PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Phototoxicity Reference Substance Database
2.2. Phototoxicity Testing Methods Used for Pharmaceuticals
2.3. Comparison of In Vivo and In Vitro Data of Phototoxicity Reference Chemicals
2.4. Photochemical Properties
3. Results
3.1. Analysis of Reference Chemicals in the PhotoChem Database
3.2. Human vs. Animal Test Results for Reference Test Chemicals
3.3. Correlation of Human Test Data with In Vivo or In Vitro Tests
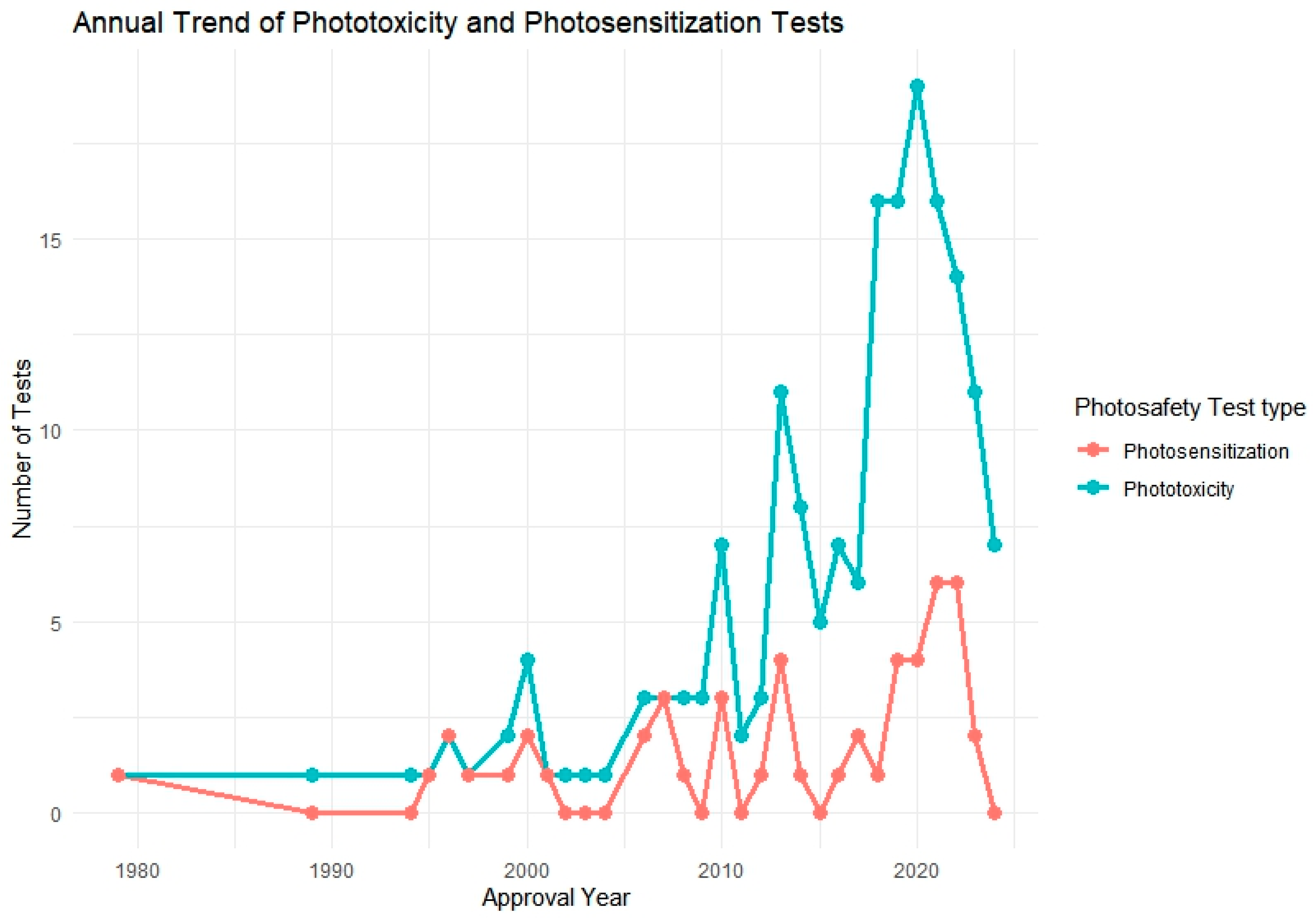
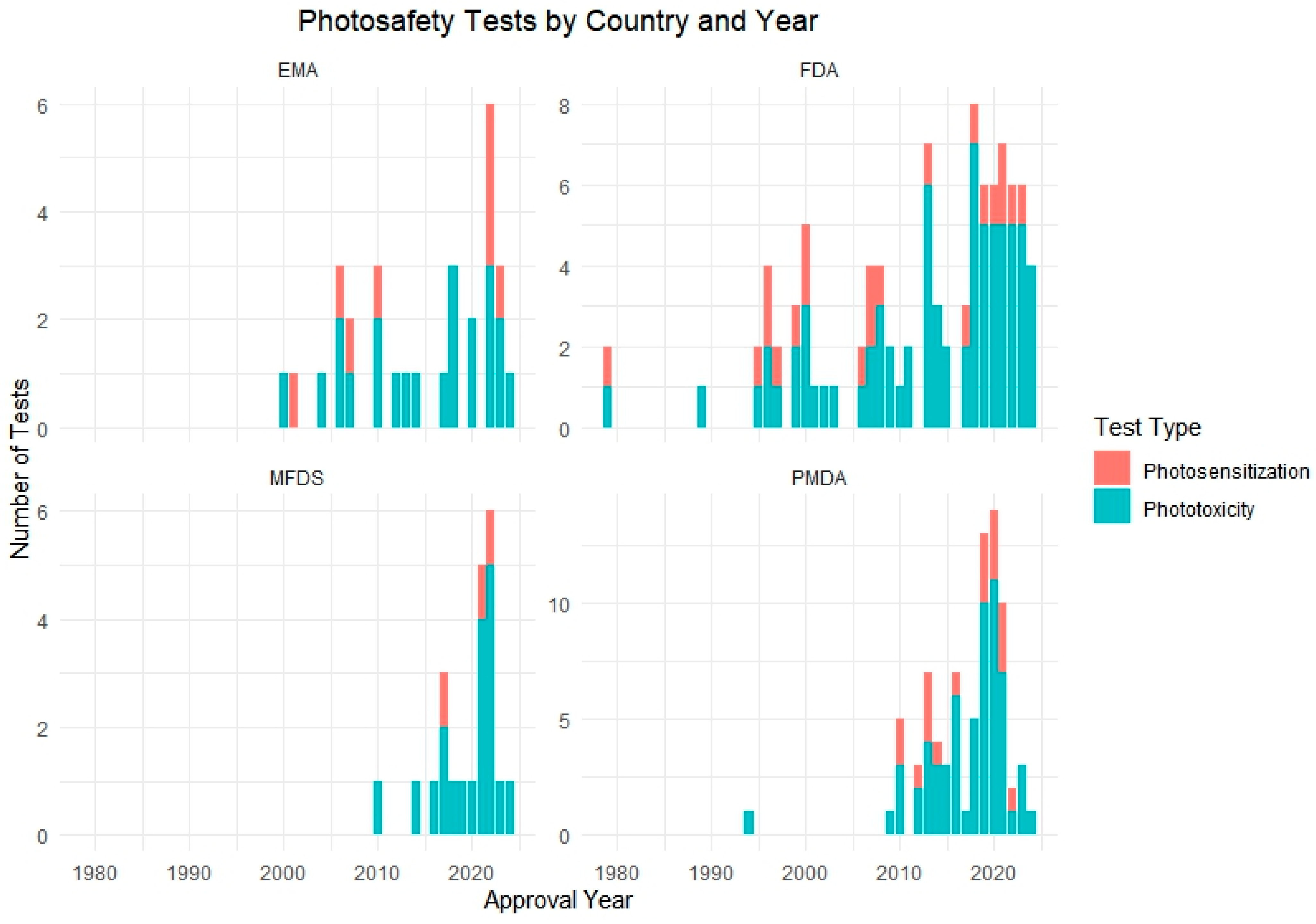
3.4. Analysis of Photosafety Testing Used in Drug Approvals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| OECD | The Organization for Economic Co-operation and Development |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| FDA | U.S. Food and Drug Administration |
| PMDA | Japan Pharmaceuticals and Medical Devices Agency |
| EMA | European Medicines Agency |
References
- U.S. Food and Drug Administration. S10 Photosafety Evaluation of Pharmaceuticals Guidance for Industry; ICH Guideline, ed.; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2015. [Google Scholar]
- Epstein, J.H. Phototoxicity and Photoallergy. Semin. Cutan. Med. Surg. 1999, 18, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Linda, J.; Loretz, J.E.B. CTFA Safety Evaluation Guidelines; The Cosmetic, Toiletry, and Fragrance Association: London, UK, 2007. [Google Scholar]
- OECD. Test No. 432: In Vitro 3T3 NRU Phototoxicity Test; OECD Publishing: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 495: Ros (Reactive Oxygen Species) Assay for Photoreactivity; OECD Publishing: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 498: In Vitro Phototoxicity-Reconstructed Human Epidermis Phototoxicity Test Method; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Kim, S.Y.; Seo, S.; Choi, K.H.; Yun, J. Evaluation of phototoxicity of tattoo pigments using the 3 T3 neutral red uptake phototoxicity test and a 3D human reconstructed skin model. Toxicol. Vitr. 2020, 65, 104813. [Google Scholar] [CrossRef]
- Lelièvre, D.; Justine, P.; Christiaens, F.; Bonaventure, N.; Coutet, J.; Marrot, L.; Cotovio, J. The EpiSkin phototoxicity assay (EPA): Development of an in vitro tiered strategy using 17 reference chemicals to predict phototoxic potency. Toxicol. Vitr. 2007, 21, 977–995. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Igarashi, N.; Yamada, S.; Tsuda, Y. High-throughput reactive oxygen species (ROS) assay: An enabling technology for screening the phototoxic potential of pharmaceutical substances. J. Pharm. Biomed. Anal. 2008, 46, 187–193. [Google Scholar] [CrossRef]
- Sohn, S.; Ju, J.H.; Son, K.H.; Lee, J.P.; Kim, J.; Lim, C.H.; Hong, S.K.; Kwon, T.R.; Chang, M.; Kim, D.S.; et al. Alternative methods for phototoxicity test using reconstructed human skin model. Altex 2009, 26, 136. [Google Scholar]
- Spielmann, H.; Balls, M.; Brand, M.; Döring, B.; Holzhütter, H.; Kalweit, S.; Klecak, G.; Eplattenier, H.; Liebsch, M.; Lovell, W.; et al. EEC/COLIPA project on in vitro phototoxicity testing: First results obtained with a Balb/c 3T3 cell phototoxicity assay. Toxicol. Vitr. 1994, 8, 793–796. [Google Scholar] [CrossRef]
- Spielmann, H.; Balls, M.; Dupuis, J.; Pape, W.J.; de Silva, O.; Holzhütter, H.G.; Gerberick, F.; Liebsch, M.; Lovell, W.W.; Pfannenbecker, U. A study on UV filter chemicals from Annex VII of European Union Directive 76/768/EEC, in the in vitro 3T3 NRU phototoxicity test. Altern. Lab. Anim. 1998, 26, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, M. Prevalidation of the “EpiDerm™ Phototoxicity Test”—FINAL REPORT(Phase I, II, III); ZEBET, German National Centre for Documentation and Evaluation of Alternatives to Animal Experiments: Berlin, Germany, 1998. [Google Scholar]
- ROS Assay Validation Management Team. Validation Report for the International Validation Study on ROS (Reactive Oxygen Species) Assay as a Test Evaluating Phototoxic Potential of Chemicals (Atlas Suntest Version). 2013. Available online: https://www.jacvam.go.jp/files/news/ROS_Assay_full%2020130920%20atlas_fourth%20data.pdf (accessed on 24 April 2025).
- Kandarova, H.; Liebsch, M. The EpiDerm™ Phototoxicity Test (EpiDerm™ H3D-PT). In Alternatives for Dermal Toxicity Testing; Springer: Berlin/Heidelberg, Germany, 2017; pp. 483–503. [Google Scholar]
- Liebsch, M.; Barrabas, C.; Traue, D.; Spielmann, H. Development of a new in vitro test for dermal phototoxicity using a model of reconstituted human epidermis. Altex 1997, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Neumanna, N.; Holzleb, E.; Plewigc, G.; Schwarzd, T.; Panizzone, R.; Breitf, R.; Ruzickaa, T.; Lehmanna, P. Photopatch testing: The 12-year experience of the German, Austrian, and Swiss photopatch test group. J. Am. Acad. Dermatol. 2000, 42, 183–192. [Google Scholar] [CrossRef]
- Jones, P.A.; Lovell, W.W.; King, A.V.; Earl, L.K. In vitro testing for phototoxic potential using the EpiDerm 3-D reconstructed human skin model. Toxicol. Methods 2001, 11, 1–19. [Google Scholar] [CrossRef]
- Spielmann, H.; Lovell, W.W.; Hölzle, E.; Johnson, B.E.; Maurer, T.; Miranda, M.A.; Pape, W.J.W.; Sapora, O.; Sladowski, D. In vitro phototoxicity testing: The report and recommendations of ECVAM workshop 2. Altern. Lab. Anim. 1994, 22, 314–348. [Google Scholar] [CrossRef]
- Spielmann, H.; Balls, M.; Dupuis, J.; Pape, W.; Pechovitch, G.; de Silva, O.; Holzhütter, H.-G.; Clothier, R.; Desolle, P.; Gerberick, F.; et al. The international EU/COLIPA in vitro phototoxicity validation study: Results of phase II (blind trial). Part 1: The 3T3 NRU phototoxicity test. Toxicol. Vitr. 1998, 12, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.-X.; Barrault, C.; Deguercy, A.; De Wever, B.; Rosdy, M. Development of a highly sensitive in vitro phototoxicity assay using the SkinEthicTM reconstructed human epidermis. Cell Biol. Toxicol. 2000, 16, 391–400. [Google Scholar] [CrossRef]
- Guillot, J.-P.; Gonnet, J.-F.; Loquerie, J.-F.; Martini, M.-C.; Convert, P.; Cotte, J. A new method for the assessment of phototoxic and photoallergic potentials by topical applications in the albino guinea pig. J. Toxicol. Cutan. Ocul. Toxicol. 1985, 4, 117–133. [Google Scholar] [CrossRef]
- Medina, J.; Elsaesser, C.; Picarles, V.; Grenet, O.; Kolopp, M.; Chibout, S.-D.; Fraissinette, A.d.B.d. Assessment of the phototoxic potential of compounds and finished topical products using a human reconstructed epidermis. Vitr. Mol. Toxicol. J. Basic Appl. Res. 2001, 14, 157–168. [Google Scholar] [CrossRef]
- Dillaha, C.J.; Jansen, G.T.; Honeycutt, W.M.; Bradford, A.C. Selective cytotoxic effect of topical 5-fluorouracil. Arch. Dermatol. 1963, 88, 247–256. [Google Scholar] [CrossRef]
- Reus, A.A.; Usta, M.; Kenny, J.D.; Clements, P.J.; Pruimboom-Brees, I.; Aylott, M.; Lynch, A.M.; Krul, C.A. The in vivo rat skin photomicronucleus assay: Phototoxicity and photogenotoxicity evaluation of six fluoroquinolones. Mutagenesis 2012, 27, 721–729. [Google Scholar] [CrossRef]
- Trevisi, P.; Vincenzi, C.; Chieregato, C.; Guerra, L.; Tosti, A. Sunscreen sensitization: A three-year study. Dermatology 1994, 189, 55–57. [Google Scholar] [CrossRef]
- Kroon, S. Standard photopatch testing with Waxtar®, para-aminobenzoic acid, potassium dichromate and balsam of Peru. Contact Dermat. 1983, 9, 5–9. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Tharmann, J.; Campos, P.M.M.; Liebsch, M. Skin phototoxicity of cosmetic formulations containing photounstable and photostable UV-filters and vitamin A palmitate. Toxicol. Vitr. 2013, 27, 418–425. [Google Scholar] [CrossRef]
- Peters, B.; Holzhütter, H.-G. Holzhütter, In vitro phototoxicity testing: Development and validation of a new concentration response analysis software and biostatistical analyses related to the use of various prediction models. Altern. Lab. Anim. 2002, 30, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, M. Prevalidation of the EpiDerm phototoxicity test. In Alternatives to Animal Testing II: Proceedings of the Second International Scientific Conference; European Cosmetic Industry: Brussels, Belgium, 1999. [Google Scholar]
- Kaidbey, K.H.; Kligman, A.M. Identification of topical photosensitizing agents in humans. J. Investig. Dermatol. 1978, 70, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Kandarova, H.; Raabe, H.; Hilberer, A.; Choksi, N.; Allen, D. Retrospective Review on In Vitro Phototoxicity Data Generated in 3D Skin Models to Support the Development of New OECD Test Guideline. Poster Presented at: Society of Toxicology USA (SOT), 2021 Virtual Annual Meeting, March. 2021. Available online: https://www.mattek.com/reference-library/retrospective-review-on-in-vitro-phototoxicity-data-generated-in-3d-skin-models-to-support-the-development-of-new-oecd-test-guideline/ (accessed on 24 April 2025).
- Kejlová, K.; Jírová, D.; Bendová, H.; Gajdoš, P.; Kolářová, H. Phototoxicity of essential oils intended for cosmetic use. Toxicol. Vitr. 2010, 24, 2084–2089. [Google Scholar] [CrossRef]
- Jones, P.; King, A.; Earl, L.; Lawrence, R. An assessment of the phototoxic hazard of a personal product ingredient using in vitro assays. Toxicol. Vitr. 2003, 17, 471–480. [Google Scholar] [CrossRef]
- Onoue, S.; Ochi, M.; Gandy, G.; Seto, Y.; Igarashi, N.; Yamauchi, Y.; Yamada, S. High-throughput screening system for identifying phototoxic potential of drug candidates based on derivatives of reactive oxygen metabolites. Pharm. Res. 2010, 27, 1610–1619. [Google Scholar] [CrossRef]
- Masuda, T.; Honda, S.; Nakauchi, Y.; Ito, H.; Kinoshitu, M. Photocontact dermatitis due to bithionol, TBS, diaphene and hexachlorophene. Jpn. J. Dermatol. (Ser. B) 1971, 81, 238–244. [Google Scholar]
- Ritacco, G.; Hilberer, A.; Lavelle, M.; Api, A.M. Use of alternative test methods in a tiered testing approach to address photoirritation potential of fragrance materials. Regul. Toxicol. Pharmacol. 2022, 129, 105098. [Google Scholar] [CrossRef]
- Review, C.I. Safety Assessment of Citrus-Derived Peel Oils as Used in Cosmetics. 2014. Available online: https://www.cir-safety.org/sites/default/files/cpeelo062014tent.pdf (accessed on 16 May 2025).
- Líšková, A.; Letašiová, S.; Jantová, S.; Brezová, V.; Kandarova, H. Evaluation of phototoxic and cytotoxic potential of TiO2 nanosheets in a 3D reconstructed human skin model. ALTEX-Altern. Anim. Exp. 2020, 37, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Jírová, D.; Kejlová, K.; Bendová, H.; Ditrichová, D.; Mezulániková, M. Phototoxicity of bituminous tars—Correspondence between results of 3T3 NRU PT, 3D skin model and experimental human data. Toxicol. Vitr. 2005, 19, 931–934. [Google Scholar] [CrossRef]
- Kejlová, K.; Jírová, D.; Bendová, H.; Kandárová, H.; Weidenhoffer, Z.; Kolářová, H.; Liebsch, M. Phototoxicity of bergamot oil assessed by in vitro techniques in combination with human patch tests. Toxicol. Vitr. 2007, 21, 1298–1303. [Google Scholar] [CrossRef]
- Jones, P.A.; King, A.V.; Lovell, W.W.; Earl, L.K. Phototoxicity testing using 3-D reconstructed human skin models. In Alternatives to Animal Testing II: Proceedings of the Second International Scientific Conference; European Cosmetic Industry: Brussels, Belgium; CPL Press: Newbury, UK, 1999. [Google Scholar]
- Kandarova, H. Evaluation and Validation of Reconstructed Human Skin Models as Alternatives to Animal Tests in Regulatory Toxicology. 2006. Available online: https://refubium.fu-berlin.de/bitstream/handle/fub188/5740/00_kandarova01.pdf?sequence=1&isAllowed=y (accessed on 24 April 2025).
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens: Review of a 15-year experience and of the literature. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; Raquet, N.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice II: Phototoxicity, photogenotoxicity, and inhibitory potency vs. cytochrome P450 3A4 activity. Food Chem. Toxicol. 2012, 50, 756–760. [Google Scholar] [CrossRef]
- Kirkup, M.; Narayan, S.; Kennedy, C. Cutaneous recall reactions with systemic fluorouracil. Dermatology 2003, 206, 175–176. [Google Scholar] [CrossRef]
- Kleinman, M.H.; Smith, M.D.; Kurali, E.; Kleinpeter, S.; Jiang, K.; Zhang, Y.; Kennedy-Gabb, S.A.; Lynch, A.M.; Geddes, C.D. An evaluation of chemical photoreactivity and the relationship to phototoxicity. Regul. Toxicol. Pharmacol. 2010, 58, 224–232. [Google Scholar] [CrossRef]
- Przybilla, B.; Ring, J.; Schwab, U.; Galosi, A.; Dorn, M.; Braun-Falco, O. Photosensitizing properties of nonsteroidal antirheumatic drugs in the photopatch test. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 1987, 38, 18–25. [Google Scholar]
- Fowler, C.L.; Campbell, T.A.; Puertolas, L.F.; Kaidbey, K.H. Photoreaction potential of orally administered levofloxacin in healthy subjects. Ann. Pharmacother. 2000, 34, 453–458. [Google Scholar]
- Seto, Y.; Inoue, R.; Ochi, M.; Gandy, G.; Yamada, S.; Onoue, S. Combined use of in vitro phototoxic assessments and cassette dosing pharmacokinetic study for phototoxicity characterization of fluoroquinolones. AAPS J. 2011, 13, 482–492. [Google Scholar] [CrossRef]
- Wagai, N.; Yoshida, M.; Takayama, S. Phototoxic potential of the new quinolone antibacterial agent levofloxacin in mice. Arzneimittelforschung 1992, 43, 404–405. [Google Scholar]
- Dam, C.; Bygum, A. Subacute cutaneous lupus erythematosus induced or exacerbated by proton pump inhibitors. Acta Derm.-Venereol. 2008, 88, 87–89. [Google Scholar] [CrossRef]
- Ljunggren, B.; Sjövall, P. Systemic quinine photosensitivity. Arch. Dermatol. 1986, 122, 909–911. [Google Scholar] [CrossRef]
- Seto, Y.; Ohtake, H.; Sato, H.; Onoue, S. Phototoxic risk assessment of dermally-applied chemicals with structural variety based on photoreactivity and skin deposition. Regul. Toxicol. Pharmacol. 2020, 113, 104619. [Google Scholar] [CrossRef] [PubMed]
- Bilaç, C.; Şahin, M.T.; Ermertcan, A.T.; Ermertcan, A.T. The Interaction of Topically Applied 2% Erythromycin, 4% Erythromycin and Tetracycline with Narrow Band UVB. J. Turk. Acad. Dermatol. 2008, 2, jtad82101a. [Google Scholar]
- Kandarova, H.; Liskova, A.; Milasova, T.; Letasiova, S. Inter-and intra-laboratory reproducibility of the in vitro photo-toxicity test using 3D reconstructed human epidermis model. Toxicol. Lett. 2018, 295, S130. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Agency. Review Report: TABRECTA Tablets 150 mg and 200 mg. 2020. Available online: https://www.pmda.go.jp/drugs/2020/P20200624005/300242000_30200AMX00494_A100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 214018. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214018Orig1s000IntegratedR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 217424. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/217424Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 209353. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/209353Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. Treatment of SARS-CoV-2 Infection in Hospitalized Patients with Moderate to Severe COVID-19 Infection Who Are at High Risk for Acute Respiratory Distress Syndrome (ARDS). 2022. Available online: https://www.fda.gov/media/162980/download (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 217417. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217417Orig1s000IntegratedR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 211964. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/211964Orig1s000IntegratedR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-038. 2007. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022038s000_PHARMR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 202211. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202211Orig1s000Pharm.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 212099. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212099Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Nubeqa. 2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/nubeqa-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: ORLADEYO Capsules 150 mg. 2020. Available online: https://www.pmda.go.jp/drugs/2020/P20201124005/252211000_30300AMX00031_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Background Information on LOQOA Tapes. 2015. Available online: https://www.pmda.go.jp/drugs/2015/P20150930001/400059000_22700AMX01021_B100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Background Information on NAILIN Capsules 100 mg. 2018. Available online: https://www.pmda.go.jp/drugs/2018/P20180117001/300089000_23000AMX00012000_B100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 216993. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216993Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 212725. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212725Orig1s000,%20212726Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Jyseleca. 2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/jyseleca-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Adempas. 2014. Available online: https://www.ema.europa.eu/en/documents/assessment-report/adempas-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Tafinlar. 2013. Available online: https://www.ema.europa.eu/en/documents/assessment-report/tafinlar-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Braftovi. 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/braftovi-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Mekinist. 2014. Available online: https://www.ema.europa.eu/en/documents/assessment-report/mekinist-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Background Information on Mekinist Tablets 0.5 mg and 2 mg. 2018. Available online: https://www.pmda.go.jp/drugs/2018/P20180320003/300242000_22800AMX00374000_B100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 204569 Pharmacology Review. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204569Orig1s000PharmR.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Lyfnua. 2023. Available online: https://www.ema.europa.eu/en/documents/assessment-report/lyfnua-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: BIKTARVY Combination Tablets. 2019. Available online: https://www.pmda.go.jp/drugs/2019/P20190423002/230867000_23100AMX00302_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: SUNLENCA Subcutaneous Injection 463.5 mg and Tablets 300 mg. 2023. Available online: https://www.pmda.go.jp/drugs/2023/P20230822001/230867000_30500AMX00183_A100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 210806. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210806Orig1s000,%20210807Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-291. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022291s000_PharmR_P3.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Visono Tape, Toa Eiyo Co., Ltd. 2019. Available online: https://www.pmda.go.jp/drugs/2019/P20190118001/480008000_22500AMX00993_A100_1.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Mektovi. 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/mektovi-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Adempas Tablets 0.5 mg, 1.0 mg, and 2.5 mg. 2013. Available online: https://www.pmda.go.jp/drugs/2013/P201300173/630004000_22600AMX00013000_A100_2.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 205834. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205834Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 204790. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204790Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 217347. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/217347Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: CRESEMBA Capsules 100 mg and Intravenous Infusion 200 mg. 2023. Available online: https://www.pmda.go.jp/drugs/2023/P20230118002/100898000_30400AMX00448_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Rebetol Capsules 200 mg. 2019. Available online: https://www.pmda.go.jp/drugs/2019/P20190116001/170050000_21300AMY00493_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Arokaris I.V. Infusion 235 mg. 2022. Available online: https://www.pmda.go.jp/drugs/2022/P20220325004/400107000_30400AMX00182_A100_2.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: EVRYSDI Dry Syrup 60 mg. 2021. Available online: https://www.pmda.go.jp/drugs/2021/P20210621001/450045000_30300AMX00294_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: ONGENTYS Tablets 25 mg. 2020. Available online: https://www.pmda.go.jp/drugs/2020/P20200619001/180188000_30200AMX00487_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Moizerto Ointment 0.3% and 1%. 2021. Available online: https://www.pmda.go.jp/drugs/2021/P20211001002/180078000_30300AMX00436_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Rapalimus Gel 0.2%. 2018. Available online: https://www.pmda.go.jp/drugs/2018/P20180402001/620095000_23000AMX00464_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Omjjara Tablets 100 mg, 150 mg, and 200 mg. 2024. Available online: https://www.pmda.go.jp/drugs/2024/P20240705002/340278000_30600AMX00155_A100_1.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Mayzent. 2019. Available online: https://www.ema.europa.eu/en/documents/assessment-report/mayzent-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 215358. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215358Orig1s000,Orig2s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: SCEMBLIX Tablets 20 mg, 40 mg. 2022. Available online: https://www.pmda.go.jp/drugs/2022/P20220330001/300242000_30400AMX00189_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Riamet Combination Tablets. 2016. Available online: https://www.pmda.go.jp/drugs/2016/P20161222001/300242000_22800AMX00727_A100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 212887. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/212887Orig1s000,212888Orig1s000IntegratedR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 207924. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: ADLUMIZ Tablets 50 mg. 2021. Available online: https://www.pmda.go.jp/drugs/2021/P20210113006/180188000_30300AMX00003_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: CORECTIM Ointment 0.5%. 2019. Available online: https://www.pmda.go.jp/drugs/2019/P20191209001/530614000_30200AMX00046_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Sirturo Tablets 100 mg. 2017. Available online: https://www.pmda.go.jp/drugs/2017/P20171228001/800155000_23000AMX00020_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Ofev Capsules 100 mg and 150 mg. 2015. Available online: https://www.pmda.go.jp/drugs/2015/P20150619001/530353000_22700AMX00693000_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Votrient Tablets 200 mg. 2012. Available online: https://www.pmda.go.jp/drugs/2012/P201200143/34027800_22400AMX01405_A100_2.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Evrenzo Tablets 20 mg, 50 mg, and 100 mg. 2019. Available online: https://www.pmda.go.jp/drugs/2019/P20191007001/800126000_30100AMX00239_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Uptravi Tablets 0.2 mg and 0.4 mg. 2016. Available online: https://www.pmda.go.jp/drugs/2016/P20161011001/530263000_22800AMX00702_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: FASLODEX Intramuscular Injection 250 mg. 2024. Available online: https://www.pmda.go.jp/drugs/2024/P20240618001/670227000_22300AMX01209_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Gonax Subcutaneous Injection 80 mg and 120 mg. 2012. Available online: https://www.pmda.go.jp/drugs/2012/P201200093/80012600_22400AMX00729000_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: ANEREM 50 mg for I.V. Injection. 2020. Available online: https://www.pmda.go.jp/drugs/2020/P20200120002/770098000_30200AMX00031_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Koselugo Capsules 10 mg and 25 mg. 2022. Available online: https://www.pmda.go.jp/drugs/2022/P20220926004/870056000_30400AMX00430000_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Jeselhy Tablets 40 mg. 2022. Available online: https://www.pmda.go.jp/drugs/2022/P20220706001/400107000_30400AMX00211_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Beleximpo Tablets 80 mg. 2020. Available online: https://www.pmda.go.jp/drugs/2020/P20200407003/180188000_30200AMX00437_A100_1.pdf (accessed on 24 April 2025).
- European Medicines Agency. CHMP Assessment Report: Zelboraf. 2012. Available online: https://www.ema.europa.eu/en/documents/assessment-report/zelboraf-epar-public-assessment-report_en.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Zelboraf Tablets 240 mg. 2014. Available online: https://www.pmda.go.jp/drugs/2014/P201400179/450045000_22600AMX01406_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Zavicefta Combination for Intravenous Infusion. 2024. Available online: https://www.pmda.go.jp/drugs/2024/P20240618004/672212000_30600AMX00154_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: Sprycel Tablets 20 mg and 50 mg. 2009. Available online: https://www.pmda.go.jp/drugs/2009/P200900013/670605000_22100AMX00395_A100_1.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Review Report: JOYCLU 30 mg Intra-Articular Injection. 2021. Available online: https://www.pmda.go.jp/drugs/2021/P20210303001/380003000_30300AMX00234_A100_1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-436. 2002. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_pharmr_P1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-749. 1997. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/20749_LAMISIL%20SOLUTION,%201%25_PHARMR.PDF (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-029. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022029s000pharmr.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 202736. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202736Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-941. 2000. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/020941s000_PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-307. 2001. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-307_Lotrimin_pharmr.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-122. 2007. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022122_PharmR_P2.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-501. 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021501s000_PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 211527. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211527Orig1s000MultidisclipineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 216632. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/216632Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 213189. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213189Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-600. 1997. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/20600_TAZORAC%200.05%25%20AND%200.1%25_PHARMR_P1.PDF (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 215272. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215272Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 19-931. 1996. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/019931ap.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-408. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022408Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 215309. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215309Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 215985. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215985Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-302. 2001. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-302_ELIDEL_pharmr_P7.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-629. 1996. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020629Orig1s000rev.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 208945 Pharmacology Review. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208945Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 208552. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208552Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-886. 1999. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20886_phrmr_P2.pdf (accessed on 24 April 2025).
- Pharmaceuticals and Medical Devices Agency. Report on the Deliberation ResultsAlabel Oral 1.5 g, Alaglio Oral 1.5 g. 2013. Available online: https://www.pmda.go.jp/files/000153482.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-055. 1999. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21055_Targretin_pharmr_P2.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 215064. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/215064Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 204708. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204708Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 22-395. 2009. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022395s000Pharmr.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-159. 2003. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21159_Loprox_pharmr.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 213433. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213433Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 21-794. 2005. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021794s000_PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 205175. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/205175Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 203567. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/203567Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 20-985. 2000. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20-985_Carac_Pharmr_P1.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 210361. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210361Orig1s000MultidisciplineR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 206255. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206255Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Center for Drug Evaluation and Research. NDA 204153. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204153Orig1s000PharmR.pdf (accessed on 24 April 2025).
- Marcin, S.; Aleksander, A. Acute toxicity assessment of nine organic UV filters using a set of biotests. Toxicol. Res. 2023, 39, 649–667. [Google Scholar] [CrossRef]




| Test Category | Test Strategy | Method | |
|---|---|---|---|
| Phototoxicity | In vitro | In vitro 3T3 Neutral Red Uptake Phototoxicity Test (OECD TG 432) | Cell viability before and after UV exposure |
| The Reactive Oxygen Species (ROS) Assay (OECD TG 495) | Measurement of ROS generation after light exposure, potentially causing cellular damage | ||
| The Reconstructed Human Epidermis Phototoxicity Test (OECD TG 498) | Tissue viability test on reconstructed human epidermis after chemical exposure with or without UV exposure | ||
| In vivo | Pigmented rats | Assessment of photoirritation responses in the skin and eyes after systemic administration of chemicals with or without UV exposure | |
| The Guinea Pig phototoxicity Test | Assessment of photoirritation responses in the shaved skin of guinea pigs after topical administration of chemicals with or without UV exposure | ||
| Photoallergy | In vivo | The Photo-LLNA (Local Lymph Node Assay) | A modified LLNA protocol measuring increased lymph node cell proliferation after UV exposure |
| The Guinea Pig Photoallergy Test | A modified version of the Guinea Pig Maximization Test (GPMT) designed to evaluate allergic responses following UV sensitization | ||
| Test Method | Prediction of Phototoxicity |
|---|---|
| OECD TG 432 [4] 3T3 Neutral Red Uptake Phototoxicity Test | Classification as phototoxic was assigned when the Photoirritation Factor (PIF) was ≥5 or the Mean Photo Effect (MPE) was ≥0.15. |
| OECD TG 495 [5] Reactive Oxygen Species Assay | Positive results were determined by the significant generation of reactive oxygen species (ROS) following UV exposure compared to non-irradiated controls. |
| OECD TG 498 [6] Reconstructed Human Epidermis Phototoxicity Test | Phototoxic if the UVA-exposed group(s) exhibit a 30% decrease in viability relative to the corresponding dark-exposed group. |
| No. | Chemical | CAS No. | Physical State | Molar Extinction Coefficient (M1 cm−1) | UV λmax | Human | Animal | TG432 (3T3) | TG495 (ROS) | TG498 (RhE Model) | Others | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chlorpromazine hydrochloride | 69-09-0 | Solid | 30,000–32,000 | 254 | PT | PT | PT | PT | PT | [7,8,9,10,11,12,13,14] | |
| 2 | Promethazine | 60-87-7 | Solid | 21,000 | 250 | PT | PT | PT | PT | [10,13,15,16,17,18,19,20] | ||
| 3 | 5-Methoxypsoralen (5-MOP or Bergapten) | 484-20-8 | Solid | 15,000 | 305 | PT | PT | PT | PT | [8,13,15,16,19,20,21,22,23] | ||
| 4 | 8-Methoxypsoralen (8-MOP or Methoxsalen) | 298-81-7 | Solid | 18,400 | 300 | PT | PT | PT | PT | PT | [8,9,11,13,14,15,16,18,19,22,23,24,25] | |
| 5 | Para-aminobenzoic acid (PABA) | 150-13-0 | Solid | 14,000 | 268 | PS (photopatch) | NPT | NPT | NPT | [11,13,14,15,16,20,21,26,27] | ||
| 6 | Benzophenone-3 | 131-57-7 | Solid | 1150 | 288 | PT | NPT | PT | NPT | [13,15,16,19,20,21,26] | ||
| 7 | Octyl methoxycinnamate | 5466-77-3 | Liquid | 22,500 | 310 | PT/NPT | NPT | NPT | NPT | NPT | [12,13,14,15,19,26] | |
| 8 | Ecamsule (Mexoryl SX) | 92761-26-7 | Solid | 32,000 | 344 | NPT | NPT | NPT | [12,13,15,16] | |||
| 9 | 6-Methylcoumarin | 92-48-8 | Solid | 9500 | 326 | PT | PT | PT | PT | NPT | [11,13,14,15,19,20,21,23,28,29] | |
| 10 | 4-tert-Butyl-3-methoxy-2,6-dinitrotoluene(Musk ambrette) | 83-66-9 | Liquid | NA | NA | PT | NPT | PT | NPT | [12,13,15,16,19,20,30] | ||
| 11 | Demeclocycline hydrochloride | 64-73-3 | Solid | 13,400 | 270 | PT | PT | PT | NPT | [8,12,13] | ||
| 12 | Bithionol | 97-18-7 | Solid | 20,000 | 258 | PT | PT | PT | PT | PT | [11,12,13,14,15,19,20,21,22,31] | |
| 13 | Bergamot oil | 8007-75-8 | Liquid | NA | NA | PT | PT | PT | PT | [8,13,15,16,19,20,23,30,31,32] | ||
| 14 | Acridine hydrochloride | 17784-47-3 | Solid | 11,800 | 355 | PT | PT | PT | PT | PT | [12,13,14,20] | |
| 15 | Anthracene | 120-12-7 | Solid | 19,300 | 251 | PT | PT | PT | PT | PT | [12,13,14,15,19,20,23] | |
| 16 | Neutral red | 553-24-2 | Solid | 8000 | 530 | PT | PT | PT | PT | PT | [11,13,15,19,20,21,30] | |
| 17 | Tetracyline | 60-54-8 | Solid | 27,000 | 276 | - | PT | - | PT | PT | [13,14,21] | |
| 18 | Penicillin G | 61-33-6 | Solid | 1700 | 264 | NPT | NPT | NPT | NPT | [10,13,15,19,30] | ||
| 19 | Sodium lauryl sulfate (SDS) | 151-21-3 | Solid | NA | NA | NPT | NPT | NPT | NPT | NPT | [8,10,11,12,13,14,15,18,19,20,21,23,29,30] | |
| 20 | Octyl salicylate | 118-60-5 | Liquid | 900–1500 | 307 | NPT | NPT | NPT | NPT | NPT | [12,13,14,15,30,32] | |
| 21 | Benzylidene Camphor Sulfonic Acid | 56039-58-8 | Solid | 28,000 | 310 | NPT | NPT | NPT | [12,13] | |||
| 22 | 4-Methylbenzylidene camphor (3-(4-Methylbenzylidene)camphor) | 36861-47-9 | Solid | 27,000 | 303 | PT/NPT | NPT | NPT | NPT | NPT | [10,12,13,15,19,30] | |
| 23 | Benzalkonium chloride | 139-07-1 | Liquid | NA | NA | NPT | NPT | NPT | [15,33] | |||
| 24 | Acridine | 260-94-6 | Solid | 11,800 | 355 | PT | PT | PT | PT | [14,15,19,20,23,30] | ||
| 25 | Amiodarone | 1951-25-3 | Solid | 25,000 | 242 | PT | PT | PT | PT | [11,12,15,19,20] | ||
| 26 | Chlorhexidine dihydrochloride | 3697-42-5 | Solid | 10,000 | 255 | NPT | NPT | [11,20] | ||||
| 27 | Chlorpromazine | 50-53-3 | Solid | 3500–4500 | 256 | PT | PT | PT | PT | [15,16,17,19,20,29,30,34] | ||
| 28 | Demeclocycline | 127-33-3 | Solid | 1200 | 350 | PT | PT | PT | [19,20] | |||
| 29 | Fenofibrate | 49562-28-9 | Solid | 9800 | 290–310 | PT | PT | PT | [8,14,19,20] | |||
| 30 | Furosemide | 54-31-9 | Solid | 2300 | 273 | PT | PT/NPT | PT | [8,9,14,19,20,29,35] | |||
| 31 | Hexachlorophene | 70-30-4 | Solid | 12,000–14,000 | 270–290 | PT/NPT | NPT | NPT | PT | [14,15,17,19,20,31,36] | ||
| 32 | Ketoprofen | 22071-15-4 | Solid | 2000–3000 | 260–280 | PT | PT/NPT | PT | PT | [8,9,12,14,19,20] | ||
| 33 | Nalidixic acid sodium salt | 3374-05-8 | Solid | 5000–7000 | 270–280 | PT | PT | PT | PT | [14,20] | ||
| 34 | Nalidixic acid | 389-08-2 | Solid | 7500 | 276 | PT | PT | PT | PT | [14,19,20] | ||
| 35 | Norfloxacin | 70458-96-7 | Solid | 8000–10,000 | 277–278 | PT | PT | PT | PT | [9,14,15,19,20,35] | ||
| 36 | Ofloxacin | 82419-36-1 | Solid | 8000–10,000 | 293 | PT | PT | PT | PT | [8,14,19,20,29] | ||
| 37 | Penicillin G sodium salt | 69-57-8 | Solid | 1000–2000 | 230–250 | NPT | NPT | NPT | NPT | [8,11,20,21] | ||
| 38 | Protoporphyrin IX | 553-12-8 | Solid | 80,000 | 400–430 | PT | PT | PT | PT | [15,19,20] | ||
| 39 | Protoporphyrin IX disodium salt | 50865-01-5 | Solid | 80,000–90,000 | 405–410 | PT | PT | PT | [12,20] | |||
| 40 | Xantryl(Rose Bengal (sodium salt)(1:2)) | 632-69-9 | Solid | 70,000 | 550 | PT | NPT | PT | PT | PT | [11,14,20,21,23] | |
| 41 | Tiaprofenic acid | 33005-95-7 | Solid | 4000–5000 | 270 | PT | PT | PT | [8,19,20] | |||
| 42 | Benzylidene camphor | 15087-24-8 | Solid | 22,000–24,000 | 300 | NPT | NPT | [12] | ||||
| 43 | 2-(Methylthio)phenol(o-(Methylthio)-phenol) | 1073-29-6 | Liquid | NA | NA | PT | [32,37] | |||||
| 44 | Polyacrylamidomethyl benzylidene camphor | 113783-61-2 | Solid | NPT | NPT | [12] | ||||||
| 45 | Sulisobenzone (Benzophenone-4) | 4065-45-6 | Solid | 20,000–30,000 | 290–360 | NPT | NPT | NPT | NPT | NPT | [9,11,12,14,15,19,29,35] | |
| 46 | Methyl-2-[(3,5,5- trimethylhexylidene) amino]benzoate | 67801-42-7 | Solid | NA | NA | NPT | PT | NPT | [32,37] | |||
| 47 | Promethazine hydrochloride | 58-33-3 | Solid | 21,000 | 257 | PT | PT | PT | [8,11,12,14,21] | |||
| 48 | Lime oil | 8008-26-2 | Liquid | 315 | PT | PT | [19,31,38] | |||||
| 49 | Lemon oil | 8008-56-8 | Liquid | PT | PT | PT/NPT | PT | [15,31,33] | ||||
| 50 | Orange oil | 8028-48-6 | Liquid | PT | PT | PT/NPT | PT | [15,31,33] | ||||
| 51 | Rue oil | 8014-29-7 | Liquid | PT | PT | [22,31] | ||||||
| 52 | Cumin oil | 8014-13-9 | Liquid | PT | PT | [22,31] | ||||||
| 53 | Angelica root oil | 8015-64-3 | Liquid | PT | PT | [22,31] | ||||||
| 54 | Methylene blue | 61-73-4 | Liquid | 74,000 | 665 | PT | [31] | |||||
| 55 | Eosin Y Disodium | 17372-87-1 | Solid | 44,000 | 515 | PT | [31] | |||||
| 56 | Disperse blue 35 | 12222-75-2 | Solid | NA | 450–600 | PT | [31] | |||||
| 57 | Bengal rose (Rose bengale) | 11121-48-5 | Solid | >5000 | 500–600 | PT | PT | PT | PT | [10,13,15,19,31] | ||
| 58 | 3,3′,4′,5-Tetrachlorosalicylanilide (TCSA) | 1154-59-2 | Solid | NA | NA | PT | PT | PT | [11,19,31] | |||
| 59 | Tribromsalan(3,5,4′-Tribromosalicylanilide,TBS) | 32055 | Solid | NA | NA | NPT | [31] | |||||
| 60 | Buclosamide(n-Butyl-4-chloro-2-hydroxybenzamide) | 575-74-6 | Solid | PT | [19,31] | |||||||
| 61 | Doxycycline hydrochloride | 10592-13-9 | Solid | 20,000 | 270–280 | PT | PT | PT | PT | PT | [9,11,14,19] | |
| 62 | Tetracycline hydrochloride | 64-75-5 | Solid | 10,000 | 270–280 | PT | PT/NPT | PT | PT/NPT | [11,13,15,19,23] | ||
| 63 | Piroxicam | 36322-90-4 | Solid | 4000 | 350 | PT | PT | NPT | PT | [11,14] | ||
| 64 | Cinnamaldehyde | 104-55-2 | Liquid | 6000 | 280 | PS | NPT | NPT | NPT | [11,15,25,39] | ||
| 65 | L-Histidine | 71-00-1 | Solid | 13,000 | 210 | NPT | NPT | NPT | weak PR | NPT | [8,11,12,14,15,19,21] | |
| 66 | Thiocarbamide(Thiourea) | 62-56-6 | Solid | 7000 | 210 | PS | NPT | [11,19] | ||||
| 67 | Carprofen | 53716-49-7 | Solid | 2000 | 254 | PT | PT | [17,19] | ||||
| 68 | Fenticlor | 97-24-5 | Solid | 1000 | 250 | PT | [17,19] | |||||
| 69 | 7-Acetyl-1,1,3,4,4,6- hexamethyltetra Hydronaphthalene | 21145-77-7 | Solid | PT | [22] | |||||||
| 70 | Galaxolide(4,6,6,7,8,8-Hexamethyl-1,3,4,6,7,8-hexahydrocyclopenta[g]isochromene) | 1222-05-5 | Liquid | NA | NA | PT | [22] | |||||
| 71 | Celestolide(4-Acetyl-6-tert-butyl-1,1-dimethylindane) | 13171-00-1 | Solid | NA | NA | NPT | [22] | |||||
| 72 | Phantolide | 15323-35-0 | Solid | NA | NA | NPT | [22] | |||||
| 73 | Versalide | 88-29-9 | Solid | NA | NA | NPT | [22] | |||||
| 74 | Ichthammol | 8029-68-3 | Liquid | PT | PT | PT | [15,40] | |||||
| 75 | 5-Aminolevulinic acid | 106-60-5 | Solid | 28,000 | 400 | PT | PT | PT | [15] | |||
| 76 | 7-Methylcoumarin | 2445-83-2 | Solid | NA | NA | PT | PT | PT | [15] | |||
| 77 | Tetrachlorosalicylanilide | 2018517 | Solid | NA | NA | PT | PT | PT | [15] | |||
| 78 | Deterpenated lemon (-) | NA | NA | PT/NPT | PT/NPT | PT | [15,41] | |||||
| 79 | Litsea cubeba oil | 68855-99-2 | Liquid | NA | NA | NPT | PT | NPT | [15,41] | |||
| 80 | ichthyolic acid, sodium salt | 1340-06-3 | Solid | NA | NA | NPT | PT | NPT | [15] | |||
| 81 | Avobenzone | 70356-09-1 | Solid | 30,000–40,000 | 320–400 | PT/NPT | NPT | PT | PT | NPT | [10,14,15,23,28,37,39,41,42,43,44] | |
| 82 | Dimethyl sulfoxide | 67-68-5 | Liquid | NA | NA | NPT | NPT | NPT | [15] | |||
| 83 | Ethanol | 64-17-5 | Liquid | NA | NA | NPT | NPT | NPT | [15,18] | |||
| 84 | Eucalyptus oil | 8000-48-4 | Liquid | NA | NA | NPT | NPT | NPT | [15] | |||
| 85 | Coumarin | 91-64-5 | Solid | 16,000–18,000 | 275 | NPT | NPT | NPT | [8,15] | |||
| 86 | Titanium (IV) oxide | 13463-67-7 | Solid | ~100,000 | 320–350 | NPT | [15,39,43] | |||||
| 87 | Cadmium sulfide | 1306-23-6 | Solid | 58,000 | 450 | PT | NPT | [7] | ||||
| 88 | Cadmium selenide | 1306-24-7 | Solid | ~100,000 | 560 | NPT | NPT | [7] | ||||
| 89 | Mercury(II) sulfide | 1344-48-5 | Solid | NPT | NPT | [7] | ||||||
| 90 | Chromium oxide | 11118-57-3 | Solid | 10,000–40,000 | visible region | NPT | NPT | [7] | ||||
| 91 | Carbazole | 86-74-8 | Solid | 6000–7000 | 290 | PT | PT | [7] | ||||
| 92 | Cobalt aluminum oxide | 13820-62-7 | Solid | 500–700 | NPT | NPT | [7] | |||||
| 93 | Benoxaprofen | 51234-28-7 | Solid | 320 | PT | PT | [19] | |||||
| 94 | Naproxen | 22204-53-1 | Solid | 19,300 | 330 | PT | PT | [19] | ||||
| 95 | Suprofen | 40828-46-4 | Solid | 244 | PT | [19] | ||||||
| 96 | Triclosan | 3380-34-5 | Solid | 4200 | 280 | PT | [19] | |||||
| 97 | Ciprofloxacin | 85721-33-1 | Solid | 33,000 | 278 | PT | PT | [19,25] | ||||
| 98 | Fleroxacin | 79660-72-3 | Solid | 25,000–35,000 | 278 | [19] | ||||||
| 99 | lomefloxacin | 98079-51-7 | Solid | 27,000–30,000 | 287 | PT | PT | [8,19] | ||||
| 100 | Bergaptol | 486-60-2 | Solid | 290–320 | NPT (V79 micronucleus assay) | [45] | ||||||
| 101 | Isopsoralen | 523-50-2 | Solid | 15,000–20,000 | 300–320 | PT | [8,19] | |||||
| 102 | 4,5′,8-Trimethylpsoralen (trioxsalen) | 3902-71-4 | Solid | 10,000–20,000 | 300–320 | PT | [19] | |||||
| 103 | 5-Fluorouracil | 51-21-8 | Solid | 8200 | 266 | NPT | NPT | [9,14,24,35,46,47] | ||||
| 104 | Amiodarone hydrochloride | 19774-82-4 | Solid | 290–350 | PT | PT | PT | PT | [9,14,19,35] | |||
| 105 | Diclofenac sodium | 15307-79-6 | Solid | 11,000–13,000 | 276 | PT | PT | PT | PT | [9,14,19,35,48] | ||
| 106 | Levofloxacin | 100986-85-4 | Solid | 16,000 | 292 | PT | PT | PT | PT | [14,25,49,50,51] | ||
| 107 | Omeprazole | 73590-58-6 | Solid | 18,000–22,000 | 302 | PT | PT/NPT | PT | [9,14,35,49,52] | |||
| 108 | Quinine hydrochloride | 130-89-2 | Solid | 5460 | 350 | PT | NPT | PT | PT | [9,14,19,35,53] | ||
| 109 | Rosiglitazone | 122320-73-4 | Solid | PT | [14] | |||||||
| 110 | Bumetrizole | 729335 | Solid | 300–400 | NPT | NPT | [14] | |||||
| 111 | Camphor sulfonic acid | 1450959 | Solid | NPT | NPT | [14] | ||||||
| 112 | Cinnamic acid | 140-10-3 | Solid | 8000–10,000 | 260 | NPT | Weak PT | [14] | ||||
| 113 | Drometrizole | 2440-22-4 | Solid | 290–320 | NPT | NPT | [14] | |||||
| 114 | Octrizole | 3147-75-9 | Solid | 290–320 | NPT | NPT | [14] | |||||
| 115 | 2-(2H-Benzotriazol-2-yl)-6-dodecyl-4-methylphenol | 125304-04-3 | Liquid | 290–400 | NPT | [14] | ||||||
| 116 | Aspirin | 50-78-2 | Solid | 15,000–20,000 | 265 | NPT | NPT | [14,35] | ||||
| 117 | Benzocaine | 34584 | Solid | 9000–11,000 | 260 | NPT | NPT | [35] | ||||
| 118 | Erythromycin | 114-07-8 | Solid | 8000–10,000 | 230 | NPT | NPT | NPT | NPT | [35,54,55] | ||
| 119 | Penicillin G potassium | 113-98-4 | Solid | 203 | NPT | NPT | [14,35] | |||||
| 120 | Phenytoin | 57-41-0 | Solid | 4500–6500 | 254 | NPT | Weak PT | [14,35] | ||||
| 121 | Chlorhexidine | 55-56-1 | Solid | 1200–1400 | 260 | NPT | Weak PT/NPT | [8,14,19] | ||||
| 122 | Octyl methacrylate | 93878 | Liquid | 210–220 | NPT | NPT | [14] | |||||
| 123 | Ethyl vanillin | 121-32-4 | Solid | 11,000–13,000 | 270 | (inconclusive) | NPT | PT | NPT | [32,37] | ||
| 124 | Vanillin isobutyrate | 20665-85-4 | Liquid | 270–280 | (inconclusive) | NPT | PT | NPT | [32,37] | |||
| 125 | Methyl 2,4-dihydroxy-3-methylbenzoate | 33662-58-7 | Solid | 270–300 | (inconclusive) | NPT | PT | PT | [32,37] | |||
| 126 | 10H-Phenothiazine | 92-84-2 | Solid | 255–265 | PT | PT | PT | [32,56] | ||||
| 127 | 4-Acetoxy-3-ethoxybenzaldehyde | 72207-94-4 | Solid | 250–300 | (inconclusive) | PT | NPT | [32,37] | ||||
| 128 | 1-phenyl-3-(4-propan-2-ylphenyl)propane-1,3-dione | 63250-25-9 | - | 250–300 | PT | [19,26] | ||||||
| 129 | Minocycline | 10118-90-8 | Solid | 7700 | 350 | NPT | [19] | |||||
| 130 | Chlordiazepoxide | 58-25-3 | Solid | 250–260 | PT | [19] | ||||||
| 131 | Diflunisal | 22494-42-4 | Solid | 252 | PT | [19] | ||||||
| 132 | Griseofulvin | 126-07-8 | Solid | 290 | PT | [19] | ||||||
| 133 | Chloroquine | 19851 | Solid | 7000–12,000 | 260 | NPT | [19] | |||||
| 134 | Chlortetracycline | 57-62-5 | Solid | 8500–12,000 | 270 | NPT | [19] | |||||
| 135 | chlorothiazide | 58-94-6 | Solid | 260 | NPT | [19] | ||||||
| 136 | Clomocycline | 1181-54-0 | - | 270–280 | NPT | [19] | ||||||
| 137 | Cyclamic acid | 100-88-9 | Solid | 250–260 | NPT | [19] | ||||||
| 138 | Fenoprofen | 29679-58-1 | Solid | 270–280 | NPT | [19] | ||||||
| 139 | Flurbiprofen | 5104-49-4 | Solid | 247–260 | NPT | [19] | ||||||
| 140 | Ibuprofen | 15687-27-1 | Solid | 264 | NPT | [19] | ||||||
| 141 | Indoprofen | 31842-01-0 | Solid | 250–280 | NPT | [19] | ||||||
| 142 | Methacycline | 914-00-1 | Solid | 270–280 | NPT | [19] | ||||||
| 143 | Oxytetracycline | 79-57-2 | Solid | 270 | NPT | [19] | ||||||
| 144 | Tolbutamide | 64-77-7 | Solid | 230–240 | NPT | [19] | ||||||
| 145 | Vanillin propylene glycol acetal | 68527-74-2 | Liquid | 270–280 | (inconclusive) | PT | NPT | [19,37] | ||||
| 146 | 4′-Hydroxy-3′-methoxyacetophenone(acetovanillone) | 498-02-2 | Solid | 280–300 | (inconclusive) | PT | NPT | [19,37] | ||||
| 147 | Vanillin | 121-33-5 | Solid | 15,000–18,000 | 270 | PT | NPT | [37] | ||||
| 148 | 2-Methoxycinnamaldehyde | 1504-74-1 | Solid | 270–290 | (inconclusive) | PT | NPT | [37] | ||||
| 149 | 5-Methylquinoxaline | 13708-12-8 | Liquid | 260–300 | (inconclusive) | PT | PT | [37] | ||||
| 150 | Capmatinib | 1029712-80-8 | Solid | PS | PT | [57] | ||||||
| 151 | Fosdenopterin hydrobromide | 2301083-34-9 | Solid | PT | PT | [58] | ||||||
| 152 | Berdazimer sodium | 1846565-00-1 | gel | NPT(UV spectrum) | [59] | |||||||
| 153 | Tretinoin | 302-79-4 | Solid | NPT | [60] | |||||||
| 154 | Sabizabulin | 1332881-26-1 | Solid | PT | [61] | |||||||
| 155 | Rezafungin acetate | 1631754-41-0 | Solid | PT | PT | [62] | ||||||
| 156 | Viloxazine hydrochloride | 35604-67-2 | Solid | 56.62 | 290 | NPT(UV spectrum) | [63] | |||||
| 157 | ESTRADIOL | 50-28-2 | Solid | NPT | [64] | |||||||
| 158 | Oxybutynin | 5633-20-5 | Solid | NPT | [65] | |||||||
| 159 | Darolutamide | 1297538-32-9 | Solid | 23,100–22,500 | 290–320 | NPT | NPT(UV spectrum) | [66,67] | ||||
| 160 | Berotralstat Hydrochloride | 1809010-52-3 | Solid | NPT | [68] | |||||||
| 161 | Esflurbiprofen | 51543-39-6 | Solid | NPT | [69] | |||||||
| 162 | Fosravuconazole L-lysine ethanolate | 914361-45-8 | Solid | NPT | [70] | |||||||
| 163 | Quizartinib | 950769-58-1 | Solid | NPT | [71] | |||||||
| 164 | Entrectinib | 1108743-60-7 | Solid | PT | PT | [72] | ||||||
| 165 | Filgotinib Maleate | 1802998-75-9 | Solid | NPT | PT | [73] | ||||||
| 166 | Riociguat | 625115-55-1 | Solid | 290–720 | NPT | PT/NPT | [74] | |||||
| 167 | Dabrafenib Mesylate | 1195768-06-9 | Solid | PT | [75] | |||||||
| 168 | Encorafenib | 1269440-17-6 | Solid | PT | [76] | |||||||
| 169 | Trametinib Dimethyl Sulfoxide | 1187431-43-1 | Solid | >1000 | 290–700 | PT | PT | [77,78] | ||||
| 170 | Suvorexant | 1030377-33-3 | Solid | NPT | [79] | |||||||
| 171 | Molnupiravir | 2349386-89-4 | Solid | >1000 | 290–700 | NPT | NPT(UV spectrum) | [79] | ||||
| 172 | Gefapixant | 1015787-98-0 | Solid | 290–350 | NPT | NPT(UV spectrum | [80] | |||||
| 173 | Bictegravir sodium | 1807988-02-8 | Solid | NPT | PT | [81] | ||||||
| 174 | Lenacapavir sodium | - | Solid | NPT | [82] | |||||||
| 175 | Doravirine | 1338225-97-0 | Solid | NPT | NPT | [83] | ||||||
| 176 | Eltrombopag olamine | 496775-62-3 | Solid | NPT | NPT | PT | [84] | |||||
| 177 | Bisoprolol | 66722-44-9 | Solid | PT/PS | [85] | |||||||
| 178 | Binimetinib | 606143-89-9 | Solid | PT | PT | [86] | ||||||
| 179 | Ibandronate Sodium | 138844-81-2 | Solid | NPS | PT | [87] | ||||||
| 180 | Ledipasvir | 1256388-51-8 | Solid | NPT | [88] | |||||||
| Sofosbuvir | 1190307-88-0 | Solid | ||||||||||
| 181 | Dolutegravir sodium | 1051375-19-9 | Solid | NPT | [89] | |||||||
| 182 | Sofpironium Bromide | 1628106-94-4 | Solid | >1000 | NPT(UV spectrum) | [90] | ||||||
| 183 | Isavuconazonium sulfate | 946075-13-4 | Solid | NPT | [91] | |||||||
| 184 | Ribavirin | 36791-04-5 | Solid | NPT | PT | [92] | ||||||
| 185 | Fosnetupitant Chloride Hydrochloride | 1643757-72-5 | Solid | PT | [93] | |||||||
| 186 | Risdiplam | 1825352-65-5 | Solid | NPT | [94] | |||||||
| 187 | Opicapone | 923287-50-7 | Solid | NPS | NPT | [95] | ||||||
| 188 | Difamilast | 937782-05-3 | Solid | NPT/NPS | NPT | [96] | ||||||
| 189 | Sirolimus((-)-Rapamycin) | 53123-88-9 | Solid | NPS | NPT | [97] | ||||||
| 190 | Momelotinib | 1056634-68-4 | Solid | NPT | [98] | |||||||
| 191 | Siponimod | 1230487-00-9 | Solid | 3309 | 260 | NPT | [99] | |||||
| 192 | Asciminib | 1492952-76-7 | Solid | PT/PS | PT | [100,101] | ||||||
| 193 | Artemether | 71963-77-4 | Solid | PT | [102] | |||||||
| Lumefantrine | 82186-77-4 | Solid | ||||||||||
| 194 | Cabotegravir | 1051375-10-0 | Solid | 2670–20,800 | 257 | NPT(UV spectrum) | [103] | |||||
| 195 | Baricitinib | 1187594-09-7 | Solid | >1000 | 290–329 | NPT | PT(UV spectrum) | [104] | ||||
| 196 | Anamorelin | 249921-19-5 | Solid | 4958 | 291 | NPT(UV spectrum) | [105] | |||||
| 197 | Delgocitinib | 1263774-59-9 | Solid | NPT/NPS | NPT | [106] | ||||||
| 198 | Bedaquiline fumarate | 845533-86-0 | Solid | NPT/NPS | PT | [107] | ||||||
| 199 | Nintedanib | 656247-17-5 | Solid | PT | [108] | |||||||
| 200 | Pazopanib | 444731-52-6 | Solid | NPT | [109] | |||||||
| 201 | Roxadustat | 808118-40-3 | Solid | NPT | [110] | |||||||
| 202 | Selexipag | 475086-01-2 | Solid | mild PT | PT | [111] | ||||||
| 203 | Fulvestrant | 129453-61-8 | Solid | NPT | [112] | |||||||
| 204 | Degarelix acetate | 934016-19-0 | Solid | NPT | [113] | |||||||
| 205 | Remimazolam besilate | 1001415-66-2 | Solid | NPT | [114] | |||||||
| 206 | Selumetinib | 606143-52-6 | Solid | 11,786 | 290 | NPT | [115] | |||||
| 207 | Pimitespib | 1260533-36-5 | Solid | NPT | [116] | |||||||
| 208 | Tirabrutinib hydrochloride | 1439901-97-9 | Solid | NPT | [117] | |||||||
| 209 | Vemurafenib | 918504-65-1 | Solid | 240–450 | NPT | PT | [118,119] | |||||
| 210 | Avibactam Sodium | 1192491-61-4 | Solid | NPT | [120] | |||||||
| Ceftazidime pentahydrate | 78439-06-2 | Solid | ||||||||||
| 211 | Dasatinib | 302962-49-8 | Solid | NPT | PT | [121] | ||||||
| 212 | Diclofenac Etalhyaluronate Sodium | 1398396-25-2 | viscous | NPT | [122] | |||||||
| 213 | Aripiprazole | 129722-12-9 | Solid | NPT | [123] | |||||||
| 214 | Terbinafine hydrochloride | 78628-80-5 | Solid | NPT | NPT/NPS | [124] | ||||||
| 215 | Methyl Salicylate | 119-36-8 | liquid | NPT/NPS | [125] | |||||||
| 216 | Ivermectin | 70288-86-7 | Solid | <290 | UV spectrum | [126] | ||||||
| 217 | Docosanol | 661-19-8 | solid | NPT/NPS | [127] | |||||||
| 218 | Butenafine hydrochloride | 101827-46-7 | solid | NPT/NPS | [128] | |||||||
| 219 | Diclofenac sodium | 15307-79-6 | solid | NPT/NPS | [129] | |||||||
| 220 | Avobenzone | 70356-09-1 | Solid | NPT/NPS | [130] | |||||||
| Ecamsule | 92761-26-7 | Solid | 32,000 | 344 | ||||||||
| Octocrylene | 6197-30-4 | liquid | ||||||||||
| 221 | Trifarotene | 895542-09-3 | solid | NPT | [131] | |||||||
| 222 | Adapalene | 106685-40-9 | solid | NPT | [132] | |||||||
| 223 | TIRBANIBUlin 1% | 897016-82-9 | solid | NPT/NPS | [133] | |||||||
| 224 | Tazarotene | 118292-40-3 | solid | NPT/NPS | [134] | |||||||
| 225 | Tapinarof | 79338-84-4 | solid | NPT | [135] | |||||||
| 226 | SULFACETAMIDE SODIUM | 127-56-0 | solid | NPT | [136] | |||||||
| 227 | SULCONAZOLE NITRATE | 61318-91-0 | solid | NPT | [137] | |||||||
| 228 | Spinosyn A | 131929-60-7 | solid | NPT | [137] | |||||||
| Spinosyn D | 131929-63-0 | solid | ||||||||||
| 229 | Sofpironium bromide | 1628106-94-4 | solid/gel | NPT(UV spectrum) | [90] | |||||||
| 230 | Ruxolitinib | 941678-49-5 | oil | NPT | NPT | [138] | ||||||
| 231 | Roflumilast | 162401-32-3 | solid | NPT | NPT | [139] | ||||||
| 232 | Pimecrolimus | 137071-32-0 | solid | NPT | PT | [140] | ||||||
| 233 | Penciclovir | 39809-25-1 | solid | NPT | NPT | NPT(UV spectrum | [141] | |||||
| 234 | Ozenoxacin | 245765-41-7 | solid | NPT | NPT | [142] | ||||||
| 235 | Oxymetazoline hydrochloride | 151615 | solid | NPT | [143] | |||||||
| 236 | Alitretinoin | 1241893 | solid | PT | PT(hemoglobin assay, histidine assay) | [144] | ||||||
| 237 | Aminolevulinic acid hydrochloride | 1297222 | solid | PS | PT | [145] | ||||||
| 238 | Benzoyl peroxide | 94-36-0 | solid | NPT | PT(hemoglobin assay, histidine assay) | [146] | ||||||
| 239 | Birch triterpenes | botanical drug | gel | NPS | NPS | NPT | [147] | |||||
| 240 | Brimonidine tartrate | 70359-46-5 | solid | NPT | [148] | |||||||
| 241 | Bexarotene | 153559-49-0 | solid | NPT | PT(hemoglobin assay, histidine assay) | [146] | ||||||
| 242 | Capsaicin | 404-86-4 | solid | NPT | [149] | |||||||
| 243 | Ciclopirox | 29342-05-0 | solid | NPT | [150] | |||||||
| 244 | Clascoterone | 19608-29-8 | solid | NPT(UV spectrum) | [151] | |||||||
| 245 | Dapsone | 80-08-0 | solid | NPT | [152] | |||||||
| 246 | Econazole nitrate | 24169-02-6 | solid | NPT | [153] | |||||||
| 247 | Efinaconazole | 164650-44-6 | solid | NPT | [154] | |||||||
| 248 | Fluorouracil | 51-21-8 | solid | 265–266 | NPT | [155] | ||||||
| 249 | Glycopyrronium tosylate | 1883451-12-4 | solid | NPT(UV spectrum) | [156] | |||||||
| 250 | Ivermectin | 70288-86-7 | solid | NPT | [157] | |||||||
| 251 | Luliconazole | 187164-19-8 | solid | NPT/NPS | [158] |
| Animal | |||
|---|---|---|---|
| PT | NPT | ||
| Human | PT | 40 | 4 |
| NPT | 1 | 13 | |
| Total (n) | 58 | ||
| Sensitivity | 90.9% | ||
| Specificity | 92.9% | ||
| Accuracy | 91.4% | ||
| TG432 (3T3 NRU) | TG495 (ROS Assay) | TG498 (Epiderm Model) | ||||
|---|---|---|---|---|---|---|
| PT | NPT | PT | NPT | PT | NPT | |
| Human vs. in vitro phototoxic test results | ||||||
| PT | 36 | 1 | 22 | 19 | 4 | |
| NPT | 2 | 13 | 5 | 7 | ||
| Total (n) | 52 | 27 | 30 | |||
| Sensitivity | 97.3% | 100% | 82.6% | |||
| Specificity | 86.7% | 100% | 100% | |||
| Accuracy | 94.2% | 100% | 86.7% | |||
| Animal vs. in vitro phototoxic test results | ||||||
| PT | 38 | 1 | 17 | 23 | 2 | |
| NPT | 16 | 19 | 4 | 7 | 2 | 19 |
| Total (n) | 74 | 28 | 46 | |||
| Sensitivity | 97.4% | 100% | 92.0% | |||
| Specificity | 54.3% | 63.6% | 90.5% | |||
| Accuracy | 77.0% | 85.7% | 91.3% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-Y.; Hwang, J.-H.; Hong, J.-H.; Bae, S.; Lim, K.-M. PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods. Toxics 2025, 13, 545. https://doi.org/10.3390/toxics13070545
Lee G-Y, Hwang J-H, Hong J-H, Bae S, Lim K-M. PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods. Toxics. 2025; 13(7):545. https://doi.org/10.3390/toxics13070545
Chicago/Turabian StyleLee, Ga-Young, Jee-Hyun Hwang, Jeong-Hyun Hong, Seungjin Bae, and Kyung-Min Lim. 2025. "PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods" Toxics 13, no. 7: 545. https://doi.org/10.3390/toxics13070545
APA StyleLee, G.-Y., Hwang, J.-H., Hong, J.-H., Bae, S., & Lim, K.-M. (2025). PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods. Toxics, 13(7), 545. https://doi.org/10.3390/toxics13070545









