Association of Urinary Cadmium and Antimony with Osteoporosis Risk in Postmenopausal Brazilian Women: Insights from a 20 Metal(loid) Biomonitoring Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. BMD Measurement
2.3. Measurement of Urinary Metal(loid) Concentrations
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Urinary Concentrations of Metals and Metalloids in Brazilian Postmenopausal Women
3.3. Intercorrelations Between Metal(loid) Concentrations in Urine
3.4. Association of Studied Variables (Clinical and Analytical) with BMD
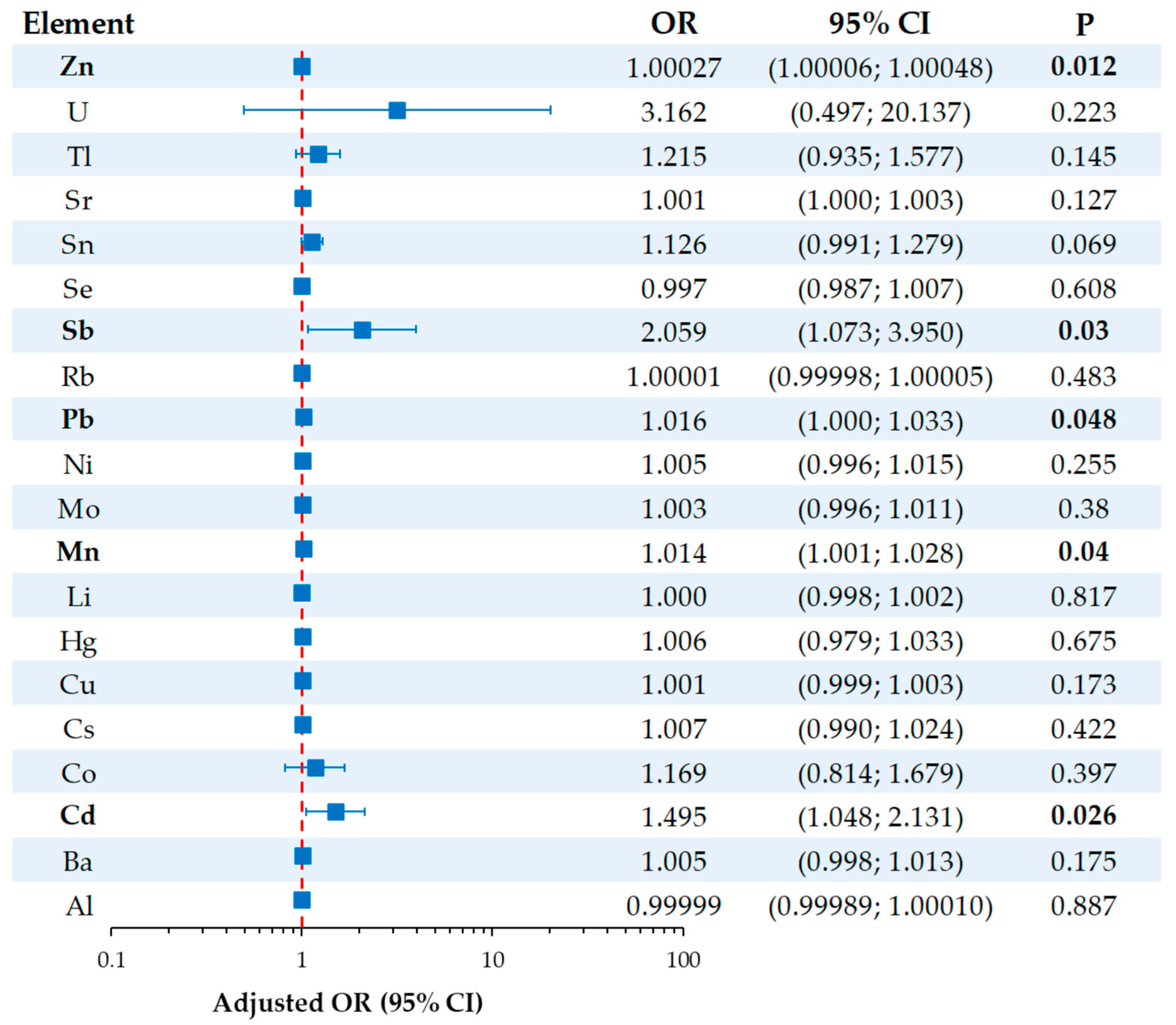
3.5. Association Between Urinary Metal(loid) Concentrations and Osteoporosis Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| BMI | Body mass index |
| DALYs | Disability-adjusted life years |
| DEXA | Dual-energy X-ray absorptiometry |
| ICP-MS | Inductively coupled plasma–mass spectrometry |
| HRT | Hormone replacement therapy |
| NHANES | U.S. National Health and Nutrition Examination Survey |
| WHO | World Health Organization |
| YLDs | Years lived with disability |
References
- Dong, Y.; Kang, H.; Peng, R.; Song, K.; Guo, Q.; Guan, H.; Zhu, M.; Ye, D.; Li, F. Global, Regional, and National Burden of Low Bone Mineral Density from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front. Endocrinol. 2022, 13, 870905. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Emmanuelle, N.E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Wang, Z.; Feng, J.; Zou, J.; Gao, B. Consequences of Aging on Bone. Aging Dis. 2023, 15, 2417–2452. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Panayi, A.C.; Wang, H.; Lin, S.; Li, G.; Liu, G. Ageing-related bone and immunity changes: Insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024, 12, 42. [Google Scholar] [CrossRef]
- Feng, J.N.; Zhang, C.G.; Li, B.H.; Zhan, S.Y.; Wang, S.F.; Song, C.L. Global burden of hip fracture: The Global Burden of Disease Study. Osteoporos. Int. 2024, 35, 41–52. [Google Scholar] [CrossRef]
- Foundation, I.O. Epidemiology of Osteoporosis and Fragility Fractures. Available online: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (accessed on 11 March 2025).
- Collaborators, G.F. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Baccaro, L.F.; Conde, D.M.; Costa-Paiva, L.; Pinto-Neto, A.M. The epidemiology and management of postmenopausal osteoporosis: A viewpoint from Brazil. Clin. Interv. Aging 2015, 10, 583–591. [Google Scholar] [CrossRef]
- Elahmer, N.R.; Wong, S.K.; Mohamed, N.; Alias, E.; Chin, K.Y.; Muhammad, N. Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines 2024, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Pivina, L.; Dadar, M.; Semenova, Y.; Chirumbolo, S.; Aaseth, J. Long-Term Accumulation of Metals in the Skeleton as Related to Osteoporotic Derangements. Curr. Med. Chem. 2020, 27, 6837–6848. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Kazemi, M.; Taheri, E.; Mohammadi, H.; Boozari, B.; Hadi, A.; Moradi, S. Exposure to heavy metals and the risk of osteopenia or osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1671–1682. [Google Scholar] [CrossRef]
- Tang, C.; Lv, X.; Zou, L.; Rong, Y.; Zhang, L.; Xu, M.; Li, S.; Chen, G. Cadmium exposure and osteoporosis: Epidemiological evidence and mechanisms. Toxicol. Sci. 2025, 205, 1–10. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.J. Toxic Metals and Metalloids in Food: Current Status, Health Risks, and Mitigation Strategies. Curr. Environ. Health Rep. 2024, 11, 468–483. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Silina, E.V.; Stupin, V.A.; Zaitsev, O.N.; Sotnikova, T.I.; Tazina, S.I.; Zhang, F.; Guo, X.; Tinkov, A.A. The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings. Biomolecules 2023, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, Y.; Long, H.; Ou, M.; Zhao, S. Relationship between blood manganese and bone mineral density and bone mineral content in adults: A population-based cross-sectional study. PLoS ONE 2022, 17, e0276551. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration-a Review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Yang, T.; Lee, S.Y.; Park, K.C.; Park, S.H.; Chung, J.; Lee, S. The Effects of Selenium on Bone Health: From Element to Therapeutics. Molecules 2022, 27, 392. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- Wei, M.H.; Cui, Y.; Zhou, H.L.; Song, W.J.; Di, D.S.; Zhang, R.Y.; Huang, Q.; Liu, J.A.; Wang, Q. Associations of multiple metals with bone mineral density: A population-based study in US adults. Chemosphere 2021, 282, 131150. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Suwazono, Y.; Wolk, A.; Vahter, M.; Akesson, A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.; Wang, L.; Huang, G.; Cheng, Y.; Hou, D.; Liu, W.; Zhang, T.; Liu, J. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients 2022, 14, 1523. [Google Scholar] [CrossRef]
- Kunioka, C.T.; Manso, M.C.; Carvalho, M. Association between Environmental Cadmium Exposure and Osteoporosis Risk in Postmenopausal Women: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 20, 485. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, C.C.; Jung, W.T.; Lin, C.Y. The associations among lead exposure, bone mineral density, and FRAX score: NHANES, 2013 to 2014. Bone 2019, 128, 115045. [Google Scholar] [CrossRef]
- Yachiguchi, K.; Sekiguchi, T.; Nakano, M.; Hattori, A.; Yamamoto, M.; Kitamura, K.; Maeda, M.; Tabuchi, Y.; Kondo, T.; Kamauchi, H.; et al. Effects of inorganic mercury and methylmercury on osteoclasts and osteoblasts in the scales of the marine teleost as a model system of bone. Zoolog. Sci. 2014, 31, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.J.; Park, H.T.; Shin, J.H.; Hur, J.Y.; Kim, S.H.; Lee, K.W.; Kim, T. The relationship between blood mercury level and osteoporosis in postmenopausal women. Menopause 2012, 19, 576–581. [Google Scholar] [CrossRef]
- Mizuno, D.; Kawahara, M.; Konoha-Mizuno, K.; Ogawara, T.; Hama, R.; Yamazaki, K. Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells. Int. J. Mol. Sci. 2023, 24, 1583. [Google Scholar] [CrossRef]
- Korotkov, S.M. Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria. Int. J. Mol. Sci. 2023, 24, 4459. [Google Scholar] [CrossRef] [PubMed]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Q.; Zhang, S.; Del Rosario, A. Lithium use and risk of fracture: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The Skeletal-Protecting Action and Mechanisms of Action for Mood-Stabilizing Drug Lithium Chloride: Current Evidence and Future Potential Research Areas. Front. Pharmacol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Zhao, S.; Luo, L.; Yan, L. Wnt/β-catenin signaling pathway: Proteins’ roles in osteoporosis and cancer diseases and the regulatory effects of natural compounds on osteoporosis. Mol. Med. 2024, 30, 193. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Gritsaenko, T.; Pierrefite-Carle, V.; Creff, G.; Simoneau, B.; Hagège, A.; Farlay, D.; Pagnotta, S.; Orange, F.; Jaurand, X.; Auwer, C.D.; et al. Low doses of uranium and osteoclastic bone resorption: Key reciprocal effects evidenced using new in vitro biomimetic models of bone matrix. Arch. Toxicol. 2021, 95, 1023–1037. [Google Scholar] [CrossRef]
- Arzuaga, X.; Gehlhaus, M.; Strong, J. Modes of action associated with uranium induced adverse effects in bone function and development. Toxicol. Lett. 2015, 236, 123–130. [Google Scholar] [CrossRef]
- Tissandie, E.; Guéguen, Y.; Lobaccaro, J.M.; Grandcolas, L.; Grison, S.; Aigueperse, J.; Souidi, M. Vitamin D metabolism impairment in the rat’s offspring following maternal exposure to 137cesium. Arch. Toxicol. 2009, 83, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F., Jr.; Devoz, P.P.; Cavalcante, M.R.N.; Gallimberti, M.; Cruz, J.C.; Domingo, J.L.; Simões, E.J.; Lotufo, P.; Liu, S.; Bensenor, I. Urinary levels of 30 metal/metalloids in the Brazilian southeast population: Findings from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Environ. Res. 2023, 225, 115624. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef]
- Esteban, M.; Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 2009, 35, 438–449. [Google Scholar] [CrossRef]
- Shilnikova, N.; Momoli, F.; Karyakina, N.; Krewski, D. Review of non-invasive biomarkers as a tool for exposure characterization in human health risk assessments. J. Toxicol. Environ. Health B Crit. Rev. 2025, 28, 122–150. [Google Scholar] [CrossRef]
- Gil, F.; Hernández, A.F. Toxicological importance of human biomonitoring of metallic and metalloid elements in different biological samples. Food Chem. Toxicol. 2015, 80, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morata, I.; Sobel, M.; Tellez-Plaza, M.; Navas-Acien, A.; Howe, C.G.; Sanchez, T.R. A State-of-the-Science Review on Metal Biomarkers. Curr. Environ. Health Rep. 2023, 10, 215–249. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.; Foldes, J.; Steinberg, R.; Menczel, J. Zinc excretion in osteoporotic women. J. Bone Miner. Res. 1990, 5, 251–257. [Google Scholar] [CrossRef]
- Kunioka, C.T.; Cruz, J.C.; Souza, V.C.O.; Rocha, B.A.; Barbosa Jr, F.; Belo, L.; Manso, M.C.; Carvalho, M. Low-Level Environmental Cadmium Exposure and Its Effects on Renal and Bone Health in Brazilian Postmenopausal Women: A Cross-Sectional Study. Expo. Health 2025. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Q.; Zhang, T.; Tang, X.; Mo, X.; Liang, Y.; Wang, X.; Cao, J.; Huang, C.; Lu, Y.; et al. Association Between Combined Polymetallic Exposure and Osteoporosis. Biol. Trace Elem. Res. 2024, 202, 3945–3958. [Google Scholar] [CrossRef]
- Haider, F.U.; Zulfiqar, U.; Ain, N.U.; Mehmood, T.; Ali, U.; Ramos Aguila, L.C.; Li, Y.; Siddique, K.H.M.; Farooq, M. Managing antimony pollution: Insights into Soil-Plant system dynamics and remediation Strategies. Chemosphere 2024, 362, 142694. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, T.; Terada, A.; Riya, S. Evaluation of Pollution Level, Spatial Distribution, and Ecological Effects of Antimony in Soils of Mining Areas: A Review. Int. J. Environ. Res. Public Health 2022, 20, 242. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Shin, S.; Lee, Y.J.; Ha, I.H. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch. Osteoporos. 2021, 16, 22. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Liu, J.; Wang, Z.; Jin, T.; Zhu, G.; Chen, X. The Association Between Cadmium Exposure and Osteoporosis: A Longitudinal Study and Predictive Model in a Chinese Female Population. Front. Public Health 2021, 9, 762475. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wei, Q.; Lv, Y.; Xue, J.; Zhang, B.; Sun, Q.; Xiao, T.; Huang, R.; Wang, P.; Dai, X.; et al. Wnt/β-Catenin Pathway Is Involved in Cadmium-Induced Inhibition of Osteoblast Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 1519. [Google Scholar] [CrossRef]
- Luo, H.; Gu, R.; Ouyang, H.; Wang, L.; Shi, S.; Ji, Y.; Bao, B.; Liao, G.; Xu, B. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-κB pathway and mitochondrial dysfunction. Environ. Pollut. 2021, 290, 118043. [Google Scholar] [CrossRef] [PubMed]
- Youness, E.R.; Mohammed, N.A.; Morsy, F.A. Cadmium impact and osteoporosis: Mechanism of action. Toxicol. Mech. Methods 2012, 22, 560–567. [Google Scholar] [CrossRef]
- Wang, J.; Xue, M.; Hu, Y.; Li, J.; Li, Z.; Wang, Y. Proteomic Insights into Osteoporosis: Unraveling Diagnostic Markers of and Therapeutic Targets for the Metabolic Bone Disease. Biomolecules 2024, 14, 554. [Google Scholar] [CrossRef]
- Galvez-Fernandez, M.; Rodriguez-Hernandez, Z.; Grau-Perez, M.; Chaves, F.J.; Garcia-Garcia, A.B.; Amigo, N.; Monleon, D.; Garcia-Barrera, T.; Gomez-Ariza, J.L.; Briongos-Figuero, L.S.; et al. Metabolomic patterns, redox-related genes and metals, and bone fragility endpoints in the Hortega Study. Free Radic. Biol. Med. 2023, 194, 52–61. [Google Scholar] [CrossRef]
- Ximenez, J.P.B.; Zamarioli, A.; Kacena, M.A.; Barbosa, R.M.; Barbosa, F., Jr. Association of Urinary and Blood Concentrations of Heavy Metals with Measures of Bone Mineral Density Loss: A Data Mining Approach with the Results from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2021, 199, 92–101. [Google Scholar] [CrossRef]
- Rana, J.; Renzetti, S.; Islam, R.; Donev, M.; Hu, K.; Oulhote, Y. Mediation Effect of Metal Mixtures in the Association Between Socioeconomic Status and Self-rated Health Among US Adults: A Weighted Quantile Sum Mediation Approach. Expo. Health 2022, 14, 609–621. [Google Scholar] [CrossRef]
- Li, H.; Li, G.; Yi, M.; Zhou, J.; Deng, Y.; Huang, Y.; He, S.; Meng, X.; Liu, L. Sex-specific associations of urinary mixed-metal concentrations with femoral bone mineral density among older people: An NHANES (2017–2020) analysis. Front. Public Health 2024, 12, 1363362. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Akesson, A.; Alfvén, T.; Järup, L.; Vahter, M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers 2005, 10, 117–126. [Google Scholar] [CrossRef] [PubMed]

| Variables | Overall n = 380 | No Osteoporosis n = 307 | With Osteoporosis n = 73 | p-Value 1 |
|---|---|---|---|---|
| Age (years) | 60.0 (56.0; 65.8) | 60.0 (56.0; 65.0) | 62.0 (59.0; 66.0) | 0.011 |
| BMI (kg/m2) | 27.0 (24.4; 30.2) | 27.6 (24.8; 30.8) | 26.1 (23.4; 28.1) | <0.001 |
| Length of menopause (years) | 13.0 (7.3; 19.0) | 12.0 (7.0; 18.0) | 16.0 (10.5; 21.5) | 0.002 |
| Prior fracture (yes) | 109 (28.7%) | 84 (27.4%) | 25 (34.2%) | 0.252 |
| Arthritis (yes) | 72 (18.9%) | 62 (20.2%) | 10 (13.7%) | 0.246 |
| Vitamin D intake (yes) | 183 (48.2%) | 149 (48.5%) | 34 (46.4%) | 0.795 |
| Corticoids (yes) | 88 (23.2%) | 70 (22.8%) | 18 (24.7%) | 0.758 |
| Prolonged bed rest (yes) | 28 (7.4%) | 21 (6.8%) | 7 (9.6%) | 0.454 |
| No exercise (yes) | 135 (35.5%) | 112 (36.5%) | 23 (31.5%) | 0.497 |
| Calcium intake (yes) | 100 (26.3%) | 68 (22.1%) | 32 (43.8%) | <0.001 |
| Alcohol intake (yes) | 1 (0.3%) | 1 (0.3%) | 0 (0%) | 1.000 |
| Smoking (yes) | 24 (6.3%) | 16 (5.2%) | 8 (11.0%) | 0.333 |
| Antiresorptive medications | <0.001 | |||
| Bisphosphonates | 10 (2.6%) | 4 (1.3%) | 6 (8.2%) | |
| Bisphosphonates and HRT | 1 (0.3%) | 0 (0%) | 1 (1.4%) | |
| HRT | 45 (11.8%) | 33 (10.7%) | 12 (16.4%) | |
| No | 324 (85.3%) | 270 (87.9%) | 54 (74.0%) | |
| Lumbar spine | ||||
| BMD (g/cm2) | 0.92 (0.82; 1.06) | 0.97 (0.88; 1.08) | 0.74 (0.70; 0.77) | <0.001 |
| T-score | −1.10 (−2.00; 0.10) | −0.70 (−1.50; 0.40) | −2.80 (−3.10; −2.50) | <0.001 |
| Diagnosis of osteoporosis | 61 (16.1%) | 0 (0%) | 61 (83.6%) | <0.001 |
| Femoral neck | ||||
| BMD (g/cm2) | 0.73 (0.65; 0.83) | 0.75 (0.68; 0.86) | 0.63 (0.56; 0.71) | <0.001 |
| T-score | −1.10 (−1.78; −0.20) | −0.90 (−1.50; 0.10) | −2.00 (−2.65; −1.35) | <0.001 |
| Diagnosis of osteoporosis | 25 (6.6%) | 0 (0%) | 25 (34.2%) | <0.001 |
| Total hip | ||||
| BMD (g/cm2) | 0.87 (0.78; 0.96) | 0.90 (0.81; 0.98) | 0.74 (0.69; 0.84) | <0.001 |
| T-score | −0.60 (−1.30; 0.10) | −0.40 (−1.00; 0.30) | −1.60 (−2.05; −0.90) | <0.001 |
| Diagnosis of osteoporosis | 10 (2.6%) | 0 (0%) | 10 (13.7%) | <0.001 |
| Urinary creatinine (mg/dL) | 52.7 (29.0; 90.9) | 52.8 (31.1; 93.1) | 52.5 (22.2; 85.4) | 0.090 |
| Elements (µg/g creat) | Overall n = 380 | No Osteoporosis n = 307 | With Osteoporosis n = 73 | p-Value 1 |
|---|---|---|---|---|
| Al | 217 (108; 448) | 202 (106; 425) | 340 (129; 638) | 0.273 |
| Ba | 16.5 (8.3; 29.0) | 15.4 (8.0; 27.8) | 19.7 (9.0; 36.8) | 0.127 |
| Cd | 0.30 (0.15; 0.55) | 0.30 (0.14; 0.49) | 0.38 (0.16; 0.71) | 0.012 |
| Co | 0.23 (0.09; 0.46) | 0.22 (0.08; 0.46) | 0.26 (0.13; 0.66) | 0.543 |
| Cs | 7.9 (4.0; 14.0) | 7.7 (4.0; 13.5) | 8.2 (4.6; 15.5) | 0.419 |
| Cu | 93.6 (53.1; 172.9) | 92.0 (52.9; 169.9) | 98.4 (57.6; 184.7) | 0.148 |
| Hg | 0.99 (0.41; 2.07) | 0.97 (0.40; 1.93) | 1.03 (0.55; 2.91) | 0.501 |
| Li | 6.9 (3.2; 14.9) | 6.7 (3.2; 14.8) | 8.5 (3.9; 17.0) | 0.578 |
| Mn | 4.4 (1.7; 9.7) | 4.1 (1.7; 8.8) | 7.0 (2.4; 15.7) | 0.014 |
| Mo | 17.0 (8.6; 33.4) | 17.0 (8.6; 32.3) | 16.5 (9.3; 37.7) | 0.378 |
| Ni | 16.0 (8.1; 30.9) | 15.8 (8.0; 28.9) | 17.5 (9.1; 35.9) | 0.219 |
| Pb | 4.0 (2.1; 8.1) | 3.7 (2.1; 7.4) | 4.8 (2.7; 11.7) | 0.020 |
| Rb | 3105 (1567; 5742) | 3026 (1557; 5627) | 3636 (1649; 6220) | 0.570 |
| Sb | 0.19 (0.10; 0.39) | 0.17 (0.10; 0.36) | 0.27 (0.13; 0.52) | 0.015 |
| Se | 11.7 (5.9; 24.4) | 12.0 (6.0; 25.6) | 10.8 (5.5; 22.9) | 0.439 |
| Sn | 0.52 (0.23; 1.09) | 0.49 (0.22; 0.92) | 0.70 (0.24; 1.86) | 0.046 |
| Sr | 76.4 (40.7; 179.3) | 75.8 (38.5; 178.7) | 87.9 (51.3; 215.3) | 0.100 |
| Tl | 0.22 (0.11; 0.46) | 0.21 (0.11; 0.45) | 0.26 (0.12; 0.54) | 0.076 |
| U | 0.017 (0.005; 0.041) | 0.016 (0.005; 0.038) | 0.021 (0.008; 0.056) | 0.109 |
| Zn | 808 (458; 1548) | 777 (460; 1510) | 860 (456; 1882) | 0.004 |
| BMD Lumbar Spine | BMD Femoral Neck | BMD Total Hip | ||
|---|---|---|---|---|
| BMD Lumbar Spine | r | 1 | 0.525 | 0.697 |
| p | <0.001 | <0.001 | ||
| BMD Femoral Neck | r | 0.525 | 1 | 0.743 |
| p | <0.001 | <0.001 | ||
| BMD Total Hip | r | 0.697 | 0.743 | 1 |
| p | <0.001 | <0.001 | ||
| Age | r | −0.182 | −0.256 | −0.278 |
| p | <0.001 | <0.001 | <0.001 | |
| BMI | r | 0.301 | 0.311 | 0.456 |
| p | <0.001 | <0.001 | <0.001 | |
| Menopause Length | r | −0.247 | −0.220 | −0.271 |
| p | <0.001 | <0.001 | <0.001 | |
| Al | r | −0.112 | −0.062 | −0.104 |
| p | 0.030 | 0.227 | 0.042 | |
| Cd | r | −0.102 | −0.064 | −0.128 |
| p | 0.048 | 0.215 | 0.013 | |
| Hg | r | −0.084 | −0.062 | −0.128 |
| p | 0.101 | 0.226 | 0.012 | |
| Mn | r | −0.128 | −0.072 | −0.097 |
| p | 0.012 | 0.159 | 0.058 | |
| Sb | r | −0.106 | −0.068 | −0.104 |
| p | 0.039 | 0.186 | 0.044 | |
| U | r | −0.107 | −0.103 | −0.116 |
| p | 0.036 | 0.044 | 0.023 |
| Dependent Variable | Model | Unstandardized Coefficients | Standardized Coefficients | t | p-Value | |
|---|---|---|---|---|---|---|
| Beta (95% CI) | Std. Error | Beta | ||||
| Ln BMD | (Constant) | −0.956 (−1.277; −0.636) | 0.163 | −5.863 | <0.001 | |
| Ln BMI | 0.310 (0.214; 0.405) | 0.049 | 0.299 | 6.366 | <0.001 | |
| Ln menopause Length | −0.052 (−0.072; −0.032) | 0.010 | −0.242 | −5.140 | <0.001 | |
| Smoking | −0.024 (−0.045; −0.004) | 0.010 | −0.111 | −2.358 | 0.019 | |
| Prolonged bed rest | −0.067 (−0.129; −0.004) | 0.032 | −0.099 | −2.096 | 0.037 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunioka, C.T.; de Oliveira Souza, V.C.; Rocha, B.A.; Júnior, F.B.; Belo, L.; Manso, M.C.; Carvalho, M. Association of Urinary Cadmium and Antimony with Osteoporosis Risk in Postmenopausal Brazilian Women: Insights from a 20 Metal(loid) Biomonitoring Study. Toxics 2025, 13, 489. https://doi.org/10.3390/toxics13060489
Kunioka CT, de Oliveira Souza VC, Rocha BA, Júnior FB, Belo L, Manso MC, Carvalho M. Association of Urinary Cadmium and Antimony with Osteoporosis Risk in Postmenopausal Brazilian Women: Insights from a 20 Metal(loid) Biomonitoring Study. Toxics. 2025; 13(6):489. https://doi.org/10.3390/toxics13060489
Chicago/Turabian StyleKunioka, Carlos Tadashi, Vanessa Cristina de Oliveira Souza, Bruno Alves Rocha, Fernando Barbosa Júnior, Luís Belo, Maria Conceição Manso, and Márcia Carvalho. 2025. "Association of Urinary Cadmium and Antimony with Osteoporosis Risk in Postmenopausal Brazilian Women: Insights from a 20 Metal(loid) Biomonitoring Study" Toxics 13, no. 6: 489. https://doi.org/10.3390/toxics13060489
APA StyleKunioka, C. T., de Oliveira Souza, V. C., Rocha, B. A., Júnior, F. B., Belo, L., Manso, M. C., & Carvalho, M. (2025). Association of Urinary Cadmium and Antimony with Osteoporosis Risk in Postmenopausal Brazilian Women: Insights from a 20 Metal(loid) Biomonitoring Study. Toxics, 13(6), 489. https://doi.org/10.3390/toxics13060489









