Abstract
Deciduous teeth accumulate toxic metals until fully mineralized, making them a stable biological matrix for assessing chronic exposure during fetal and early postnatal life. Their metal content is influenced by environmental factors (e.g., industrial areas, mining sites) and individual factors (e.g., maternal diet, early nutrition, passive smoking). The aim of this study was to evaluate the toxic metal content in deciduous teeth and to identify factors contributing to its accumulation, as well as possible health implications. A systematic review was conducted in accordance with the PRISMA guidelines and following the PICO framework. Quality assessment was assessed using the Joanna Briggs Institute (JBI) checklist for quasi-experimental studies. The literature search was carried out in the PubMed, Scopus, and Web of Science databases using the following keywords: deciduous, milk, primary, decidua, teeth, dentition, heavy metal, toxic metals. A total of 134 articles were initially identified, with 95 remaining after duplicate removal. After screening, 75 articles were excluded: 71 did not meet the inclusion criteria, 3 were not available in English, and 1 lacked full-text access. Ultimately, 20 studies were included in the review. Toxic metal concentrations were determined using various analytical techniques, mainly inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectrometry (AAS). Higher levels of metals, especially lead, were observed in the teeth of children residing in industrial areas, near mines, or in regions affected by armed conflict. Although two out of five studies indicated a possible link between fathers’ smoking habits and elevated lead concentrations, no definitive relationship was established between secondhand smoke exposure and the levels of lead and cadmium found in dental tissue. Similarly, no definitive relationship was identified between mercury and lead content and the prevalence of autism. However, lower manganese levels were associated with the presence of autistic traits, weaker verbal performance, and reduced memory capacity. In conclusion, deciduous teeth represent a valuable biological material for assessing chronic prenatal and early postnatal exposure to toxic metals, which may serve as a starting point for further research into diseases of unknown etiology, such as autism, and in the future may have clinical significance in their prevention and treatment. And it is also important for monitoring environmental pollution levels.
1. Introduction
Biological matrices such as blood, urine, hair, nails and teeth are commonly used in environmental studies [1]. Among these biological matrices, deciduous teeth are particularly noteworthy, as their mineralization process begins as early as 14 weeks of fetal life and continues until about 3 years of age [2,3]. The elemental composition of deciduous teeth reflects long-term individual exposure to toxic substances, such as toxic metals. During mineralization, toxic metals accumulate in dental tissues, making teeth a particularly useful biological material for assessing chronic exposure to toxic metals during both fetal and early postnatal life [4,5,6,7,8,9,10]. Moreover, due to the physiological process of replacing deciduous teeth with permanent ones, deciduous teeth can be collected in a non-invasive manner [11]. The use of deciduous teeth to assess exposure to toxic metals enables, among other things, the identification of sources of exposure and assessment of the effectiveness of preventive measures [5].
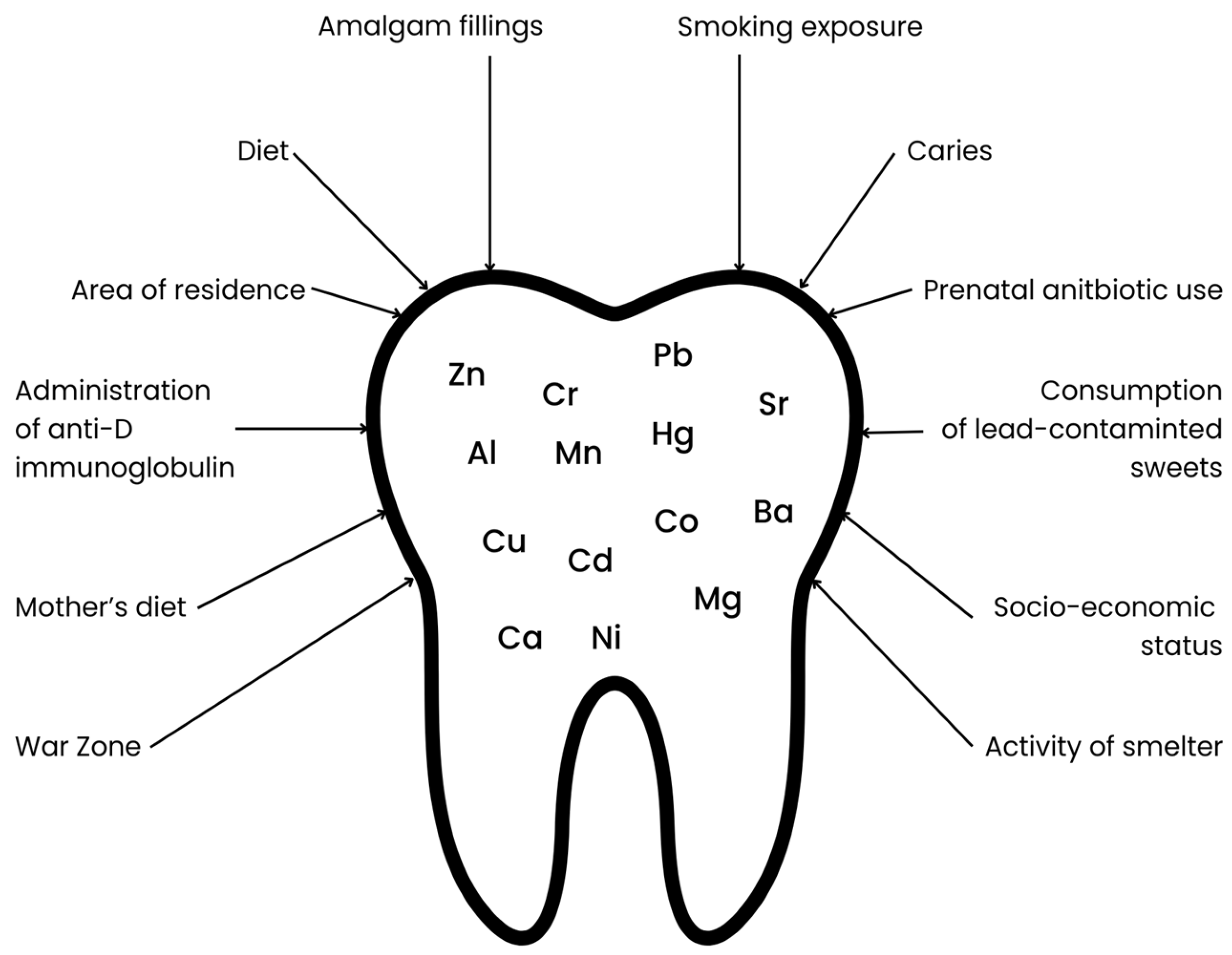
The mineral composition of deciduous teeth is influenced by many factors, beginning with the mother’s lifestyle during pregnancy, the child’s living environment during early development, medications used by the mother during pregnancy, and medications taken by the child at an early age, as well as the child’s diet (see Figure 1). Increased retention of toxic metals is particularly promoted by maternal smoking during pregnancy, the child’s exposure to tobacco smoke, and living in industrial areas (near steel mills, factories, mines, or chemical plants) [12]. The use of antibiotics, which disrupt the gut microbiota, may contribute to increased accumulation and decreased excretion of toxic metals through the gastrointestinal tract [13,14]. Furthermore, diet is another critical factor influencing toxic metal content. A dietary pattern characterized by a low intake of calcium, iron, and zinc may contribute to an increased accumulation of toxic metals due to competition for common intestinal transporters. In addition, consumption of contaminated foods including fish and seafood (Hg), as well as rice and grain products (As), may further elevate toxic metal levels [15,16,17,18].
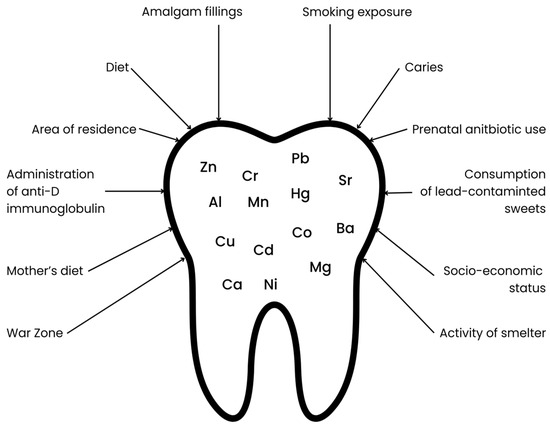
Figure 1.
Factors influencing the content of selected elements in primary teeth.
Of the many toxic substances and compounds found on Earth, those containing toxic metals have a particularly significant impact on human health and the environment. It is worth noting that toxic metals occur naturally in the Earth’s crust [19,20]. The greatest threats are posed by mercury, aluminum, chromium, arsenic, lead and cadmium, even in trace amounts. Their global distribution to the degree that threatens living organisms is primarily due to industry, agriculture, mining, and power plant operations [21,22,23]. They also enter the environment naturally, e.g., as a result of volcanic erosion [19]. Toxic metals present in water, soil and air lead to food contamination, which is how they enter the body [24,25]. They cause disorders affecting the nervous, digestive and cardiovascular systems and impair the functioning of internal organs such as the kidneys, lungs, liver and brain [19,26,27]. Toxic metals accumulate in the human body [28,29,30,31,32,33], which is why their effects can be both acute and chronic. Furthermore, toxic metals have been shown to have carcinogenic effects and have been classified as group 1 carcinogens [21,34,35].
This systematic review aims to assess the toxic metal content of primary teeth. There are a number of studies that have examined the content of toxic metals in primary teeth, taking into account various factors, but no review has been published to date. Examining the available literature revealed a need for a comprehensive review on this topic. Consolidating information on the accumulation of toxic metals in children’s teeth could raise awareness of global environmental pollution caused by toxic metals and encourage action to reduce human activities that contribute to environmental contamination.
2. Materials and Methods
2.1. Focused Question
The systematic review followed the PICO framework as follows: In the case of deciduous teeth (population), will exposure to environmental or lifestyle-related factors (investigated condition) result in differences in toxic metal content (outcome) compared to teeth from children not exposed to such factors (comparison condition)?
2.2. Protocol
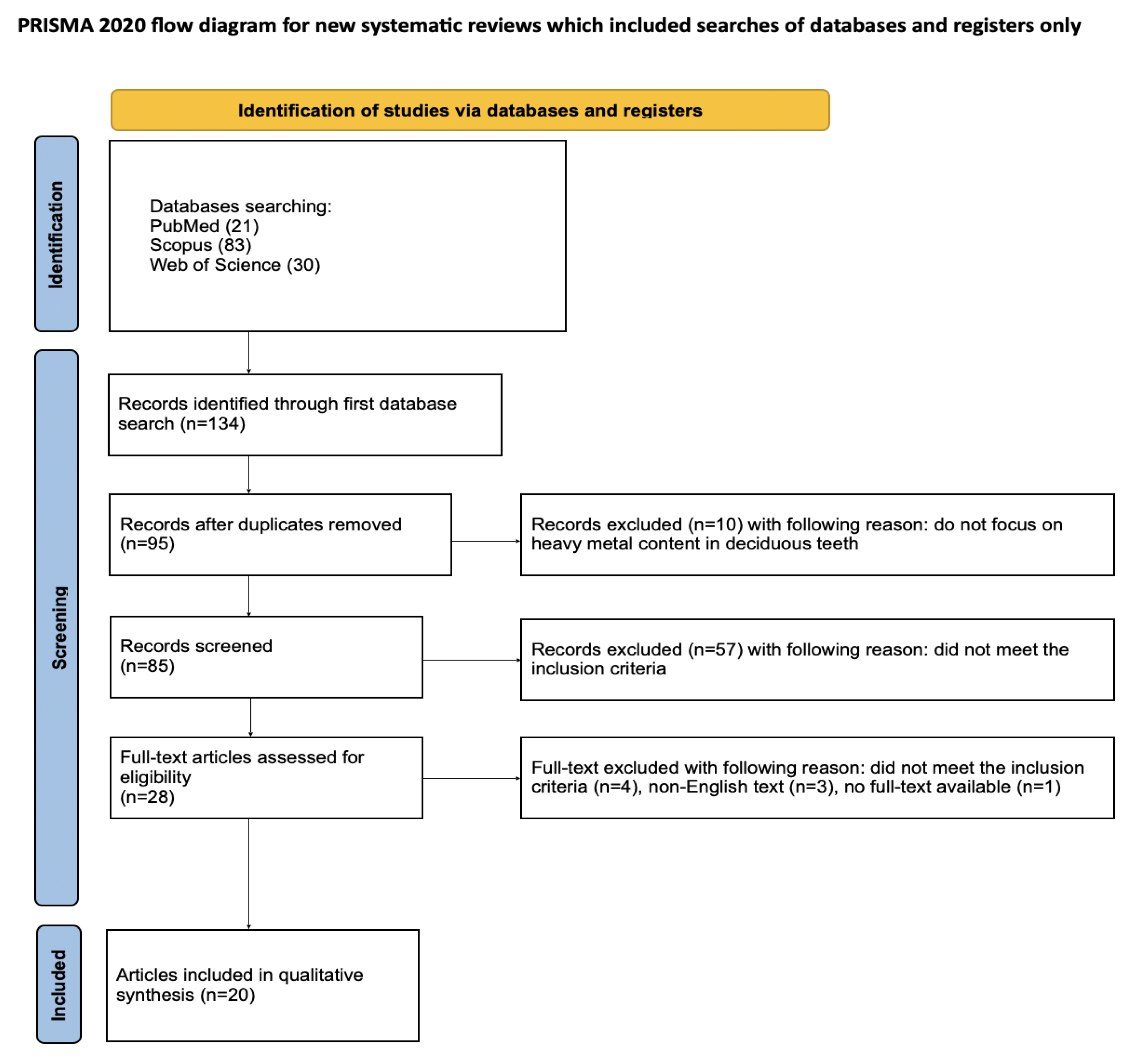
Article selection for the systematic review followed a detailed methodology outlined through the PRISMA flow chart (Figure 2). PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is a set of guidelines for reporting systematic literature reviews and meta-analyses, intended to ensure clarity and completeness of reports [36]. Registration of the systematic review was completed on the Open Science Framework at the specified link: https://osf.io/jywrn (accessed on 7 May 2025).
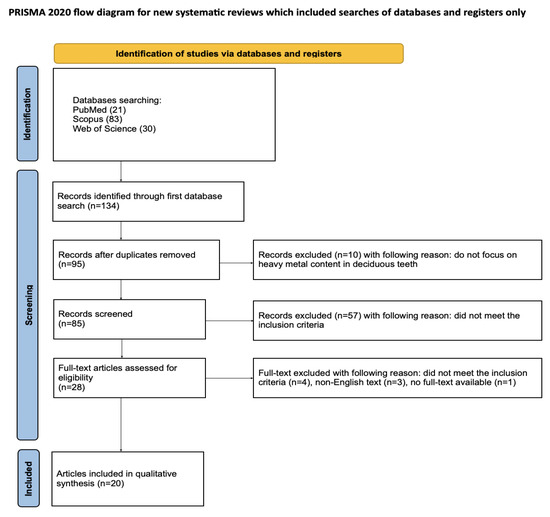
Figure 2.
The PRISMA 2020 flow diagram [36].
2.3. Eligibility Criteria
The following standards determined which investigations qualified for acceptance into the review:
- Studies conducted on deciduous teeth;
- Measurement of toxic metal concentrations;
- In vitro studies;
- Studies in English;
- Full-text articles.
The reviewers reached consensus on the following elimination standards:
- Studies focusing or permanent teeth or bones;
- Studies which did not examine the concentration of toxic metals;
- Non-English papers;
- Systematic review papers;
- Review articles;
- Not full-text accessible;
- Duplicated publications.
No restrictions were applied with regard to the year of publication.
2.4. Information Sources, Search Strategy, and Study Selection
In March 2025, a thorough literature search was conducted across PubMed, Scopus, and Web of Science (WoS) databases to identify studies that fulfilled the established inclusion criteria. The search targeted research related to concentration of toxic metals in deciduous teeth and was limited to titles and abstracts containing the keywords: deciduous OR milk OR primary OR decidua AND teeth OR dentition AND heavy metal OR toxic metals. All collected studies were assessed using predetermined selection criteria, and the final review contained solely those publications with obtainable full manuscripts.
2.5. Data Collection Process and Data Items
Six independent reviewers (I.Z., J.K., A.K., J.K., S.K. and M.M.) systematically selected studies that met the inclusion criteria. From each eligible article, data such as the first author’s name, year of publication, study design, article title, a type of toxic metal and its concentration were extracted. All information was organized and documented using a standardized Excel spreadsheet.
2.6. Risk of Bias and Quality Assessment
In the initial phase of study selection, each reviewer independently evaluated the titles and abstracts to minimize potential bias. The degree of agreement between reviewers was measured using Cohen’s kappa statistic. Any conflicts regarding the inclusion or exclusion of studies were addressed through discussion until consensus was reached among the authors.
2.7. Quality Assessment
The methodological quality of each included study was independently evaluated by two blinded reviewers (J.M. and M.D.) using the Joanna Briggs Institute (JBI) checklist for quasi-experimental studies (i.e., nonrandomized designs). This assessment tool consists of nine targeted questions designed to assess key aspects of study design and execution:
- Is it clear in the study what is the “cause” and what is the “effect”?
- Were the participants included in any similar comparisons?
- Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? Was there a control group?
- Were there multiple measurements of the outcome both before and after the intervention/exposure?
- Was a follow up completed, and if not, were differences between groups in terms of their follow up adequately described and analyzed? Were the outcomes of participants included in any comparisons measured in the same way?
- Were the outcomes measured in a reliable way?
- Was an appropriate statistical analysis used?
Each item was rated using one of four choices: “yes,” “no,” “not clear,” or “does not apply.” When evaluators provided different answers, they discussed the differences until they reached a shared conclusion. Inter-rater agreement was measured with Cohen’s kappa statistic, computed via MedCalc software (v23.1.7, MedCalc Software Ltd., Ostend, Belgium). The analysis yielded a kappa score of 0.86 (p < 0.001), reflecting strong agreement and high evaluator consistency.
3. Results
3.1. Study Selection
An initial database search of PubMed, Scopus, and WoS yielded 134 articles potentially relevant to the review. After removing duplicates, 95 articles were screened. After the initial screening of titles and abstracts, 67 articles that did not consider toxic metal content in deciduous teeth were excluded. It was not possible to access the full text of one article. Of the remaining 27 articles, 4 were excluded as they did not meet the inclusion criteria and 3 were excluded because full text was not available in English. Ultimately, a total of 20 articles were included in the qualitative synthesis of this review. The considerable heterogeneity among the included studies prevents the possibility of conducting a meta-analysis.
3.2. General Characteristics of the Included Studies
Most of the qualifying studies examined the content of more than one element [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53], but only Friedman et al. did not decide to examine the amount of lead [54]. The level of lead in tissues was measured using various measurement techniques, including flame atomic absorption spectrometry (FAAS) [48], graphite furnace atomic absorption spectrometry (GFAAS) [37,38,43,52], inductively coupled plasma mass spectrometry (ICP MS) [40,41,42,44,45,46,47,51,55] and secondary-ion mass spectrometry (SIMS) [56]. Many authors have taken into account that the content of toxic metals in the body is influenced by the state of the environment in which the studied individuals live [39,41,43,49,50,51,52,54,55,56]; therefore, the place of conducting the studies is a significant heterogeneity of the studies. Thus, Twinnereim et al. [38] conducted studies on the teeth of children from Norway, three studies were based on samples from Iran [39,47,52], four from the USA [37,53,55,56], two from Mexico [45,56], two from Romania [41,51]; Amr et al. [42] studied children from Egypt, Gomes et al. [43] from Brazil, Yalcin et al. [46] from Turkey, Friedman et al. [54] from Italy, Savabiesfahani et al. [47] from Iraq and Lebanon, Fischer et al. [48] from Poland, and Bayo et al. [49,50] from Spain. Additional factors taken into account from the eligible studies were: presence of dental caries [38], distance from factories [39,53,54], socioeconomic status [39,49,50,52,53], serological conflict [37], mother’s diet during pregnancy [37,55], presence of amalgam fillings in both the mother during pregnancy and the examined child [37], child’s diet [52,56], and passive smoking [46,49,50,55] (see Table 1).

Table 1.
General characteristics of included studies.
3.3. Main Study Outcomes
3.3.1. Differences by Tooth Type and Presence of Caries
Tvinnereim et al. [38] demonstrated that deciduous teeth with caries exhibit higher concentrations of lead (Pb), mercury (Hg), and zinc (Zn) compared to healthy teeth. The study also highlighted a variation in metal accumulation by tooth type: incisors contained the highest levels of Pb, while molars had greater amounts of Hg and Zn. Moreover, the presence of dental roots was associated with increased Pb and Zn concentrations. These findings suggest that both the anatomical characteristics of the tooth and its pathological state influence its potential for metal accumulation. Contradictory results were reported by Motevaselian et al. [52], who found higher Pb levels in posterior teeth than in anterior ones, possibly due to differences in environmental or dietary exposure.
3.3.2. Environmental Exposure: Industrial, Mining, and Conflict Zones
Children residing in polluted environments consistently showed higher concentrations of toxic metals in their deciduous teeth. For instance, Gomes et al. [43] reported significantly elevated Pb levels in children from industrial areas of Brazil, averaging 275.5 µg/g, compared to 183.1 µg/g in children from non-industrial regions. Similarly, Dinca et al. [51] found higher concentrations of copper, cadmium, chromium, and zinc in the teeth of children from the more polluted city of Slatina, Romania, relative to those from a less polluted city. In Iran, Nazemisalman et al. [39] observed elevated levels of Pb and Zn in children living near metal mines, regardless of tooth type or jaw location. Moreover, Savabieasfahani et al. [47] demonstrated that children living in war zones—specifically in Iraq and Iran—had high levels of Pb, particularly among those with congenital defects. These studies provide robust evidence that anthropogenic environmental contamination, whether due to industrial activities, mining operations, or armed conflict, contributes significantly to the bioaccumulation of toxic metals in primary dentition.
3.3.3. Socioeconomic and Behavioral Factors
Socioeconomic status and individual behaviors were also found to influence metal concentrations in primary teeth. Bayo et al. [49,50] identified that children from lower-income families and older homes, especially in more polluted urban zones, had higher levels of Pb and Cd. Thumb sucking and nail biting were associated with increased Pb in upper jaw teeth. Furthermore, Cd concentrations were higher in non-carious teeth and in children not exposed to school-based fluoride programs. These findings suggest a complex interplay between social determinants and environmental exposure.
3.3.4. Prenatal and Postnatal Exposure Patterns
Lead accumulation in deciduous teeth also reflects prenatal environmental exposure. Sitarik et al. [55] found that children with low birth weight and African American ethnicity had higher Pb levels in their teeth. A noteworthy finding was the correlation between prenatal Pb exposure and altered gut microbiota profiles, suggesting that even subclinical exposure may have systemic effects. Johnston et al. [53] confirmed that prenatal Pb concentrations in children’s teeth closely corresponded with soil contamination levels in areas surrounding a lead-acid battery recycling facility in California, underlining the value of teeth as biological matrices for assessing in utero exposure.
3.3.5. Age and Dentition Differences
Several studies have shown that toxic metal concentrations in teeth decrease with age. Fischer et al. [48] and Bayo et al. [50] reported a decline in Pb and Cd levels as children grew older. Additionally, primary teeth differed significantly from permanent teeth in elemental composition. Tacail et al. [40] and Amr et al. [42] found lower levels of calcium, magnesium, strontium, and zinc in primary teeth, which may reflect both physiological differences in mineralization and varied timings of exposure.
3.3.6. Associations with Neurodevelopmental Disorders
The evidence on the relationship between toxic metals and neurodevelopmental disorders is mixed. Adams et al. [37] reported elevated Hg levels in children with autism, though Pb and Zn did not differ significantly between groups. Abdullah et al. [44] did not find a clear association between autism and levels of Pb, Hg, or Mn. However, Yalcin et al. [46] identified significantly lower levels of strontium in children with autism and ADHD, suggesting a possible link between trace element deficiencies and neurodevelopmental outcomes. Additionally, Friedman et al. [54] associated elevated prenatal Mn levels with poorer cognitive and memory performance in adolescence, particularly among males (see Table 2).

Table 2.
Detailed characteristic of included studies.
3.4. Quality Assessment
For all of the 9 questions, 7 papers received a positive answer to 8 questions [37,38,41,43,46,51,53], 10 papers received a positive answer to 7 of them [39,42,44,45,48,49,50,52,54,55] and 3 received a positive answer to 6 questions [40,47,56] (see Table 3).

Table 3.
Quality assessment—JBI checklist for quasi-experimental studies (nonrandomized experimental studies).
4. Discussion
Exposure to toxic metals can occur orally through the consumption of contaminated food or water [19,20], inhalation through inhaling dust containing metals in industrial areas or smoking [27], and percutaneously through skin contact with contaminated surfaces, cosmetics, paints, or metals in the work environment [20,57,58]. Regardless of the way they enter the body, their removal is not easy, which is why they accumulate in various tissues. The aim of this systematic review was to assess the presence of toxic metals in primary teeth. Of the 20 studies analyzed, 17 investigated more than one metal [37,38,39,40,41,42,46,48,49,50,51,52,53,56]. The most frequently studied elements were Pb [37,38,39,40,41,42,43,44,45,46,47,48,49,50,55,56], Zn [37,38,39,40,41,42,46,47], Cd [38,39,41,42,46,48,49,50], Cu [39,40,41,42,45,46,48] and Hg [37,38,44,46]. Particular attention was paid to analyzing metal concentrations in teeth [37,38,39,41,42,44,45,46,48,49,50,51,52,53,54,55,56], determining the origin of factors causing the presence of toxic metals in teeth [37,38,39,41,43,46,47,49,50,51,52,53,54,55,56], and investigating the effects of poisoning by these metals. The results indicate a strong relationship between environmental pollution and the accumulation of toxic metals in teeth. Increased concentrations have been noted among people living in industrial areas [41,43,51], mining areas [39], war zones [47] and those with a low socioeconomic status [49]. Elevated levels of toxic metals have also been found to cause neurological disorders [54], autism [37], lung function disorders [45] and low birth weight [55].
Twelve publications have demonstrated the impact of the living environment on the concentration of toxic metals in primary teeth [39,41,43,47,49,50,51,52,53,54,55,56]. Seven of these papers [39,41,43,47,51,53,54] focused on anthropogenic pollution from mines [39], smelters [41,54], warfare [47], a lead-acid battery recycling plant [53], and industrial areas [43,51]. All of the researchers involved in these studies found a positive correlation between environmental pollution and the concentration of toxic metals in primary teeth. In children from industrialized areas, the concentration of lead (Pb) was found to be more than 1.5 times higher than in children living in non-industrial areas, as demonstrated by Gomes et al. [43]. Furthermore, children in industrial areas were found to be more exposed to other toxic metals. Dinca et al. [51] noted increased concentrations of Cu, Cd, Cr and, in particular, Zn, while Nedelescu [41] proved that living in an area with smelter activity results in a Pb accumulation in primary teeth that is almost twice as high. In general, concentrations of other metals were found to be 60–95% higher, such as: Cd, Zn, Mn, Ni, Co and Cr. Despite the lack of a comparison with an unpolluted area, Nazemisalman et al. [39] believe that the concentrations of lead, cadmium, copper and zinc in the studied area are high and can only be attributed to the activity of lead and zinc mines. Savabieasfahani’s [47] observations suggest that warfare is also a significant source of exposure to toxic metals, particularly Pb. Similar concentrations of this metal have been found in the teeth of children in various locations around the world where wars are taking place.
The accumulation of toxic metals in primary teeth is not uniform. This issue was investigated in five studies [38,39,49,50,52]. Differences in metal accumulation were observed between incisors and molars, and, in one study [49], between the maxilla and mandible. Three out of four studies [38,49,50] observed that lead (Pb) accumulated mainly in upper arch teeth and incisors. According to two studies by Bayo et al. [49,50], cadmium (Cd) accumulated in greater amounts in incisors than in molars. In two other studies [38,39], no significant differences in the concentration of this metal were observed depending on the tooth. In contrast, according to Tvinnereim et al. [38], Hg and Zn accumulated more in posterior teeth. Mineralization of hard dental tissues is a process that, in the case of primary dentition, takes place from the fourth month of fetal life to one year after tooth eruption. The presence of toxic metals in teeth is associated with this process of mineralization. It is mainly then that elements can be incorporated into the tooth structure. Therefore, the presence of specific metals in specific teeth should be associated with exposure to toxic metals in the body during tooth mineralization. Differences in metal content have also been observed in primary and permanent teeth [40,42]. The lower content of Mg and Ca in primary teeth can be associated with physiologically lower mineralization; however, differences in the content of toxic metals should also be sought in the exposure to them during the mineralization period.
Most of the studies reviewed did not find a statistically significant correlation between lead and mercury content in deciduous teeth and the occurrence of neuropsychological disorders such as autism spectrum disorder or increased disruptive behavior [44,46]. However, a study by Yalçın et al. [46] showed that blood samples from children with autism spectrum disorder (ASD) had significantly higher concentrations of lead and cadmium, and lower concentrations of strontium, both in deciduous teeth and blood, compared to controls [46]. An exception to this trend was observed in the study by Adams et al. [37], which found statistically significantly higher mercury concentrations in the deciduous teeth of children with autism, while no such relationship was observed for lead. The discrepancy regarding the effect of mercury may be due to the preponderance of children with autism relative to the control group in the study by Adams et al. [37]. Additionally, the authors of the Yalçın et al. [46] study suggest that current exposure to toxic metals may exert a greater impact on the development of autism spectrum disorders than prenatal or early postnatal exposure. In addition, it was shown that the deciduous teeth of children with autism contained significantly lower concentrations of manganese compared to neurotypical children. Furthermore, it has been observed that children who had higher dentin manganese concentrations prenatally performed better in terms of cognitive function [5]. Most of the available studies focus on the toxic effects of excess manganese, neglecting the potential consequences of manganese deficiency [59,60]. Meanwhile, the work reviewed suggests that manganese deficiency during fetal development may adversely affect neurodevelopment, likely due to manganese’s role as a cofactor for numerous enzymes essential for normal body function [61]. The Savabieasfahani et al. [47] study also showed that the deciduous teeth of children with congenital malformations contained significantly higher lead concentrations compared to controls. Much previous work confirms the association between prenatal lead exposure and the occurrence of congenital malformations, particularly those involving the nervous, cardiovascular and skeletal systems [62,63,64,65,66]. This is probably related to the fact that lead readily crosses the placental barrier and exhibits teratogenic properties. The mechanisms of lead toxicity include the induction of oxidative stress, inhibition of key enzyme activity, mitochondrial damage and disruption of DNA methylation [67,68,69]. Another study showed that exposure to cadmium, manganese and, in the case of boys, lead at 12–15 weeks of fetal life significantly correlated with reduced lung function at 8–14 years of age [45]. This may be related to the overlap of two key stages of lung development at this time, the pseudomembranous phase and the tubular phase, during which the branching of terminal bronchioles into respiratory bronchioles and the formation of alveoli occur [70]. Exposure to toxic metals during this period may increase oxidative stress, resulting in abnormalities in lung development, which may have key implications for lung function [71]. Furthermore, it has been shown that lead content in deciduous teeth correlated significantly positively with Malassezia and Saccharomyces abundance and negatively with Candida, Penicillium and Aspergillus abundance in the gut microbiome [55]. Based on previous data, it can be speculated that higher lead concentrations favor the proliferation of Malassezia and Saccharomyces, while limiting the growth of Candida, Penicillium and Aspergillus through a mechanism of interspecies competition, thus influencing the relative composition of microorganisms in the gut [14,72].
There are several limitations to this systematic review that should be considered when interpreting the findings. The studies included employed a variety of analytical techniques—such as inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS), and laser ablation—each with differing levels of sensitivity and specificity, which hinders the comparability of results. Moreover, discrepancies in sample preparation methods (e.g., full mineralization vs. surface ablation) and small sample sizes in some studies may reduce statistical power and increase the risk of Type II error. The exclusion of non-English publications is another limitation, although it helped ensure full comprehension and accurate analysis of included studies. To improve future research, it is strongly recommended to adopt standardized protocols for sample preparation, metal quantification, and data reporting. Studies should aim to include large, well-characterized cohorts and report both relative and absolute concentrations of metals. Consistent testing and reporting standards would greatly enhance the comparability and reliability of findings across studies. In terms of biological mechanisms, several heavy metals such as lead, mercury, and cadmium are known neurotoxicants that can interfere with neurotransmitter systems, oxidative stress pathways, and calcium-dependent signaling during critical periods of brain development. For example, lead can disrupt synapse formation and plasticity, contributing to cognitive deficits and behavioral problems. Cadmium has been associated with impaired lung development and function through mechanisms involving inflammation and oxidative damage. These pathways underscore the relevance of assessing metal accumulation in deciduous teeth as an early indicator of potential developmental risks. Future research could benefit from incorporating advanced statistical approaches such as Monte Carlo simulations to better estimate individual and population-level risk. These tools allow for the integration of variability and uncertainty across multiple exposure factors, enhancing the reliability of risk assessments based on toxic metal concentrations in primary teeth.
5. Conclusions
In conclusion, deciduous teeth can be used as a reliable and non-invasive biological matrix reflecting chronic exposure to toxic metals during both the prenatal and early postnatal periods. Their mineralized structure allows for the long-term retention of toxic metals, providing a valuable archive of environmental exposure during critical stages of development. The results of this review highlight the significant influence of environmental and lifestyle-related factors, such as industrial pollution and proximity to mining areas, on the accumulation of toxic metals in deciduous teeth. While associations with specific health outcomes, including autism, remain inconclusive, observed correlations with neurodevelopmental disorders, particularly those linked to manganese deficiency, underscore the need for further research. Monitoring the elemental composition of deciduous teeth may support the early detection of environmental hazards and contribute to the development of public health strategies aimed at reducing exposure to toxic metals among vulnerable populations.
Author Contributions
Conceptualization, I.Z., J.M. and M.D.; methodology, I.Z.; software, M.M.; validation, J.M.; formal analysis, I.Z. and J.K. (Jan Kiryk); investigation, I.Z., M.M., A.K., S.K., J.K. (Jan Kiryk), J.K. (Julia Kensy) and J.M.; resources, M.M.; data curation, M.M., A.K., S.K., J.K. (Jan Kiryk) and J.K. (Julia Kensy); writing—original draft preparation, I.Z., M.M., A.K., S.K., J.K. (Jan Kiryk) and J.K. (Julia Kensy); writing—review and editing, J.M. and M.D.; visualization, I.Z. and J.K. (Jan Kiryk); supervision, J.M.; project administration, J.M. and M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by a subsidy from Wroclaw Medical University, number SUBZ.B180.25.091.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gil, F.; Hernández, A.F. Toxicological Importance of Human Biomonitoring of Metallic and Metalloid Elements in Different Biological Samples. Food Chem. Toxicol. 2015, 80, 287–297. [Google Scholar] [CrossRef]
- Seow, W.K. Dental Enamel Defects in the Primary Dentition: Prevalence and Etiology. In Planning and Care for Children and Adolescents with Dental Enamel Defects: Etiology, Research and Contemporary Management; Drummond, B.K., Kilpatrick, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–14. ISBN 978-3-662-44800-7. [Google Scholar]
- Sunderland, E.P.; Smith, C.J.; Sunderland, R. A Histological Study of the Chronology of Initial Mineralization in the Human Deciduous Dentition. Arch. Oral. Biol. 1987, 32, 167–174. [Google Scholar] [CrossRef]
- Bauer, J.A.; Punshon, T.; Barr, M.N.; Jackson, B.P.; Weisskopf, M.G.; Bidlack, F.B.; Coker, M.O.; Peacock, J.L.; Karagas, M.R. Deciduous Teeth from the New Hampshire Birth Cohort Study: Early Life Environmental and Dietary Predictors of Dentin Elements. Environ. Res. 2024, 256, 119170. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Bauer, J.A.; Austin, C.; Downs, T.J.; Tripodis, Y.; Heiger-Bernays, W.; White, R.F.; Arora, M.; Claus Henn, B. Multiple Metals in Children’s Deciduous Teeth: Results from a Community-Initiated Pilot Study. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gerbi, L.; Austin, C.; Pedretti, N.F.; McRae, N.; Amarasiriwardena, C.J.; Mercado-García, A.; Torres-Olascoaga, L.A.; Tellez-Rojo, M.M.; Wright, R.O.; Arora, M.; et al. Biomarkers of Maternal Lead Exposure during Pregnancy Using Micro-Spatial Child Deciduous Dentine Measurements. Environ. Int. 2022, 169, 107529. [Google Scholar] [CrossRef] [PubMed]
- Gunier, R.B.; Mora, A.M.; Smith, D.; Arora, M.; Austin, C.; Eskenazi, B.; Bradman, A. Biomarkers of Manganese Exposure in Pregnant Women and Children Living in an Agricultural Community in California. Environ. Sci. Technol. 2014, 48, 14695–14702. [Google Scholar] [CrossRef]
- Rojas-Torres, J.; Quijón, M.E.G.; Henríquez-Vidal, A.; Devia-Rubio, L.; Martínez-Duran, L. Permanent and Decidua Dentition as Chronological Biomarkers of Heavy Metal Contamination: A Review of the Forensic Literature. J. Trace Elem. Med. Biol. 2024, 84, 127435. [Google Scholar] [CrossRef]
- Dantham, P.; Nuvvula, S.; Ismail, A.F.; Akkilagunta, S.; Mallineni, S.K. Association between Passive Smoking and Dental Caries Status in Children: A Cross-Sectional Analytical Study. Dent. Med. Probl. 2024, 61, 209–216. [Google Scholar] [CrossRef]
- Rayad, S.; Klimas, S.; Janeczek, M.; Małyszek, A.; Bort, M.; Małysa, A.; Dominiak, M.; Dobrzyński, M. Studies on the Content of Toxic Metals in Teeth: A Narrative Review of Literature. Dent. Med. Probl. 2024, 61, 943–961. [Google Scholar] [CrossRef]
- Jarka, J.; Angerman, A. The Sequence of Replacement of Deciduous Teeth by Permanent Teeth in the Lateral Maxillary Segments and Its Clinical Significance. Authors’ Own Observation. Forum Ortod. 2021, 17, 93–105. [Google Scholar] [CrossRef]
- Punshon, T.; Bauer, J.A.; Karagas, M.R.; Coker, M.O.; Weisskopf, M.G.; Mangano, J.J.; Bidlack, F.B.; Barr, M.N.; Jackson, B.P. Quantified Retrospective Biomonitoring of Fetal and Infant Elemental Exposure Using LA-ICP-MS Analysis of Deciduous Dentin in Three Contrasting Human Cohorts. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, S.-Z.; Li, Y.-Y.; Xue, R.-Y.; Duan, X.; Lin, X.-Y.; Chen, S.; Zhou, D.; Li, H.-B. Gut Dysbiosis Exacerbates Intestinal Absorption of Cadmium and Arsenic from Cocontaminated Rice in Mice Due to Impaired Intestinal Barrier Functions. Environ. Sci. Technol. 2025, 59, 3459–3471. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and Essential Metals: Metabolic Interactions with the Gut Microbiota and Health Implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef] [PubMed]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Maerten, A.; Bannon, D. Metal Transporters in Intestine and Brain: Their Involvement in Metal-Associated Neurotoxicities. Hum. Exp. Toxicol. 2007, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bzikowska-Jura, A.; Wesołowska, A.; Sobieraj, P.; Nawrocka, A.; Filipek, A.; Durkalec, M.; Katryńska, D.; Jedziniak, P. Essential and Non-Essential Element Concentrations in Human Milk Samples and the Assessment of Infants’ Exposure. Sci. Rep. 2024, 14, 8140. [Google Scholar] [CrossRef]
- Furman, J.; Ćwieląg-Drabek, M. The Content of Metallic Trace Elements in Rice-Containing Products Used in the Diet of Infants and Young Children—Health Risks for Consumers. Food Chem. Toxicol. 2025, 197, 115310. [Google Scholar] [CrossRef]
- Małyszek, A.; Kiryk, S.; Kensy, J.; Kotela, A.; Michalak, M.; Kiryk, J.; Janeczek, M.; Matys, J.; Dobrzyński, M. Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Appl. Sci. 2025, 15, 5974. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Wiatrak, B.; Rayad, S.; Gębarowski, T.; Hadzik, J.; Styczyńska, M.; Gedrange, T.; Dobrzyński, M.; Barg, E.; Dominiak, M. Comparative Analysis of Heavy Metal Content in Impacted Third Molars from Industrial and Non-Industrial Areas and Its Effect on the Isolation, Culture, and Proliferation of Dental Stem Cells (DSCs). J. Clin. Med. 2024, 13, 5465. [Google Scholar] [CrossRef]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Zou, B.; Zhao, J. Combined Toxicity of Heavy Metal Mixtures in Liver Cells. J. Appl. Toxicol. 2016, 36, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Bryła, E.; Dobrzyński, M.; Konkol, D.; Kuropka, P.; Styczyńska, M.; Korczyński, M. Toxic Metals Content in Impacted Third Molars and Adjacent Bone Tissue in Different Groups of Patients. Materials 2021, 14, 793. [Google Scholar] [CrossRef]
- Rayad, S.; Dobrzyński, M.; Kuźniarski, A.; Styczyńska, M.; Diakowska, D.; Gedrange, T.; Klimas, S.; Gębarowski, T.; Dominiak, M. Mercury Content in Impacted Wisdom Teeth from Patients of the Legnica–Głogów Copper Area—An In Vitro Pilot Study. J. Xenobiot. 2023, 13, 463–478. [Google Scholar] [CrossRef]
- Rayad, S.; Dobrzyński, M.; Kuźniarski, A.; Styczyńska, M.; Diakowska, D.; Gedrange, T.; Klimas, S.; Gębarowski, T.; Dominiak, M. An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study. Appl. Sci. 2023, 13, 2904. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef]
- Rygas, J.; Matys, J.; Wawrzyńska, M.; Szymonowicz, M.; Dobrzyński, M. The Use of Graphene Oxide in Orthodontics—A Systematic Review. J. Funct. Biomater. 2023, 14, 500. [Google Scholar] [CrossRef]
- Sztyler, K.; Wiglusz, R.J.; Dobrzynski, M. Review on Preformed Crowns in Pediatric Dentistry—The Composition and Application. Materials 2022, 15, 2081. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Congiargiu, A.; Azara, E.; Mammani, I.M.A.; De Miglio, M.R.; Zinellu, A.; Carru, C.; Medici, S. Heavy Metals in Biological Samples of Cancer Patients: A Systematic Literature Review. Biometals 2024, 37, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Adams, J.B.; Romdalvik, J.; Ramanujam, V.M.S.; Legator, M.S. Mercury, Lead, and Zinc in Baby Teeth of Children with Autism Versus Controls. J. Toxicol. Environ. Health Part A 2007, 70, 1046–1051. [Google Scholar] [CrossRef]
- Tvinnereim, H.M.; Eide, R.; Riise, T. Heavy Metals in Human Primary Teeth: Some Factors Influencing the Metal Concentrations. Sci. Total Environ. 2000, 255, 21–27. [Google Scholar] [CrossRef]
- Nazemisalman, B.; Bayat, N.; Darvish, S.; Nahavandi, S.; Mohseni, M.; Luchian, I. Polarography Can Successfully Quantify Heavy Metals in Dentistry. Medicina 2022, 58, 448. [Google Scholar] [CrossRef]
- Tacail, T.; Kovačiková, L.; Brůžek, J.; Balter, V. Spatial Distribution of Trace Element Ca-Normalized Ratios in Primary and Permanent Human Tooth Enamel. Sci. Total Environ. 2017, 603–604, 308–318. [Google Scholar] [CrossRef]
- Nedelescu, M.; Baconi, D.; Ciobanu, A.-M.; Manda, G. Heavy Metal Levels in Teeth and Hair Samples of Children Living in an Industrial Area. J. Environ. Prot. Ecol. 2015, 16, 926–932. [Google Scholar]
- Amr, M.A. Trace Elements in Egyptian Teeth. Int. J. Phys. Sci. 2011, 6, 6241–6245. [Google Scholar] [CrossRef]
- Gomes, V.E.; De Sousa, M.D.L.R.; Barbosa Jr Jr, F.; Krug, F.J.; Saraiva, M.D.C.P.; Cury, J.A.; Gerlach, R.F. In Vivo Studies on Lead Content of Deciduous Teeth Superficial Enamel of Preschool Children. Sci. Total Environ. 2004, 320, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.; Ly, A.R.; Goldberg, W.A.; Clarke-Stewart, K.A.; Dudgeon, J.V.; Mull, C.G.; Chan, T.J.; Kent, E.E.; Mason, A.Z.; Ericson, J.E. Heavy Metal in Children’s Tooth Enamel: Related to Autism and Disruptive Behaviors? J. Autism Dev. Disord. 2012, 42, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Gennings, C.; Curtin, P.; Alcala, C.S.; Lamadrid-Figueroa, H.; Tamayo-Ortiz, M.; Mercado-Garcia, A.; Torres-Olascoaga, L.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Associations between Prenatal Metal and Metalloid Mixtures in Teeth and Reductions in Childhood Lung Function. Sci. Total Environ. 2024, 938, 173352. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.S.; Çak, T.; Yalçın, S. Lower Strontium in Two Different Body Matrices in Neurodevelopmental Disorders: A Preliminary Report. J. Trace Elem. Med. Biol. 2020, 62, 126553. [Google Scholar] [CrossRef]
- Savabieasfahani, M.; Ali, S.S.; Bacho, R.; Savabi, O.; Alsabbak, M. Prenatal Metal Exposure in the Middle East: Imprint of War in Deciduous Teeth of Children. Environ. Monit. Assess. 2016, 188, 505. [Google Scholar] [CrossRef]
- Fischer, A.; Wiechuła, D.; Przybyła-Misztela, C. Changes of Concentrations of Elements in Deciduous Teeth with Age. Biol. Trace Elem. Res. 2013, 154, 427–432. [Google Scholar] [CrossRef]
- Bayo, J.; Moreno-Grau, S.; Martinez, M.J.; Moreno, J.; Angosto, J.M.; Pérez, J.J.G.; Marcos, L.G.; Moreno-Clavel, J. Environmental and Physiological Factors Affecting Lead and Cadmium Levels in Deciduous Teeth. Arch. Environ. Contam. Toxicol. 2001, 41, 247–254. [Google Scholar] [CrossRef]
- Bayo, J.; Moreno-Grau, S.; Martínez-García, M.J.; Moreno, J.M.; Angosto, J.M.; Guillén-Pérez, J.J.; García-Marcos, L.; Moreno-Clavel, J. Contributions of Risk Factors to High Lead and Cadmium Levels in Deciduous Teeth; WIT Press: Cardiff, UK, 2001; pp. 3–12. [Google Scholar]
- Dincă, M.; Daciana, P.; Ionita, D. About Oral Health of Romanian Children from Various Polluted Area Due to Heavy Metals. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2012, 74, 171–182. [Google Scholar]
- Motevasselian, F.; Abdi, K.; Ghodarati, H.; Shamshiri, A.R.; Lippert, F.; Hessari, H. The Role of Lead and Cadmium in Deciduous Teeth and Saliva on Dental Caries in Children Residing in Tehran, Iran. J. Trace Elem. Med. Biol. 2023, 79, 127209. [Google Scholar] [CrossRef]
- Johnston, J.E.; Franklin, M.; Roh, H.; Austin, C.; Arora, M. Lead and Arsenic in Shed Deciduous Teeth of Children Living Near a Lead-Acid Battery Smelter. Environ. Sci. Technol. 2019, 53, 6000–6006. [Google Scholar] [CrossRef]
- Friedman, A.; Schildroth, S.; Bauer, J.A.; Coull, B.A.; Smith, D.R.; Placidi, D.; Cagna, G.; Krengel, M.H.; Tripodis, Y.; White, R.F.; et al. Early-Life Manganese Exposure during Multiple Developmental Periods and Adolescent Verbal Learning and Memory. Neurotoxicol. Teratol. 2023, 100, 107307. [Google Scholar] [CrossRef] [PubMed]
- Sitarik, A.R.; Arora, M.; Austin, C.; Bielak, L.F.; Eggers, S.; Johnson, C.C.; Lynch, S.V.; Kyun Park, S.; Hank Wu, K.-H.; Yong, G.J.M.; et al. Fetal and Early Postnatal Lead Exposure Measured in Teeth Associates with Infant Gut Microbiota. Environ. Int. 2020, 144, 106062. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.E. Enamel Lead Biomarker for Prenatal Exposure Assessment. Environ. Res. 2001, 87, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hostynek, J.J. Factors Determining Percutaneous Metal Absorption. Food Chem. Toxicol. 2003, 41, 327–345. [Google Scholar] [CrossRef]
- Larese Filon, F.; Maina, G.; Adami, G.; Venier, M.; Coceani, N.; Bussani, R.; Massiccio, M.; Barbieri, P.; Spinelli, P. In Vitro Percutaneous Absorption of Cobalt. Int. Arch. Occup. Environ. Health 2004, 77, 85–89. [Google Scholar] [CrossRef]
- Conley, T.E.; Beaudin, S.A.; Lasley, S.M.; Fornal, C.A.; Hartman, J.; Uribe, W.; Khan, T.; Strupp, B.J.; Smith, D.R. Early Postnatal Manganese Exposure Causes Arousal Dysregulation and Lasting Hypofunctioning of the Prefrontal Cortex Catecholaminergic Systems. J. Neurochem. 2020, 153, 631–649. [Google Scholar] [CrossRef]
- Bałasz, M.; Szkilnik, R.; Brus, R.; Malinowska-Borowska, J.; Kasperczyk, S.; Nowak, D.; Kostrzewa, R.M.; Nowak, P. Perinatal Manganese Exposure and Hydroxyl Radical Formation in Rat Brain. Neurotox. Res. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Dhole, P. Heavy Metal Exposure and Its Health Implications: A Comprehensive Review. Ind. J. Clin. Biochem. 2025, 1–29. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschläger, M. The Role of the Placenta in Fetal Exposure to Heavy Metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P. Developmental Neurotoxicity of Industrial Chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Nyanza, E.C.; Mhana, R.J.; Asori, M.; Thomas, D.S.K.; Kisoka, A.P. Effects of Prenatal Lead, Mercury, Cadmium, and Arsenic Exposure on Children’s Neurodevelopment in an Artisanal Small-Scale Gold Mining Area in Northwestern Tanzania Using a Multi-Chemical Exposure Model. PLoS Glob. Public Health 2025, 5, e0004577. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Barone, S. Critical Periods of Vulnerability for the Developing Nervous System: Evidence from Humans and Animal Models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M. Molecular Mechanisms of Lead Toxicity. BioTechnologia 2015, 95, 137–149. [Google Scholar] [CrossRef]
- Virgolini, M.B.; Aschner, M. Molecular Mechanisms of Lead Neurotoxicity. In Advances in Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 5, pp. 159–213. ISBN 978-0-12-823775-5. [Google Scholar]
- Gonzalez-Villalva, A.; Marcela, R.-L.; Nelly, L.-V.; Patricia, B.-N.; Guadalupe, M.-R.; Brenda, C.-T.; Maria Eugenia, C.-V.; Martha, U.-C.; Isabel, G.-P.; Fortoul, T.I. Lead Systemic Toxicity: A Persistent Problem for Health. Toxicology 2025, 515, 154163. [Google Scholar] [CrossRef]
- Copland, I.; Post, M. Lung Development and Fetal Lung Growth. Paediatr. Respir. Rev. 2004, 5, S259–S264. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Persky, V.; Pappalardo, A.; Argos, M. Association of Heavy Metals with Measures of Pulmonary Function in Children and Youth: Results from the National Health and Nutrition Examination Survey (NHANES). Environ. Int. 2018, 121, 871–878. [Google Scholar] [CrossRef]
- Wylie, A.C.; Murgueitio, N.; Carlson, A.L.; Fry, R.C.; Propper, C.B. The Role of the Gut Microbiome in the Associations between Lead Exposure and Child Neurodevelopment. Toxicol. Lett. 2025, 408, 95–104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).