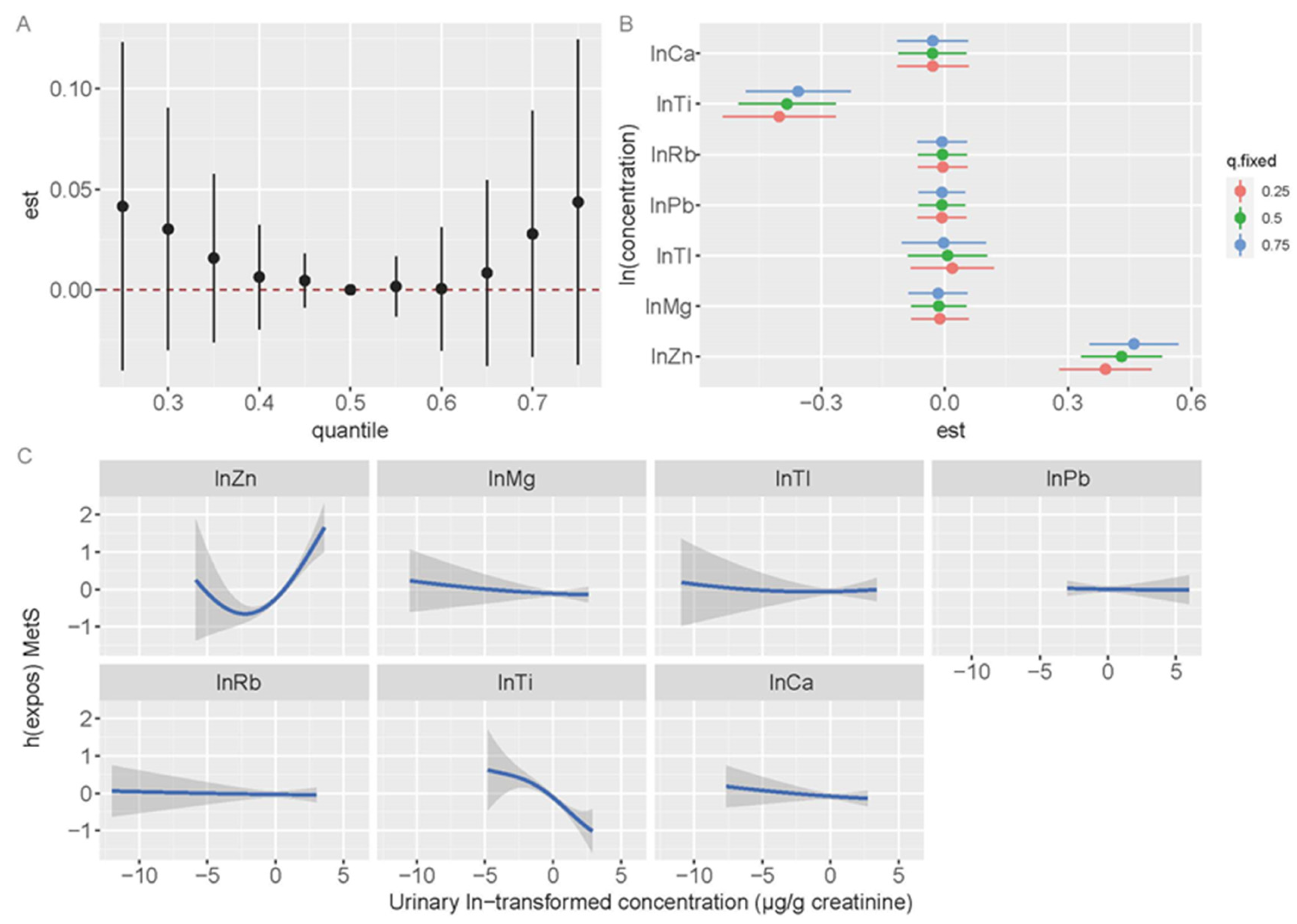
| Variables | Reference Area | Exposed Area | ||
|---|---|---|---|---|
| MetS (n = 170) | No MetS (n = 851) | MetS (n = 99) | No MetS (n = 624) | |
| Age, years | 58.4 ± 8.7 | 55.4 ± 10.7 | 57.2 ± 10.3 | 54.5 ± 12.7 |
| Sex, n | ||||
| Male | 69 (40.6%) | 260 (30.6%) | 40 (40.4%) | 231 (37.0%) |
| Female | 101 (59.4%) | 591 (69.4%) | 59 (59.6%) | 393 (63.0%) |
| Ethnicity, n | ||||
| Han | 115 (67.6%) | 458 (53.8%) | 20 (20.2%) | 126 (20.2%) |
| Zhuang | 17 (10.0%) | 197 (23.1%) | 74 (74.7%) | 480 (76.9%) |
| Others | 38 (22.4%) | 196 (23.0%) | 5 (5.1%) | 18 (2.9%) |
| Education, n | ||||
| <high school | 158 (92.9%) | 771 (90.6%) | 89 (89.9%) | 512 (82.1%) |
| ≥high school | 12 (7.1%) | 80 (9.4%) | 10 (10.1%) | 112 (17.9%) |
| Smoking status, n | ||||
| Never | 126 (74.1%) | 653 (76.7%) | 73 (73.7%) | 474 (76.0%) |
| Former | 26 (15.3%) | 70 (8.2%) | 7 (7.1%) | 58 (9.3%) |
| Current | 18 (10.6%) | 128 (15.0%) | 19 (19.2%) | 92 (14.7%) |
| Drinking status, n | ||||
| Never | 74 (43.5%) | 385 (45.2%) | 45 (45.5%) | 335 (53.7%) |
| Former | 31 (18.2%) | 130 (15.3%) | 14 (14.1%) | 65 (10.4%) |
| Current | 65 (38.2%) | 336 (39.5%) | 40 (40.4%) | 224 (35.9%) |
| Physical activity, n | ||||
| Low | 5 (2.9%) | 29 (3.4%) | 2 (2.0%) | 25 (4.0%) |
| Moderate | 36 (21.2%) | 105 (12.3%) | 28 (28.3%) | 82 (13.1%) |
| High | 129 (75.9%) | 717 (84.3%) | 69 (69.7%) | 517 (82.9%) |
| BMI, kg/m2 | 27.1 ± 3.1 | 23.0 ± 3.2 | 26.4 ± 3.1 | 22.3 ± 3.0 |
| DBP, mmHg | 89.5 ± 12.3 | 78.7 ± 12.1 | 87.0 ± 15.2 | 74.9 ± 12.5 |
| SBP, mmHg | 152.7 ± 20.3 | 135.1 ± 20.5 | 154.1 ± 22.3 | 133.2 ± 22.2 |
| TG, mmol/L | 2.25 (1.82, 3.44) | 1.34 (0.97, 1.87) | 2.34 (1.75, 3.32) | 1.08 (0.80, 1.49) |
| TC, mmol/L | 5.61 (4.89, 6.52) | 5.21 (4.60, 5.86) | 5.85 (5.29, 6.34) | 5.08 (4.51, 5.89) |
| HDL-c, mmol/L | 1.25 (1.11, 1.42) | 1.52 (1.30, 1.80) | 1.30 (1.07, 1.57) | 1.44 (1.24, 1.71) |
| LDL-c, mmol/L | 3.02 (2.54, 3.84) | 2.89 (2.35, 3.52) | 3.03 (2.55, 3.53) | 2.74 (2.26, 3.32) |
| FPG, mmol/L | 6.42 (5.63, 7.88) | 5.46 (5.16, 5.79) | 6.38 (5.80, 7.14) | 5.44 (5.07, 5.85) |
| Metals (μg/g Creatinine) | <LOD (%) | Reference (n = 1021) | Exposed (n = 723) | Total (n = 1744) | p-Value |
|---|---|---|---|---|---|
| Mg (mg/g creatinine) | 0.00 | 38.38 (25.15, 53.35) | 37.10 (17.51, 57.37) | 37.68 (23.02, 55.48) | 0.026 |
| Ca (mg/g creatinine) | 0.06 | 85.478 (51.75, 130.81) | 86.07 (51.25, 138.59) | 85.81 (51.53, 134.20) | 0.300 |
| Ti | 0.00 | 22.96 (16.81, 30.82) | 21.61 (15.25, 28.89) | 22.50 (16.27, 30.05) | 0.004 |
| V | 0.00 | 0.24 (0.16, 0.34) | 0.21 (0.15, 0.29) | 0.22 (0.16, 0.32) | <0.001 |
| Cr | 0.17 | 0.26 (0.16, 0.48) | 0.50 (0.28, 1.25) | 0.34 (0.19, 0.74) | <0.001 |
| Mn | 0.00 | 0.25 (0.13, 0.56) | 0.44 (0.21, 0.92) | 0.32 (0.15, 0.72) | <0.001 |
| Fe | 0.00 | 13.86 (9.24, 23.88) | 15.48 (9.20, 28.56) | 14.36 (9.23, 25.59) | 0.036 |
| Co | 0.00 | 0.25 (0.16, 0.51) | 0.29 (0.17, 0.63) | 0.27 (0.16, 0.55) | 0.013 |
| Ni | 0.34 | 1.62 (1.00, 2.70) | 1.56 (0.91, 2.68) | 1.61 (0.97, 2.69) | 0.200 |
| Cu | 0.00 | 12.26 (9.55, 16.22) | 13.50 (10.18, 19.56) | 12.63 (9.77, 17.52) | <0.001 |
| Zn | 0.17 | 272.43 (197.58, 383.70) | 272.04 (180.04, 396.00) | 272.35 (188.94, 388.68) | 0.464 |
| Ga | 0.57 | 0.24 (0.13, 0.46) | 0.27 (0.14, 0.53) | 0.25 (0.14, 0.48) | 0.053 |
| As | 0.00 | 34.31 (24.53, 49.10) | 37.89 (25.09, 54.42) | 35.58 (24.66, 51.29) | 0.004 |
| Se | 0.00 | 23.73 (18.83, 29.89) | 18.21 (13.32, 23.74) | 21.33 (16.63, 27.80) | <0.001 |
| Rb (mg/g creatinine) | 0.00 | 1.75 (1.28, 2.578) | 1.66 (1.17, 2.24) | 1.71 (1.24, 2.42) | <0.001 |
| Sr | 0.06 | 80.89 (52.16, 122.05) | 46.48 (27.96, 72.91) | 65.82 (38.64, 103.21) | <0.001 |
| Mo | 0.00 | 68.87 (45.75, 104.90) | 91.19 (55.14, 150.85) | 76.66 (48.79, 126.29) | <0.001 |
| Cd | 0.00 | 2.34 (1.39, 3.83) | 4.15 (2.12, 8.08) | 2.86 (1.60, 5.22) | <0.001 |
| Sn | 0.11 | 0.48 (0.34, 0.74) | 0.62 (0.42, 0.93) | 0.53 (0.37, 0.84) | <0.001 |
| Sb | 0.11 | 0.09 (0.06, 0.14) | 0.07 (0.05, 0.11) | 0.08 (0.06, 0.13) | <0.001 |
| Cs | 0.00 | 8.85 (6.71, 11.87) | 7.58 (5.59, 9.77) | 8.21 (6.21, 10.85) | <0.001 |
| W | 0.52 | 0.23 (0.11, 0.59) | 0.39 (0.15, 1.25) | 0.28 (0.12, 0.85) | <0.001 |
| Tl | 0.00 | 0.47 (0.33, 0.70) | 0.45 (0.29, 0.67) | 0.46 (0.31, 0.69) | 0.007 |
| Pb | 0.00 | 2.08 (1.35, 3.55) | 3.06 (1.31, 7.40) | 2.34 (1.34, 4.87) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Xu, Q.; Qin, L.; He, T.; Zhang, Y.; Chen, R.; Tao, L.; Chen, T.; Zhong, Q. Exposure to Metal Mixtures and Metabolic Syndrome in Residents Living near an Abandoned Lead–Zinc Mine: A Cross-Sectional Study. Toxics 2025, 13, 565. https://doi.org/10.3390/toxics13070565
Zhao M, Xu Q, Qin L, He T, Zhang Y, Chen R, Tao L, Chen T, Zhong Q. Exposure to Metal Mixtures and Metabolic Syndrome in Residents Living near an Abandoned Lead–Zinc Mine: A Cross-Sectional Study. Toxics. 2025; 13(7):565. https://doi.org/10.3390/toxics13070565
Chicago/Turabian StyleZhao, Min, Qi Xu, Lingqiao Qin, Tufeng He, Yifan Zhang, Runlin Chen, Lijun Tao, Ting Chen, and Qiuan Zhong. 2025. "Exposure to Metal Mixtures and Metabolic Syndrome in Residents Living near an Abandoned Lead–Zinc Mine: A Cross-Sectional Study" Toxics 13, no. 7: 565. https://doi.org/10.3390/toxics13070565
APA StyleZhao, M., Xu, Q., Qin, L., He, T., Zhang, Y., Chen, R., Tao, L., Chen, T., & Zhong, Q. (2025). Exposure to Metal Mixtures and Metabolic Syndrome in Residents Living near an Abandoned Lead–Zinc Mine: A Cross-Sectional Study. Toxics, 13(7), 565. https://doi.org/10.3390/toxics13070565







