Modeling Desorption Rates and Background Concentrations of Heavy Metals Using a One-Dimensional Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Cartagena Bay: A Reference System for Estuarine Conditions
2.2. Mathematical Model
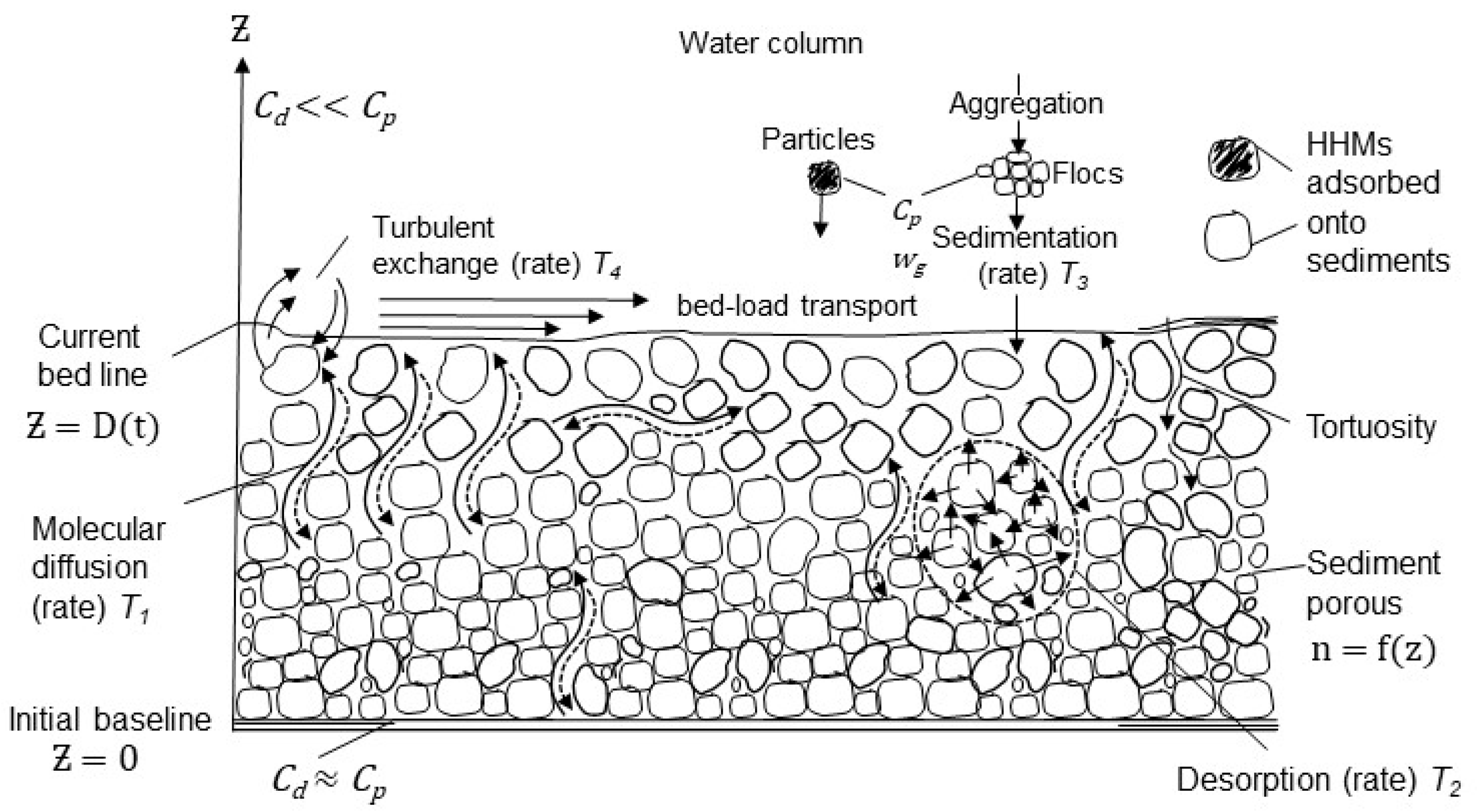
2.2.1. Definition of the Physical Problem
2.2.2. Governing Equations and Boundary Conditions
2.2.3. Numerical Solution Under Variable Boundary Conditions
3. Results
3.1. Estimation of Molecular Diffusion (T1), Desorption (T2), and Sedimentation (T3)
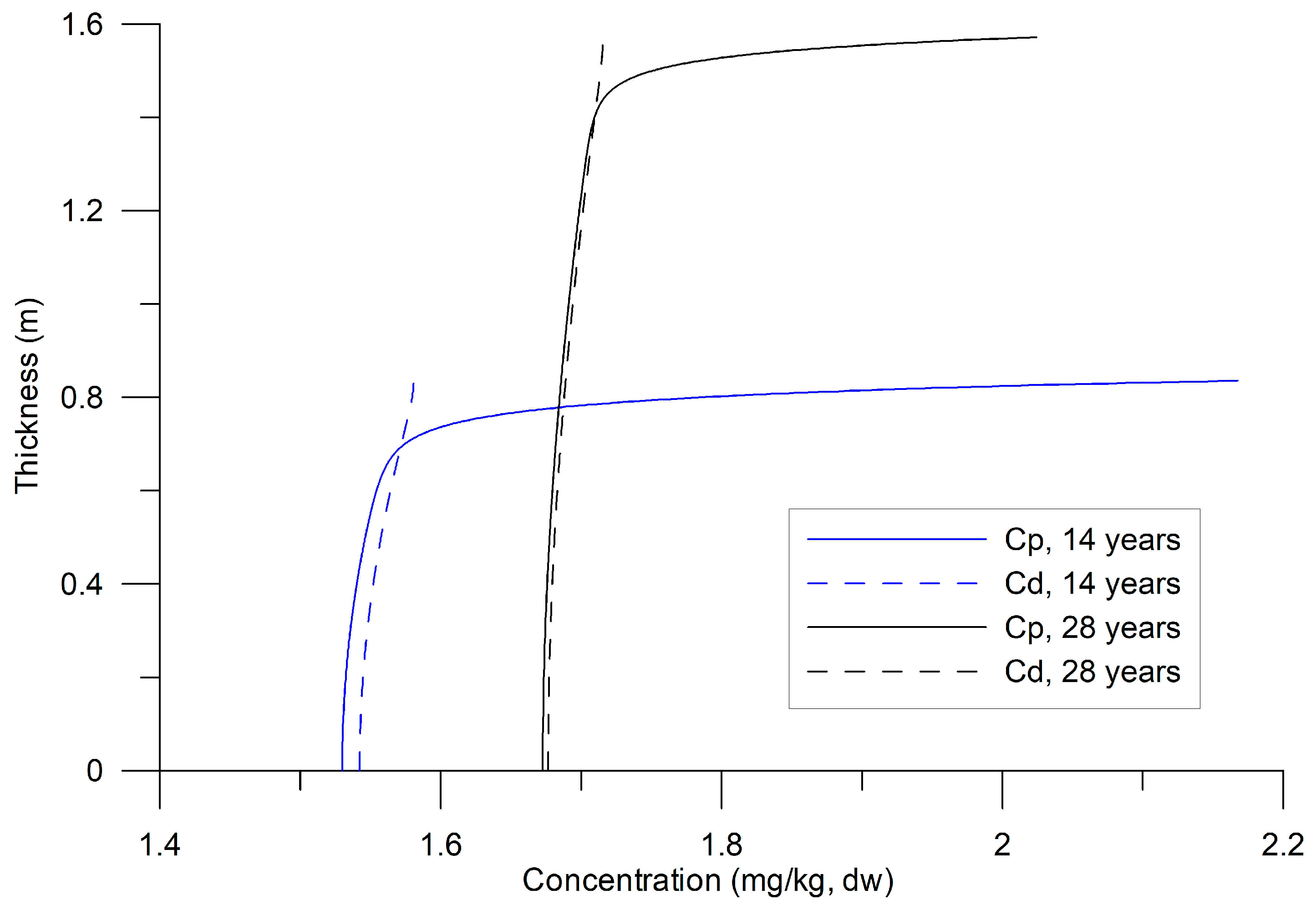
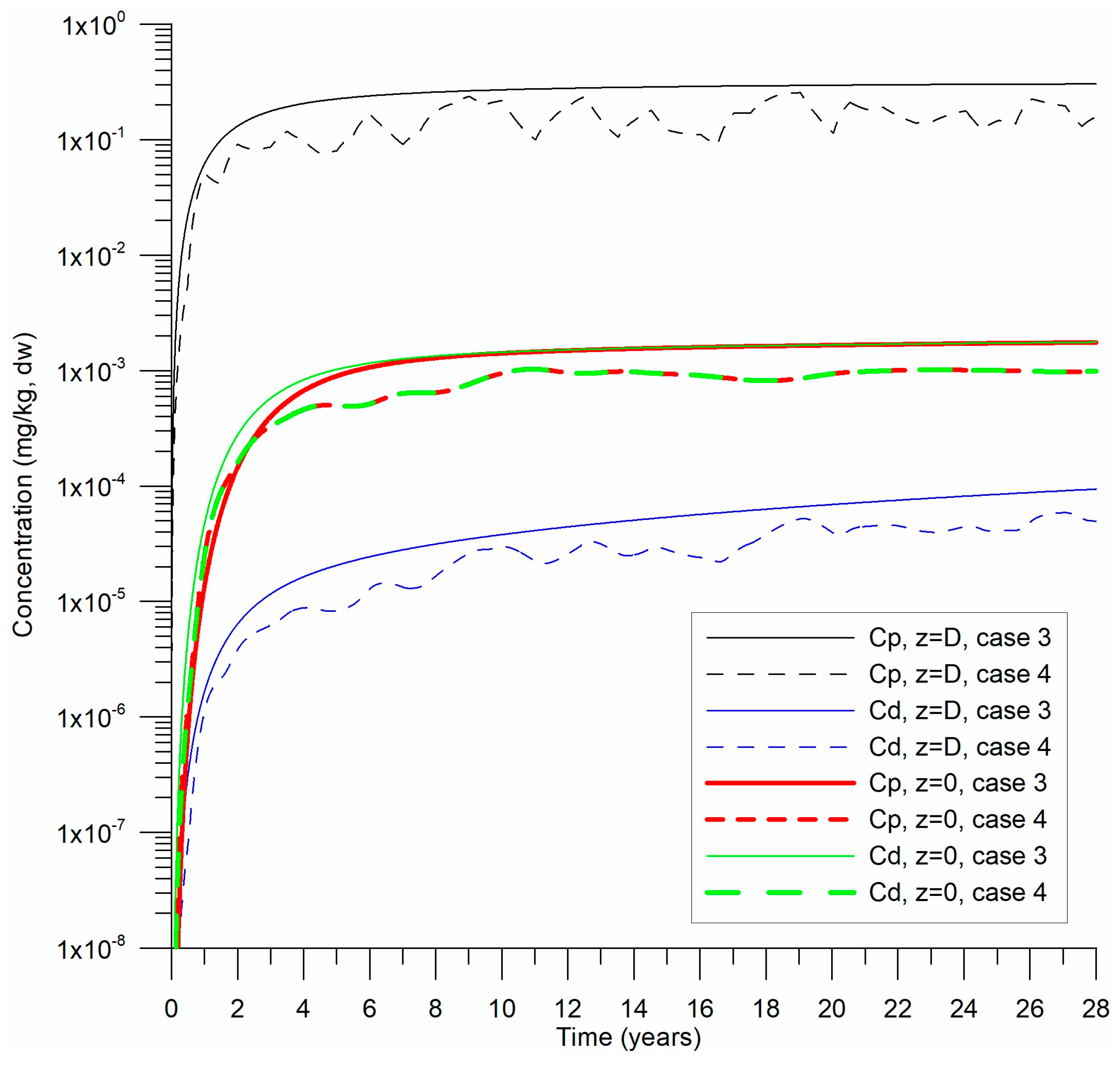
3.2. Numerical Experiments
4. Discussion
4.1. Temporal Evolution of Background Concentrations Estimated by the Model
4.2. Dimensionless Analysis and HHM Dynamics
4.3. Model Assumptions, Limitations, and Ecological Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Abdelmonem, B.H.; Kamal, L.T.; Elbaz, R.M.; Khalifa, M.R.; Abdelnaser, A. From Contamination to Detection: The Growing Threat of Heavy Metals. Heliyon 2025, 11, e41713. [Google Scholar] [CrossRef] [PubMed]
- Proshad, R.; Asha, S.M.A.A.; Tan, R.; Lu, Y.; Abedin, M.A.; Ding, Z.; Zhang, S.; Li, Z.; Chen, G.; Zhao, Z. Machine Learning Models with Innovative Outlier Detection Techniques for Predicting Heavy Metal Contamination in Soils. J. Hazard. Mater. 2025, 481, 136536. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, C.; Zhang, G.; Guo, Y.; Song, Y.; Thangaraj, S.; Zhang, X.; Sun, J. Dissolved and Particulate Heavy Metal Pollution Status in Seawater and Sedimentary Heavy Metals of the Bohai Bay. Mar. Environ. Res. 2023, 191, 106158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, P.; Hu, Z.; Shu, Q.; Chen, Y. Identification of the Sources and Influencing Factors of the Spatial Variation of Heavy Metals in Surface Sediments along the Northern Jiangsu Coast. Ecol. Indic. 2022, 137, 108716. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Meng, Y.; Byrne, P.; Ghomshei, M.; Abbaspour, K.C. Modeling Transport and Fate of Heavy Metals at the Watershed Scale: State-of-the-Art and Future Directions. Sci. Total Environ. 2023, 878, 163087. [Google Scholar] [CrossRef]
- Schnoor, J.; Sato, C.; McKechnie, D.; Sahoo, D. Processes, Coefficients, and Models for Simulating Toxic Organics and Heavy Metals in Surface Waters; EPA/600/3-87/015; Department of Civil and Environmental Engineering, University of Iowa: Iowa City, IA, USA, 1987. [Google Scholar]
- Sun, Y.; Zhao, Y.; Hao, L.; Zhao, X.; Lu, J.; Shi, Y.; Ma, C.; Li, Q. Application of the Partial Least Square Regression Method in Determining the Natural Background of Soil Heavy Metals: A Case Study in the Songhua River Basin, China. Sci. Total Environ. 2024, 918, 170695. [Google Scholar] [CrossRef]
- Luo, M.; Kang, X.; Liu, Q.; Yu, H.; Tao, Y.; Wang, H.; Niu, Y.; Niu, Y. Research on the Geochemical Background Values and Evolution Rules of Lake Sediments for Heavy Metals and Nutrients in the Eastern China Plain from 1937 to 2017. J. Hazard. Mater. 2022, 436, 129136. [Google Scholar] [CrossRef]
- Kuang, Z.; Shi, Z.; Wang, H.; Du, S.; Gong, H.; Liu, Q.; Gu, Y.; Fan, Z.; Huang, H.; Wang, S. Bioavailability of Trace Metals in Sediments from Daya Bay Nature Reserve: Spatial Variation, Controlling Factors and the Exposure Risk Assessment for Aquatic Biota. Ecol. Indic. 2024, 169, 112789. [Google Scholar] [CrossRef]
- Wu, Y.; Falconer, R.; Lin, B. Modelling Trace Metal Concentration Distributions in Estuarine Waters. Estuar. Coast. Shelf Sci. 2005, 64, 699–709. [Google Scholar] [CrossRef]
- Chang, S.; Han, L.; Chen, R.; Liu, Z.; Fan, Y.; An, X.; Zhai, Y.; Wu, P.; Wang, T. Vulnerability Assessment of Soil Cadmium with Adsorption–Desorption Coupling Model. Ecol. Indic. 2023, 146, 109904. [Google Scholar] [CrossRef]
- Ristea, E.; Pârvulescu, O.C.; Lavric, V.; Oros, A. Assessment of Heavy Metal Contamination of Seawater and Sediments Along the Romanian Black Sea Coast: Spatial Distribution and Environmental Implications. Sustainability 2025, 17, 2586. [Google Scholar] [CrossRef]
- Yao, J.; Liu, W.; Chen, Z. Numerical Solution of a Moving Boundary Problem of One-Dimensional Flow in Semi-Infinite Long Porous Media with Threshold Pressure Gradient. Math. Probl. Eng. 2013, 2013, 384246. [Google Scholar] [CrossRef]
- Bhandari, A.; Surampalli, R.Y.; Champagne, P.; Ong, S.K.; Tyagi, R.D.; Lo, I.M.C. Remediation Technologies for Soils and Groundwater; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2007; Volume 60, pp. 1–449. [Google Scholar] [CrossRef]
- Liang, H.Y.; Zhang, Y.H.; Du, S.L.; Cao, J.L.; Liu, Y.F.; Zhao, H.; Ding, T.T. Heavy Metals in Sediments of the River-Lake System in the Dianchi Basin, China: Their Pollution, Sources, and Risks. Sci. Total Environ. 2024, 957, 177652. [Google Scholar] [CrossRef]
- Lonin, S.; Andrade, C.A.; Monroy, J. Wave Climate and the Effect of Induced Currents over the Barrier Reef of the Cays of Alburquerque Island, Colombia. Sustainability 2022, 14, 6069. [Google Scholar] [CrossRef]
- Boudreau, B.P. Diagenetic Models and Their Implementation; Springer: Berlin/Heidelberg, Germany, 1998; Volume 15, ISBN 978-3-642-64399-6. [Google Scholar]
- Lynch, D.R.; Officer, C.B. Nonlinear Parameter Estimation for Sediment Cores. Chem. Geol. 1984, 44, 203–225. [Google Scholar] [CrossRef]
- Nicolis, C. Tracer Dynamics in Ocean Sediments and the Deciphering of Past Climates. Math. Comput. Model. 1995, 21, 27–38. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Li, K.; Gong, J.; Gao, J.; Wang, Z.; Cai, Y.; Zhao, K.; Hu, S.; Fu, Y.; et al. Differentiating Environmental Scenarios to Establish Geochemical Baseline Values for Heavy Metals in Soil: A Case Study of Hainan Island, China. Sci. Total Environ. 2023, 898, 165634. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, C.J.; Chung, C.S.; Kim, T.; Gu, H.S.; Kim, H.E.; Choi, K.Y. Applying New Regional Background Concentration Criteria to Assess Heavy Metal Contamination in Deep-Sea Sediments at an Ocean Dumping Site, Republic of Korea. Mar. Pollut. Bull. 2024, 200, 116065. [Google Scholar] [CrossRef]
- Olivero-Verbel, R.; Eljarrat, E.; Johnson-Restrepo, B. Organophosphate Ester Flame Retardants in Sediments and Marine Fish Species in Colombia: Occurrence, Distribution, and Implications for Human Risk Assessment. Mar. Pollut. Bull. 2025, 213, 117654. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Alcala-Orozco, M.; Barraza-Quiroz, D.; De la Rosa, J.; Olivero-Verbel, J. Environmental Risks Associated with Trace Elements in Sediments from Cartagena Bay, an Industrialized Site at the Caribbean. Chemosphere 2020, 242, 125173. [Google Scholar] [CrossRef]
- Tosic, M.; Restrepo, J.D.; Lonin, S.; Izquierdo, A.; Martins, F. Water and Sediment Quality in Cartagena Bay, Colombia: Seasonal Variability and Potential Impacts of Pollution. Estuar. Coast. Shelf Sci. 2019, 216, 187–203. [Google Scholar] [CrossRef]
- Romero-Murillo, P.; Gallego, J.L.; Leignel, V. Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics 2023, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Tosic, M.; Ángel, J.D.R. Sustainability Impacts of Sediments on the Estuary, Ports, and Fishing Communities of Cartagena Bay, Colombian Caribbean. Wiley Interdiscip. Rev. Water 2023, 11, e1709. [Google Scholar] [CrossRef]
- Cano, W.T.G.; Kim, K. How to Achieve Sustainably Beneficial Uses of Marine Sediments in Colombia ? Sustainability 2022, 14, 14821. [Google Scholar] [CrossRef]
- Tosic, M.; Martins, F.; Lonin, S.; Izquierdo, A.; Restrepo, J.D. Hydrodynamic Modelling of a Polluted Tropical Bay: Assessment of Anthropogenic Impacts on Freshwater Runoff and Estuarine Water Renewal. J. Environ. Manag. 2019, 236, 695–714. [Google Scholar] [CrossRef]
- Andrade, C.; Thomas, Y.F.; Lonin, S.; Parra, C.; Kunesch, S.; Menanteau, L.; Andriau, A.; Piñeres, C.; Velasco, S. Aspectos morfodinámicos de la bahía de Cartagena de Indias. Bol. Cient. CIOH 2004, 22, 90–104. [Google Scholar] [CrossRef]
- Thomas, Y.F.; Cesaraccio, M.; Kunesch, S.; Andrieu, A.; Ménanteau, L.; Andrade, C.; Lonin, S.; Parra, C.; Pineres, C.; Velasco, S.P. Étude Morphodynamique de La Baie de Carthagène Des Indes, Colombie. In Milieux Littoraux, Nouvelles Perspectives D’ Études; L’Harmattan: Paris, France, 2005; pp. 171–191. [Google Scholar]
- Thomas, Y.F.; Ménanteau, L.; Kunesch, S.; Cesaraccio, M.; Andrade, C.; Lonin, S.; Parra, C. Le delta du canal du Dique (baie de Carthagène des Indes, Colombie). Modélisation géomorphologique et sédimentologique. In Proceedings of the Colloque International Interactions-Nature-Sociétés, Analyses et Modèles, La Baule-Escoublac, France, 3–6 May 2006; pp. 1–7. [Google Scholar]
- Van Rijn, L.C. Principles of Sediment Transport in Rivers, Estuaries and Coastal Seas; Aqua Publications: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Bartlett, P.; Craig, P. Total Mercury and Methyl Mercury Levels in British Estuarine Sediments—II. Water Res. 1981, 15, 37–47. [Google Scholar] [CrossRef]
- Boudreau, B.P. The Diffusive Tortuosity of Fine-Grained Unlithified Sediments. Geochim. Cosmochim. Acta 1996, 60, 3139–3142. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Ewing, R.P.; Sahimi, M. Tortuosity in Porous Media: A Critical Review. Soil Sci. Soc. Am. J. 2013, 77, 1461–1477. [Google Scholar] [CrossRef]
- Monin, A.S.; Yaglom, A.M. Statistical Fluid Mechanics: The Mechanics of Turbulence, Volume 1; MIT Press: Cambridge, MA, USA, 1973; Volume 60. [Google Scholar]
- Orani, A.M.; Vassileva, E.; Azemard, S.; Alonso-Hernandez, C. Trace Elements Contamination Assessment in Marine Sediments from Different Regions of the Caribbean Sea. J. Hazard. Mater. 2020, 399, 122934. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Caballero-Gallardo, K.; Torres-Fuentes, N. Assessment of Mercury in Muscle of Fish from Cartagena Bay, a Tropical Estuary at the North of Colombia. Int. J. Environ. Health Res. 2009, 19, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Marchuk, G.I.; Kagan, B.A. Ocean Tides: Mathematical Models and Numerical Experiments; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Pintilie, S.; Brânză, L.; Beţianu, C.; Pavel, L.V.; Ungureanu, F.; Gavrilescu, M. Modelling and Simulation of Heavy Metals Transport in Water and Sediments. Environ. Eng. Manag. J. 2007, 6, 153–161. [Google Scholar] [CrossRef]
- Luo, P.; Luo, M.; Li, F.; Qi, X.; Huo, A.; Wang, Z.; He, B.; Takara, K.; Nover, D.; Wang, Y. Urban Flood Numerical Simulation: Research, Methods and Future Perspectives. Environ. Model. Softw. 2022, 156, 105478. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, Z.; Liu, G.; Li, S.; Hu, J. Assessment of Heavy Metals Remobilization and Release Risks at the Sediment-Water Interface in Estuarine Environment. Mar. Pollut. Bull. 2023, 187, 114517. [Google Scholar] [CrossRef]
- He, L.; Chen, G.; Wang, X.; Shen, J.; Zhang, H.; Lin, Y.; Shen, Y.; Lang, F.; Gong, C. Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China. Toxics 2024, 12, 322. [Google Scholar] [CrossRef]
- Fukue, M.; Yanai, M.; Sato, Y.; Fujikawa, T.; Furukawa, Y.; Tani, S. Background Values for Evaluation of Heavy Metal Contamination in Sediments. J. Hazard. Mater. 2006, 136, 111–119. [Google Scholar] [CrossRef]
- Maerki, M.; Wehrli, B.; Dinkel, C.; Müller, B. The Influence of Tortuosity on Molecular Diffusion in Freshwater Sediments of High Porosity. Geochim. Cosmochim. Acta 2004, 68, 1519–1528. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Z. Critical Review of the Impact of Tortuosity on Diffusion. Chem. Eng. Sci. 2007, 62, 3748–3755. [Google Scholar] [CrossRef]



| Parameter | Description | Unit | Value | Reference |
|---|---|---|---|---|
| HHM background concentration | g L−1, mg kg−1 (dw) * | see Table 2 | calculated | |
| Drag coefficient | / | 2 × 10−3 | [39] | |
| Dissolved-phase HHM concentration | g L−1, mg kg−1 (dw) * | / | calculated | |
| Cm | Suspended-sediment mass concentration | g L−1 | / | [40] |
| Particulate-phase HHM concentration | g L−1, mg kg−1 (dw) * | / | calculated | |
| Initial particulate HHM at precipitation | g L−1 | / | assumed | |
| Suspended-sediment volumetric concentration | / | 10−4–10−5 | assumed | |
| d50 | Median grain diameter of sediment | m | / | measured |
| D | Sediment thickness | m | 0–1.6 ** | calculated |
| F | Porosity–tortuosity factor | / | / | calculated |
| HHMs | Harmful heavy metals | g L−1, mg kg−1 (dw) * | varies | measured |
| Coefficient of equilibrium distribution | / | / | assumed | |
| m | Exponent in the relationship of Sc and n | / | / | literature |
| N | Number of computational nodes | / | 100 | assumed |
| n | Porosity | / | 0.4 | [34] |
| Q | Molecular diffusion flux | kg m−2 s−1 | varies | calculated |
| S | Salinity | / | 0.06–35.7 | assumed |
| Schmidt number | / | 10–100 | [6] | |
| t | Time | s | 0–8.64 × 108 s | assumed |
| T1 | Molecular diffusion rate | yr | 0.3–3 | calculated |
| T2 | Desorption rate | yr | 3.15 (for γ = 5 × 10−8) | calculated |
| T3 | Sediment rate | yr | >31 | calculated |
| T4 | Turbulent exchange rate | yr | / | calculated |
| Friction (dynamic) velocity | m s−1 | 0–0.01 | assumed | |
| Settling velocity of sediments due to gravity | m s−1 | 10−5 | assumed | |
| Y | Dimensionless vertical coordinate | / | 0–1 | calculated |
| Vertical level within the substrate | m | 0–1.6 | calculated | |
| Roughness parameter | m | / | literature | |
| Inverse Schmidt number | / | 0.01–0.1 | [36] | |
| γ | Desorption coefficient | s−1 | 5 × 10−8–1 × 10−9 | [41] |
| Vertical grid size in dimensionless coordinates | / | 1/(N − 1) | calculated | |
| θ | Tortuosity | / | / | [35] |
| Karman constant | / | 0.41 | literature | |
| ν | Kinematic molecular viscosity of water | m2 s−1 | 10−6 | constant |
| Sediment–particle density | kg m−3 | 2650 | [39] | |
| Molecular diffusion coefficient (water only) | m2 s−1 | / | [17] | |
| Molecular diffusion coefficients (with sediments) | m2 s−1 | / | calculated |
| Case | Description | mg kg−1 (dw) | Observation |
|---|---|---|---|
| 1 | γ = 5 × 10−8 1/s | 1.4–1.7 | Long-term equilibrium at z = 0 |
| 2 | γ = 10−8 1/s | 1.0–1.2 | Slower equilibrium from low γ |
| 3 | increasing over 28 yr | 2.0–2.4 | Closest to observed CB field data |
| 4 | Variable sediment input * | 2.0–2.2 | Dynamic but consistent at z = 0 |
| - | Average Hg (model) | 0.2 ± 1.7 | Variability across all cases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Cano, W.T.; Lonin, S.; Kim, K. Modeling Desorption Rates and Background Concentrations of Heavy Metals Using a One-Dimensional Approach. Toxics 2025, 13, 421. https://doi.org/10.3390/toxics13060421
Gonzalez Cano WT, Lonin S, Kim K. Modeling Desorption Rates and Background Concentrations of Heavy Metals Using a One-Dimensional Approach. Toxics. 2025; 13(6):421. https://doi.org/10.3390/toxics13060421
Chicago/Turabian StyleGonzalez Cano, Wendy Tatiana, Serguei Lonin, and Kyoungrean Kim. 2025. "Modeling Desorption Rates and Background Concentrations of Heavy Metals Using a One-Dimensional Approach" Toxics 13, no. 6: 421. https://doi.org/10.3390/toxics13060421
APA StyleGonzalez Cano, W. T., Lonin, S., & Kim, K. (2025). Modeling Desorption Rates and Background Concentrations of Heavy Metals Using a One-Dimensional Approach. Toxics, 13(6), 421. https://doi.org/10.3390/toxics13060421








