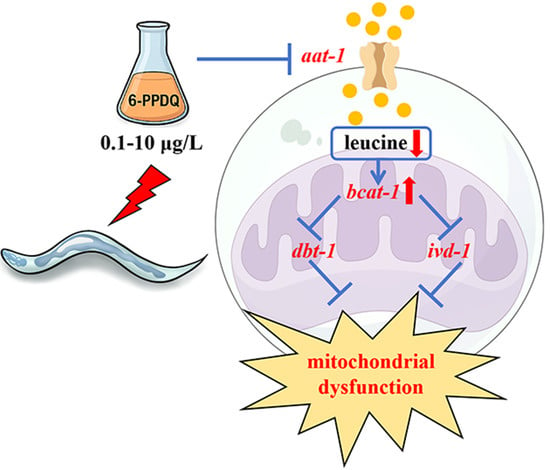
Exposure to 6-PPD Quinone Disrupts Adsorption and Catabolism of Leucine and Causes Mitochondrial Dysfunction in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. 6-PPDQ Exposure
2.3. Leucine Content Assay
2.4. Transcriptional Expression
2.5. NADH and FADH2 Contents
2.6. Mitochondrial Function
2.7. Toxicity Assessments
2.8. RNA Interference (RNAi)
2.9. Pharmacological Treatment
2.10. Data Analysis
3. Results
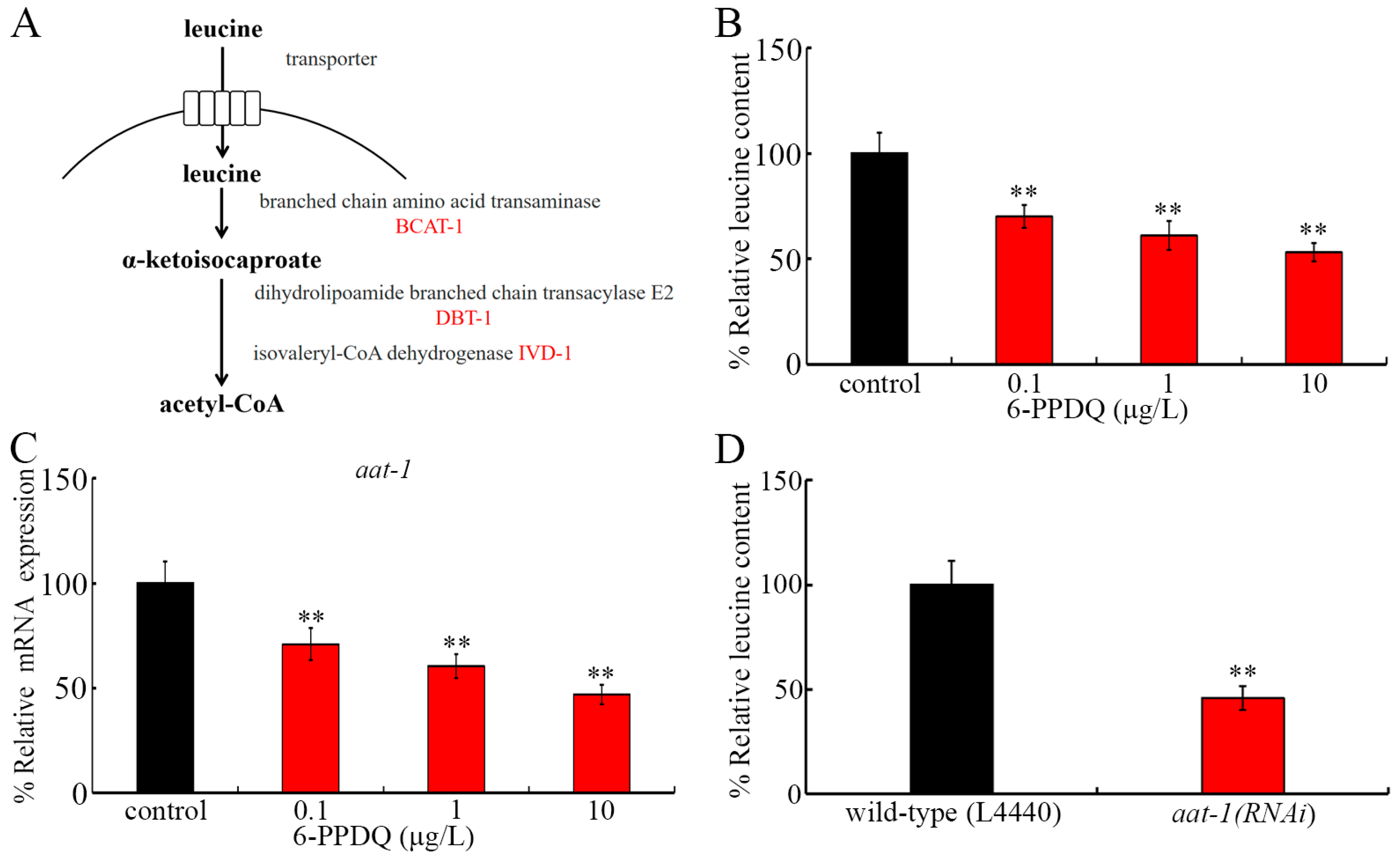
3.1. 6-PPDQ Reduced Leucine Content
3.2. Exposure to 6-PPDQ Inhibited Leucine Adsorption
3.3. 6-PPDQ Accelerated 6-PPDQ Catabolism
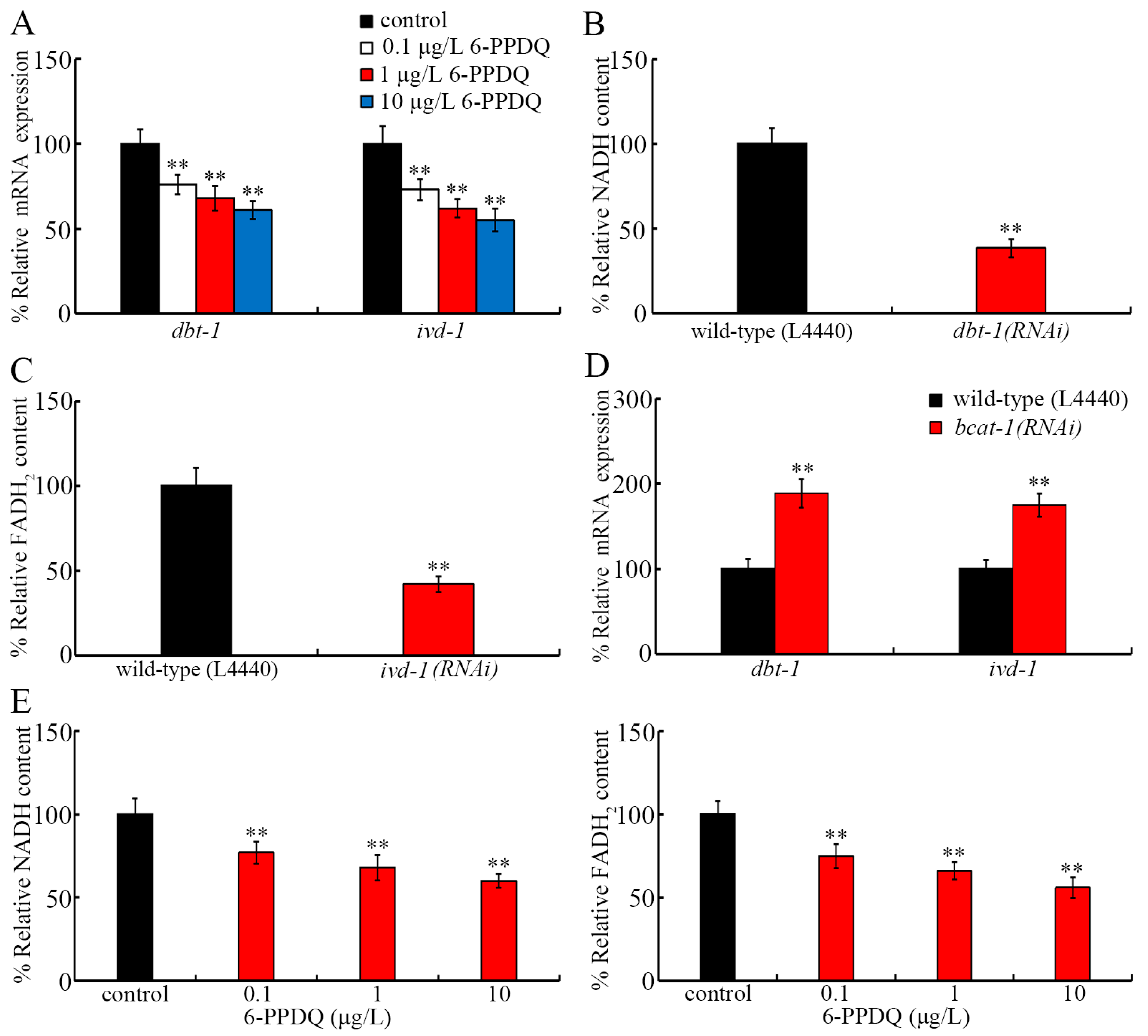
3.4. 6-PPDQ Affected Expressions of dbt-1 and ivd-1 and Their Functions
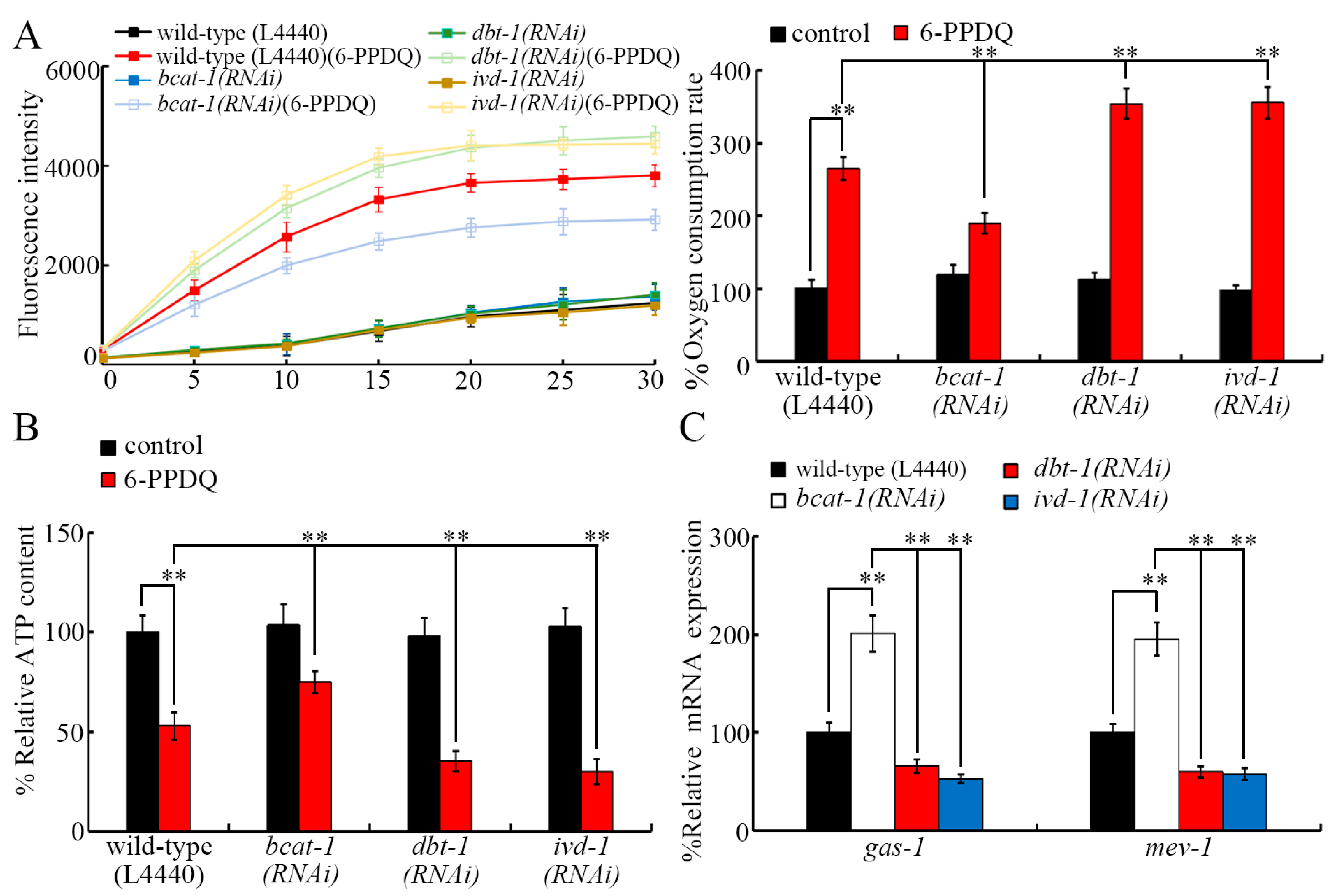
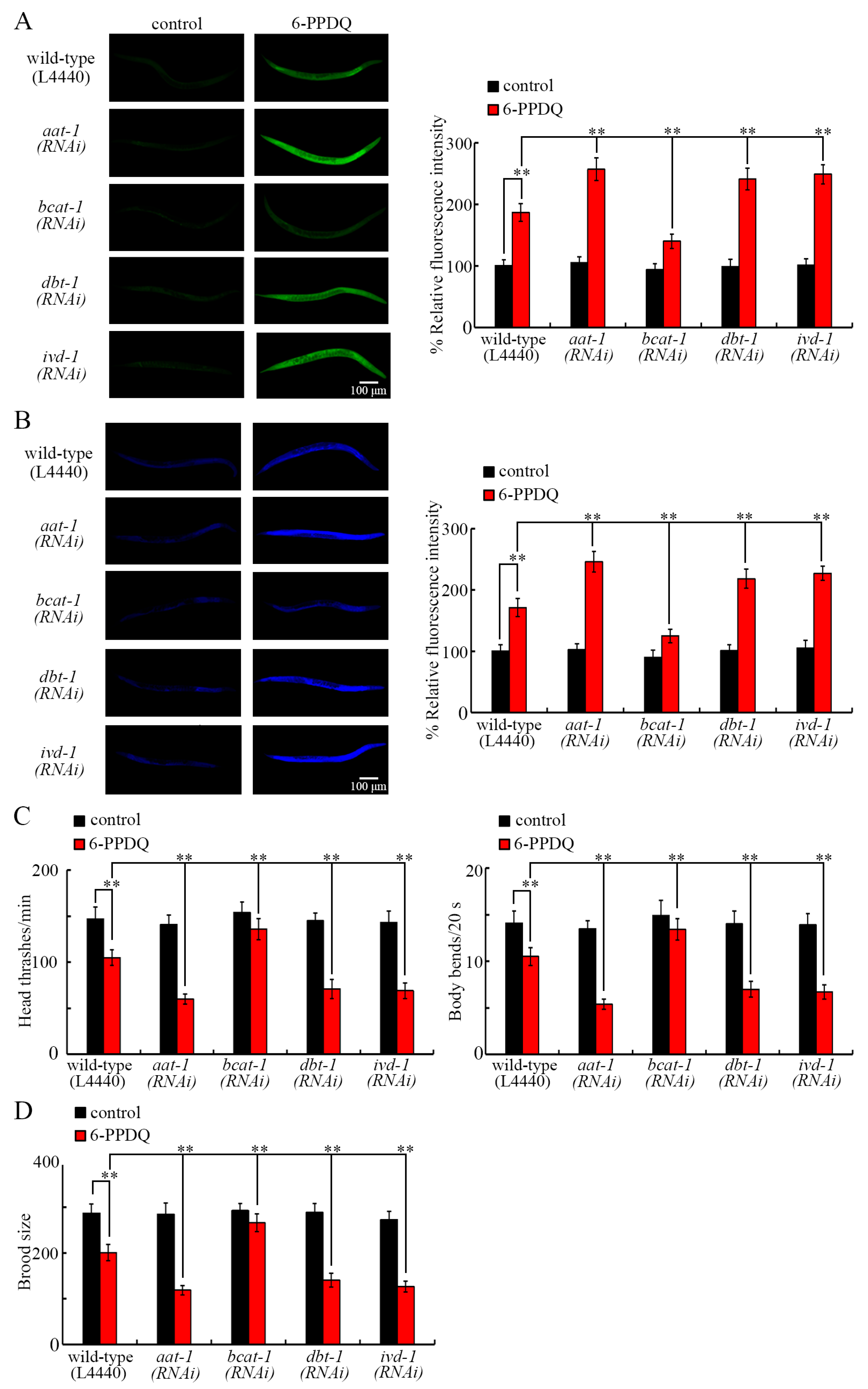
3.5. RNAi of bcat-1, dbt-1, and ivd-1 Affected 6-PPDQ-Induced Mitochondrial Dysfunction
3.6. RNAi of aat-1, bcat-1, dbt-1, and ivd-1 Affected Induction of 6-PPDQ Toxicity
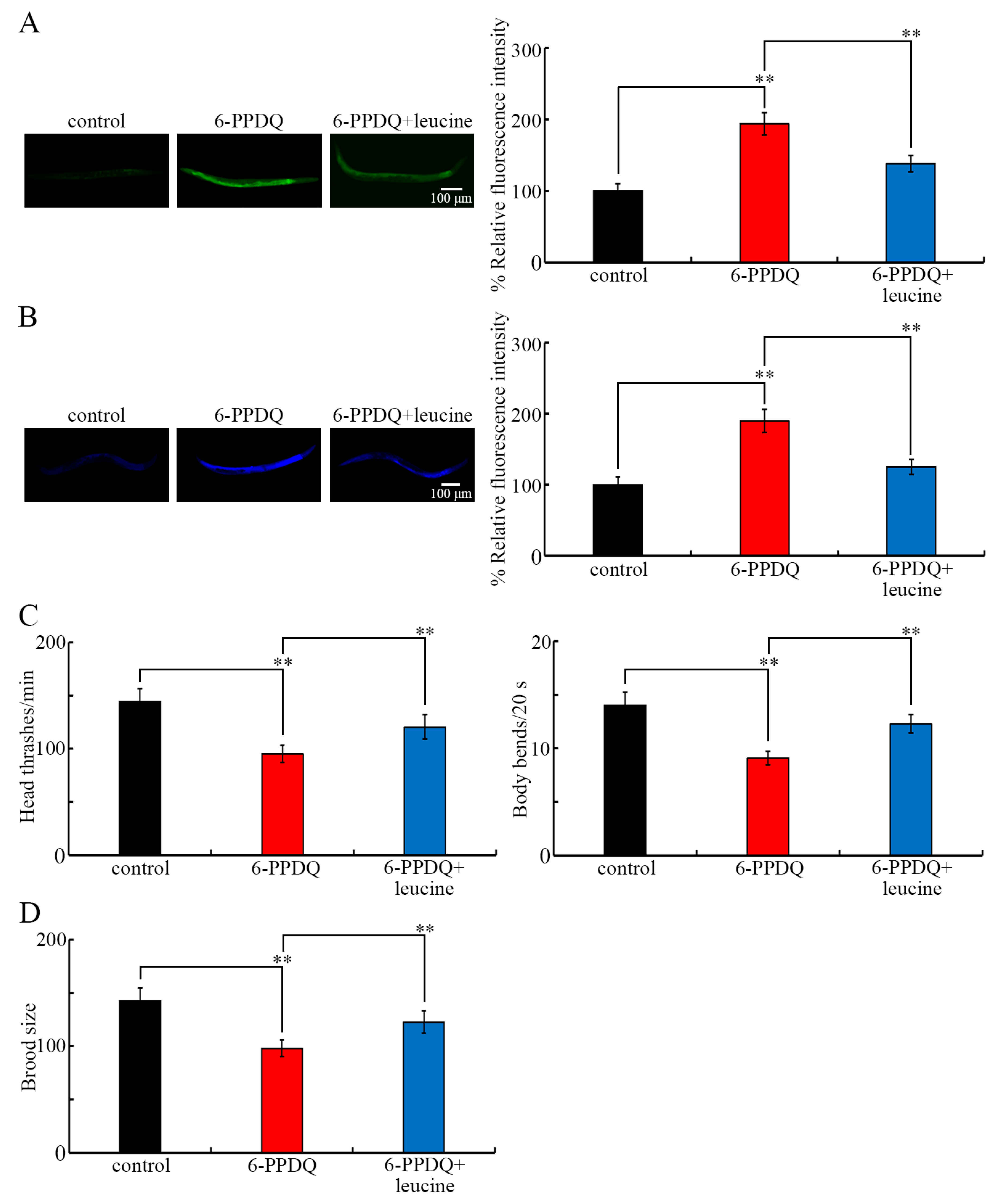
3.7. Beneficial Effect of Leucine Treatment Against 6-PPDQ-Induced Mitochondrial Dysfunction
3.8. Beneficial Effect of Leucine Treatment Against Other Aspects of 6-PPDQ Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, L.; Li, Y.; Sun, Y.; Liu, L.Y.; Shen, M.; Du, B. Widespread occurrence and transport of p-phenylenediamines and their quinones in sediments across urban rivers, estuaries, coasts, and deep-sea regions. Environ. Sci. Technol. 2023, 57, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. Tire-rubber related pollutant 6-PPD quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 459, 132265. [Google Scholar] [CrossRef]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-PPD and 6-PPD quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, F.; Li, J.; Wan, X.; Ge, Y.; Liang, G.; Zhou, Y. P-phenylenediamine antioxidants and their quinone derivatives: A review of their environmental occurrence, accessibility, potential toxicity, and human exposure. Sci. Total Environ. 2024, 948, 174449. [Google Scholar] [CrossRef]
- Lane, R.F.; Smalling, K.L.; Bradley, P.M.; Greer, J.B.; Gordon, S.E.; Hansen, J.D.; Kolpin, D.W.; Spanjer, A.R.; Masoner, J.R. Tire-derived contaminants 6PPD and 6PPD-Q: Analysis, sample handling, and reconnaissance of United States stream exposures. Chemosphere 2024, 363, 142830. [Google Scholar] [CrossRef]
- Jaeger, A.; Monaghan, J.; Tomlin, H.; Atkinson, J.; Gill, C.G.; Krogh, E.T. Intensive spatiotemporal characterization of the tire wear toxin 6PPD quinone in urban waters. ACS ES T Water 2024, 4, 5566–5574. [Google Scholar] [CrossRef]
- Yi, J.; Ruan, J.; Yu, H.; Wu, B.; Zhao, J.; Wang, H.; Chen, R.; Yang, Q.; Chen, J.; Sun, D. Environmental fate, toxicity, and mitigation of 6PPD and 6PPD-Quinone: Current understanding and future directions. Environ. Pollut. 2025, 375, 126352. [Google Scholar] [CrossRef]
- Rauert, C.; Charlton, N.; Okoffo, E.D.; Stanton, R.S.; Agua, A.R.; Pirrung, M.C.; Thomas, K.V. Concentrations of tire additive chemicals and tire road wear particles in an Australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421–2431. [Google Scholar] [CrossRef]
- Varshney, S.; Gora, A.H.; Siriyappagouder, P.; Kiron, V.; Olsvik, P.A. Toxicological effects of 6PPD and 6PPD quinone in zebrafish larvae. J. Hazard. Mater. 2022, 424, 127623. [Google Scholar] [CrossRef]
- Wan, X.; Liang, G.-Y.; Wang, D.-Y. Potential human health risk of the emerging environmental contaminant 6-PPD quinone. Sci. Total Environ. 2024, 949, 175057. [Google Scholar] [CrossRef] [PubMed]
- Black, G.P.; De Parsia, M.; Uychutin, M.; Lane, R.; Orlando, J.L.; Hladik, M.L. 6PPD-quinone in water from the San Francisco-San Joaquin Delta, California, 2018-2024. Environ. Monit. Assess. 2025, 197, 369. [Google Scholar] [CrossRef]
- Roberts, C.; Lin, J.; Kohlman, E.; Jain, N.; Amekor, M.; Alcaraz, A.J.; Hogan, N.; Hecker, M.; Brinkmann, M. Acute and subchronic toxicity of 6PPD-quinone to early life stage lake trout (Salvelinus namaycush). Environ. Sci. Technol. 2025, 59, 791–797. [Google Scholar] [CrossRef]
- Ricarte, M.; Prats, E.; Montemurro, N.; Bedrossiantz, J.; Bellot, M.; Gómez-Canela, C.; Raldúa, D. Environmental concentrations of tire rubber-derived 6PPD-quinone alter CNS function in zebrafish larvae. Sci. Total Environ. 2023, 896, 165240. [Google Scholar] [CrossRef]
- Liao, X.L.; Chen, Z.F.; Liu, Q.Y.; Zhou, J.M.; Cai, W.X.; Wang, Y.; Cai, Z. Tissue accumulation and biotransformation of 6PPD-quinone in adult zebrafish and its effects on the intestinal microbial community. Environ. Sci. Technol. 2024, 58, 10275–10286. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Sun, W.; Wang, B.; Shi, C.; Ran, R.; Zhang, Y.; Lu, L.; Zhang, H. Environmental concentrations of 6PPD and 6PPD-quinone induce hepatic lipid metabolism disorders in male black-spotted frogs. J. Hazard. Mater. 2024, 480, 136400. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Ma, L.; Gao, T.; Wāng, Y. Environmental profiles, hazard identification, and toxicological hallmarks of emerging tire rubber-related contaminants 6PPD and 6PPD-quinone. Environ. Int. 2024, 187, 108677. [Google Scholar] [CrossRef]
- Yao, K.; Kang, Q.; Liu, W.; Chen, D.; Wang, L.; Li, S. Chronic exposure to tire rubber-derived contaminant 6PPD-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024, 470, 134165. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Lv, J.; Chen, H.; Wang, H.; Xu, J.; Hu, J.; Tao, L.; Fang, M.; Huang, Y. Environmentally realistic dose of tire-derived metabolite 6PPD-Q exposure causes intestinal jejunum and ileum damage in mice via cannabinoid receptor-activated inflammation. Sci. Total Environ. 2024, 918, 170679. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Sun, N.; Lv, J.; Hu, J.; Tao, L.; Zhang, C.; Wang, H.; Chen, L.; Xu, D.X.; et al. Low-dose tire wear chemical 6PPD-Q exposure elicit fatty liver via promoting fatty acid biosynthesis in ICR mice. J. Hazard. Mater. 2025, 489, 137574. [Google Scholar] [CrossRef]
- Di, S.; Xu, H.; Yu, Y.; Qi, P.; Wang, Z.; Liu, Z.; Zhao, H.; Jin, Y.; Wang, X. Environmentally relevant concentrations of S-6PPD-quinone caused more serious hepatotoxicity than r-enantiomer and racemate in Oncorhynchus mykiss. Environ. Sci. Technol. 2024, 58, 17617–17628. [Google Scholar] [CrossRef]
- Chen, H.; Xie, M.; Li, W.; Tan, L.; Cai, X.; Shen, M.; Li, R. Detection of 6-PPD and 6-PPDQ in airborne particulates and assessment of their toxicity in lung cells. Chemosphere 2024, 364, 143205. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Cao, G.; Wang, F.; Ru, Y.; Wang, B.; Zhang, Y.; Zhang, D.; Yan, J.; Xu, J.; et al. 6PPD-quinone exposure induces neuronal mitochondrial dysfunction to exacerbate Lewy neurites formation induced by alpha-synuclein preformed fibrils seeding. J. Hazard. Mater. 2024, 465, 133312. [Google Scholar] [CrossRef]
- Fang, C.; Di, S.; Yu, Y.; Qi, P.; Wang, X.; Jin, Y. 6PPD induced cardiac dysfunction in zebrafish associated with mitochondrial damage and inhibition of autophagy processes. J. Hazard. Mater. 2024, 471, 134357. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Li, H.; Zeng, L.; Wang, C.; Shi, C.; Li, Y.; Peng, Y.; Chen, H.; Zhang, J.; Cheng, B.; Chen, C.; et al. Review of the toxicity and potential molecular mechanisms of parental or successive exposure to environmental pollutants in the model organism Caenorhabditis elegans. Environ. Pollut. 2022, 311, 119927. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, T.; Tang, M. A critical review of advances in reproductive toxicity of common nanomaterials to Caenorhabditis elegans and influencing factors. Environ. Pollut. 2022, 306, 119270. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wang, Z. Long-term exposure to polystyrene nanoparticles at environmentally relevant concentration causes suppression in heme homeostasis signal associated with transgenerational toxicity induction in Caenorhabditis elegans. J. Hazard. Mater. 2023, 459, 132124. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Bian, Q.; Wang, D.-Y. 6-PPD quinone induces response of nuclear hormone receptors in the germline associated with formation of reproductive toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2025, 495, 138815. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zheng, J.; Zhang, D.; Ding, Y.; Yu, H.Q. Environmentally relevant exposure to tetrabromobisphenol A induces reproductive toxicity via regulating glucose-6-phosphate 1-dehydrogenase and sperm activation in Caenorhabditis elegans. Sci. Total Environ. 2024, 907, 167820. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, D.-Y. Transgenerational intestinal toxicity of 6-PPD quinone in causing ROS production, enhancement in intestinal permeability and suppression in innate immunity in C. elegans. Environ. Pollut. 2024, 363, 125208. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Li, Y.-H.; Wang, D.-Y. Transgenerational response of glucose metabolism in Caenorhabditis elegans exposed to 6-PPD quinone. Chemosphere 2024, 367, 143653. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, D.-Y. Exposure to 6-PPD quinone enhances glycogen accumulation in Caenorhabditis elegans. Environ. Pollut. 2024, 359, 124600. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Li, Y.-H.; Wang, D.-Y. Exposure to 6-PPD quinone disrupts glucose metabolism associated with lifespan reduction by affecting insulin and AMPK signals in Caenorhabditis elegans. Chemosphere 2024, 363, 142975. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. Environmentally relevant concentration of 6-PPD quinone inhibits two types of mitophagy to cause mitochondrial dysfunction and lifespan reduction in Caenorhabditis elegans. Environ. Sci. Process. Impacts 2025. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. 6-PPD quinone at environmentally relevant concentrations induced damage on longevity in C. elegans: Mechanistic insight from inhibition in mitochondrial UPR response. Sci. Total Environ. 2024, 954, 176275. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched chain amino acids: Beyond nutrition metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. y+LAT1 and y+LAT2 contribution to arginine uptake in different human cell models: Implications in the pathophysiology of Lysinuric Protein Intolerance. J. Cell Mol. Med. 2020, 24, 921–929. [Google Scholar] [CrossRef]
- Díaz-Pérez, F.; Radojkovic, C.; Aguilera, V.; Veas, C.; González, M.; Lamperti, L.; Escudero, C.; Aguayo, C. L-arginine transport and nitric oxide synthesis in human endothelial progenitor cells. J. Cardiovasc. Pharmacol. 2012, 60, 439–449. [Google Scholar] [CrossRef]
- Mansfeld, J.; Urban, N.; Priebe, S.; Groth, M.; Frahm, C.; Hartmann, N.; Gebauer, J.; Ravichandran, M.; Dommaschk, A.; Schmeisser, S.; et al. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun. 2015, 6, 10043. [Google Scholar] [CrossRef]
- Ravanelli, S.; Li, Q.; Annibal, A.; Trifunovic, A.; Antebi, A.; Hoppe, T. Reprograming of proteasomal degradation by branched chain amino acid metabolism. Aging Cell 2022, 21, e13725. [Google Scholar] [CrossRef]
- Reifenberg, P.; Zimmer, A. Branched-chain amino acids: Physico-chemical properties, industrial synthesis and role in signaling, metabolism and energy production. Amino Acids 2024, 56, 51. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Y.; Liu, M.; Huang, Y.; Wang, S.; An, J.; Wang, Y.; Shang, Y. The joint effects of nanoplastics and TBBPA on neurodevelopmental toxicity in Caenorhabditis elegans. Toxicol. Res. 2023, 12, 76–85. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wu, J.-W.; Wang, D.-Y. 6-PPD quinone causes lipid accumulation across multiple generations differentially affected by metabolic sensors and components of COMPASS complex in Caenorhabditis elegans. Environ. Pollut. 2025, 366, 125539. [Google Scholar] [CrossRef]
- Song, M.-X.; Ruan, Q.-L.; Wang, D.-Y. Comparison of transgenerational toxicity on locomotion behavior and development of D-type motor neurons between pristine and amino modified nanoplastics at environmentally relevant doses in C. elegans. Toxics 2024, 12, 555. [Google Scholar] [CrossRef]
- Zheng, F.; Aschner, M.; Li, H. Evaluations of environmental pollutant-induced mitochondrial toxicity using Caenorhabditis elegans as a model system. Methods Mol. Biol. 2021, 2326, 33–46. [Google Scholar]
- Guo, X.; Li, Q.; Shi, J.; Shi, L.; Li, B.; Xu, A.; Zhao, G.; Wu, L. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 2016, 155, 115–126. [Google Scholar] [CrossRef]
- Luo, X.; Tu, J.; Xu, H.; Xia, E.; Yao, S.; Han, Y.; Niu, C.; Chen, L. Biological effects of graphene oxide-induced fluorescent marker perturbation in Caenorhabditis elegans via food chain exposure. Ecotoxicol. Environ. Saf. 2025, 302, 118524. [Google Scholar] [CrossRef]
- Wu, J.-W.; Shao, Y.-T.; Hua, X.; Wang, D.-Y. Activated hedgehog and insulin ligands by decreased transcriptional factor DAF-16 mediate transgenerational nanoplastic toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2024, 480, 135909. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.; Liang, Y.; Cao, X.; Jiang, J.; Liu, Y.; Cao, X.; Qiu, Y.; He, D. Polymer chain extenders induce significant toxicity through DAF-16 and SKN-1 pathways in Caenorhabditis elegans: A comparative analysis. J. Hazard. Mater. 2024, 473, 134730. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Shi, W.; Lu, L.; Zhou, Q.; Pu, Y.; Yin, L. Copper exposure induces neurotoxicity through ferroptosis in C. elegans. Chem. Biol. Interact. 2025, 407, 111369. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhang, L.; Wang, D.-Y. Nanoplastic at environmentally relevant concentrations activates germline mir-240-rab-5 signaling cascade to affect secreted ligands associated with transgenerational toxicity induction in C. elegans. Environ. Sci. Nano 2024, 11, 3615. [Google Scholar] [CrossRef]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 8. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. 6-PPD quinone causes alteration in ubiquinone-mediated complex III associated with toxicity on mitochondrial function and longevity in Caenorhabditis elegans. J. Environ. Chem. Engineer. 2025, 13, 116571. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Powell, J.D.; Hutson, S.M. Leucine Metabolism in T Cell Activation: mTOR Signaling and Beyond. Adv. Nutr. 2016, 7, 798S–805S. [Google Scholar] [CrossRef]
- Souza, H.F.S.; Marsiccobetre, S.; Souza, R.O.O.; Luévano-Martínez, L.A.; Silber, A.M. Branched chain amino acids catabolism as a source of new drug targets in pathogenic protists. Exp. Parasitol. 2023, 249, 108499. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a treatment for muscle wasting: A critical review. Clin. Nutr. 2014, 33, 937–945. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine supplementation in cancer cachexia: Mechanisms and a review of the pre-clinical literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef]
- Chang, M.C.; Choo, Y.J. Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: A systematic review and meta-analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.-H.; Wang, D.-Y. Long-term exposure to 6-PPD quinone inhibits glutamate synthesis and glutamate receptor function associated with its toxicity induction in Caenorhabditis elegans. Toxics 2025, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, E.; Stasiuk, S.; Skelly, P.J.; Shoemaker, C.B.; Verrey, F. Functional characterization of Caenorhabditis elegans heteromeric amino acid transporters. J. Biol. Chem. 2004, 279, 7655–7662. [Google Scholar] [CrossRef]
- Lesnik, C.; Kaletsky, R.; Ashraf, J.M.; Sohrabi, S.; Cota, V.; Sengupta, T.; Keyes, W.; Luo, S.; Murphy, C.T. Enhanced branched-chain amino acid metabolism improves age-related reproduction in C. elegans. Nat. Metab. 2024, 6, 724–740. [Google Scholar] [CrossRef]
- Zdraljevic, S.; Fox, B.W.; Strand, C.; Panda, O.; Tenjo, F.J.; Brady, S.C.; Crombie, T.A.; Doench, J.G.; Schroeder, F.C.; Andersen, E.C. Natural variation in C. elegans arsenic toxicity is explained by differences in branched chain amino acid metabolism. Elife 2019, 8, e40260. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, C.; Guan, K.; Xu, S.; Chen, X.; Lin, Y.; Li, X.; Shan, Y. Protective role of ghrelin against 6PPD-quinone-induced neurotoxicity in zebrafish larvae (Danio rerio) via the GHSR pathway. Ecotoxicol. Environ. Saf. 2024, 285, 117031. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, Y.; Wang, D. Exposure to 6-PPD Quinone Disrupts Adsorption and Catabolism of Leucine and Causes Mitochondrial Dysfunction in Caenorhabditis elegans. Toxics 2025, 13, 544. https://doi.org/10.3390/toxics13070544
Wang W, Li Y, Wang D. Exposure to 6-PPD Quinone Disrupts Adsorption and Catabolism of Leucine and Causes Mitochondrial Dysfunction in Caenorhabditis elegans. Toxics. 2025; 13(7):544. https://doi.org/10.3390/toxics13070544
Chicago/Turabian StyleWang, Wei, Yunhui Li, and Dayong Wang. 2025. "Exposure to 6-PPD Quinone Disrupts Adsorption and Catabolism of Leucine and Causes Mitochondrial Dysfunction in Caenorhabditis elegans" Toxics 13, no. 7: 544. https://doi.org/10.3390/toxics13070544
APA StyleWang, W., Li, Y., & Wang, D. (2025). Exposure to 6-PPD Quinone Disrupts Adsorption and Catabolism of Leucine and Causes Mitochondrial Dysfunction in Caenorhabditis elegans. Toxics, 13(7), 544. https://doi.org/10.3390/toxics13070544