Removal of the Antibiotic Amoxicillin from Polluted Freshwater by Biosorption Using the Living Biomass of the Microalga Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of the Microalga
2.2. Amoxicillin Stock
2.3. Reagents
2.4. Biosorption Assays
2.4.1. Stability of AMX
2.4.2. Determination of the Effect of pH on Biosorption Capacity
2.5. Analytical Methods
2.5.1. Determination of AMX Concentration
2.5.2. Determination of the Amount of AMX Removed
2.5.3. Analysis of Kinetics and Equilibrium Isotherms
2.6. Determination of the Biomass Zero Charge Point
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Statistical Analysis
3. Results
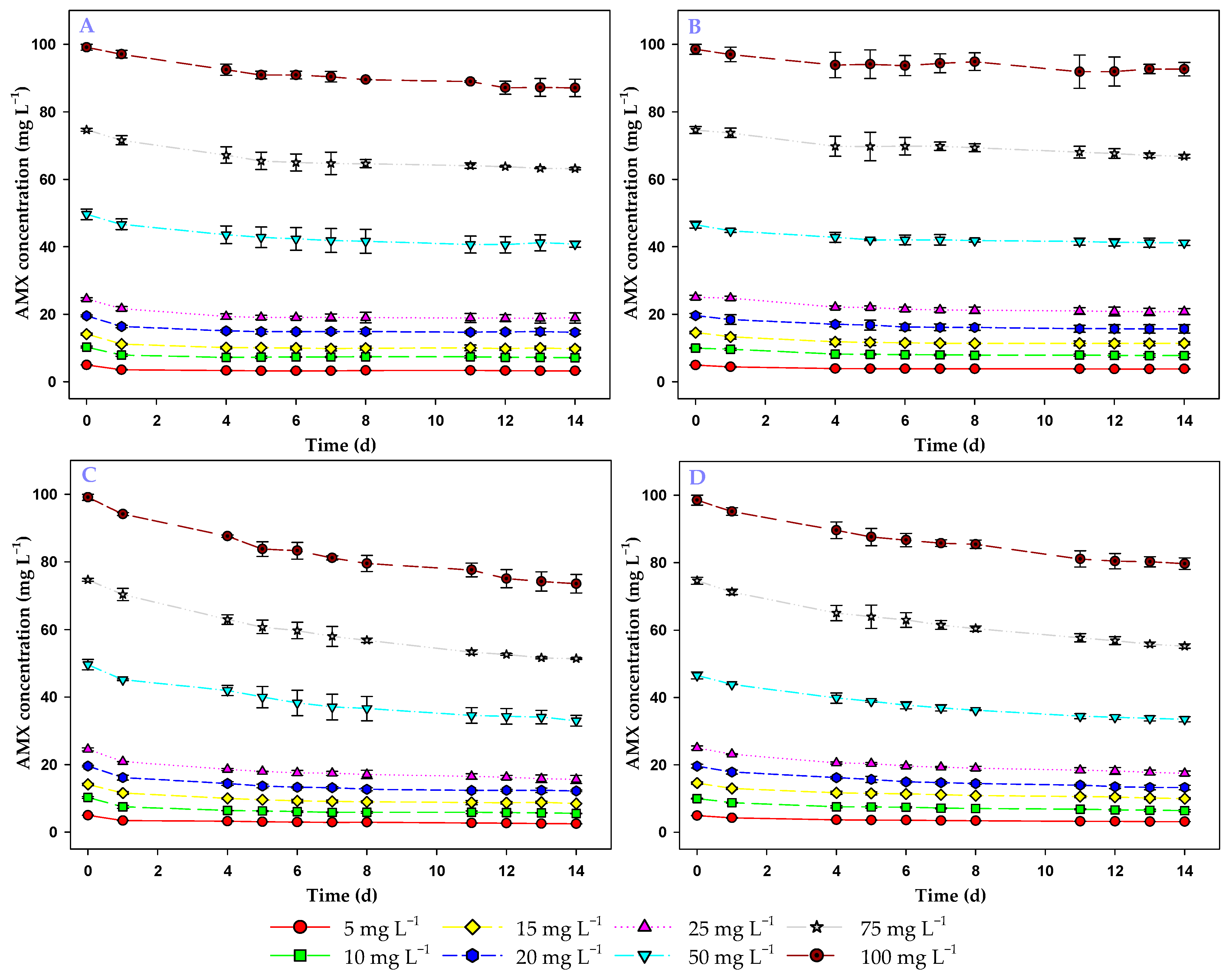
3.1. Study of AMX Stability Under Experimental Conditions
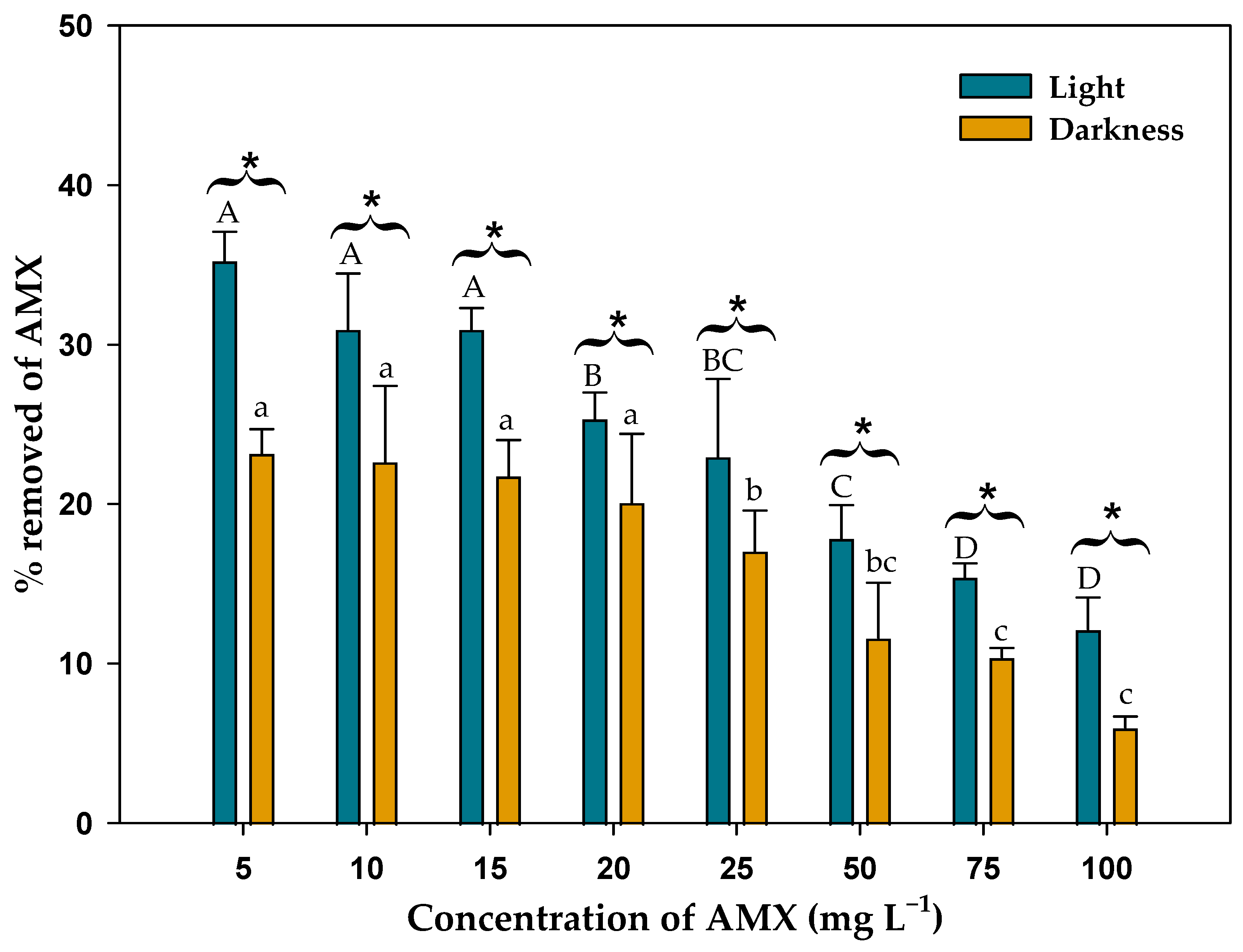
3.2. Effect of Microalgal Biomass on AMX Removal in Light and Darkness
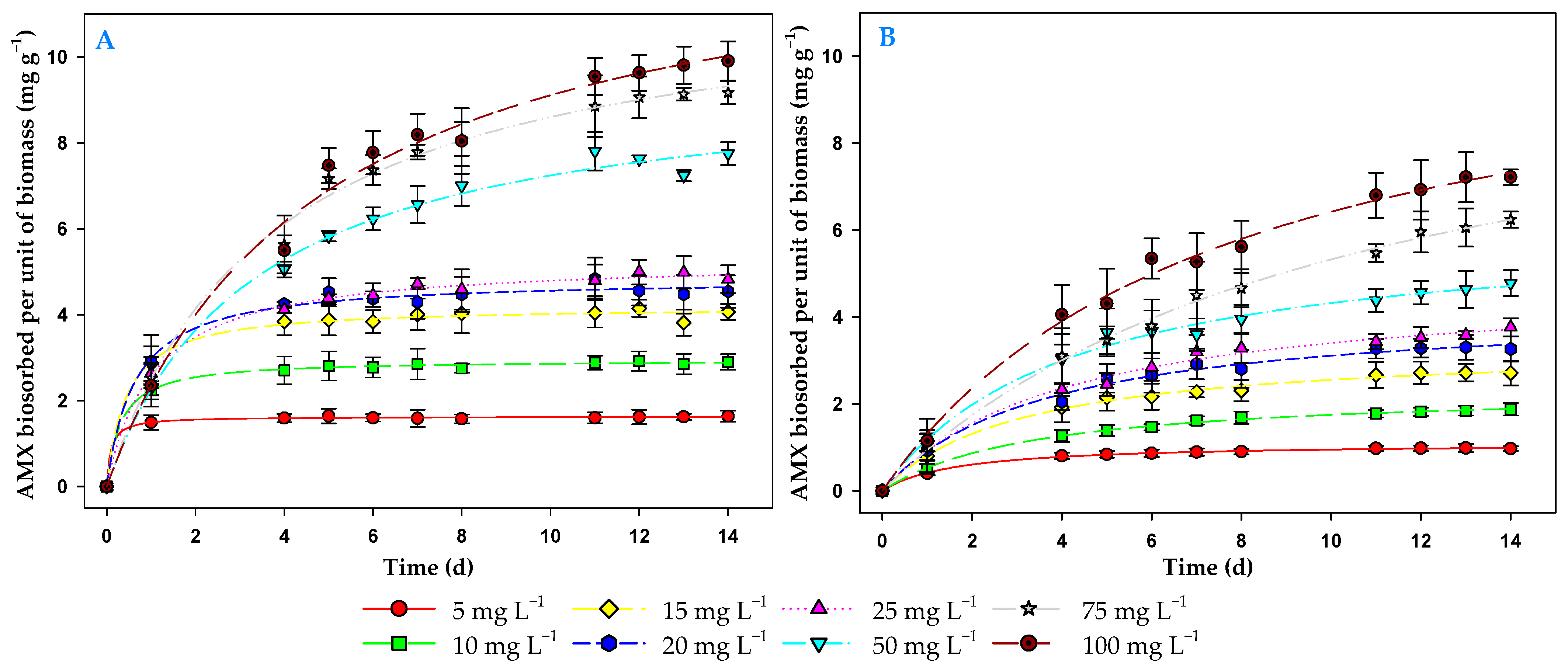
3.3. Effect of Contact Time and Initial Antibiotic Concentration on the Biosorption Process
3.4. Kinetic Models
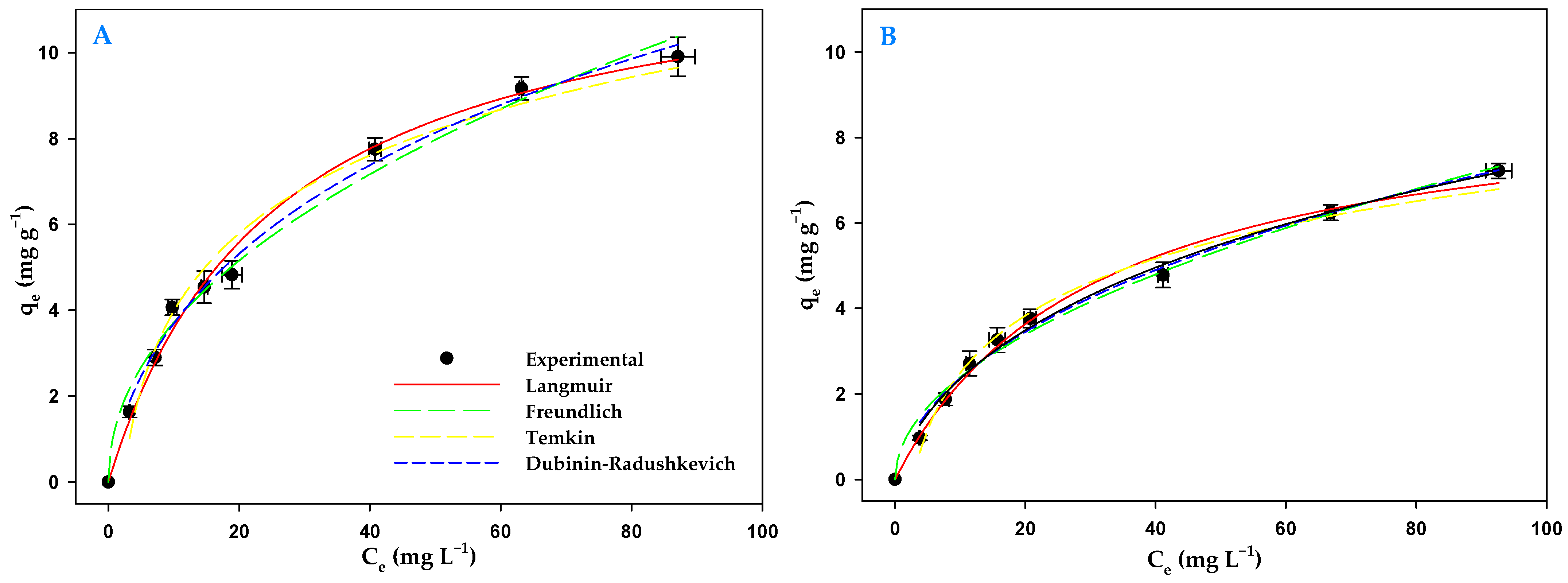
3.5. Biosorption Isotherms
3.6. Biomass Characterization
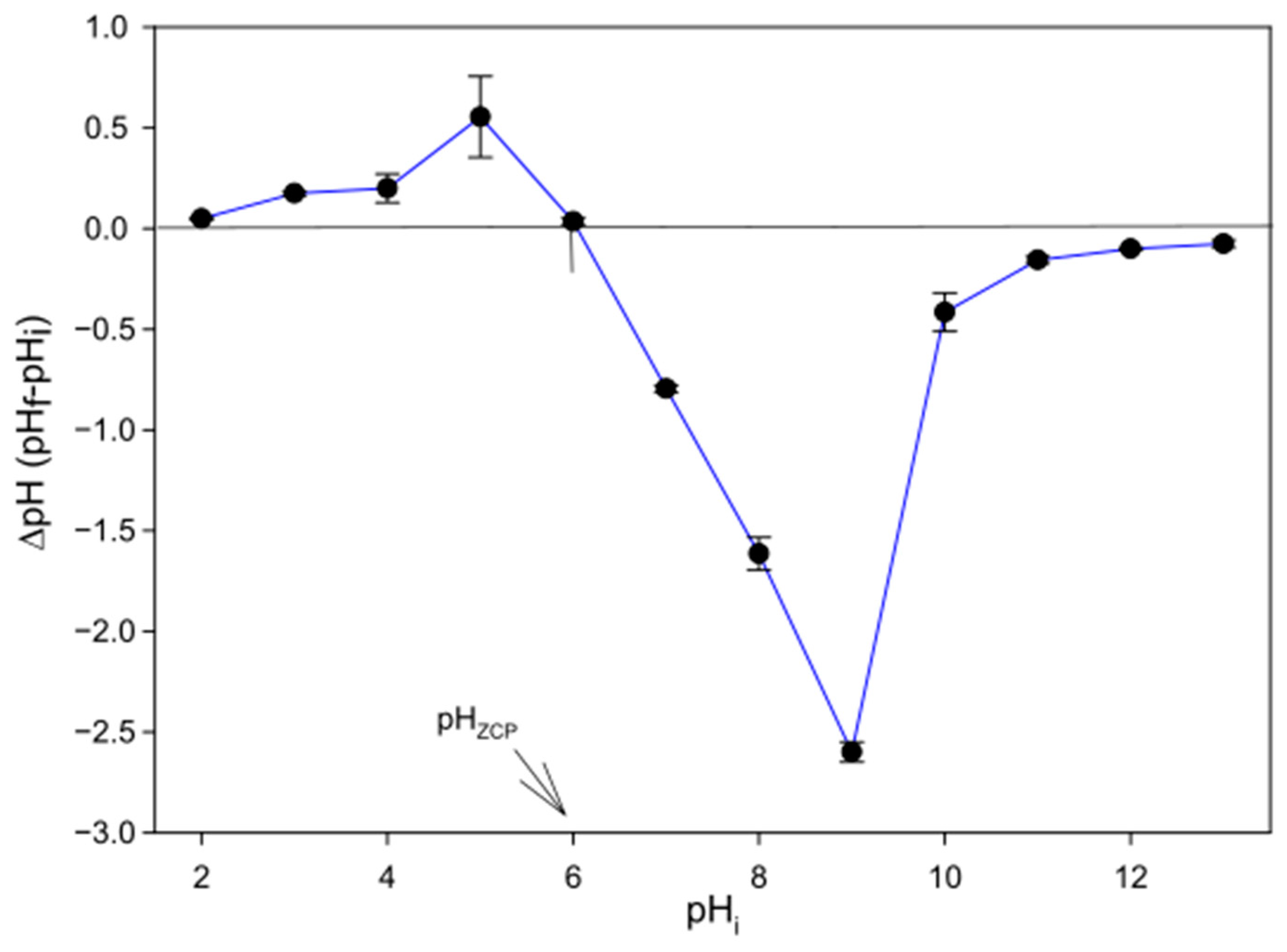
3.6.1. Determination of pHzcp
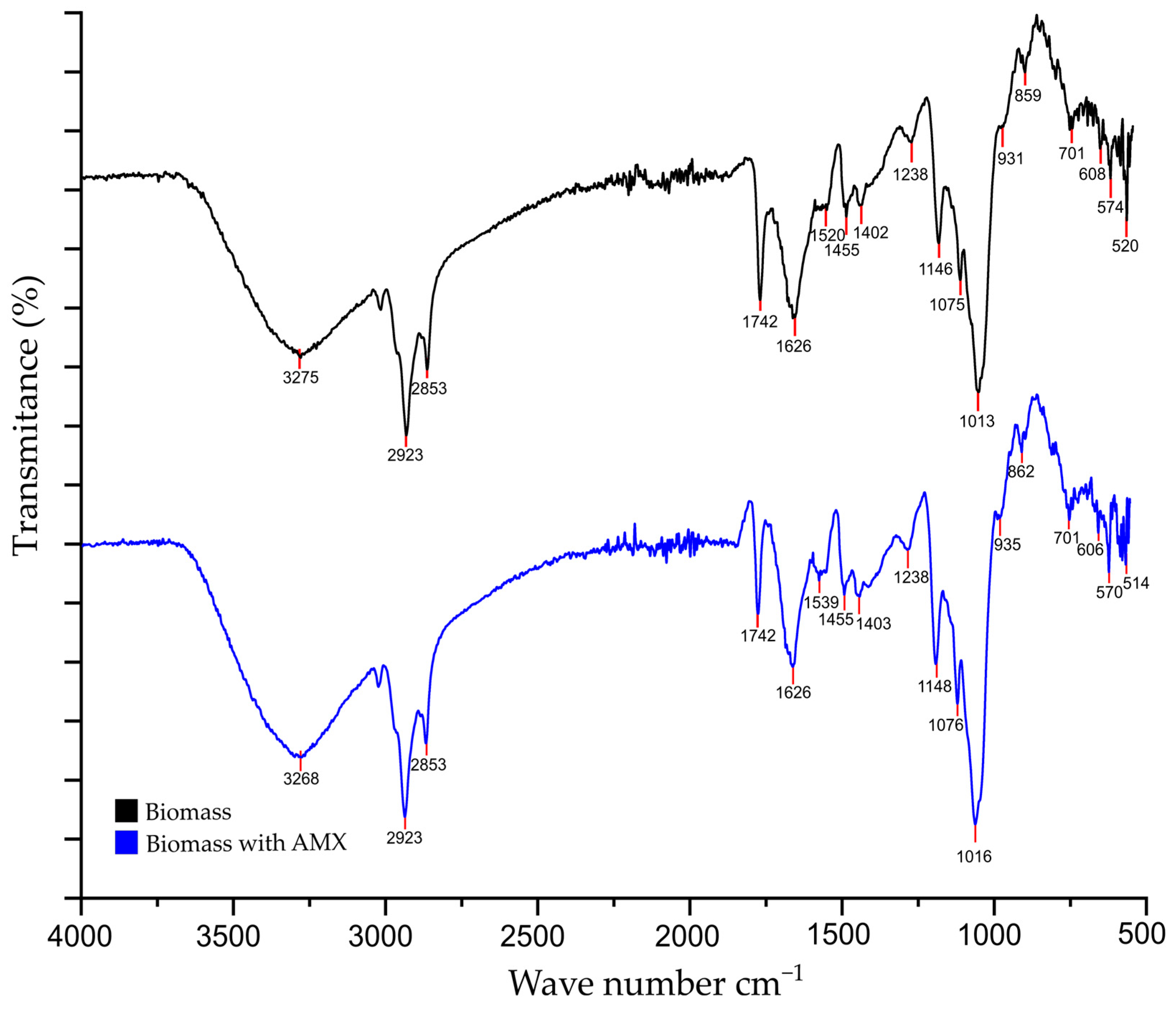
3.6.2. FTIR
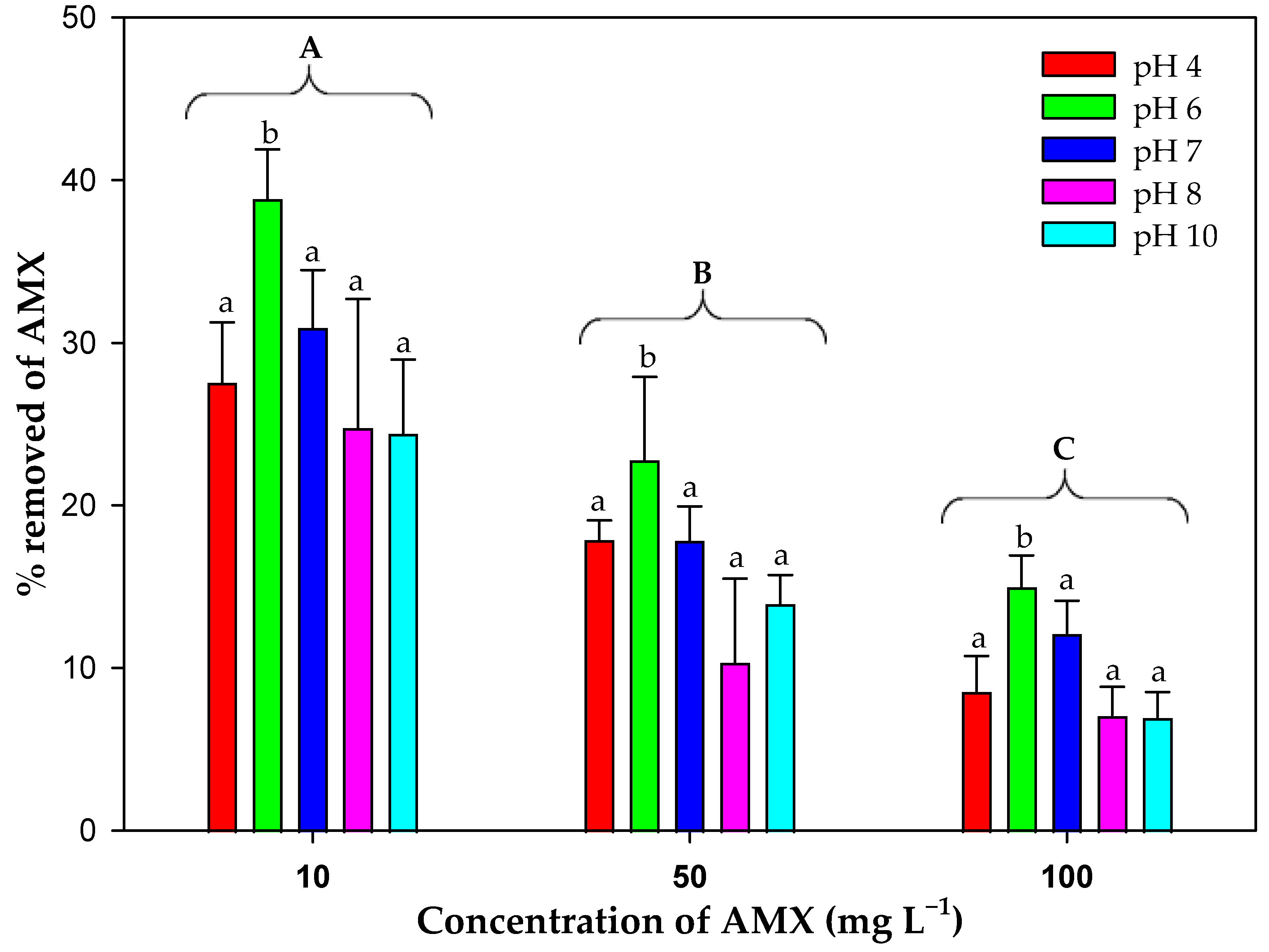
3.7. Effect of pH on Biosorption Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean–Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- World Health Organization. WHO Model List of Essential Medicines 2023. 12 July 2023. Available online: https://iris.who.int/bitstream/handle/10665/371090/WHO-MHP-HPS-EML-2023.02-eng.pdf (accessed on 2 May 2025).
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef]
- Velpandian, T.; Halder, N.; Nath, M.; Das, U.; Moksha, L.; Gowtham, L.; Batta, S.P. Un-segregated waste disposal: An alarming threat of antimicrobials in surface and ground water sources in Delhi. Environ. Sci. Pollut. Res. 2018, 25, 29518–29528. [Google Scholar] [CrossRef]
- Buxton, H.T.; Kolpin, D.W. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in US Streams; US Geological Survey: Reston, VA, USA, 2002. [Google Scholar]
- Calamari, D.; Zuccato, E.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Strategic survey of therapeutic drugs in the rivers Po and Lambro in northern Italy. Environ. Sci. Technol. 2003, 37, 1241–1248. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Hektoen, H.; Berge, J.A.; Hormazabal, V.; Yndestad, M. Persistence of antibacterial agents in marine sediments. Aquaculture 1995, 133, 175–184. [Google Scholar] [CrossRef]
- Gilliver, M.A.; Bennett, M.; Begon, M.; Hazel, S.M.; Hart, C.A. Antibiotic resistance found in wild rodents. Nature 1999, 401, 233–234. [Google Scholar] [CrossRef]
- Flaherty, C.M.; Dodson, S.I. Effects of pharmaceuticals on Daphnia survival, growth, and reproduction. Chemosphere 2005, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.A.; Han, R.; Qu, L. Occurrence, detection and removal of amoxicillin in wastewater: A review. J. Clean. Prod. 2022, 368, 1–25. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef]
- Moussavi, G.; Alahabadi, A.; Yaghmaeian, K.; Eskandari, M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem. Eng. J. 2013, 217, 119–128. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.-W.; Hong, Y.M.; Kim, H.W. Removal of amoxicillin by UV and UV/H2O2 processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef]
- Fernández, D.; Abalde, J.; Torres, E. The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater. Toxics 2024, 12, 277. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Fan, Y.V.; Perry, S.; Klemeš, J.J.; Lee, C.T. A review on air emissions assessment: Transportation. J. Clean. Prod. 2018, 194, 673–684. [Google Scholar] [CrossRef]
- Sangwan, S.; Dukare, A. Microbe-mediated bioremediation: An eco-friendly sustainable approach for environmental clean-up. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 1: Soil-Microbe Interaction; Springer: Singapore, 2018; pp. 145–163. [Google Scholar] [CrossRef]
- Wang, N.; Peng, L.; Gu, Y.; Liang, C.; Pott, R.W.M.; Xu, Y. Insights into biodegradation of antibiotics during the biofilm-based wastewater treatment processes. J. Clean. Prod. 2023, 393, 136321. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Solisio, C.; Converti, A.; Casazza, A.A. Efficient removal of tetracycline, ciprofloxacin, and amoxicillin by novel magnetic chitosan/microalgae biocomposites. Sep. Purif. Technol. 2024, 329, 125115. [Google Scholar] [CrossRef]
- Ricky, R.; Chiampo, F.; Shanthakumar, S. Efficacy of Ciprofloxacin and Amoxicillin Removal and the Effect on the Biochemical Composition of Chlorella vulgaris. Bioengineering 2022, 9, 134. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A review of the latest advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Grossman, A.R.; Lohr, M.; Im, C.S. Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 2004, 38, 119–173. [Google Scholar] [CrossRef]
- Jamers, A.; Blust, R.; De Coen, W.; Griffin, J.L.; Jones, O.A.H. Copper toxicity in the microalga Chlamydomonas reinhardtii: An integrated approach. BioMetals 2013, 26, 731–740. [Google Scholar] [CrossRef]
- Liakh, I.; Harshkova, D.; Hrouzek, P.; Bišová, K.; Aksmann, A.; Wielgomas, B. Green alga Chlamydomonas reinhardtii can effectively remove diclofenac from the water environment—A new perspective on biotransformation. J. Hazard. Mater. 2023, 455, 131570. [Google Scholar] [CrossRef] [PubMed]
- Al-Abachi, M.Q.; Haddi, H.; Al-Abachi, A.M. Spectrophotometric determination of amoxicillin by reaction with N, N-dimethyl-p-phenylenediamine and potassium hexacyanoferrate (III). Anal. Chim. Acta 2005, 554, 184–189. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Huang, W.; Chen, J.; He, F.; Tang, J.; Li, D.; Zhu, Y.; Zhang, Y. Effective phosphate adsorption by Zr/Al-pillared montmorillonite: Insight into equilibrium, kinetics and thermodynamics. Appl. Clay Sci. 2015, 104, 252–260. [Google Scholar] [CrossRef]
- Seoane, R.; Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of methylene blue using living biomass of the microalga Chlamydomonas moewusii: Kinetics and equilibrium studies. Int. J. Environ. Res. Public Health 2022, 19, 2653. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. URSS 1940, 12, 217–225. [Google Scholar]
- Chu, K.H. Revisiting the Temkin isotherm: Dimensional inconsistency and approximate forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of activated charcoal. In Proccedings of Academic Od Science Physical Chemistry Sections; Physical Chemistry Section USSR: Moscow, Russia, 1947; Volume 55, pp. 331–333. [Google Scholar]
- Singh, A.; Singh, S.; Singh, S.; Prasad, N.; Asthana, R.K. Harnessing bioenergy potential of Chlamydomonas reinhardtii: A comprehensive characterization and valorization of biomass towards energy conversion under circular bioeconomy. Renew. Energy 2025, 240. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Singh, P.; Mohan, S.V.; Mohanty, K. Dairy wastewater treatment using Monoraphidium sp. KMC4 and its potential as hydrothermal liquefaction feedstock. Bioresour. Technol. 2023, 376, 128877. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Rapid determination of lipid accumulation under sulfur starvation in Chlamydomonas reinhardtii microalga using Fourier Transform Infrared (FTIR) spectroscopy. Int. J. Pharm. Clin. Res. 2016, 8, 1356–1360. [Google Scholar]
- Khamayseh, M.M.; Kidak, R. Biosorption of reactive Amoxicillin antibiotic on Pithophora macroalgae in aqueous solution: Equilibrium and Kinetic Studies. Desalination Water Treat. 2024, 100669. [Google Scholar] [CrossRef]
- Williams, N.E.; Oba, O.A.; Aydinlik, N.P. Modification, production, and methods of KOH-activated carbon. Chem. Biochem. Eng. Rev. 2022, 9, 164–189. [Google Scholar] [CrossRef]
- Bebu, A.; Szabó, L.; Leopold, N.; Berindean, C.; David, L. IR, Raman, SERS and DFT study of amoxicillin. J. Mol. Struct. 2011, 993, 52–56. [Google Scholar] [CrossRef]
- González, V.; Abalde, J.; Torres, E. Discoloration and biosorption of Brilliant green dye in seawater using living biomass of the microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2024, 36, 1823–1835. [Google Scholar] [CrossRef]
- Ecke, A.; Westphalen, T.; Retzmann, A.; Schneider, R.J. Factors affecting the hydrolysis of the antibiotic amoxicillin in the aquatic environment. Chemosphere 2023, 311, 136921. [Google Scholar] [CrossRef]
- Naidoo, K.; Nompuku, P.; Mkalali, S.; Shabangu, K.; Nkabinde, L.; Singh, V. Post-marketing stability surveillance: Amoxicillin. S. Afr. Fam. Pract. 2006, 48, 14–14e. [Google Scholar] [CrossRef]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Xie, P.; Chen, C.; Zhang, C.; Su, G.; Ren, N.; Ho, S.-H. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020, 172, 115475. [Google Scholar] [CrossRef]
- Kotrba, P.; Mackova, M.; Macek, T. Microbial Biosorption of Metals—General Introduction; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Ali, M.E.; Abd El-Aty, A.M.; Badawy, M.I.; Ali, R.K. Removal of pharmaceutical pollutants from synthetic wastewater using chemically modified biomass of green alga Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2018, 151, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Santaeufemia, S.; Torres, E.; Mera, R.; Abalde, J. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J. Hazard. Mater. 2016, 320, 315–325. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; de Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the environment: Occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]
- Janssen, M.; Janssen, M.; de Winter, M.; Tramper, J.; Mur, L.R.; Snel, J.; Wijffels, R.H. Efficiency of light utilization of Chlamydomonas reinhardtii under medium-duration light/dark cycles. J. Biotechnol. 2000, 78, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lin, X.; Xie, Q.; Liu, J.; Luo, L. Effects of temperature and light on microalgal growth and nutrient removal in turtle aquaculture wastewater. Biology 2024, 13, 901. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B. Application of isotherms models and error functions in activated carbon CO2 sorption processes. Microporous Mesoporous Mater. 2023, 354, 112513. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Erah, P.; Goddard, A.; Barrett, D.; Shaw, P.; Spiller, R. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: Relevance to the treatment of Helicobacter pylori infection. J. Antimicrob. Chemother. 1997, 39, 5–12. [Google Scholar] [CrossRef]
- Aranda, F.L.; Rivas, B.L. Removal of amoxicillin through different methods, emphasizing removal by biopolymers and its derivatives. An overview. J. Chil. Chem. Soc. 2022, 67, 5643–5655. [Google Scholar] [CrossRef]
- Githinji, L.J.; Musey, M.K.; Ankumah, R.O. Evaluation of the fate of ciprofloxacin and amoxicillin in domestic wastewater. Water Air Soil Pollut. 2011, 219, 191–201. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Biosorption of antimony by brown algae S. muticum and A. nodosum. Environ. Eng. Manag. J. 2015, 14, 455–463. [Google Scholar]
- Rodrigues, D.L.C.; Machado, F.M.; Osório, A.G.; de Azevedo, C.F.; Lima, E.C.; da Silva, R.S.; Lima, D.R.; Gonçalves, F.M. Adsorption of amoxicillin onto high surface area–activated carbons based on olive biomass: Kinetic and equilibrium studies. Environ. Sci. Pollut. Res. 2020, 27, 41394–41404. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Asgari, G.; Shokoohi, R.; Dargahi, A.; Arabkouhsar, A. Removing amoxicillin antibiotic from aqueous solutions by Saccharomyces cerevisiae bioadsorbent: Kinetic, thermodynamic and isotherm studies. Desalination Water Treat 2019, 152, 306–315. [Google Scholar] [CrossRef]
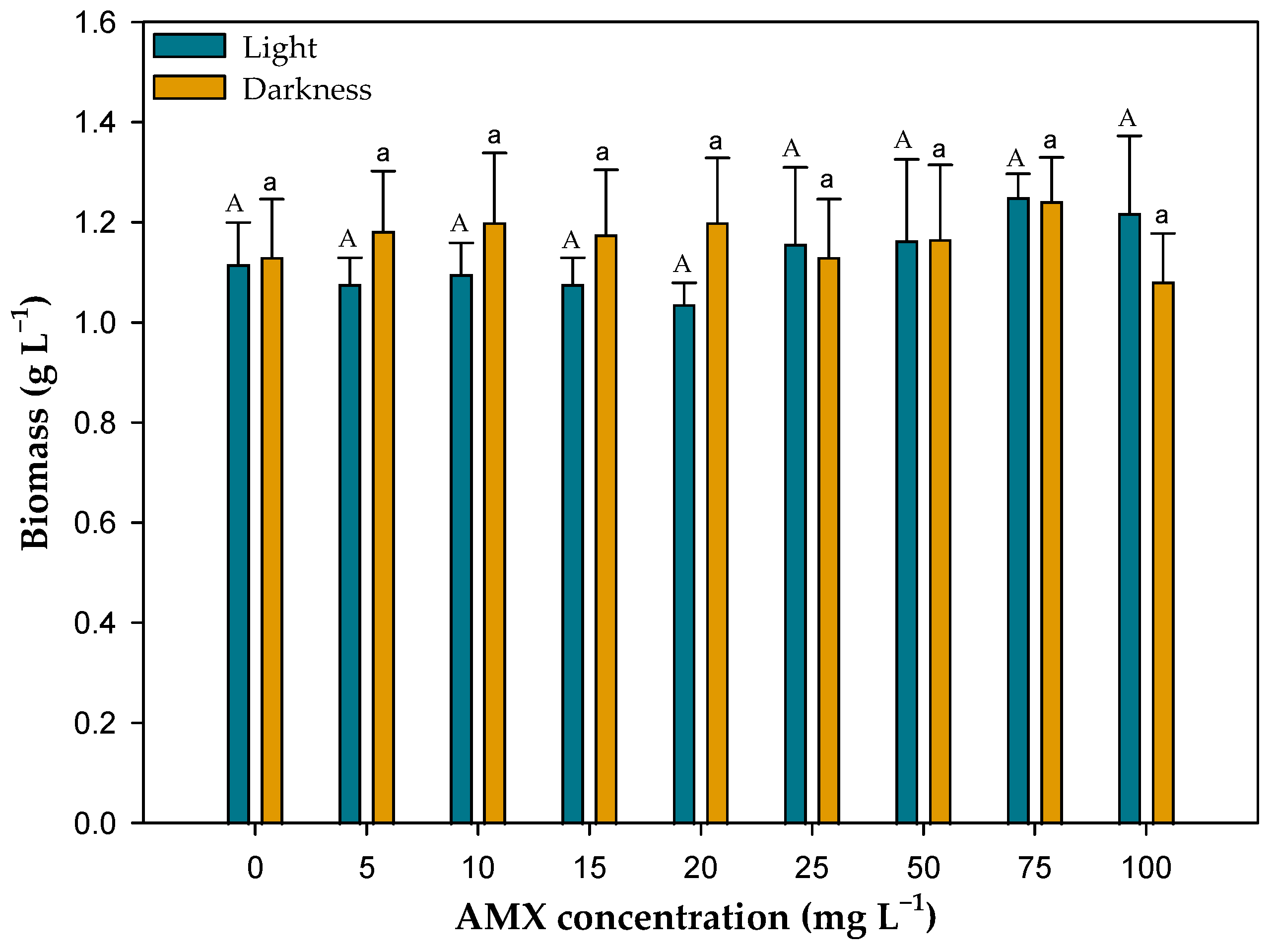
| Kinetics | Isotherms |
|---|---|
|
|
| (3) | (6) |
|
|
| (4) | (7) |
| (5) | (8) |
| |
| (9) (10) | |
| q (mg g−1) is the mass of AMX biosorbed per unit of biomass over time t (d), qe (mg g−1) is the mass of AMX biosorbed per unit of biomass at equilibrium, k1 (d−1) is the constant of the pseudo-first order kinetic model, k2 (g mg−1 d−1) is the constant of the pseudo-second order kinetic model and k3 (g2 mg−2 d−1) is the constant of the pseudo-third order kinetic model. | qe (mg g−1) is the mass of AMX biosorbed at equilibrium per unit of biomass, qmax (mg g−1) is the maximum sorption capacity, qT (mg g−1) is the mass of AMX biosorbed per unit of mass over time, KL (L mg−1) is the affinity constant of the material, Ce (mg L−1) is the concentration of AMX at equilibrium, KF (L mg−1) is the Freundlich constant, n the intensity of the sorption, AT (L g−1) is the binding energy constant, R is the gas constant (0.008314 KJ mol−1 K−1), T is temperature at 291 K, BD is the free energy of sorption per mole of sorbate (mol2 KJ−2), ED (KJ mol−1) is the apparent energy of biosorption and sol is the solubility of the antibiotic (mg/L). |
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | Pseudo-Third Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg L−1) | k1 (h−1) | radj2 | qe (mg L−1) | k2 (L mg−1 h−1) | radj2 | qe (mg L−1) | k3 (L2 mg−2 d−1) | radj2 | |
| 5 | 2.18 ± 0.08 | 1.16 ± 0.36 | 0.8817 | 2.42 ± 0.11 | 0.50 ± 0.19 | 0.9367 | 2.75 ± 0.15 | 0.19 ± 0.09 | 0.9536 |
| 10 | 4.38 ± 0.08 | 0.95 ± 0.14 | 0.9702 | 4.80 ± 0.07 | 0.27 ± 0.03 | 0.9930 | 5.41 ± 0.09 | 0.05 ± 8 × 10−7 | 0.9958 |
| 15 | 5.36 ± 0.13 | 0.45 ± 0.06 | 0.9730 | 6.12 ± 0.12 | 0.10 ± 0.01 | 0.9935 | 7.17 ± 0.15 | 0.01 ± 2 × 10−3 | 0.9953 |
| 20 | 7.11 ± 0.22 | 0.40 ± 0.06 | 0.9612 | 8.12 ± 0.23 | 0.07 ± 0.01 | 0.9870 | 9.55 ± 0.29 | 7 × 10−3 ± 1 × 10−3 | 0.9916 |
| 25 | 8.44 ± 0.33 | 0.32 ± 0.05 | 0.9566 | 9.87 ± 0.38 | 0.04 ± 8 × 10−3 | 0.9829 | 11.78 ± 0.45 | 3 × 10−3 ± 7 × 10−4 | 0.9894 |
| 50 | 18.15 ± 1.00 | 0.16 ± 0.02 | 0.9806 | 24.93 ± 1.96 | 5 × 10−3 ± 1 × 10−3 | 0.9828 | 32.13 ± 2.87 | 1 × 10−4 ± 4 × 10−5 | 0.9836 |
| 75 | 27.19 ± 0.65 | 0.14 ± 7 × 10−3 | 0.9976 | 38.87 ± 1.00 | 3 × 10−3 ± 2 × 10−4 | 0.9987 | 51.18 ± 1.37 | 3 × 10−5 ± 4 × 10−6 | 0.9989 |
| 100 | 30.11 ± 1.52 | 0.13 ± 0.01 | 0.9909 | 43.61 ± 2.92 | 2 × 10−3 ± 4 × 10−4 | 0.9922 | 57.74 ± 4.30 | 2 × 10−5 ± 7 × 10−6 | 0.9926 |
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | Pseudo-Third Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (h−1) | radj2 | qe (mg g−1) | k2 (g mg−1 h−1) | radj2 | qe (mg g−1) | k3 (g2 mg−2 d−1) | radj2 | |
| 5 | 1.61 ± 0.01 | 2.61 ± 0.19 | 0.9981 | 1.63 ± 0.01 | 6.79 ± 1.37 | 0.9982 | 1.67 ± 0.02 | 17.07 ± 6.76 | 0.9980 |
| 10 | 2.83 ± 0.02 | 1.57 ± 0.11 | 0.9937 | 2.95 ± 0.02 | 1.07 ± 0.10 | 0.9978 | 3.17 ± 0.03 | 0.57 ± 0.09 | 0.9976 |
| 15 | 3.97 ± 0.04 | 1.27 ± 0.10 | 0.9917 | 4.19 ± 0.05 | 0.53 ± 0.07 | 0.9934 | 4.56 ± 0.09 | 0.17 ± 0.05 | 0.9905 |
| 20 | 4.49 ± 0.05 | 1.02 ± 0.10 | 0.9881 | 4.84 ± 0.08 | 0.33 ± 0.05 | 0.9899 | 5.38 ± 0.15 | 0.07 ± 0.02 | 0.9859 |
| 25 | 4.73 ± 0.08 | 0.69 ± 0.09 | 0.9789 | 5.29 ± 0.06 | 0.18 ± 0.02 | 0.9965 | 6.06 ± 0.10 | 0.03 ± 4 × 10−3 | 0.9963 |
| 50 | 7.82 ± 0.16 | 0.28 ± 0.02 | 0.9910 | 9.63 ± 0.32 | 0.03 ± 4 × 10−3 | 0.9919 | 11.81 ± 0.52 | 2 × 10−3 ± 5 × 10−4 | 0.9908 |
| 75 | 9.42 ± 0.17 | 0.26 ± 0.02 | 0.9941 | 11.78 ± 0.34 | 0.02 ± 2 × 10−3 | 0.9946 | 14.58 ± 0.57 | 1 × 10−3 ± 2 × 10−4 | 0.9935 |
| 100 | 10.34 ± 0.31 | 0.22 ± 0.02 | 0.9888 | 13.39 ± 0.65 | 0.02 ± 2 × 10−3 | 0.9889 | 16.84 ± 1.03 | 7 × 10−4 ± 2 × 10−4 | 0.9881 |
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | Pseudo-Third Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg L−1) | k1 (h−1) | radj2 | qe (mg L−1) | k2 (L mg−1 h−1) | radj2 | qe (mg L−1) | k3 (L2 mg−2 d−1) | radj2 | |
| 5 | 1.74 ± 0.06 | 0.31 ± 0.04 | 0.9702 | 2.06 ± 0.06 | 0.19 ± 0.03 | 0.9907 | 2.47 ± 0.07 | 0.06 ± 0.01 | 0.9951 |
| 10 | 3.46 ± 0.14 | 0.27 ± 0.04 | 0.9683 | 4.19 ± 0.18 | 0.08 ± 0.01 | 0.9858 | 5.09 ± 0.22 | 0.01 ± 2 × 10−3 | 0.9901 |
| 15 | 4.45 ± 0.21 | 0.24 ± 0.04 | 0.9609 | 5.45 ± 0.30 | 0.05 ± 0.01 | 0.9788 | 6.65 ± 0.39 | 6 × 10−3 ± 2 × 10−3 | 0.9842 |
| 20 | 6.78 ± 0.27 | 0.19 ± 0.02 | 0.9857 | 8.98 ± 0.47 | 0.02 ± 3 × 10−3 | 0.9898 | 11.38 ± 0.66 | 1 × 10−3 ± 3 × 10−4 | 0.9911 |
| 25 | 7.70 ± 0.25 | 0.20 ± 0.02 | 0.9886 | 10.05 ± 0.38 | 0.02 ± 3 × 10−3 | 0.9940 | 12.67 ± 0.51 | 1 × 10−3 ± 2 × 10−4 | 0.9952 |
| 50 | 14.77 ± 0.32 | 0.15 ± 7 × 10−3 | 0.9974 | 20.67 ± 0.57 | 6 × 10−3 ± 5 × 10−4 | 0.9982 | 26.95 ± 0.82 | 1 × 10−4 ± 2 × 10−5 | 0.9984 |
| 75 | 23.25 ± 0.88 | 0.12 ± 9 × 10−3 | 0.9957 | 34.06 ± 1.42 | 3 × 10−3 ± 3 × 10−4 | 0.9974 | 45.33 ± 1.94 | 4 × 10−5 ± 6 × 10−6 | 0.9978 |
| 100 | 23.46 ± 1.28 | 0.12 ± 0.01 | 0.9919 | 34.66 ± 2.42 | 3 × 10−3 ± 5 × 10−4 | 0.9932 | 46.29 ± 3.54 | 3 × 10−5 ± 9 × 10−6 | 0.9935 |
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | Pseudo-Third Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (h−1) | radj2 | qe (mg g−1) | k2 (g mg−1 h−1) | radj2 | qe (mg g−1) | k3 (g2 mg−2 d−1) | radj2 | |
| 5 | 0.96 ± 0.01 | 0.45 ± 0.03 | 0.9901 | 1.11 ± 0.01 | 0.54 ± 0.04 | 0.9980 | 1.31 ± 0.03 | 0.37 ± 0.05 | 0.9956 |
| 10 | 1.89 ± 0.02 | 0.27 ± 0.01 | 0.9976 | 2.35 ± 0.04 | 0.13 ± 8 × 10−3 | 0.9984 | 2.89 ± 0.07 | 0.03 ± 4 × 10−3 | 0.9970 |
| 15 | 2.74 ± 0.06 | 0.29 ± 0.02 | 0.9900 | 3.35 ± 0.08 | 0.10 ± 0.01 | 0.9958 | 4.10 ± 0.12 | 0.02 ± 3 × 10−3 | 0.9955 |
| 20 | 3.39 ± 0.08 | 0.26 ± 0.02 | 0.9904 | 4.23 ± 0.16 | 0.07 ± 0.01 | 0.9909 | 5.22 ± 0.25 | 0.01 ± 2 × 10−3 | 0.9898 |
| 25 | 3.79 ± 0.09 | 0.24 ± 0.02 | 0.9917 | 4.82 ± 0.18 | 0.05 ± 7 × 10−3 | 0.9927 | 6.00 ± 0.28 | 6 × 10−3 ± 1 × 10−3 | 0.9921 |
| 50 | 4.80 ± 0.13 | 0.24 ± 0.02 | 0.9886 | 6.13 ± 0.26 | 0.04 ± 7 × 10−3 | 0.9904 | 7.67 ± 0.42 | 4 × 10−3 ± 1 × 10−4 | 0.9895 |
| 75 | 7.51 ± 0.25 | 0.13 ± 8 × 10−3 | 0.9963 | 10.99 ± 0.47 | 0.01 ± 1 × 10−3 | 0.9975 | 14.63 ± 0.70 | 3 × 10−4 ± 6 × 10−5 | 0.9974 |
| 100 | 8.12 ± 0.26 | 0.16 ± 0.01 | 0.9942 | 11.30 ± 0.54 | 0.01 ± 1 × 10−3 | 0.9943 | 14.73 ± 0.84 | 3 × 10−4 ± 1 × 10−4 | 0.9941 |
| Isotherm | Parameters | Light | Darkness |
|---|---|---|---|
| Langmuir | qmax (mg g−1) | 12.72 ± 0.57 | 9.25 ± 0.48 |
| KL (L mg−1) | 0.04 ± 4 × 10−3 | 0.03 ± 4 × 10−3 | |
| radj2 | 0.9909 | 0.9896 | |
| Freundlich | KF (L mg−1) | 1.24 ± 0.14 | 0.75 ± 0.08 |
| 1/n | 0.48 ± 0.03 | 0.50 ± 0.03 | |
| radj2 | 0.9860 | 0.9871 | |
| Temkin | bT (J mol−1) | 2.62 ± 0.17 | 1.93 ± 0.10 |
| AT (L mg−1) | 0.46 ± 0.07 | 0.36 ± 0.04 | |
| radj2 | 0.9720 | 0.9798 | |
| Dubinin– Radushkevich | qmax (mg g−1) | 25.20 ± 1.52 | 18.51 ± 1.13 |
| BD (mol2 KJ−2) | 6.9 × 10−3 ± 3 × 10−4 | 7.4 × 10−3 ± 3 × 10−4 | |
| ED (KJ mol−1) | 8.51 ± 0.13 | 8.22 ± 0.12 | |
| radj2 | 0.9892 | 0.9897 |
| Removed AMX (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial AMX Concentration (mg L−1) | Light (pH = 7) | Darkness (pH = 7) | pH (Light, Microalgal Biomass) | |||||
| Abiotic Degradation + Biomass | Microalgal Biomass | Abiotic Degradation + Biomass | Microalgal Biomass | 4 | 6 | 8 | 10 | |
| 5 | 50.38 ± 0.79 | 35.20 ± 1.87 | 36.21 ± 0.43 | 23.16 ± 1.54 | N.D. | N.D. | N.D. | N.D. |
| 10 | 46.29 ± 1.38 | 30.92 ± 2.55 | 35.81 ± 0.85 | 22.64 ± 3.79 | 27.56 ± 3.70 | 38.82 ± 3.07 | 24.78 ± 2.64 | 24.42 ± 4.53 |
| 15 | 40.22 ± 1.58 | 30.92 ± 1.38 | 31.49 ± 0.97 | 21.74 ± 0.28 | N.D. | N.D. | N.D. | N.D. |
| 20 | 37.64 ± 0.52 | 25.32 ± 0.67 | 32.32 ± 1.85 | 20.07 ± 4.34 | N.D. | N.D. | N.D. | N.D. |
| 25 | 36.37 ± 3.91 | 22.96 ± 4.89 | 30.16 ± 2.71 | 17.04 ± 2.56 | N.D. | N.D. | N.D. | N.D. |
| 50 | 33.43 ± 3.63 | 17.83 ± 2.11 | 27.93 ± 1.22 | 11.61 ± 3.45 | 17.88 ± 1.20 | 22.79 ± 5.10 | 10.35 ± 3.96 | 13.97 ± 1.74 |
| 75 | 31.14 ± 0.48 | 15.40 ± 0.87 | 25.87 ± 1.54 | 10.38 ± 0.60 | N.D. | N.D. | N.D. | N.D. |
| 100 | 25.82 ± 2.90 | 12.13 ± 2.01 | 19.14 ± 1.91 | 5.97 ± 0.71 | 8.56 ± 2.18 | 14.99 ± 1.93 | 7.08 ± 1.75 | 6.98 ± 1.53 |
| Adsorbents | qmax † (mg g−1) | KF †† (L mg−1) | Pseudo-Second Order Kinetic Constant (g mg−1 d−1) | Biomass (g L−1) | Initial AMX Concentration (mg L−1) | References |
|---|---|---|---|---|---|---|
| Activated carbon with induced NH4Cl | 438.6 | 76.3 | 48.0–0.24 | 0.8 | 10–100 | [19] |
| Olive biomass | 237.0 | 64.4 | 2 × 10−2–9 × 10−2 | 0.6 | 400–800 | [69] |
| Pithophora | 25.8 | 1.2 | 1.4–7 × 10−2 | 5 | 10–150 | [46] |
| Chlamydomonas reinhardtii | 12.7 | 1.2 | 6.8–2 × 10−2 | 1.1 | 5–100 | This work |
| Saccharomyces cerevisiae | 6.3 | 0.5 | 0.1 | 5 | 5–25 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, D.; Abalde, J.; Torres, E. Removal of the Antibiotic Amoxicillin from Polluted Freshwater by Biosorption Using the Living Biomass of the Microalga Chlamydomonas reinhardtii. Toxics 2025, 13, 520. https://doi.org/10.3390/toxics13070520
Fernández D, Abalde J, Torres E. Removal of the Antibiotic Amoxicillin from Polluted Freshwater by Biosorption Using the Living Biomass of the Microalga Chlamydomonas reinhardtii. Toxics. 2025; 13(7):520. https://doi.org/10.3390/toxics13070520
Chicago/Turabian StyleFernández, David, Julio Abalde, and Enrique Torres. 2025. "Removal of the Antibiotic Amoxicillin from Polluted Freshwater by Biosorption Using the Living Biomass of the Microalga Chlamydomonas reinhardtii" Toxics 13, no. 7: 520. https://doi.org/10.3390/toxics13070520
APA StyleFernández, D., Abalde, J., & Torres, E. (2025). Removal of the Antibiotic Amoxicillin from Polluted Freshwater by Biosorption Using the Living Biomass of the Microalga Chlamydomonas reinhardtii. Toxics, 13(7), 520. https://doi.org/10.3390/toxics13070520











