Coupled Effects of Polyethylene Microplastics and Cadmium on Soil–Plant Systems: Impact on Soil Properties and Cadmium Uptake in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials Preparation
2.2. Experimental Design and Procedure
2.3. Determination and Analysis of Plant and Soil Indexes
2.3.1. Plant Indexes
2.3.2. Soil Indexes
2.4. Statistical Analysis
3. Results
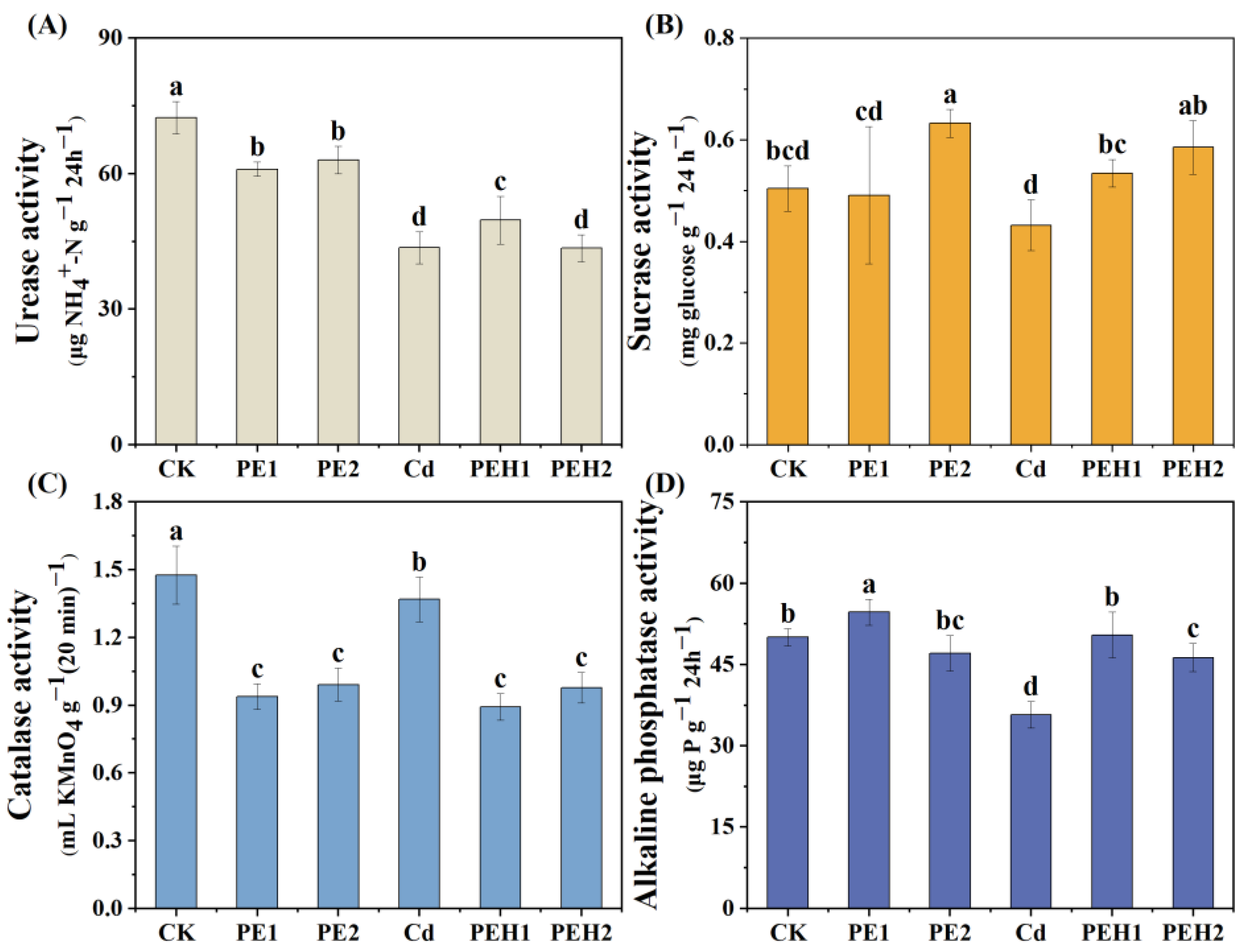
3.1. Soil Physicochemical Properties and Enzyme Activities
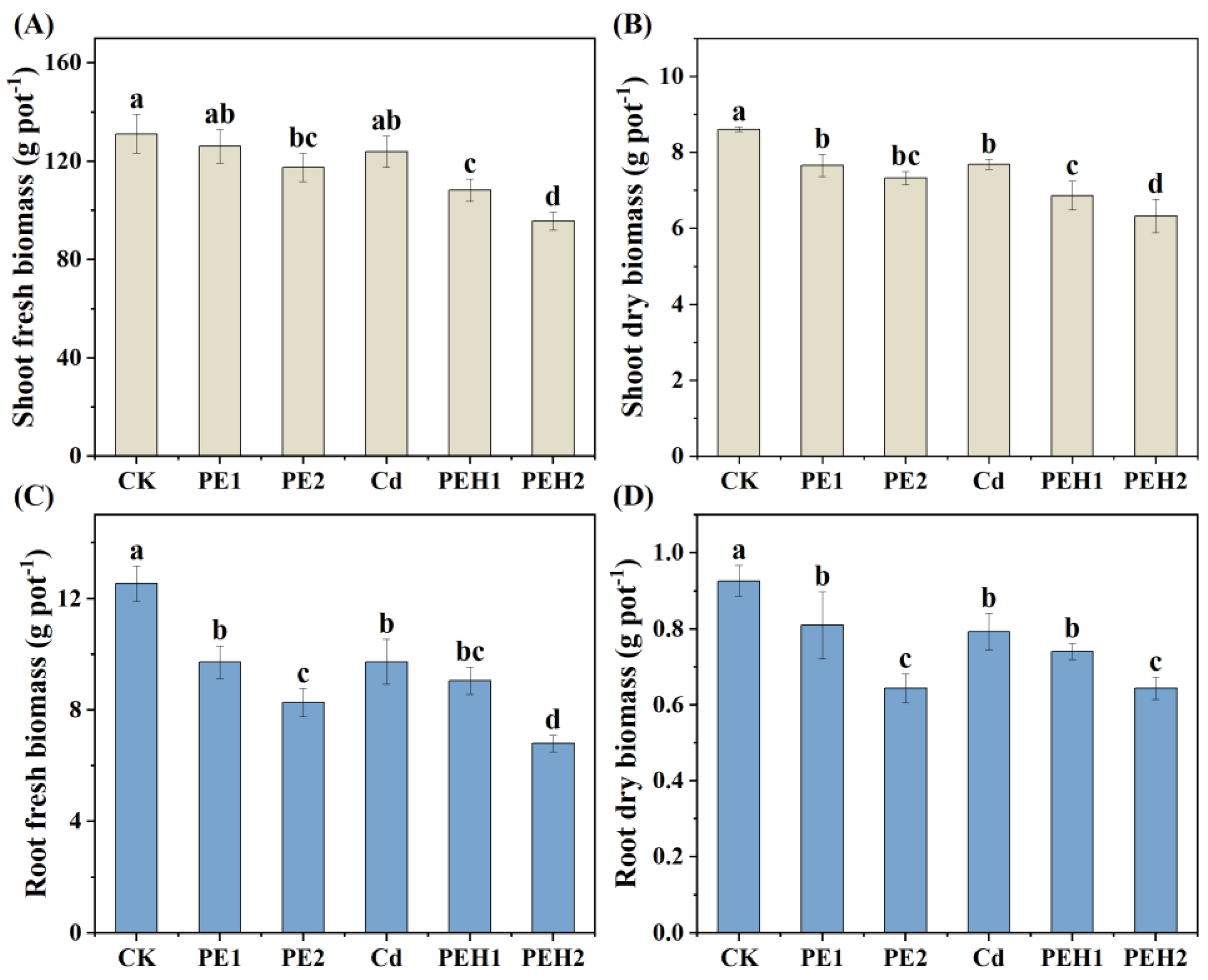
3.2. Plant Biomass and Photosynthesis Parameters
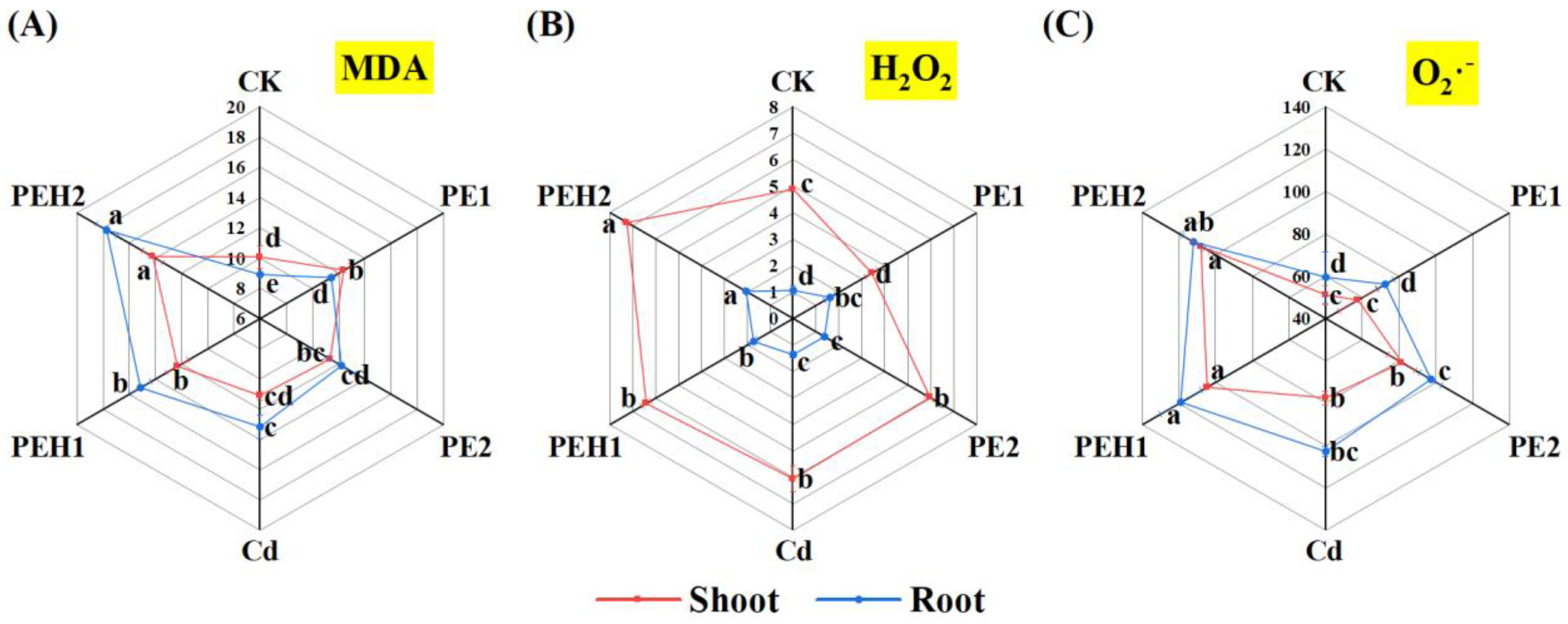
3.3. Plant Oxidative Damage and Antioxidant Enzyme Activity
3.4. Cadmium Concentration in Plant and Soil
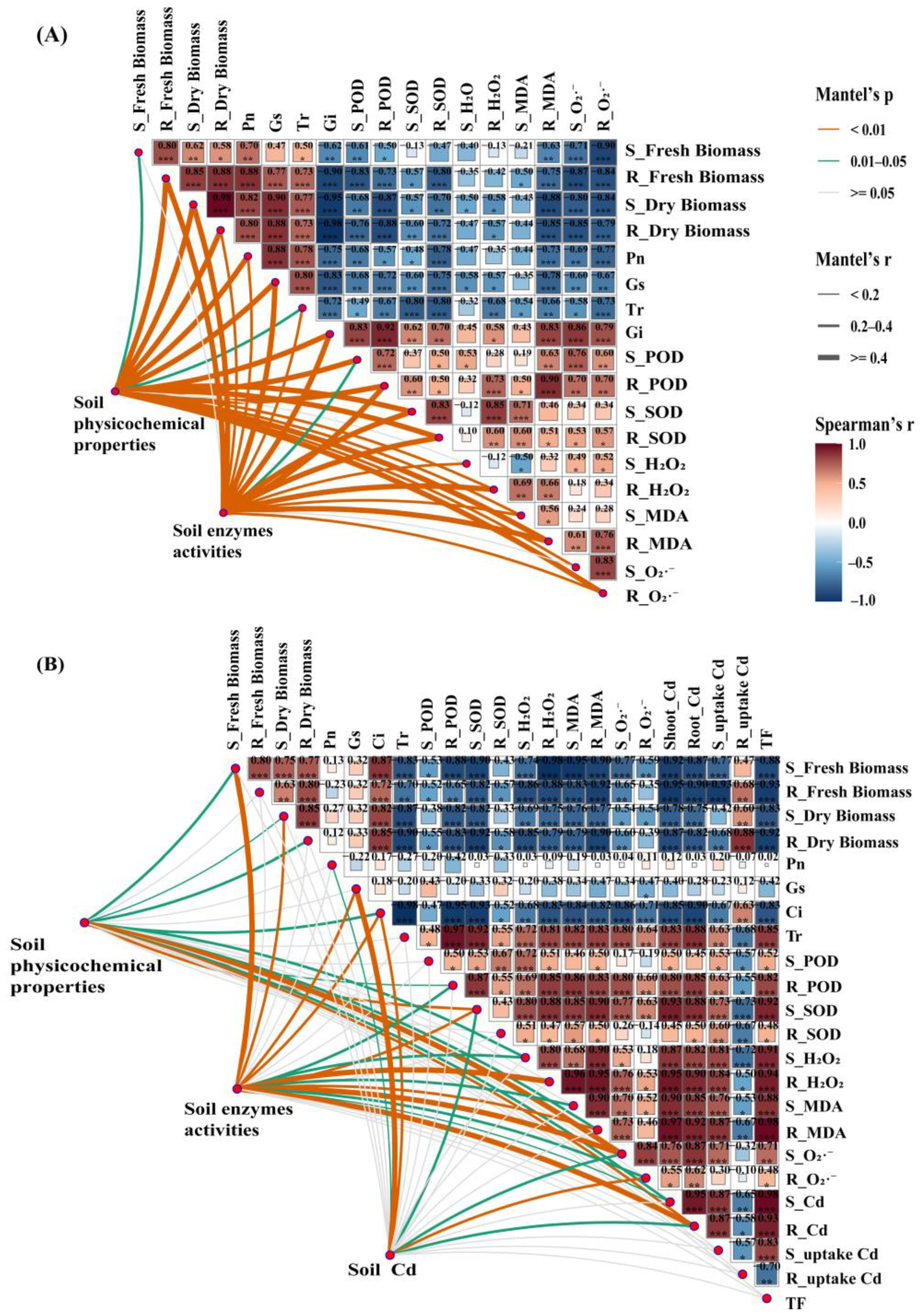
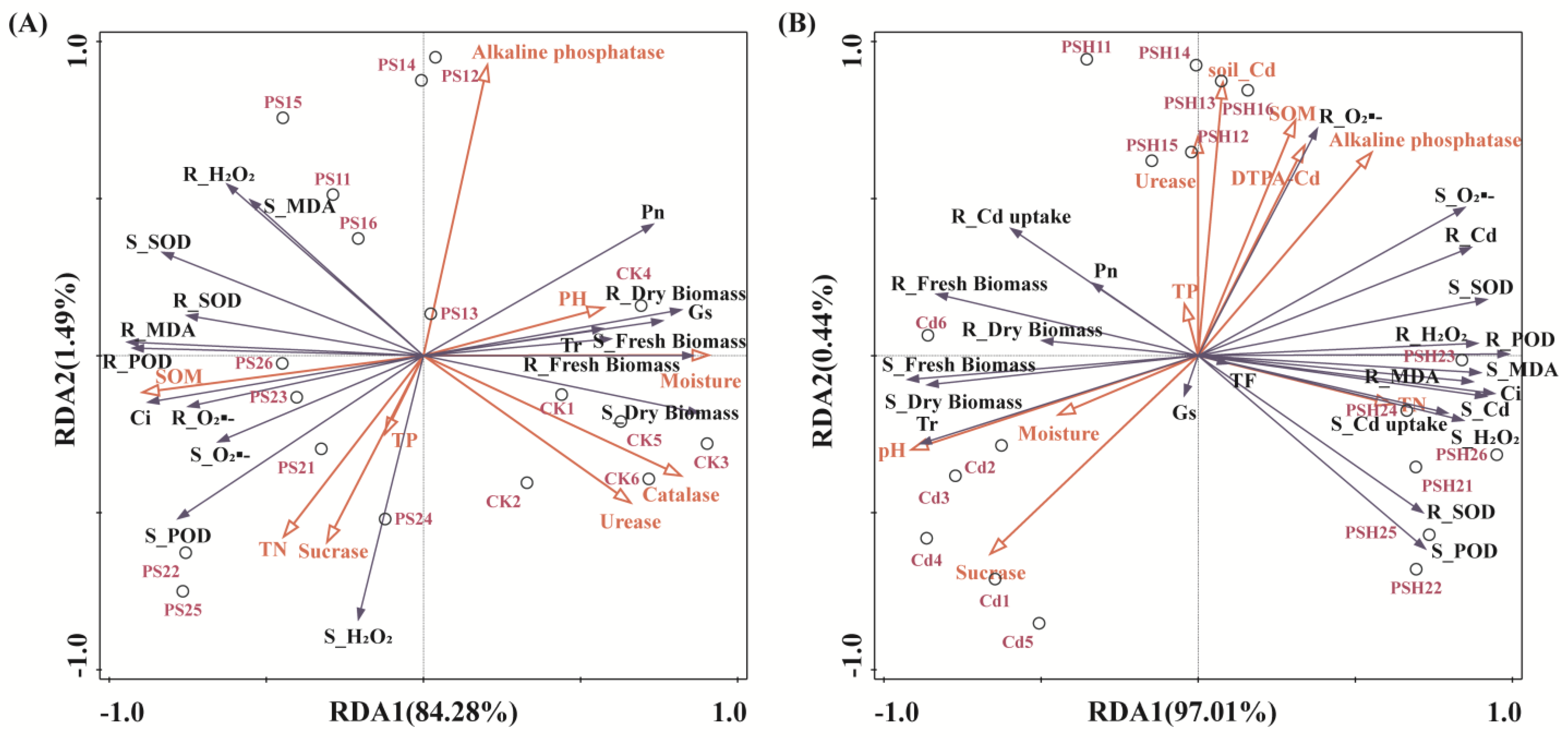
3.5. The Relationship Between Soil Environmental Factors and Plant Growth Characteristics
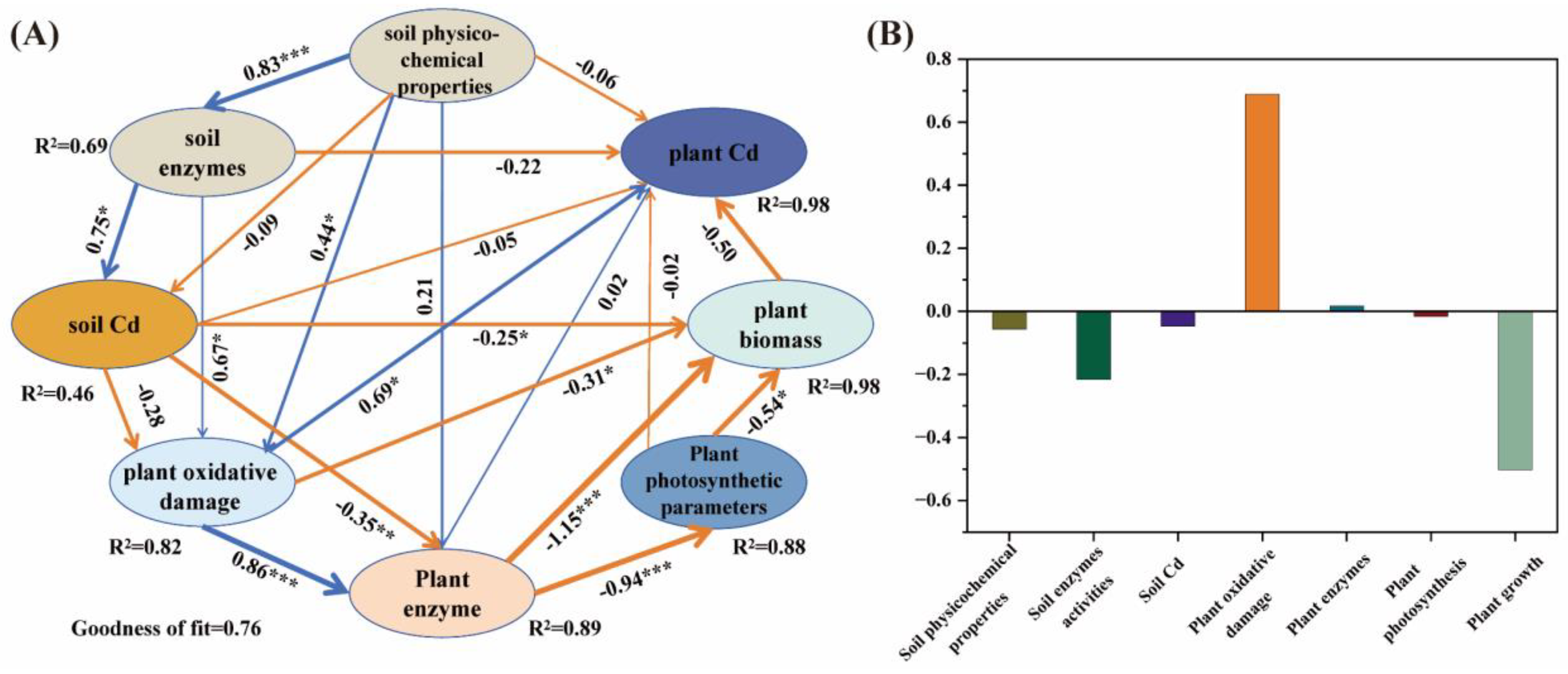
3.6. Factors Affecting Cd Uptake and Enrichment in Plants
4. Discussion
4.1. Effect of MPs Alone or Combined with Cd on Soil Properties
4.2. Effect of MPs Alone or Combined with Cd on Plant Growth
4.3. Effect of MPs Alone or Combined with Cd on Plant Cd Uptake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Cui, Q.L.; Chen, L.; Zhu, X.Z.; Zhao, S.L.; Duan, C.J.; Zhang, X.C.; Song, D.X.; Fang, L.C. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Wang, Q.L.; Adams, C.A.; Sun, Y.H.; Zhang, S.W. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhao, S.L.; Chen, L.; Duan, C.J.; Zhang, X.C.; Fang, L.C. A review of microplastics in soil: Occurrence, analytical methods, combined contamination and risks. Environ. Pollut. 2022, 306, 119374. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.C.; Cheng, L.; Li, Z.Q.; Cui, Y.L.; Liu, J.W.; Xu, D.; Liu, S.J.; Lin, Z.; Chen, J.G.; Zhang, Y.Q. Mechanism of microplastics in the reduction of cadmium toxicity in tomato. Ecotoxicol. Environ. Saf. 2025, 289, 117621. [Google Scholar] [CrossRef] [PubMed]
- Li, F.P.; Yang, X.Y.; Zhang, Z.M.; Jiang, Y.C.; Gong, Y.F. Behaviour, ecological impacts of microplastics and cadmium on soil systems: A systematic review. Environ. Technol. Innov. 2024, 35, 103637. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic disguising as soil carbon storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Penuelas, J.; Sardans, J.; Liu, Y.; Yao, B.; Li, Y. Effects of microplastics exposure on soil inorganic nitrogen: A comprehensive synthesis. J. Hazard. Mater. 2023, 460, 132514. [Google Scholar] [CrossRef]
- Qi, Y.L.; Ossowicki, A.; Yang, X.M.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2019, 387, 121711. [Google Scholar] [CrossRef]
- Su, X.X.; Yang, K. Exploring the blindspot: The soil plastisphere. Soil Ecol. Lett. 2023, 6, 230209. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.D.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Liang, R.; He, J.; Sun, F.H.; Zhang, R.F.; Wang, X.X. Effects of Polyethylene Microplastics on Soil Nutrients and Enzyme Activities. Environ. Sci. 2024, 45, 3679–3687. [Google Scholar]
- Zhang, Z.Y.; Wang, W.F.; Liu, J.P.; Wu, H.T. Discrepant responses of bacterial community and enzyme activities to conventional and biodegradable microplastics in paddy soil. Sci. Total Environ. 2024, 909, 168513. [Google Scholar] [CrossRef]
- Zhao, S.L.; Zhang, Z.Q.; Chen, L.; Cui, Q.L.; Cui, Y.X.; Song, D.X.; Fang, L.C. Review on migration, transformation and ecological impacts of microplastics in soil. Appl. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Zhu, F.X.; Zhu, C.Y.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Huang, D.L.; Wang, X.Y.; Yin, L.S.; Chen, S.; Tao, J.X.; Zhou, W.; Chen, H.J.; Zhang, G.X.; Xiao, R.H. Research progress of microplastics in soil-plant system: Ecological effects and potential risks. Sci. Total Environ. 2022, 812, 151487. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, A.H.; Zhao, B.W.; Ma, F.F.; Zhang, X.; Zhang, Y.; Duan, K.X.; Li, Y.Q. Effects of microplastics and cadmium co-contamination on soil properties, maize (Zea mays L.) growth characteristics, and cadmium accumulation in maize in loessial soil-maize systems. Environ. Pollut. 2024, 356, 124363. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Kun, F.Y.; Qun, F.Z.; Lei, J.; Can, L.; Ling, J.C. Aged polylactic acid microplastics with ultraviolet irradiation stunted pakchoi (Brassica chinensis L.) germination and growth with cadmium in hydroponics. Ecotoxicol. Environ. Saf. 2025, 289, 117696. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Zhang, Y.Q.; Wang, Y.; Feng, J.; Xu, T.Q.; Han, S.Q.; Liu, J.X.; Song, T.J.; Li, L.; Lin, Y.B. Dynamic responses of soil microbial communities to long-term co-contamination with PBAT and cadmium. J. Hazard. Mater. 2025, 492, 138151. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Liu, W.T.; Meng, L.Z.; Lian, J.P.; Wang, Q.; Lian, Y.H.; Chen, C.H.; Wu, J.N. Effects of polyester microfibers (PMFs) and cadmium on lettuce (Lactuca sativa) and the rhizospheric microbial communities: A study involving physio-biochemical properties and metabolomic profiles. J. Hazard. Mater. 2022, 424, 127405. [Google Scholar] [CrossRef]
- Li, Q.J.; Yan, J.; Li, Y.L.; Liu, Y.W.; Andom, O.; Li, Z.J. Microplastics alter cadmium accumulation in different soil-plant systems: Revealing the crucial roles of soil bacteria and metabolism. J. Hazard. Mater. 2024, 474, 134768. [Google Scholar] [CrossRef]
- Wang, F.L.; Wang, X.X.; Song, N.N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Bethanis, J.; Golia, E.E. Revealing the Combined Effects of Microplastics, Zn, and Cd on Soil Properties and Metal Accumulation by Leafy Vegetables: A Preliminary Investigation by a Laboratory Experiment. Soil Syst. 2023, 7, 65. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhang, X.Q.; Zhang, S.Q.; Zhang, S.W.; Sun, Y.H. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- An, Q.R.; Zheng, N.; Chen, C.C.; Li, X.Q.; Ji, Y.N.; Peng, L.Y.; Xiu, Z.F.; Lin, Q.Y. Regulation strategies of microplastics with different particle sizes on cadmium migration processes and toxicity in soil-pakchoi system. J. Hazard. Mater. 2025, 488, 137505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Li, Y.; Qiu, T.Y.; Duan, C.J.; Chen, L.; Zhao, S.L.; Zhang, X.C.; Fang, L.C. Microplastics addition reduced the toxicity and uptake of cadmium to Brassica chinensis L. Sci. Total Environ. 2022, 852, 158353. [Google Scholar] [CrossRef]
- Huang, F.Y.; Hu, J.Z.; Chen, L.; Wang, Z.; Sun, S.Y.; Zhang, W.M.; Jiang, H.; Luo, Y.; Wang, L.; Zeng, Y.; et al. Microplastics may increase the environmental risks of Cd via promoting Cd uptake by plants: A meta-analysis. J. Hazard. Mater. 2023, 448, 130887. [Google Scholar] [CrossRef]
- Bashir, M.S.; Saeed, U.; Khan, J.A.; Saeed, M.; Mustafa, G.; Malik, R.N. Mitigating potential of polystyrene microplastics on bioavailability, uptake, and toxicity of copper in maize (Zea mays L.). Environ. Pollut. 2024, 356, 124299. [Google Scholar] [CrossRef]
- Cui, Y.X.; Wang, X.; Zhang, X.C.; Ju, W.L.; Duan, C.J.; Guo, X.B.; Wang, Y.Q.; Fang, L.C. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis; Part 2, Chemical and Microbial Properties. Agronomy Monograph; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Duan, C.J.; Fang, L.C.; Yang, C.L.; Chen, W.B.; Cui, Y.X.; Li, S.Q. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018, 156, 106–115. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bacheher, J.B.; Faltin, E.; Becker, R.; Goerlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, C.X.; Xue, Q.; Hui, X.M.N. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Qi, R.M.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C.R. Behavior of microplastics and plastic film residues in the soil environment: A critical review—ScienceDirect. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, Q.L.; Sun, Y.H.; Zhang, S.W.; Wang, F.Y. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef]
- Li, G.L.; Cui, X.R.; Tariq, M.; Khan, I.; Khan, A.R.; Al Obaid, S.; Ansari, M.J.; Zhou, H.; Iqbal, B.; Zhao, X. Microplastic and cadmium contamination: Impact on the soil by inhibiting the growth of pak choi (Brassica rapa subsp. chinensis). Process Saf. Environ. Prot. 2024, 189, 714–727. [Google Scholar] [CrossRef]
- Bozkurt, H.S.; Yörüklü, H.C.; Bozkurt, K.; Denktaş, C.; Bozdoğan, A.; Özdemir, O.; Özkaya, B. Biodegradation of microplastic by probiotic bifidobacterium. Int. J. Glob. Warm. 2022, 26, 429–443. [Google Scholar] [CrossRef]
- Kim, S.W.; Jeong, S.W.; An, Y.J. Microplastics disrupt accurate soil organic carbon measurement based on chemical oxidation method. Chemosphere 2021, 276, 130178. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.F.; Huang, S.Y.; Zhang, H.B.; Tong, Y.Z.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2019, 707, 135634. [Google Scholar] [CrossRef]
- Chen, H.P.; Wang, Y.H.; Sun, X.; Peng, Y.K.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Liang, Y.; Lehmann, A.; Yang, G.W.; Leifheit, E.; Rillig, M. Effects of Microplastic Fibers on Soil Aggregation and Enzyme Activities Are Organic Matter Dependent. Front. Environ. Sci. 2021, 9, 650155. [Google Scholar] [CrossRef]
- Xu, B.L.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.J.; He, Y.; Wang, H.Z.; Lu, Z.M.; Brookes, P.C.; Tang, C.X.; et al. Microplastics in the soil environment: Occurrence, risks, interactions and fate-A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Li, K.; He, Q.H.; Zhu, J.; Wang, J.Y.; Sun, C.; Tan, A.; Zhao, X.Q.; Peng, Y.H.; Huang, C.; Cai, J.J.; et al. Responses of microbial communities to the addition of different types of microplastics in agricultural soils. Environ. Pollut. 2025, 364, 125220. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Yang, X.M.; Liu, G.B.; Liang, C.T.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Li, Y.J.; Xu, G.H.; Yu, Y. Effects of microplastics on black soil health: A global meta-analysis. J. Hazard. Mater. 2025, 490, 137850. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, J.L.; Cheng, X.Y.; Cai, Z.H.; Wang, Z.K.; Zhou, J. Biodegradable microplastics reduce the effectiveness of biofertilizers by altering rhizospheric microecological functions. J. Environ. Manage. 2024, 352, 120071. [Google Scholar] [CrossRef]
- Yang, M.; Huang, D.Y.; Tian, Y.B.; Zhu, Q.H.; Zhang, Q.; Zhu, H.H.; Xu, C. Influences of different source microplastics with different particle sizes and application rates on soil properties and growth of Chinese cabbage (Brassica chinensis L.). Ecotoxicol. Environ. Saf. 2021, 222, 112480. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.L.; Ji, X.H.; Luo, Z.X.; Wu, G.; Luo, X.G. Alterations of the rhizosphere soil microbial community composition and metabolite profiles of Zea mays by polyethylene-particles of different molecular weights. J. Hazard. Mater. 2022, 423, 127062. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.L.; Yang, X.M.; Mejia Pelaez, A.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Xu, G.H.; Wang, Y.; Yu, Y. Impacts of polyethylene microplastics on bioavailability and toxicity of metals in soil. Sci. Total Environ. 2021, 760, 144037. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; Lopez, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined effect of microplastics and cd alters the enzymatic activity of soil and the productivity of strawberry plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Guo, P.Y.; Zhang, X.Y.; Zhang, Y.X.; Xie, S.T.; Den, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.Y.; Xian, S.T.; Wang, J.W.; Zheng, X.B.; Song, X.L. Phytotoxic effects of polyethylene microplastics combined with cadmium on the photosynthetic performance of maize (Zea mays L.). Plant Physiol. Biochem. 2023, 203, 108065. [Google Scholar] [CrossRef]
- Liao, Y.L.; Tang, Q.X.; Yang, J.Y. Microplastic characteristics and microplastic-heavy metal synergistic contamination in agricultural soil under different cultivation modes in Chengdu, China. J. Hazard. Mater. 2023, 459, 132270. [Google Scholar] [CrossRef]
- Ju, W.L.; Liu, L.; Jin, X.L.; Duan, C.J.; Cui, Y.X.; Wang, J.; Ma, D.K.; Zhao, W.; Wang, Y.Q.; Fang, L.C. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere 2020, 254, 126724. [Google Scholar] [CrossRef]
- Dong, Y.M.; Gao, M.L.; Qiu, W.W.; Song, Z.G. Uptake of microplastics by carrots in presence of As (III): Combined toxic effects. J. Hazard. Mater. 2021, 411, 125055. [Google Scholar] [CrossRef]
- Gao, M.L.; Liu, Y.; Song, Z.G. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Alp, F.N.; Elbasan, F.; Zengin, G.; Cavusoglu, H.; Sakalak, H. The hormetic dose-risks of polymethyl methacrylate nanoplastics on chlorophyll a fluorescence transient, lipid composition and antioxidant system in Lactuca sativa. Environ. Pollut. 2022, 308, 119651. [Google Scholar] [CrossRef]
- Yin, L.S.; Wen, X.F.; Huang, D.L.; Zeng, G.M.; Deng, R.; Liu, R.; Zhou, Z.Y.; Tao, J.X.; Xiao, R.H.; Pan, H.M. Microplastics retention by reeds in freshwater environment. Sci. Total Environ. 2021, 790, 148200. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Xiang, Y.J.; Jiang, L.; Zhou, Y.Y.; Luo, Z.R.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, W.T.; Wang, X.; Zeb, A.; Wang, Q.; Mo, F.; Shi, R.Y.; Liu, J.Z.; Yu, M.; Li, J.T. Assessing stress responses in potherb mustard (Brassica juncea var. multiceps) exposed to a synergy of microplastics and cadmium: Insights from physiology, oxidative damage, and metabolomics. Sci. Total Environ. 2024, 907, 167920. [Google Scholar] [CrossRef] [PubMed]
- An, Q.Y.; Zhou, T.; Wen, C.; Yan, C.Z. The effects of microplastics on heavy metals bioavailability in soils: A meta-analysis. J. Hazard. Mater. 2023, 460, 132369. [Google Scholar] [CrossRef]
- Huang, L.K.; Wang, Q.; Zhou, Q.Y.; Ma, L.Y.; Wu, Y.J.; Liu, Q.Z.; Wang, S.; Feng, Y. Cadmium uptake from soil and transport by leafy vegetables: A meta-analysis. Environ. Pollut. 2020, 264, 114677. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Yang, Y.Y.; Liu, G.H.; He, G.; Liu, W.Z. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Chen, L.; Beiyuan, J.Z.; Hu, W.F.; Zhang, Z.Q.; Fang, L.C. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577. [Google Scholar] [CrossRef]
| Treatments | Pn | Gs | Tr | Ci |
|---|---|---|---|---|
| (μmol CO2 m−2 s−1) | (mol H2O m−2 s−1) | (Mmol H2O m−2 s−1) | (μmol CO2 mol−1) | |
| CK | 16.86 ± 0.32 a | 0.31 ± 0.02 a | 11.70 ± 0.89 a | 204.01 ± 1.89 d |
| PE1 | 16.56 ± 0.09 ab | 0.24 ± 0.04 bc | 10.75 ± 0.30 ab | 222.32 ± 0.50 c |
| PE2 | 15.57 ± 0.25 bc | 0.19 ± 0.01 d | 10.12 ± 0.80 bc | 242.98 ± 4.37 a |
| Cd | 14.65 ± 0.81 c | 0.27 ± 0.01 b | 10.15 ± 0.49 bc | 226.34 ± 2.69 c |
| PEH1 | 14.51 ± 1.07 c | 0.22 ± 0.01 cd | 9.18 ± 0.08 cd | 231.46 ± 0.73 b |
| PEH2 | 14.63 ± 0.46 c | 0.25 ± 0.02 bc | 8.62 ± 0.14 d | 245.17 ± 1.23 a |
| Factor (Df) | 7.17 | 9.21 | 12.14 | 127.1 |
| p value | ** | *** | *** | *** |
| Treatments | Cd Concentration (mg kg−1) | Total Uptake (μg pot−1) | TF | Soil Total Cd Concentration | DTPA-Cd Concentration | ||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | (mg kg−1) | (mg kg−1) | ||
| Cd | 3.12 ± 0.27 c | 45.74 ± 0.39 b | 24.01 ± 2.46 b | 36.26 ± 2.10 a | 0.07 ± 0.01 b | 3.16 ± 0.02 b | 4.05 ± 0.02 b |
| PEH1 | 3.70 ± 0.09 b | 49.66 ± 0.85 a | 25.44 ± 1.80 b | 36.77± 1.28 a | 0.07 ± 0.01 b | 3.53 ± 0.06 a | 4.18 ± 0.05 a |
| PEH2 | 4.88 ± 0.20 a | 50.80 ± 0.41 a | 30.78 ± 1.20 a | 32.73 ± 1.68 b | 0.10 ± 0.01 a | 3.24 ± 0.08 b | 4.12 ± 0.07 ab |
| Factor (Df) | 60.58 | 61.44 | 10.71 | 4.94 | 38.74 | 14.07 | 4.82 |
| p | *** | ** | * | ns | *** | ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Bi, B. Coupled Effects of Polyethylene Microplastics and Cadmium on Soil–Plant Systems: Impact on Soil Properties and Cadmium Uptake in Lettuce. Toxics 2025, 13, 555. https://doi.org/10.3390/toxics13070555
Zhang Z, Bi B. Coupled Effects of Polyethylene Microplastics and Cadmium on Soil–Plant Systems: Impact on Soil Properties and Cadmium Uptake in Lettuce. Toxics. 2025; 13(7):555. https://doi.org/10.3390/toxics13070555
Chicago/Turabian StyleZhang, Zhiqin, and Boyuan Bi. 2025. "Coupled Effects of Polyethylene Microplastics and Cadmium on Soil–Plant Systems: Impact on Soil Properties and Cadmium Uptake in Lettuce" Toxics 13, no. 7: 555. https://doi.org/10.3390/toxics13070555
APA StyleZhang, Z., & Bi, B. (2025). Coupled Effects of Polyethylene Microplastics and Cadmium on Soil–Plant Systems: Impact on Soil Properties and Cadmium Uptake in Lettuce. Toxics, 13(7), 555. https://doi.org/10.3390/toxics13070555