Occurrence and Drivers of Antibiotic Resistance Genes Carried by Bacteriophages in Soils Following Different Fertilization Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. Soil Physicochemical Analysis
2.4. Total DNA Extraction and 16S rRNA Gene Sequencing
2.5. Bacterial and Phage DNA Extraction and ARG Quantification
2.6. Statistical Analysis
3. Results and Discussion
3.1. Diversity and Abundance of pARGs in Different Fertilization Treatment Soils
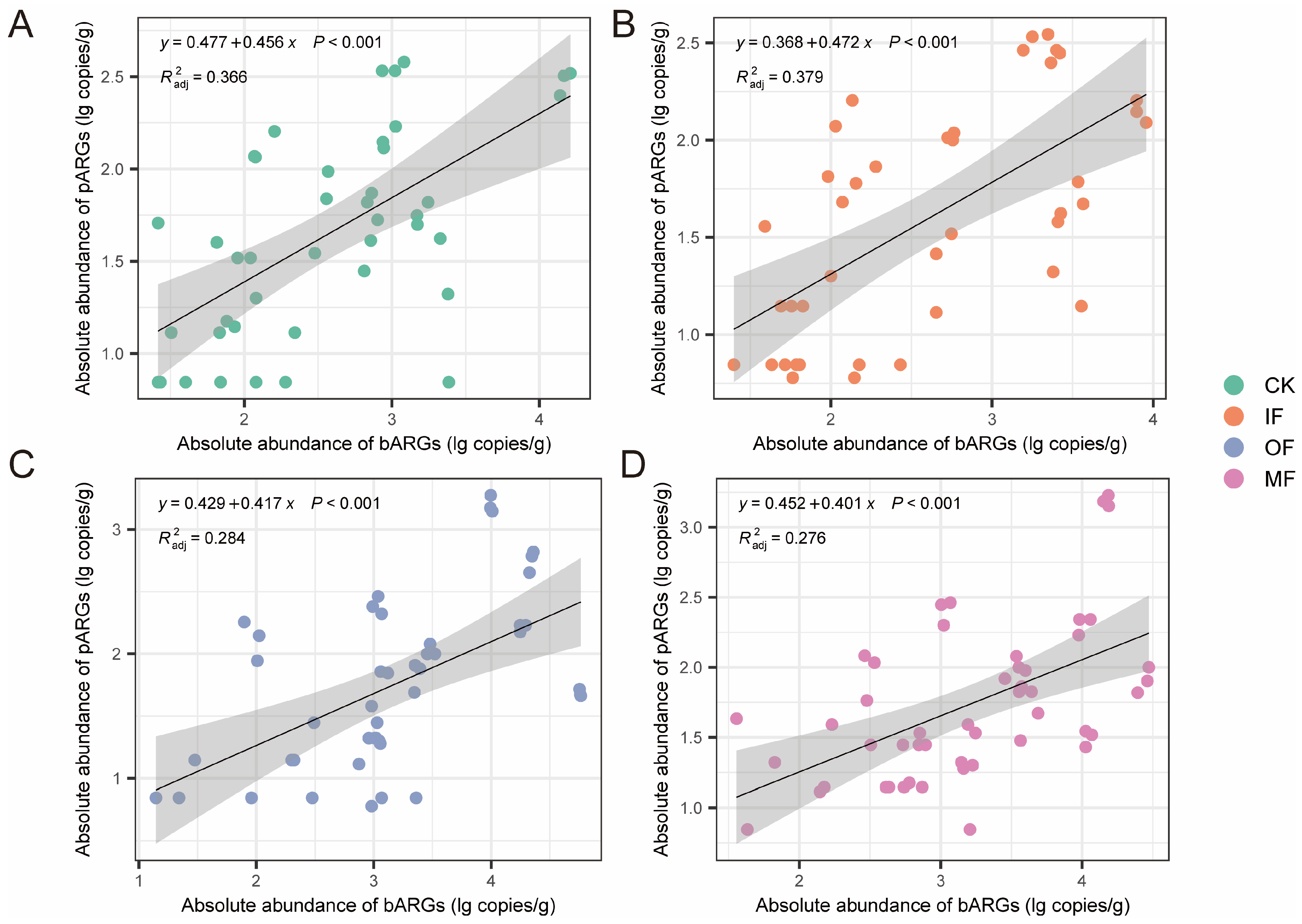
3.2. Relationship Between bARGs and pARGs
3.3. Co-Occurrence of Bacterial Community and pARGs
3.4. Relationship Between pARGs and Environmental Factors
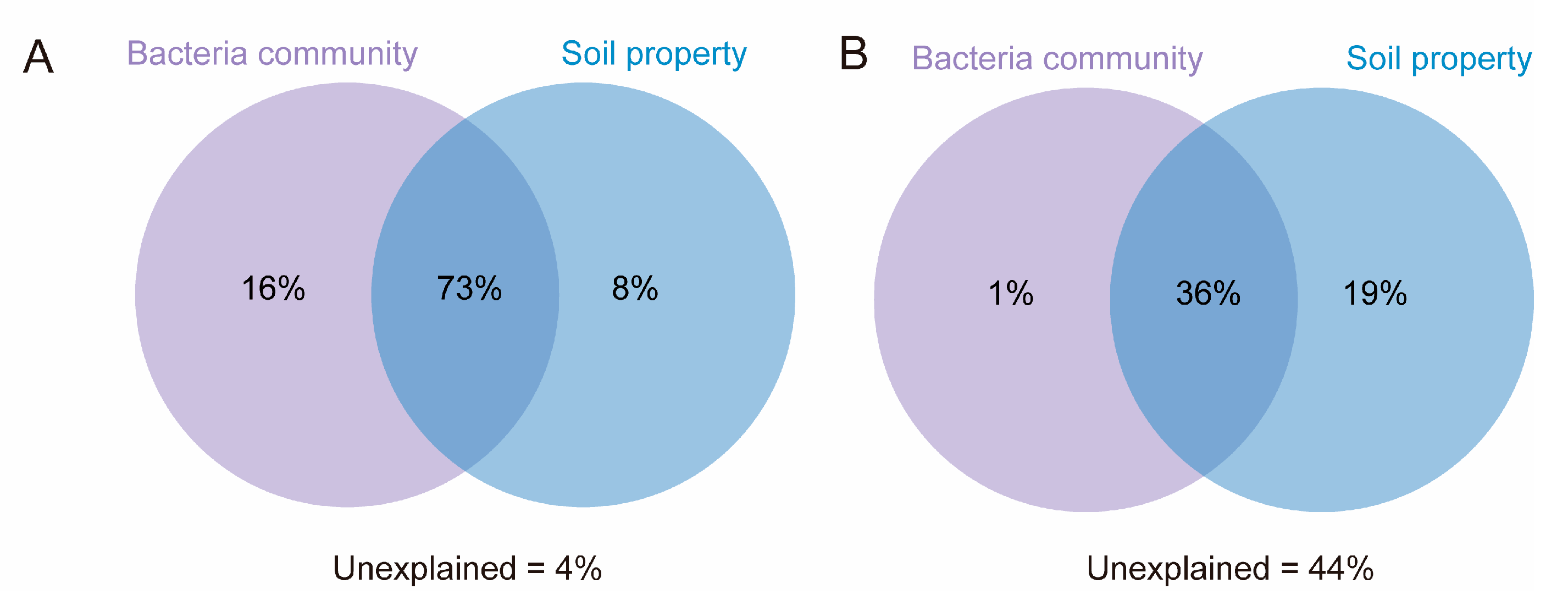
3.5. Contribution of Different Factors to ARG Profile Variation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fillol-Salom, A.; Bacigalupe, R.; Humphrey, S.; Chiang, Y.N.; Chen, J.; Penadés, J.R. Lateral Transduction Is Inherent to the Life Cycle of the Archetypical Salmonella Phage P22. Nat. Commun. 2021, 12, 6510. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal Transfer of Antibiotic Resistance Genes in Clinical Environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, S.A.; Sutar, P.P.; Chen, C.; Ni, J.-B.; Wang, J.; Mujumdar, A.S.; Zhang, J.-S.; Xu, M.-Q.; Fang, X.-M.; Zhang, C.; et al. Antibiotic Resistant Bacteria in Food Systems: Current Status, Resistance Mechanisms, and Mitigation Strategies. Agric. Commun. 2024, 2, 100027. [Google Scholar] [CrossRef]
- Kavagutti, V.S.; Andrei, A.-Ş.; Mehrshad, M.; Salcher, M.M.; Ghai, R. Phage-Centric Ecological Interactions in Aquatic Ecosystems Revealed through Ultra-Deep Metagenomics. Microbiome 2019, 7, 135. [Google Scholar] [CrossRef]
- Kauffman, K.M.; Chang, W.K.; Brown, J.M.; Hussain, F.A.; Yang, J.; Polz, M.F.; Kelly, L. Resolving the Structure of Phage–Bacteria Interactions in the Context of Natural Diversity. Nat. Commun. 2022, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Chevallereau, A.; Pons, B.J.; Van Houte, S.; Westra, E.R. Interactions between Bacterial and Phage Communities in Natural Environments. Nat. Rev. Microbiol. 2021, 20, 49–62. [Google Scholar] [CrossRef]
- Anand, T.; Bera, B.C.; Vaid, R.K.; Barua, S.; Riyesh, T.; Virmani, N.; Hussain, M.; Singh, R.K.; Tripathi, B.N. Abundance of Antibiotic Resistance Genes in Environmental Bacteriophages. J. Gen. Virol. 2016, 97, 3458–3466. [Google Scholar] [CrossRef]
- Modi, S.R.; Lee, H.H.; Spina, C.S.; Collins, J.J. Antibiotic Treatment Expands the Resistance Reservoir and Ecological Network of the Phage Metagenome. Nature 2013, 499, 219–222. [Google Scholar] [CrossRef]
- Petrovich, M.L.; Zilberman, A.; Kaplan, A.; Eliraz, G.R.; Wang, Y.; Langenfeld, K.; Duhaime, M.; Wigginton, K.; Poretsky, R.; Avisar, D.; et al. Microbial and Viral Communities and Their Antibiotic Resistance Genes Throughout a Hospital Wastewater Treatment System. Front. Microbiol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Blanco-Picazo, P.; Gómez-Gómez, C.; Morales-Cortes, S.; Muniesa, M.; Rodríguez-Rubio, L. Antibiotic Resistance in the Viral Fraction of Dairy Products and a Nut-Based Milk. Int. J. Food Microbiol. 2022, 367, 109590. [Google Scholar] [CrossRef]
- Yosef, I.; Goren, M.G.; Globus, R.; Molshanski-Mor, S.; Qimron, U. Extending the Host Range of Bacteriophage Particles for DNA Transduction. Mol. Cell 2017, 66, 721–728.e3. [Google Scholar] [CrossRef]
- Calero-Cáceres, W.; Muniesa, M. Persistence of Naturally Occurring Antibiotic Resistance Genes in the Bacteria and Bacteriophage Fractions of Wastewater. Water Res. 2016, 95, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Göller, P.C.; Elsener, T.; Lorgé, D.; Radulovic, N.; Bernardi, V.; Naumann, A.; Amri, N.; Khatchatourova, E.; Coutinho, F.H.; Loessner, M.J.; et al. Multi-Species Host Range of Staphylococcal Phages Isolated from Wastewater. Nat. Commun. 2021, 12, 6965. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, C.; Zhou, S.; Liu, C.; Eldridge, D.J.; Ai, C.; Wilhelm, S.W.; Singh, B.K.; Liang, X.; Radosevich, M.; et al. Prophage-Encoded Antibiotic Resistance Genes Are Enriched in Human-Impacted Environments. Nat. Commun. 2024, 15, 8315. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, T.; Gao, M.; Ru, S.; Gao, H.; Wang, X. Does Increasing the Organic Fertilizer Application Rate Always Boost the Antibiotic Resistance Level in Agricultural Soils? Environ. Pollut. 2023, 322, 121251. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Topp, E. Abundance of Antibiotic Resistance Genes in Bacteriophage Following Soil Fertilization with Dairy Manure or Municipal Biosolids, and Evidence for Potential Transduction. Appl. Environ. Microbiol. 2015, 81, 7905–7913. [Google Scholar] [CrossRef]
- Sun, M.; Ye, M.; Jiao, W.; Feng, Y.; Yu, P.; Liu, M.; Jiao, J.; He, X.; Liu, K.; Zhao, Y.; et al. Changes in Tetracycline Partitioning and Bacteria/Phage-Comediated ARGs in Microplastic-Contaminated Greenhouse Soil Facilitated by Sophorolipid. J. Hazard. Mater. 2018, 345, 131–139. [Google Scholar] [CrossRef]
- Chen, M.-L.; An, X.-L.; Liao, H.; Yang, K.; Su, J.-Q.; Zhu, Y.-G. Viral Community and Virus-Associated Antibiotic Resistance Genes in Soils Amended with Organic Fertilizers. Environ. Sci. Technol. 2021, 55, 13881–13890. [Google Scholar] [CrossRef]
- Sun, Y.; Qiu, T.; Gao, M.; Shi, M.; Zhang, H.; Wang, X. Inorganic and Organic Fertilizers Application Enhanced Antibiotic Resistome in Greenhouse Soils Growing Vegetables. Ecotoxicol. Environ. Saf. 2019, 179, 24–30. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Shi, M.; Qiu, T.; Gao, M.; Tian, S.; Wang, X. Effect of Antibiotic Type and Vegetable Species on Antibiotic Accumulation in Soil-Vegetable System, Soil Microbiota, and Resistance Genes. Chemosphere 2021, 263, 128099. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, T.; Gao, M.; Sun, Y.; Cheng, S.; Gao, H.; Wang, X. Diversity and Abundance of Antibiotic Resistance Genes in Rhizosphere Soil and Endophytes of Leafy Vegetables: Focusing on the Effect of the Vegetable Species. J. Hazard. Mater. 2021, 415, 125595. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Jia, Y.; Qiu, T.; Gao, M.; Cheng, S.; Wang, X.; Sun, Y. Poly-γ-Glutamic Acid Improves the Drought Resistance of Maize Seedlings by Adjusting the Soil Moisture and Microbial Community Structure. Appl. Soil Ecol. 2018, 129, 128–135. [Google Scholar] [CrossRef]
- Morella, N.M.; Yang, S.C.; Hernandez, C.A.; Koskella, B. Rapid Quantification of Bacteriophages and Their Bacterial Hosts in Vitro and in Vivo Using Droplet Digital PCR. J. Virol. Methods 2018, 259, 18–24. [Google Scholar] [CrossRef]
- Bert, F. Identification of PSE and OXA beta-lactamase genes in Pseudomonas aeruginosa using PCR-restriction fragment length polymorphism. J. Antimicrob. Chemoth. 2002, 50, 11–18. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Yang, L.; Stedtfeld, R.D.; Peng, A.; Gu, C.; Boyd, S.A.; Li, H. Antibiotic resistance genes and bacterial communities in cornfield and pasture soils receiving swine and dairy manures. Environ. Pollut. 2019, 248, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Lluch, M.; Jofre, J.; Muniesa, M. Quinolone resistance genes (qnrA and qnrS) in bacteriophage particles from wastewater samples and the effect of inducing agents on packaged antibiotic resistance genes. J. Antimicrob. Chemoth. 2014, 69, 1265–1274. [Google Scholar] [CrossRef]
- Ouyang, W.; Huang, F.; Zhao, Y.; Li, H.; Su, J. Increased levels of antibiotic resistance in urban stream of Jiulongjiang River, China. Appl. Microbiol. Biot. 2015, 99, 5697–5707. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, W.; Lu, S.; Liu, J.; Liang, H.; Yang, Y.; Duan, G.; Li, Y.; Wang, H.; Zhang, A. Prevalence of antibiotic resistance genes in bacteriophage DNA fraction from Funan River water in Sichuan, China. Sci. Total Environ. 2018, 626, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Effect of Fertilization on the Antibiotic Resistance Genes in Soil Bacteria and Bacteriophages; University of Chinese Academy of Science: Beijing, China, 2019. (in Chinese) [Google Scholar]
- Yang, Y.; Xing, S.; Chen, Y.; Wu, R.; Wu, Y.; Wang, Y.; Mi, J.; Liao, X. Profiles of Bacteria/Phage-Comediated ARGs in Pig Farm Wastewater Treatment Plants in China: Association with Mobile Genetic Elements, Bacterial Communities and Environmental Factors. J. Hazard. Mater. 2021, 404, 124149. [Google Scholar] [CrossRef]
- Calero-Cáceres, W.; Ye, M.; Balcázar, J.L. Bacteriophages as Environmental Reservoirs of Antibiotic Resistance. Trends Microbiol. 2019, 27, 570–577. [Google Scholar] [CrossRef]
- Debroas, D.; Siguret, C. Viruses as Key Reservoirs of Antibiotic Resistance Genes in the Environment. ISME J. 2019, 13, 2856–2867. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L. How Do Bacteriophages Promote Antibiotic Resistance in the Environment? Clin. Microbiol. Infec. 2018, 24, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.-G. Long-Term Field Application of Sewage Sludge Increases the Abundance of Antibiotic Resistance Genes in Soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Tong, C.; Xiao, D.; Xie, L.; Yang, J.; Zhao, R.; Hao, J.; Huo, Z.; Zeng, Z.; Xiong, W. Swine Manure Facilitates the Spread of Antibiotic Resistome Including Tigecycline-Resistant Tet(X) Variants to Farm Workers and Receiving Environment. Sci. Total Environ. 2022, 808, 152157. [Google Scholar] [CrossRef]
- Hardy, A.; Sharma, V.; Kever, L.; Frunzke, J. Genome Sequence and Characterization of Five Bacteriophages Infecting Streptomyces Coelicolor and Streptomyces Venezuelae: Alderaan, Coruscant, Dagobah, Endor1 and Endor2. Viruses 2020, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gliesche, C.G.; Holm, N.C.; Beese, M.; Neumann, M.; Volker, H.; Gebers, R.; Hirsch, P. New Bacteriophages Active on Strains of Hyphomicrobium. J. Gen. Microb. 1988, 134, 1339–1353. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, S.; Ding, S.; Shen, J.; Zhu, K. Toxins and Mobile Antimicrobial Resistance Genes in Bacillus Probiotics Constitute a Potential Risk for One Health. J. Hazard. Mater. 2020, 382, 121266. [Google Scholar] [CrossRef]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of Antibiotic Resistance Genes from Antibiotic Producers to Pathogens. Nat. Commun. 2017, 8, 15784. [Google Scholar] [CrossRef]
- Wang, H.; Tao, X.; Yin, H.; Xing, X.; Shi, B. The Perfluorooctanoic Acid Accumulation and Release from Pipelines Promoted Growth of Bacterial Communities and Opportunistic Pathogens with Different Antibiotic Resistance Genes in Drinking Water. J. Hazard. Mater. 2024, 478, 135600. [Google Scholar] [CrossRef]
- Bernard, C.; Li, Y.; Lopez, P.; Bapteste, E. Beyond Arbitrium: Identification of a Second Communication System in Bacillus Phage phi3T That May Regulate Host Defense Mechanisms. ISME J. 2020, 15, 545–549. [Google Scholar] [CrossRef]
- Ma, B.; Lv, X.; Cai, Y.; Chang, S.X.; Dyck, M.F. Liming Does Not Counteract the Influence of Long-Term Fertilization on Soil Bacterial Community Structure and Its Co-Occurrence Pattern. Soil Biol. Biochem. 2018, 123, 45–53. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-Term Organic Fertilizer Substitution Increases Rice Yield by Improving Soil Properties and Regulating Soil Bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Qaswar, M.; Jing, H.; Ahmed, W.; Dongchu, L.; Shujun, L.; Lu, Z.; Cai, A.; Lisheng, L.; Yongmei, X.; Jusheng, G.; et al. Yield Sustainability, Soil Organic Carbon Sequestration and Nutrients Balance under Long-Term Combined Application of Manure and Inorganic Fertilizers in Acidic Paddy Soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Shi, H.; Hu, X.; Li, W.; Zhang, J.; Hu, B.; Lou, L. Soil Component: A Potential Factor Affecting the Occurrence and Spread of Antibiotic Resistance Genes. Antibiotics 2023, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.H.; Li, X.; Rashid, A.; Su, J.; Xu, J.; Brejnrod, A.D.; Su, J.-Q.; Wu, Y.; Zhu, Y.-G.; Zhou, S.G.; et al. Co-Selection of Antibiotic Resistance Genes, and Mobile Genetic Elements in the Presence of Heavy Metals in Poultry Farm Environments. Sci. Total Environ. 2021, 755, 142702. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Ma, R.-A.; Zhu, D.; Konstantinidis, K.T.; Zhu, Y.-G.; Zhang, S.-Y. Organic Fertilization Co-Selects Genetically Linked Antibiotic and Metal(Loid) Resistance Genes in Global Soil Microbiome. Nat. Commun. 2024, 15, 5168. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, M.; Wang, F.-H.; Zhu, Y.-G. Use of Commercial Organic Fertilizer Increases the Abundance of Antibiotic Resistance Genes and Antibiotics in Soil. Environ. Sci. Pollut. Res. 2016, 24, 701–710. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Guo, Y.; Zhang, Y.; Hu, X.; Cheng, S.; Wang, X. Occurrence and Drivers of Antibiotic Resistance Genes Carried by Bacteriophages in Soils Following Different Fertilization Treatments. Toxics 2025, 13, 495. https://doi.org/10.3390/toxics13060495
Zhang M, Guo Y, Zhang Y, Hu X, Cheng S, Wang X. Occurrence and Drivers of Antibiotic Resistance Genes Carried by Bacteriophages in Soils Following Different Fertilization Treatments. Toxics. 2025; 13(6):495. https://doi.org/10.3390/toxics13060495
Chicago/Turabian StyleZhang, Mingdi, Yajie Guo, Yue Zhang, Xueying Hu, Shoutao Cheng, and Xuming Wang. 2025. "Occurrence and Drivers of Antibiotic Resistance Genes Carried by Bacteriophages in Soils Following Different Fertilization Treatments" Toxics 13, no. 6: 495. https://doi.org/10.3390/toxics13060495
APA StyleZhang, M., Guo, Y., Zhang, Y., Hu, X., Cheng, S., & Wang, X. (2025). Occurrence and Drivers of Antibiotic Resistance Genes Carried by Bacteriophages in Soils Following Different Fertilization Treatments. Toxics, 13(6), 495. https://doi.org/10.3390/toxics13060495





