Physicochemical Exploration and Computational Analysis of Bone After Subchronic Exposure to Kalach 360 SL in Female Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
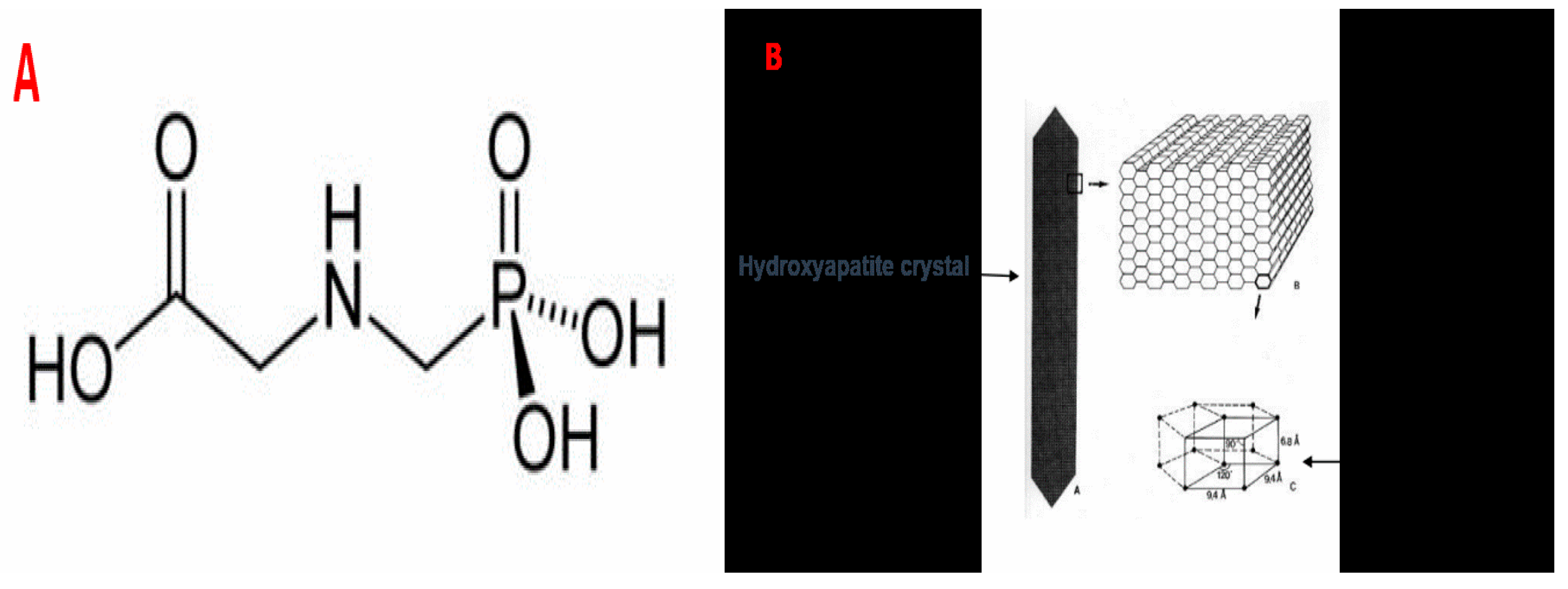
2.1. Chemicals Used in Our Study
2.2. Animals
2.3. Experimental Design
2.4. Euthanasia
2.5. Infrared Spectroscopic (FTIR) Analysis of Bone Sample
2.6. X-Ray Diffraction (XRD) Analysis
2.7. Optical Emission Spectrometry (ICP-OES)
2.8. Computational Study
2.9. Statistical Analyses
3. Results
3.1. FTIR Results
- The 1637 cm−1 band corresponding to the C=O bond;
- The 1400 cm−1 band corresponding to the C-O bond;
- The 1218 cm−1 band corresponding to the C-N bond;
- The 720 cm−1 band corresponding to the C-H bond
3.2. XRD Results
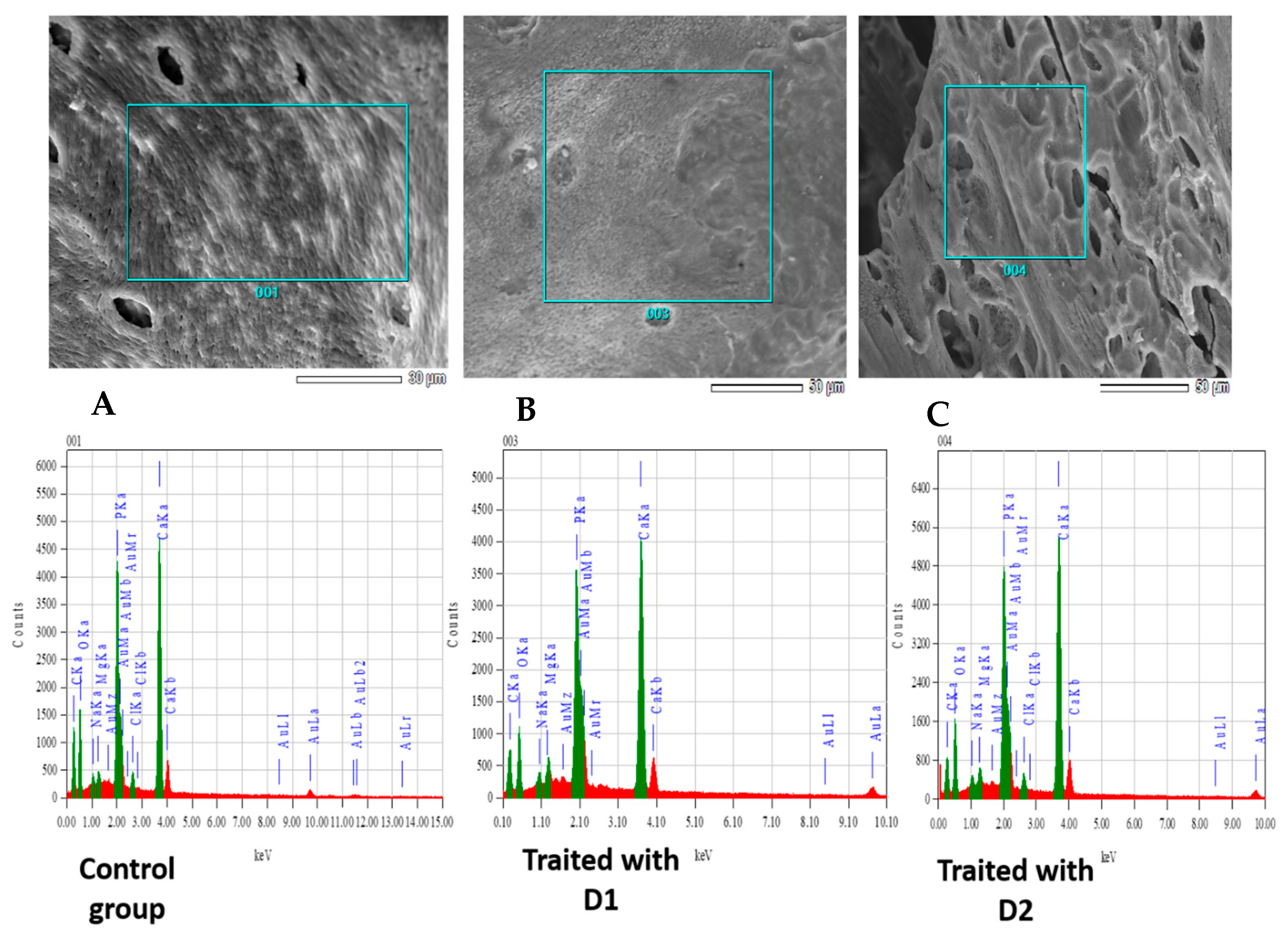
3.3. Bone Microanalysis: Energy-Dispersive X-Ray Spectroscopy (EDX) and ICP Results
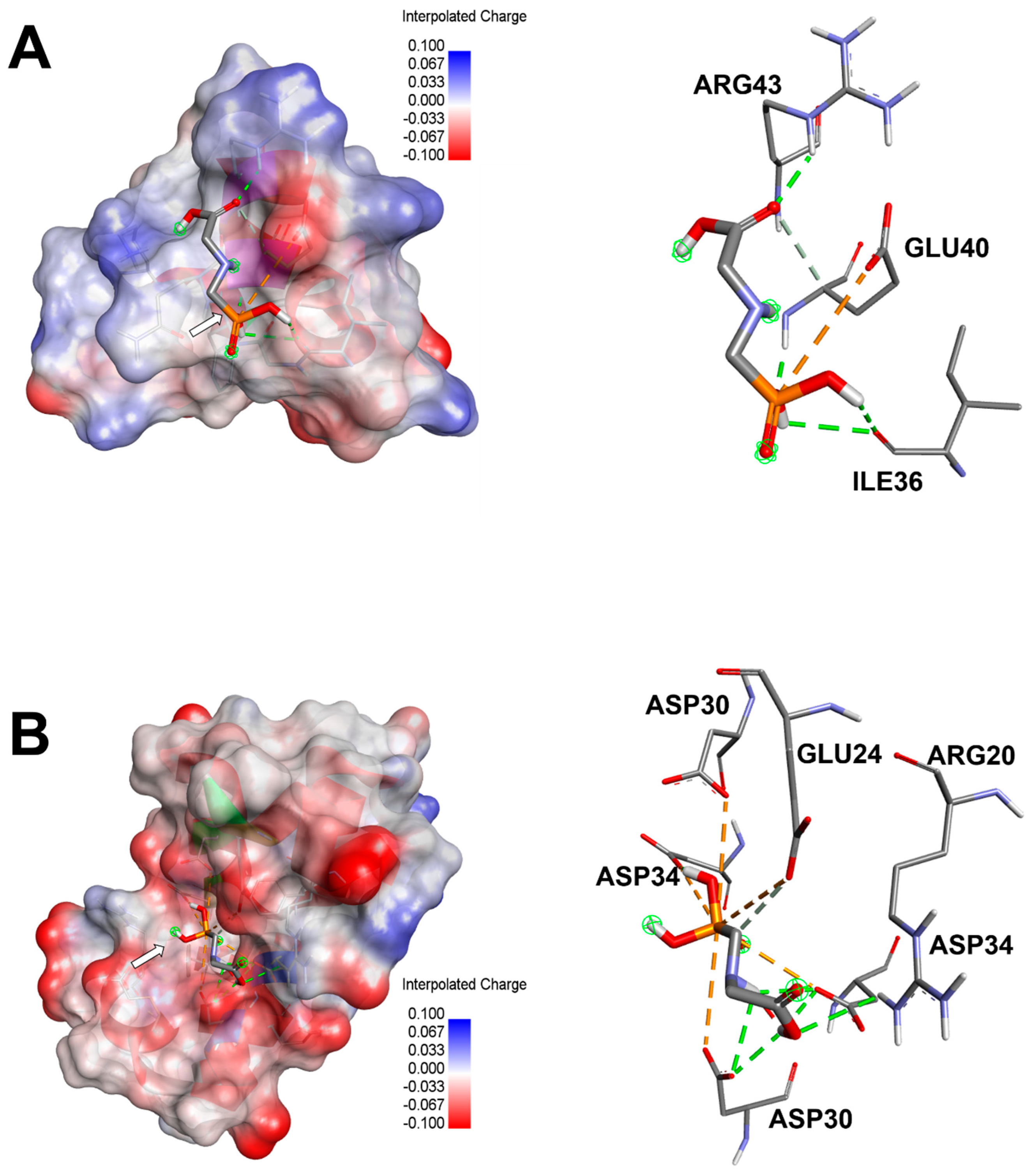
3.4. Computational Results and Interaction Assays
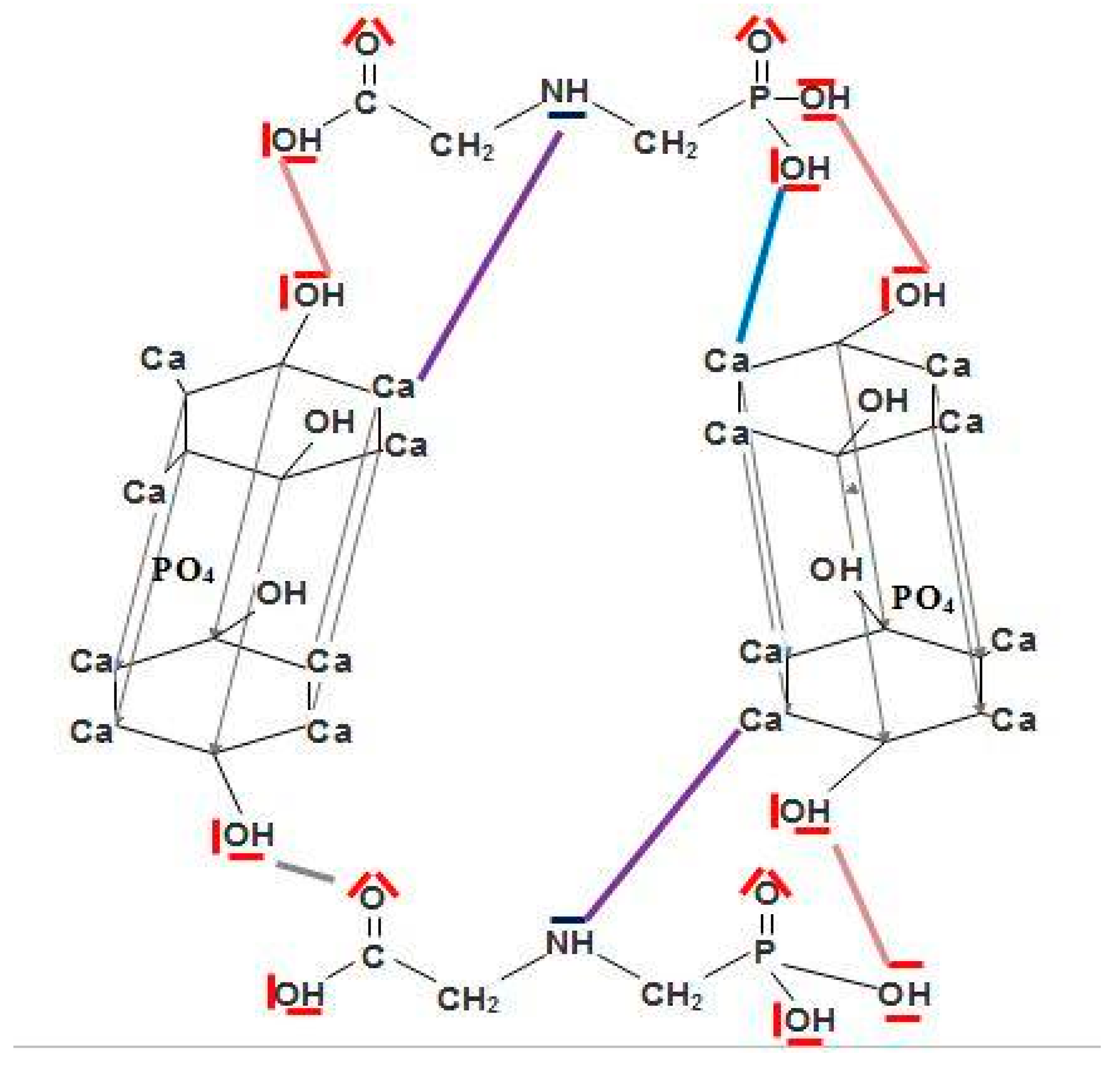
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Amor, M.; Hamdaoui, L.; Daoud, S.; Ammar, M.; Louati, N.; Elleuch, A.; Badraoui, R.; Ben Mahmoud, L.; Ben Amor, I.; Sellami, A.; et al. Impact of sub-chronic exposure to Kalach on male reproductive system and sperm function: In silico modelling and in vivo study in rats. Reprod. Toxicol. 2025, 132, 108853. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, L.; Naifar, M.; Mzid, M.; Ben Salem, M.; Chtourou, A.; Makni-Ayadi, F.; Sahnoun, Z.; Rebai, T. Nephrotoxicity of Kalach 360 SL: Biochemical and histopathological findings. Toxicol. Mech. Methods 2016, 26, 685–691. [Google Scholar] [CrossRef]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and Health Effects of Pesticide Residues. In Sustainable Agriculture Reviews 48; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 48, pp. 311–336. [Google Scholar] [CrossRef]
- Agas, D.; Sabbieti, M.G.; Marchetti, L. Endocrine disruptors and bone metabolism. Arch. Toxicol. 2013, 87, 735–751. [Google Scholar] [CrossRef]
- Elonheimo, H.; Lange, R.; Tolonen, H.; Kolossa-Gehring, M. Environmental Substances Associated with Osteoporosis–A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 738. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, L.; Oudadesse, H.; Lefeuvre, B.; Mahmoud, A.; Naifer, M.; Badraoui, R.; Ayadi, F.; Rebai, T. Sub-chronic exposure to Kalach 360 SL, Glyphosate-based Herbicide, induced bone rarefaction in female Wistar rats. Toxicology 2020, 436, 152412. [Google Scholar] [CrossRef]
- Gupta, P.K. Herbicides and fungicides. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 665–689. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323897730000357 (accessed on 17 January 2025).
- Kini, U.; Nandeesh, B.N. Physiology of Bone Formation, Remodeling, and Metabolism. In Radionuclide and Hybrid Bone Imaging [Internet]; Fogelman, I., Gnanasegaran, G., Van Der Wall, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–57. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Narayan, R.K.; Satyam, A.; Naaz, S. Prevalence of anomalous or ectopic insertion of pectoralis minor: A systematic review and meta-analysis of 4146 shoulders. Surg. Radiol. Anat. 2021, 43, 631–643. [Google Scholar] [CrossRef]
- Asghar, A.; Kumar, A.; Kant Narayan, R.; Naaz, S. Is the cortical capillary renamed as the transcortical vessel in diaphyseal vascularity? Anat. Rec. 2020, 303, 2774–2784. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Du, W.; Hu, J.; Quan, R. Association between urinary glyphosate exposure and bone mineral density in adults. Medicine 2023, 102, e36506. [Google Scholar] [CrossRef]
- Kaur, M.; Nagpal, M.; Singh, M. Osteoblast-n-Osteoclast: Making Headway to Osteoporosis Treatment. Curr. Drug Targets 2020, 21, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. What is the function of osteocalcin? J. Oral. Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Mendelsohn, R. Infrared analysis of bone in health and disease. J. Biomed. 2005, 10, 031102. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Ghorbel, H.; Feki, I.; Bouallagui, Z.; Guermazi, F.; Ayadi, L.; Sayadi, S. Oleuropein and hydroxytyrosol protect rats’ pups against bisphenol A induced hypothyroidism. Biomed. Pharmacother. 2018, 103, 1115–1126. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux De Vendômois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Serra, L.; Estienne, A.; Vasseur, C.; Froment, P.; Dupont, J. Review: Mechanisms of Glyphosate and Glyphosate-Based Herbicides Action in Female and Male Fertility in Humans and Animal Models. Cells 2021, 10, 3079. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ji, Y.; Song, X.; Guo, H.; Han, L.; Zhang, F.; Liu, X.; Zhang, H.; Zhu, B.; Xu, M. Effects of glyphosate exposure on sperm concentration in rodents: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2017, 55, 148–155. [Google Scholar] [CrossRef]
- Mabrouk, M.; Mostafa, A.A.; Oudadesse, H.; Mahmoud, A.A.; El-Gohary, M.I. Effect of ciprofloxacin incorporation in PVA and PVA bioactive glass composite scaffolds. Ceram. Int. 2014, 40, 4833–4845. [Google Scholar] [CrossRef]
- Jebahi, S.; Nsiri, R.; Boujbiha, M.; Bouroga, E.; Rebai, T.; Keskes, H.; El Feki, A.; Oudadesse, H.; El Feki, H. The impact of orthopedic device associated with carbonated hydroxyapatite on the oxidative balance: Experimental study of bone healing rabbit model. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 759–766. [Google Scholar] [CrossRef]
- Montaser, A.; Golightly, D.W. (Eds.) Inductively Coupled Plasmas in Analytical Atomic Spectrometry; VCH: New York, NY, USA, 1992. [Google Scholar]
- Badraoui, R.; Adnan, M.; Bardakci, F.; Alreshidi, M.M. Chloroquine and Hydroxychloroquine Interact Differently with ACE2 Domains Reported to Bind with the Coronavirus Spike Protein: Mediation by ACE2 Polymorphism. Molecules 2021, 26, 673. [Google Scholar] [CrossRef]
- Rahmouni, F.; Hamdaoui, L.; Saoudi, M.; Badraoui, R.; Rebai, T. Antioxidant and antiproliferative effects of Teucrium polium extract: Computational and in vivo study in rats. Toxicol. Mech. Methods 2024, 34, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. [Google Scholar] [CrossRef] [PubMed]
- Jedli, O.; Ben-Nasr, H.; Zammel, N.; Rebai, T.; Saoudi, M.; Elkahoui, S.; Jamal, A.; Siddiqui, A.J.; Sulieman, A.E.; Alreshidi, M.M.; et al. Attenuation of ovalbumin-induced inflammation and lung oxidative injury in asthmatic rats by Zingiber officinale extract: Combined in silico and in vivo study on antioxidant potential, STAT6 and TNF-α pathways. 3 Biotech 2022, 12, 191. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Dgachi, S.; Belgacem, S.; Ahmed, A.B.; Henry, N.; Loiseau, T.; Nasr, S.; Badraoui, R.; Naϊli, H. Design, theoretical study, druggability, pharmacokinetics and properties evolution of a new organo-bromocadmate compound as prospective anticancer agent. J. Mol. Struct. 2023, 1274, 134439. [Google Scholar] [CrossRef]
- Chira, A.B.; Kadmi, Y.; Badraoui, R.; Aouadi, K.; Alhawday, F.; Boudaya, M.; Jamoussi, K.; Kallel, C.; El Feki, A.; Kadri, A.; et al. GC-MS/MS Analysis and Wound Repair Potential of Urtica dioica Essential Oil: In silico Modeling and In vivo Study in Rats. Curr. Pharm. Biotechnol. 2025, 26, 591–607. [Google Scholar] [CrossRef]
- Badraoui, R.; Abdelmoula, N.B.; Sahnoun, Z.; Fakhfakh, Z.; Rebai, T. Effect of subchronic exposure to tetradifon on bone remodelling and metabolism in female rat. Comptes Rendus Biol. 2007, 330, 897–904. [Google Scholar] [CrossRef]
- Pesce, V.; Speciale, D.; Sammarco, G.; Patella, S.; Spinarelli, A.; Patella, V. Surgical approach to bone healing in osteoporosis. Clin. Cases Min. Bone Metab. 2009, 6, 131–135. [Google Scholar]
- Mzid, M.; Badraoui, R.; Khedir, S.B.; Sahnoun, Z.; Rebai, T. Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomed. Pharmacother. 2017, 91, 1022–1041. [Google Scholar] [CrossRef]
- Dallegrave, E.; Mantese, F.D.; Coelho, R.S.; Pereira, J.D.; Dalsenter, P.R.; Langeloh, A. The teratogenic potential of the herbicide glyphosate-Roundup® in Wistar rats. Toxicol. Lett. 2003, 142, 45–52. [Google Scholar] [CrossRef]
- Robinson, C. Teratogenic Effects of Glyphosate-Based Herbicides: Divergence of Regulatory Decisions from Scientific Evidence. J. Environ. Anal. Toxicol. 2012. Available online: https://www.researchgate.net/publication/258409685_Teratogenic_Effects_of_Glyphosate-Based_Herbicides_Divergence_of_Regulatory_Decisions_from_Scientific_Evidence (accessed on 23 March 2025). [CrossRef]
- Roy, N.M.; Ochs, J.; Zambrzycka, E.; Anderson, A. Glyphosate induces cardiovascular toxicity in Danio rerio. Environ. Toxicol. Pharmacol. 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Boulila, S.; Oudadesse, H.; Elfeki, H.; Kallel, R.; Lefeuvre, B.; Mabrouk, M.; Tounsi, S.; Mhalla, D.; Mostafa, A.; Chaabouni, K.; et al. Antibacterial and in vivo reactivity of bioactive glass and poly(vinyl alcohol) composites prepared by melting and sol-gel techniques. Korean J. Chem. Eng. 2016, 33, 1659–1668. [Google Scholar] [CrossRef]
- Mzid, M.; El Feki, H.; Oudadesse, H.; Lefeuvre, B.; Rebai, T. Physico-chemical exploration of pesticide (imidacloprid)-induced osteoporosis in female rats the protective effect of Urtica urens L. leaves. Biochem. Biotechnol. Res. 2020, 8, 1–20. [Google Scholar]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Mackay, A.L. A preliminary examination of the structure of α-Ca3(PO4)2. Acta Crystallogr. 1953, 6, 743–744. [Google Scholar] [CrossRef]
- Jasper, R.; Locatelli, G.O.; Pilati, C.; Locatelli, C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup®. Interdiscip. Toxicol. 2012, 5, 133–140. [Google Scholar] [CrossRef]
- Badraoui, R.; Allouche, M.; El Ouaer, D.; Siddiqui, A.J.; Ishak, S.; Hedfi, A.; Beyrem, H.; Pacioglu, O.; Rudayni, H.A.; Boufahja, F. Ecotoxicity of chrysene and phenanthrene on meiobenthic nematodes with a case study of Terschellingia longicaudata: Taxonomics, toxicokinetics, and molecular interactions modelling. Environ. Pollut. 2023, 316, 120459. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Struct. Dyn. 2022, 40, 4341–4351. [Google Scholar] [CrossRef]
- Dellali, M.; Mardassi, K.; Harrath, A.H.; Mansour, L.; Pacioglu, O.; Aldahmash, W.; Nahdi, S.; Badraoui, R.; Alrefaei, A.F.; Boufahja, F. Physiological Responses of the Bivalves Mytilus galloprovincialis and Ruditapes decussatus Following Exposure to Phenanthrene: Toxicokinetics, Dynamics and Biomarkers Study. Animals 2022, 13, 151. [Google Scholar] [CrossRef]
- Badraoui, R.; Ben-Nasr, H.; Bardakçi, F.; Rebai, T. Pathophysiological impacts of exposure to an endocrine disruptor (tetradifon) on α–amylase and lipase activities associated metabolic disorders. Pestic. Biochem. Physiol. 2020, 167, 104606. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, M.; Badraoui, R.; Rahmouni, F.; Jamoussi, K.; El Feki, A. Antioxidant and Protective Effects of Artemisia campestris Essential Oil Against Chlorpyrifos-Induced Kidney and Liver Injuries in Rats. Front. Physiol. 2021, 12, 618582. [Google Scholar] [CrossRef] [PubMed]
- Manservisi, F.; Lesseur, C.; Panzacchi, S.; Mandrioli, D.; Falcioni, L.; Bua, L.; Manservigi, M.; Spinaci, M.; Galeati, G.; Mantovani, A.; et al. The Ramazzini Institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: Effects on development and endocrine system. Environ. Health 2019, 18, 1–16. Available online: https://ehjournal.biomedcentral.com/articles/10.1186/s12940-019-0453-y (accessed on 1 May 2025). [CrossRef] [PubMed]
- McGehee, J. The Effects of Chronic Glyphosate Exposure on Osteoblast Cell Function. Ph.D. Thesis, The University of North Carolina at Greensboro, Greensboro, NC, USA, 2020. [Google Scholar]
 The newly appeared bands;
The newly appeared bands;  The bands disappeared compared to the control group. FT: Female rats of the control group; FD1: Female rats treated with Dose 1 of KL; FD2: Female rats treated with Dose 2 of KL. The green asterisk represent the newly appeared bands. (F): FTIR analysis allowed us to detect groups specific to KL in the bones of treated rats.
The bands disappeared compared to the control group. FT: Female rats of the control group; FD1: Female rats treated with Dose 1 of KL; FD2: Female rats treated with Dose 2 of KL. The green asterisk represent the newly appeared bands. (F): FTIR analysis allowed us to detect groups specific to KL in the bones of treated rats.
 The newly appeared bands;
The newly appeared bands;  The bands disappeared compared to the control group. FT: Female rats of the control group; FD1: Female rats treated with Dose 1 of KL; FD2: Female rats treated with Dose 2 of KL. The green asterisk represent the newly appeared bands. (F): FTIR analysis allowed us to detect groups specific to KL in the bones of treated rats.
The bands disappeared compared to the control group. FT: Female rats of the control group; FD1: Female rats treated with Dose 1 of KL; FD2: Female rats treated with Dose 2 of KL. The green asterisk represent the newly appeared bands. (F): FTIR analysis allowed us to detect groups specific to KL in the bones of treated rats.



| Wave Numbers (cm−1)/Group | CO32− | PO43− | OH- Displaced | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1563 | 1556 | 1536 | 1510 | - | 816 | 1253 | 1201 | - | 1147 | - | 602 | 659 | 546 | 718 | 751 |
| Group 2 (126 mg of GLP/Kg) | - | - | 1543 | - | 1417 | - | 1240 | - | 1161 | - | - | 605 | - | 561 | 723 | - |
| Group 3 (315 mg of GLP/Kg) | - | - | 1538 | - | 1417 | - | 1237 | - | 1160 | - | 1116 | 605 | - | 558 | 720 | - |
| Kalach | - | - | 1523 | - | 1399 | 767 | 1247 | 1219 | 1163 | - | - | 589 | 720 | 1523 | 687 | |
| Group/Peak | DRX | ||
|---|---|---|---|
| (002) | (211) | (310) | |
| HPA | 26 | 33 | 41 |
| Control | 26 | 33 | 41 |
| Group 2 (126 mg of GLP/Kg) | 25.97 | 32.16 | 39.81 |
| Group 3 (315 mg of GLP/Kg) | 25.12 | 32.33 | 39.65 |
| Ca mg/g of Tissu | P mg/g of Tissu | Ca/P | |
|---|---|---|---|
| Control | 35.91 ± 0.07 | 13.80 ± 0.18 | 2.60 ± 0.03 |
| Group 2 (126 mg of GLP/Kg) | 37.10 ± 1.16 | 13.27 ± 0.19 ** | 2.79 ± 0.11 * |
| Group 3 (315 mg of GLP/Kg) | 33.86 ± 1.20 * | 12.66 ± 0.41 ** | 2.67 ± 0.13 * |
| Ca ppm | P ppm | Ca/P | |
| Kalach | 11.96 ± 1.28 | 31.66 ± 22.36 | 0.78 ± 0.87 |
| Entry | Affinity (kcal/mol) | RMSD (Lower–Upper) | Interacting Residues | Closest Interacting Residue (Distance, Å) |
|---|---|---|---|---|
| 4mzz | −3.5 | 0.0–10.49 | Attractive Charge: GLU40 Conventional H-Bond: GLU40, ARG43, ARG43, ILE36, ILe36 Carbon H-Bond: GLU40 | Glu40:HN (1.966) |
| 8i75 | −4.2 | 0.0–10.9 | Attractive Charge: GLU24, ASP30, ASP34, ASP30, ASP34 Conventional H-Bond: ARG20, ASP30, ASP34, ASP34, ASP30, ASP34 Carbon H-Bond: GLU24 | ASP30:OD2 (2.401) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdaoui, L.; El Feki, H.; Ben Amor, M.; Oudadesse, H.; Badraoui, R.; Khalil, N.; Brahmi, F.; Jilani, S.; Aloufi, B.; Ben Amara, I.; et al. Physicochemical Exploration and Computational Analysis of Bone After Subchronic Exposure to Kalach 360 SL in Female Wistar Rats. Toxics 2025, 13, 456. https://doi.org/10.3390/toxics13060456
Hamdaoui L, El Feki H, Ben Amor M, Oudadesse H, Badraoui R, Khalil N, Brahmi F, Jilani S, Aloufi B, Ben Amara I, et al. Physicochemical Exploration and Computational Analysis of Bone After Subchronic Exposure to Kalach 360 SL in Female Wistar Rats. Toxics. 2025; 13(6):456. https://doi.org/10.3390/toxics13060456
Chicago/Turabian StyleHamdaoui, Latifa, Hafedh El Feki, Marwa Ben Amor, Hassane Oudadesse, Riadh Badraoui, Naila Khalil, Faten Brahmi, Saoussen Jilani, Bandar Aloufi, Ibtissem Ben Amara, and et al. 2025. "Physicochemical Exploration and Computational Analysis of Bone After Subchronic Exposure to Kalach 360 SL in Female Wistar Rats" Toxics 13, no. 6: 456. https://doi.org/10.3390/toxics13060456
APA StyleHamdaoui, L., El Feki, H., Ben Amor, M., Oudadesse, H., Badraoui, R., Khalil, N., Brahmi, F., Jilani, S., Aloufi, B., Ben Amara, I., & Rebai, T. (2025). Physicochemical Exploration and Computational Analysis of Bone After Subchronic Exposure to Kalach 360 SL in Female Wistar Rats. Toxics, 13(6), 456. https://doi.org/10.3390/toxics13060456








