Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS)
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Study Design
2.2. Analytical Chemistry
2.2.1. Sample Preparation
2.2.2. Non-Targeted Analysis (NTA)
2.2.3. NTA Data Analysis
2.2.4. Glucuronide Formation Assay
3. Results
3.1. Data Processing and Filtering
3.2. Dosing Solution and PFHxSA Standard NTA Results Summary
3.3. Feature Annotation and Identification of Predicted Biotransformation Products PFHxS, PFHxSi, and PFHxSA-N-glucuronide
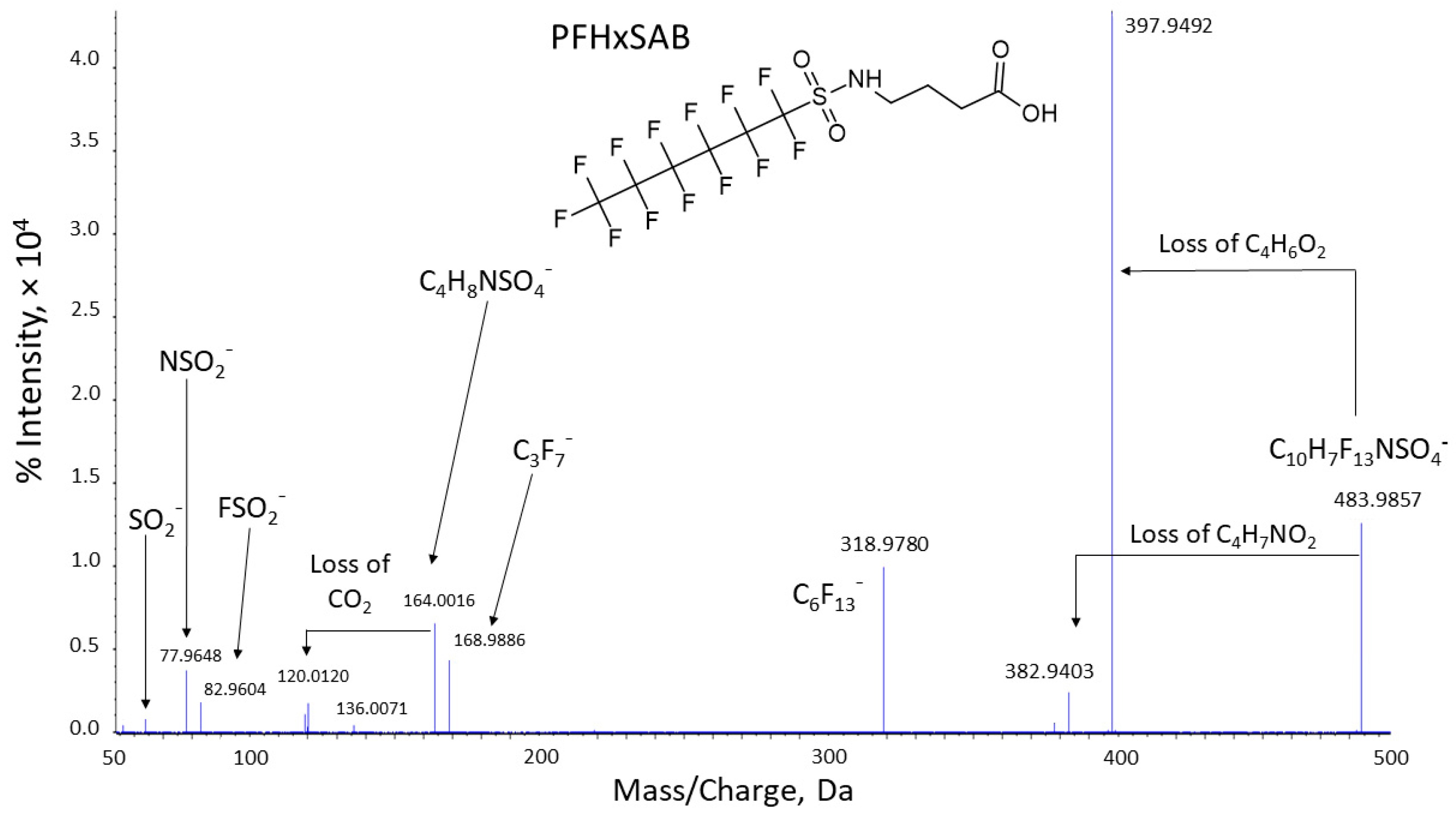
3.4. Feature Annotation and Identification of Precursor Perfluoroalkyl Ether Sulfonamide Impurities and Perfluoroalkyl Ether Sulfonic Acid Biotransformation Products
3.5. Feature Annotation and Identification of Proposed Polyfluorinated Biotransformation Products
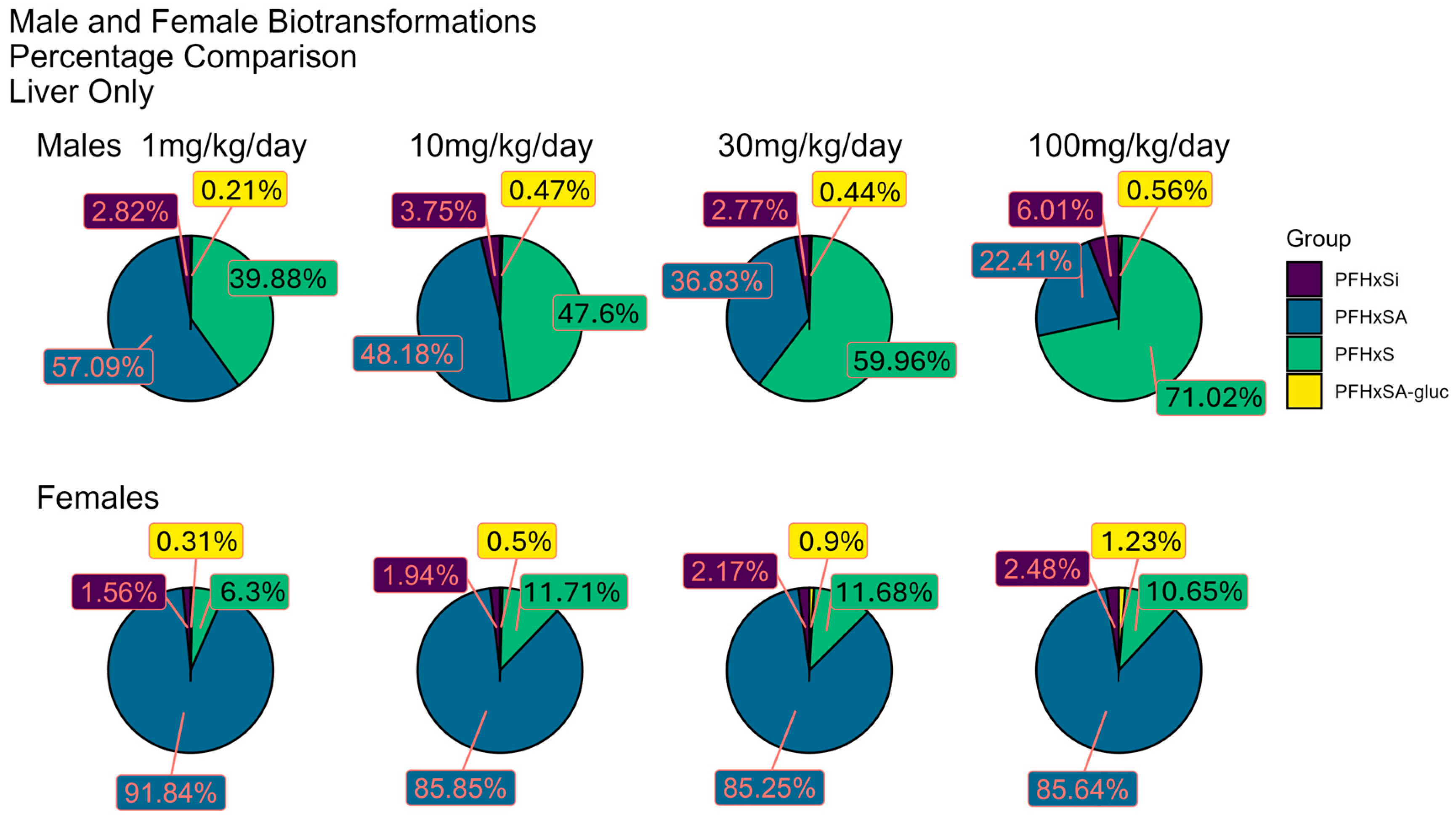
3.6. Distribution of PFHxSA and Predicted BPs
4. Discussion
4.1. Predicted PFHxSA Biotransformation Products
4.2. Dosing Solution Impurities and Their Biotransformation Products
4.3. Novel Potential Biotransformation Products PFPeSAB, PFHxSAA, and PFHxSAB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strynar, M.J. A paradigm shift in environmental monitoring—The time for non-targeted analysis (NTA) is now. Environ. Int. 2025, 197, 109332. [Google Scholar] [CrossRef] [PubMed]
- Black, G.; Lowe, C.; Anumol, T.; Bade, J.; Favela, K.; Feng, Y.-L.; Knolhoff, A.; McEachran, A.; Nuñez, J.; Fisher, C.; et al. Exploring chemical space in non-targeted analysis: A proposed ChemSpace tool. Anal. Bioanal. Chem. 2023, 415, 35–44. [Google Scholar] [CrossRef]
- Reymond, J.-L. The Chemical Space Project. Acc. Chem. Res. 2015, 48, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Strynar, M.J.; Dagnino, S.; McMahen, R.; Liang, S.; Lindstrom, A.; Andersen, E.; McMillan, L.; Thurman, M.; Ferrer, I.; Ball, C. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ. Sci. Tech. 2015, 49, 11622–11630. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.P.; Strynar, M.J.; Washington, J.W.; Bergman, E.L.; Goodrow, S.M. Emerging Chlorinated Polyfluorinated Polyether Compounds Impacting the Waters of Southwestern New Jersey Identified by Use of Nontargeted Analysis. Environ. Sci. Technol. Lett. 2020, 7, 903–908. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.s.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Megson, D.; Niepsch, D.; Spencer, J.; dos Santos, C.; Florance, H.; MacLeod, C.; Ross, I. Non-targeted analysis reveals hundreds of per- and polyfluoroalkyl substances (PFAS) in UK freshwater in the vicinity of a fluorochemical plant. Chemosphere 2024, 367, 143645. [Google Scholar] [CrossRef]
- Bangma, J.; Guillette, T.C.; Strynar, M.J.; Lindstrom, A.B.; McCord, J.P.; Hill, D.; Lau, C.; Chernoff, N.; Lang, J.R. A rapid assessment bioaccumulation screening (RABS) study design for emerging per-and polyfluoroalkyl substances in mice exposed to industrially impacted surface water. Chemosphere 2022, 308, 136159. [Google Scholar] [CrossRef]
- Boettger, J.D.; DeLuca, N.M.; Zurek-Ost, M.A.; Miller, K.E.; Fuller, C.; Bradham, K.D.; Ashley, P.; Friedman, W.; Pinzer, E.A.; Cox, D.C.; et al. Emerging Per- and Polyfluoroalkyl Substances in Tap Water from the American Healthy Homes Survey II. Environ. Sci. Technol. 2025, 59, 2686–2698. [Google Scholar] [CrossRef]
- Manz, K.E.; Feerick, A.; Braun, J.M.; Feng, Y.-L.; Hall, A.; Koelmel, J.; Manzano, C.; Newton, S.R.; Pennell, K.D.; Place, B.J.; et al. Non-targeted analysis (NTA) and suspect screening analysis (SSA): A review of examining the chemical exposome. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 524–536. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, G.; Li, W.; Mejia Avendaño, S. Isomer-specific biotransformation of perfluoroalkyl sulfonamide compounds in aerobic soil. Sci. Tot. Environ. 2019, 651, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, H.; Buckley, T.; Sobus, J.; Bangma, J.; MacMillan, D.; Williams, A.; Janesch, G.; Coombs, J.; Newman, E.; Dahlmeier, A.; et al. Non-Targeted Analysis of Surface and Groundwaters Impacted by Historic PFAS Waste Sites. Environ. Sci. Technol. Accept. 2025. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, H.D.; Buckley, T.J.; Sobus, J.R.; Bangma, J.; MacMillan, D.K.; Ferland, T.M.; Chao, A.; Williams, A.J.; Janesch, G.; Cofield, K.; et al. The ENTAiLS Toolkit: An Integrated Workflow to Perform Non-Targeted Analysis of Per- and Polyfluoroalkyl Substances. Anal. Bioanal. Chem. 2025. [Google Scholar] [CrossRef] [PubMed]
- Curtzwiler, G.W.; Silva, P.; Hall, A.; Ivey, A.; Vorst, K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packagin. Integr. Environ. Assess. Manag. 2021, 17, 7–12. [Google Scholar] [CrossRef]
- Seltenrich, N. PFAS in Food Packaging: A Hot, Greasy Exposure. Environ. Health Perspect. 2020, 128. [Google Scholar] [CrossRef]
- Harris, K.J.; Munoz, G.; Woo, V.; Sauvé, S.; Rand, A.A. Targeted and Suspect Screening of Per- and Polyfluoroalkyl Substances in Cosmetics and Personal Care Products. Environ. Sci. Technol. 2022, 56, 14594–14604. [Google Scholar] [CrossRef]
- Annunziato, K.M.; Doherty, J.; Lee, J.; Clark, J.M.; Liang, W.; Clark, C.W.; Nguyen, M.; Roy, M.A.; Timme-Laragy, A.R. Chemical Characterization of a Legacy Aqueous Film-Forming Foam Sample and Developmental Toxicity in Zebrafish (Danio rerio). Environ. Health Perspect. 2020, 128, 97006. [Google Scholar] [CrossRef]
- Dubocq, F.; Wang, T.; Yeung, L.W.Y.; Sjöberg, V.; Kärrman, A. Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol. 2020, 54, 245–254. [Google Scholar] [CrossRef]
- Megson, D.; Bruce-Vanderpuije, P.; Idowu, I.G.; Ekpe, O.D.; Sandau, C.D. A systematic review for non-targeted analysis of per- and polyfluoroalkyl substances (PFAS). Sci. Tot. Environ. 2025, 960, 178240. [Google Scholar] [CrossRef]
- Ruyle, B.J.; Thackray, C.P.; McCord, J.P.; Strynar, M.J.; Mauge-Lewis, K.A.; Fenton, S.E.; Sunderland, E.M. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett. 2021, 8, 59–65. [Google Scholar] [CrossRef]
- Choi, Y.J.; Helbling, D.E.; Liu, J.; Olivares, C.I.; Higgins, C.P. Microbial biotransformation of aqueous film-forming foam derived polyfluoroalkyl substances. Sci. Tot. Environ. 2022, 824, 153711. [Google Scholar] [CrossRef] [PubMed]
- Renyer, A.; Ravindra, K.; Wetmore, B.A.; Ford, J.L.; DeVito, M.; Hughes, M.F.; Wehmas, L.C.; MacMillan, D.K. Dose Response, Dosimetric, and Metabolic Evaluations of Replacement PFAS Perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic) Acid (HFPO-TeA). Toxics 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Li, J.; Gong, S.; Herczegh, S.M.; Zhang, Q.; Letcher, R.J.; Su, G. Established and emerging organophosphate esters (OPEs) and the expansion of an environmental contamination issue: A review and future directions. J. Haz. Mater. 2023, 459, 132095. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Cowden, J.; Davidson-Fritz, S.; Dean, J.; Devito, M.; Everett, L.; Harrill, A.; Hester, S.; Hughes, M.; Lambert, J.; et al. Scientific Studies Supporting Development of Transcriptomic Points of Departure for EPA Transcriptomic Assessment Products (ETAPs). 2024. Available online: https://epa.figshare.com/articles/online_resource/Scientific_Studies_Supporting_Development_of_Transcriptomic_Points_of_Departure_for_EPA_Transcriptomic_Assessment_Products_ETAPs_/25365550 (accessed on 16 June 2025).
- Brennan, A.; Chang, D.; Cowden, J.; Davidson-Fritz, S.; Dean, J.; Devito, M.; Ford, J.; Everett, L.; Harrill, A.; Hester, S.; et al. Standard Methods for Development of EPA Transcriptomic Assessment Products (ETAPs). 2024. Available online: https://epa.figshare.com/articles/online_resource/Standard_Methods_for_Development_of_EPA_Transcriptomic_Assessment_Products_ETAPs_/25365496/1 (accessed on 16 June 2025).
- Patlewicz, G.; Richard, A.M.; Williams, A.J.; Grulke, C.M.; Sams, R.; Lambert, J.; Noyes, P.D.; DeVito, M.J.; Hines, R.N.; Strynar, M.; et al. A Chemical Category-Based Prioritization Approach for Selecting 75 Per- and Polyfluoroalkyl Substances (PFAS) for Tiered Toxicity and Toxicokinetic Testing. Environ. Health Perspect. 2019, 127, 14501. [Google Scholar] [CrossRef]
- Patlewicz, G.; Richard, A.M.; Williams, A.J.; Judson, R.S.; Thomas, R.S. Towards reproducible structure-based chemical categories for PFAS to inform and evaluate toxicity and toxicokinetic testing. Comput. Toxicol. 2022, 24, 100250. [Google Scholar] [CrossRef]
- Patlewicz, G.; Shah, I. Towards systematic read-across using Generalised Read-Across (GenRA). Comput. Toxicol. 2023, 25, 100258. [Google Scholar] [CrossRef]
- Mutlu, E.; Wehmas, L.C.; Harrill, A.H.; DeVito, M.J.; Thomas, R.S.; Hughes, M.F.; MacMillan, D.K.; Brennan, A.A.; Bounds, J.G.; Weitekemp, C.A.; et al. Transcriptomic dose response of PFAS chemicals 3:3 fluorotelomer carboxylic acid, 7:3 fluorotelomer alcohol, and perfluorohexanesulfonamide. Toxicology, 2025; Submitted. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- McDonough, C.A.; Choyke, S.; Barton, K.E.; Mass, S.; Starling, A.P.; Adgate, J.L.; Higgins, C.P. Unsaturated PFOS and Other PFASs in Human Serum and Drinking Water from an AFFF-Impacted Community. Environ. Sci. Technol. 2021, 55, 8139–8148. [Google Scholar] [CrossRef]
- Munoz, G.; Mercier, L.; Duy, S.V.; Liu, J.; Sauvé, S.; Houde, M. Bioaccumulation and trophic magnification of emerging and legacy per- and polyfluoroalkyl substances (PFAS) in a St. Lawrence River food web. Environ. Pollut. 2022, 309, 119739. [Google Scholar] [CrossRef]
- All POPs Listed in the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 1 May 2023).
- Carrizo, J.C.; Munoz, G.; Vo Duy, S.; Liu, M.; Houde, M.; Amé, M.V.; Liu, J.; Sauvé, S. PFAS in fish from AFFF-impacted environments: Analytical method development and field application at a Canadian international civilian airport. Sci. Tot. Environ. 2023, 879, 163103. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.C.; Saley, M.R.; Serrano, J.A.; Daley, S.M.; Tapper, M.A. PFAS Biotransformation Pathways: A Species Comparison Study. Toxics 2023, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.D.; Dukes, D.A.; Koelmel, J.P.; Stelben, P.; Finch, J.; Okeme, J.; Lowe, C.; Williams, A.; Godri, D.; Rennie, E.E.; et al. Expanding PFAS Identification with Transformation Product Libraries: Nontargeted Analysis Reveals Biotransformation Products in Mice. Environ. Sci. Technol. 2025, 59, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, Y.; Wang, Z.; Xie, H.; Chen, J. Transformation Pathways of Isomeric Perfluorooctanesulfonate Precursors Catalyzed by the Active Species of P450 Enzymes: In Silico Investigation. Chem. Res. Toxicol. 2015, 28, 482–489. [Google Scholar] [CrossRef]
- Benskin, J.P.; De Silva, A.O.; Martin, L.J.; Arsenault, G.; McCrindle, R.; Riddell, N.; Mabury, S.A.; Martin, J.W. Disposition of perfluorinated acid isomers in Sprague-Dawley rats; part 1: Single dose. Environ. Toxicol. Chem. 2009, 28, 542–554. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, W.; Li, N.; Ma, D.; Zhao, C.; Li, J.; Wang, Y.; Jiang, G. Assessment of perfluorohexane sulfonic acid (PFHxS)-related compounds degradation potential: Computational and experimental approaches. J. Haz. Mater. 2022, 436, 129240. [Google Scholar] [CrossRef]
- Han, J.; Gu, W.; Barrett, H.; Yang, D.; Tang, S.; Sun, J.; Liu, J.; Krause, H.M.; Houck, K.A.; Peng, H.A. A Roadmap to the Structure-Related Metabolism Pathways of Per- and Polyfluoroalkyl Substances in the Early Life Stages of Zebrafish (Danio rerio). Environ. Health Perspect. 2021, 129, 007174. [Google Scholar] [CrossRef]
- Tomy, G.T.; Tittlemier, S.A.; Palace, V.P.; Budakowski, W.R.; Braekevelt, E.; Brinkworth, L.; Friesen, K. Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes. Environ. Sci. Technol. 2004, 38, 758–762. [Google Scholar] [CrossRef]
- Xu, L.; Krenitsky, D.M.; Seacat, A.M.; Butenhoff, J.L.; Anders, M.W. Biotransformation of N-ethyl-N-(2-hydroxyethyl)perfluorooetanesulfonamide by rat liver microsomes, cytosol, and slices and by expressed rat and human cytochromes P450. Chem. Res. Toxicol. 2004, 17, 766–775. [Google Scholar] [CrossRef]
- Xu, L.; Krenitsky, D.M.; Seacat, A.M.; Butenhoff, J.L.; Tephly, T.R.; Anders, M.W. N-glucuronidation of perfluorooctanesulfonamide by human, rat, dog, and monkey liver microsomes and by expressed rat and human UDP-glucuronosyltransferases. Drug Metab. Dispos. Biol. Fate Chem. 2006, 34, 1406–1410. [Google Scholar] [CrossRef]
- Ross, M.S.; Wong, C.S.; Martin, J.W. Isomer-specific biotransformation of perfluorooctane sulfonamide in Sprague-Dawley rats. Environemental Sci. Technol. 2012, 46, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.J.; Chu, S.; McKinney, M.A.; Tomy, G.T.; Sonne, C.; Dietz, R. Comparative hepatic in vitro depletion and metabolite formation of major perfluorooctane sulfonate precursors in arctic polar bear, beluga whale and ringed seal. Chemosphere 2014, 112, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, B.; Zhong, Z.; Liu, T.; Liang, T.; Zhan, J. Contributions of enzymes and gut microbes to biotransformation of perfluorooctane sulfonamide in earthworms (Eisneia fetida). Chemosphere 2020, 238, 124619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liang, T.; Zhou, T.; Li, D.; Wang, B.; Zhan, J.; Liu, L. Biotransformation and responses of antioxidant enzymes in hydroponically cultured soybean and pumpkin exposed to perfluorooctane sulfonamide (FOSA). Ecotoxicol. Environ. Saf. 2018, 161, 669–675. [Google Scholar] [CrossRef]
- Zhao, S.; Liang, T.; Zhu, L.; Yang, L.; Liu, T.; Fu, J.; Wang, B.; Zhan, J.; Liu, L. Fate of 6:2 fluorotelomer sulfonic acid in pumpkin (Cucurbita maxima L.) based on hydroponic culture: Uptake, translocation and biotransformation. Environ. Pollut. 2019, 252, 804–812. [Google Scholar] [CrossRef]
- Lange, C.C. Biodegradation Study Report: The Aerobic Biodegradation of N-EtFOSE Alcohol by the Microbial Activity Present in Municipal Wastewater Treatment Sludge. 2000. Available online: https://static.ewg.org/reports/2003/pfcs/226-1030a078.pdf (accessed on 16 June 2025).
- Rhoads, K.R.; Janssen, E.M.-L.; Luthy, R.G.; Criddle, C.S. Aerobic Biotransformation and Fate of N-Ethyl Perfluorooctane Sulfonamidoethanol (N-EtFOSE) in Activated Sludge. Environ. Sci. Technol. 2008, 42, 2873–2878. [Google Scholar] [CrossRef]
- Avendano, S.J.; Liu, J. Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 2015, 119, 1084–1090. [Google Scholar] [CrossRef]
- Hill, N.I.; Becanova, J.; Vojta, S.; Barber, L.B.; LeBlanc, D.R.; Vajda, A.M.; Pickard, H.M.; Lohmann, R. Bioconcentration of Per- and Polyfluoroalkyl Substances and Precursors in Fathead Minnow Tissues Environmentally Exposed to Aqueous Film-Forming Foam–Contaminated Waters. Environ. Toxicol. Chem. 2024, 43, 1795–1806. [Google Scholar] [CrossRef]
- Dooley, M.R.; Nixon, S.P.; Payton, B.E.; Hudak, M.A.; Odei, F.; Vyas, S. Atmospheric Oxidation of PFAS by Hydroxyl Radical: A Density Functional Theory Study. Environ. Sci. Technol. Air 2024, 1, 1352–1361. [Google Scholar] [CrossRef]
- Seger, S.T.; Rydberg, P.; Olsen, L. Mechanism of the N-hydroxylation of primary and secondary amines by cytochrome P450. Chem. Res. Toxicol. 2015, 28, 597–603. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Song, R.; Ding, W.; Li, F.; Ji, L. Emerging Metabolic Profiles of Sulfonamide Antibiotics by Cytochromes P450: A Computational–Experimental Synergy Study on Emerging Pollutants. Environ. Sci. Technol. 2023, 57, 5368–5379. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.T.; Wathieu, H.; Ojo, A.; Byers, S.W.; Dakshanamurthy, S. Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools. Curr. Drug Metab. 2017, 18, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.M.; Roberts, S.M.; Gordon, D.S.; Stuchal, L.D. Derivation of a chronic reference dose for perfluorohexane sulfonate (PFHxS) for reproductive toxicity in mice. Regul. Toxicol. Pharmacol. 2019, 108, 104452. [Google Scholar] [CrossRef]
- Dukes, D.A.; McDonough, C.A. N-glucuronidation and Excretion of Perfluoroalkyl Sulfonamides in Mice Following Ingestion of Aqueous Film-Forming Foam. Environ. Toxicol. Chem. 2024, 43, 2274–2284. [Google Scholar] [CrossRef]
- Pickard, H.M.; Haque, F.; Sunderland, E.M. Bioaccumulation of Perfluoroalkyl Sulfonamides (FASA). Environ. Sci. Technol. Lett. 2024, 11, 350–356. [Google Scholar] [CrossRef]
- Holder, C.; DeLuca, N.; Luh, J.; Alexander, P.; Minucci, J.M.; Vallero, D.A.; Thomas, K.; Cohen Hubal, E.A. Systematic Evidence Mapping of Potential Exposure Pathways for Per- and Polyfluoroalkyl Substances Based on Measured Occurrence in Multiple Media. Environ. Sci. Technol. 2023, 57, 5107–5116. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals: Eighth Edition; National Research Council: Washington, DC, USA, 2011; p. 246.
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Guidelines for the Euthanasia of Animals; American Veterinary Medical Association (AVMA): Schaumburg, IL, USA, 2020.
- Bounds, J.G.; Renyer, A.; Brennan, A.A.; Ford, J.L.; Ravindra, K.; Wetmore, B.A.; Devito, M.; Hughes, M.F.; Wehmas, L.C.; MacMillan, D.K. Evaluations of Thyroid Effects, Dosimetry, and Metabolism following Perfluorohexanesulfonamide Exposure in Sprague Dawley Rats. Toxics, 2025; To Be Submitted. [Google Scholar]
- Lambert, J.P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Roemmelt, A.T.; Steuer, A.E.; Poetzsch, M.; Kraemer, T. Liquid Chromatography, in Combination with a Quadrupole Time-of-Flight Instrument (LC QTOF), with Sequential Window Acquisition of All Theoretical Fragment-Ion Spectra (SWATH) Acquisition: Systematic Studies on Its Use for Screenings in Clinical and Forensic Toxicology and Comparison with Information-Dependent Acquisition (IDA). Anal. Chem. 2014, 86, 11742–11749. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.T.; Phillips, A.L.; Knolhoff, A.M.; Gardinali, P.R.; Manzano, C.A.; Miller, K.E.; Pristner, M.l.; Sabourin, L.; Sumarah, M.W.; Warth, B.; et al. Nontargeted Analysis Study Reporting Tool: A Framework to Improve Research Transparency and Reproducibility. Anal. Chem. 2021, 93, 13870–13879. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Chemical Transformation Simulator. Available online: https://qed.epa.gov/cts/ (accessed on 13 June 2022).
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Tebes-Stevens, C.; Washington, J.W.; Gladstone, R. Development of a PFAS reaction library: Identifying plausible transformation pathways in environmental and biological systems. Env. Sci. Process Impacts 2022, 24, 689–753. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef]
- Ruttkies, C.; Neumann, S.; Posch, S. Improving MetFrag with statistical learning of fragment annotations. BMC Bioinform. 2019, 20, 376. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Heuckeroth, S.; Damiani, T.; Smirnov, A.; Mokshyna, O.; Brungs, C.; Korf, A.; Smith, J.D.; Stincone, P.; Dreolin, N.; Nothias, L.-F.; et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nat. Protoc. 2024, 19, 2597–2641. [Google Scholar] [CrossRef]
- Deutsch, E.W. Mass Spectrometer Output File Format mzML. Methods Mol. Biol. 2010, 604, 319–331. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amode, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Sobus, J.R.; Sayre-Smith, N.A.; Chao, A.; Ferland, T.M.; Minucci, J.M.; Carr, E.T.; Brunelle, L.D.; Batt, A.L.; Whitehead, H.D.; Cathey, T.; et al. Automated QA/QC reporting for non-targeted analysis: A demonstration of “INTERPRET NTA” with de facto water reuse data. Anal. Bioanal. Chem. 2025, 417, 1897–1914. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Chemoinform. 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Janesch, G.; Carr, E.T.; Sivasupramaniam, S.; Charest, N.; Tkachenko, V.; Williams, A.J. Applying Chemoinformatics to Develop a Structure Searchable Database of Analytical Methods. JoVE 2025, e68194. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Igarashi, K. The Koenigs-Knorr Reaction. In Advances in Carbohydrate Chemistry and Biochemistr; Elsevier: Amsterdam, The Netherlands, 1977; Volume 34, pp. 243–283. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Auerbach, S.S.; Ballin, J.D.; Blake, J.C.; Browning, D.B.; Collins, B.J.; Cora, M.C.; Fernando, R.A.; Fostel, J.M.; Liu, Y.F.; Luh, J.; et al. Report on the In Vivo Repeat Dose Biological Potency Study of Perfluorohexanesulfonamide (CASRN 41997-13-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats (Gavage Studies). NIEHS Report 10; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2023. [Google Scholar]
- Berthiaume, J.; Wallace, K.B. Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicol. Lett. 2002, 129, 23–32. [Google Scholar] [CrossRef]
- Derbel, M.; Hosokawa, M.; Satoh, T. Differences in the induction of carboxylesterase RL4 in rat liver microsomes by various perfluorinated fatty acids, metabolically inert derivatives of fatty acids. Biol. Pharm. Bull. 1996, 19, 765–767. [Google Scholar] [CrossRef]
- Crizer, D.M.; Rice, J.R.; Smeltz, M.G.; Lavrich, K.S.; Ravindra, K.; Wambaugh, J.F.; DeVito, M.; Wetmore, B.A. In Vitro Hepatic Clearance Evaluations of Per- and Polyfluoroalkyl Substances (PFAS) across Multiple Structural Categories. Toxics 2024, 12, 672. [Google Scholar] [CrossRef]
- Ryu, S.; Yamaguchi, E.; Sadegh Modaresi, S.M.; Agudelo, J.; Costales, C.; West, M.A.; Fischer, F.; Slitt, A.L. Evaluation of 14 PFAS for permeability and organic anion transporter interactions: Implications for renal clearance in humans. Chemosphere 2024, 361, 142390. [Google Scholar] [CrossRef]
- Louisse, J.; Dellafiora, L.; van den Heuvel, J.J.M.W.; Rijkers, D.; Leenders, L.; Dorne, J.C.M.; Punt, A.; Russel, F.G.M.; Koenderink, J.B. Perfluoroalkyl substances (PFASs) are substrates of the renal human organic anion transporter 4 (OAT4). Arch. Toxicol. 2023, 97, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Katakura, M.; Sato, Y.; Kawashima, Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem.-Biol. Interact. 2002, 139, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Dzierlenga, A.L.; Robinson, V.G.; Waidyanatha, S.; DeVito, M.J.; Eifrid, M.A.; Gibbs, S.T.; Granville, C.A.; Blystone, C.R. Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd:Sprague dawley SD rats following intravenous or gavage administration. Xenobiotica 2020, 50, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Dzierlenga, A.L.; Robinson, V.G.; Waidyanatha, S.; DeVito, M.J.; Eifrid, M.A.; Granville, C.A.; Gibbs, S.T.; Blystone, C.R. Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd:Sprague Dawley SD rats after intravenous and gavage administration. Toxicol. Rep. 2019, 6, 645–655. [Google Scholar] [CrossRef]
- Ohmori, K.; Kudo, N.; Katayama, K.; Kawashima, Y. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 2003, 184, 135–140. [Google Scholar] [CrossRef]
- Sundström, M.; Chang, S.-C.; Noker, P.E.; Gorman, G.S.; Hart, J.A.; Ehresman, D.J.; Bergman, A.; Butenhoff, J.L. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reprod. Toxicol. 2012, 13, 441–451. [Google Scholar] [CrossRef]
- Lehmler, H.-J. Synthesis of environmentally relevant fluorinated surfactants—A review. Chemosphere 2005, 58, 1471–1496. [Google Scholar] [CrossRef]
- Lehmler, H.-J.; Rao, V.V.V.N.S.R.; Nauduri, D.; Vargo, J.D.; Parkin, S. Synthesis and Structure of Environmentally Relevant Perfluorinated Sulfonamides. J. Fluor. Chem. 2007, 128, 595–607. [Google Scholar] [CrossRef]
- Liwara, D.J.; Pavlov, A.; Johansen, J.E.; Leonards, P.E.G.; Brandsma, S.; de Boer, J.; Liu, H. Synthesis of reference standards for emerging sulfonamide PFAS precursors suspected in aqueous film-forming foams via a benzyl intermediate. Tetrahedron 2025, 169, 134347. [Google Scholar] [CrossRef]
- Qiu, Z.-M. Water-and Oil-Repellency Imparting Ester Oligomers Comprising Perfluoroalkyl Moieties. CN1492854, 8 October 2005. [Google Scholar]
- Brown-Leung, J.M.; Cannon, J.R. Neurotransmission Targets of Per- and Polyfluoroalkyl Substance Neurotoxicity: Mechanisms and Potential Implications for Adverse Neurological Outcomes. Chem. Res. Toxicol. 2022, 35, 1312–1333. [Google Scholar] [CrossRef]
- Manojkumar, Y.; Pilli, S.; Rao, P.V.; Tyagi, R.D. Sources, occurrence and toxic effects of emerging per- and polyfluoroalkyl substances (PFAS). Neurotoxicol. Teratol. 2023, 97, 107174. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect. 2020, 128, 47005. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Bonilla, K.M.; Aga, D.S.; Lee, J.; König, M.; Qin, W.; Cristobal, J.R.; Atilla-Gokcumen, G.E.; Escher, B.I. Neurotoxic Effects of Mixtures of Perfluoroalkyl Substances (PFAS) at Environmental and Human Blood Concentrations. Environ. Sci. Technol. 2024, 58, 16774–16784. [Google Scholar] [CrossRef] [PubMed]
- Lagostena, L.; Rotondo, D.; Gualandris, D.; Calisi, A.; Lorusso, C.; Magnelli, V.; Dondero, F. Impact of Legacy Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) on GABA Receptor-Mediated Currents in Neuron-Like Neuroblastoma Cells: Insights into Neurotoxic Mechanisms and Health Implications. J. Xenobiot. 2024, 14, 1771–1783. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- Kanwal, S.; Incharoensakdi, A. The role of GAD pathway for regulation of GABA accumulation and C/N balance in Synechocystis sp. PCC6803. J. Appl. Phycol. 2019, 31, 3503–3514. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacMillan, D.K.; Bounds, J.G.; Willis, W.A.; Strynar, M.J.; Wetmore, B.A.; Liberatore, R.J.; McCord, J.P.; Devito, M.J. Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS). Toxics 2025, 13, 523. https://doi.org/10.3390/toxics13070523
MacMillan DK, Bounds JG, Willis WA, Strynar MJ, Wetmore BA, Liberatore RJ, McCord JP, Devito MJ. Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS). Toxics. 2025; 13(7):523. https://doi.org/10.3390/toxics13070523
Chicago/Turabian StyleMacMillan, Denise K., Jackson G. Bounds, William A. Willis, Mark J. Strynar, Barbara A. Wetmore, Richard J. Liberatore, James P. McCord, and Michael J. Devito. 2025. "Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS)" Toxics 13, no. 7: 523. https://doi.org/10.3390/toxics13070523
APA StyleMacMillan, D. K., Bounds, J. G., Willis, W. A., Strynar, M. J., Wetmore, B. A., Liberatore, R. J., McCord, J. P., & Devito, M. J. (2025). Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS). Toxics, 13(7), 523. https://doi.org/10.3390/toxics13070523










