Polarization of THP-1-Derived Human M0 to M1 Macrophages Exposed to Flavored E-Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Supplies
2.2. E-Liquids Preparation
2.3. THP-1 Culturing, Differentiation, and Polarization
2.4. THP-1 Growth and Viability with E-Liquids
2.5. Polarization of M0 to M1 Macrophages with 1% E-Liquids
2.6. E-Liquid Interference with LDH Enzymatic Activity
2.7. LDH Cytotoxicity Assay
2.8. RNA Extraction, cDNA Reverse Transcription and qPCR
2.9. Cytokine Production
2.10. Statistical Analysis
3. Results
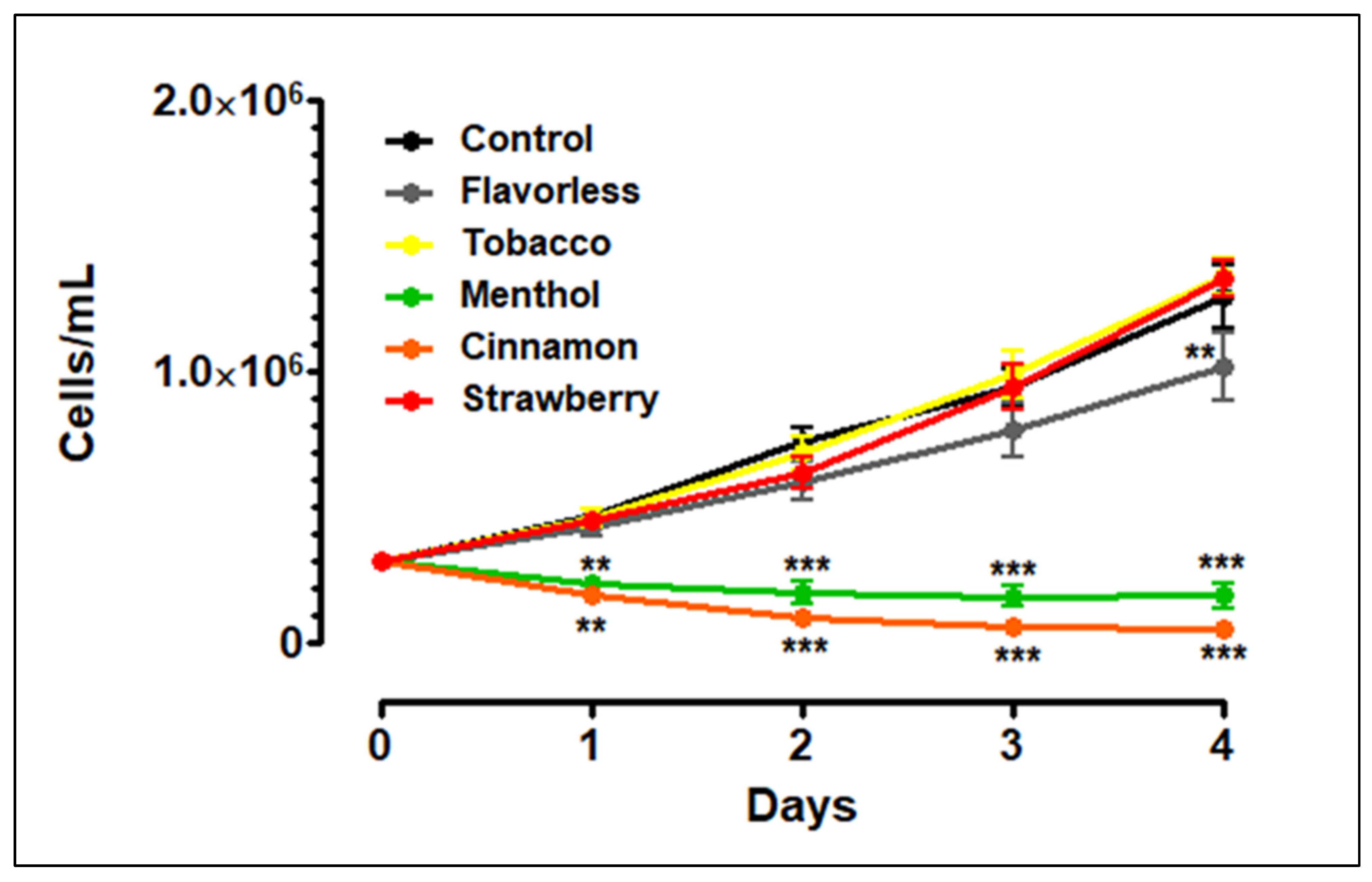
3.1. THP-1 Growth and Viability with E-Liquids
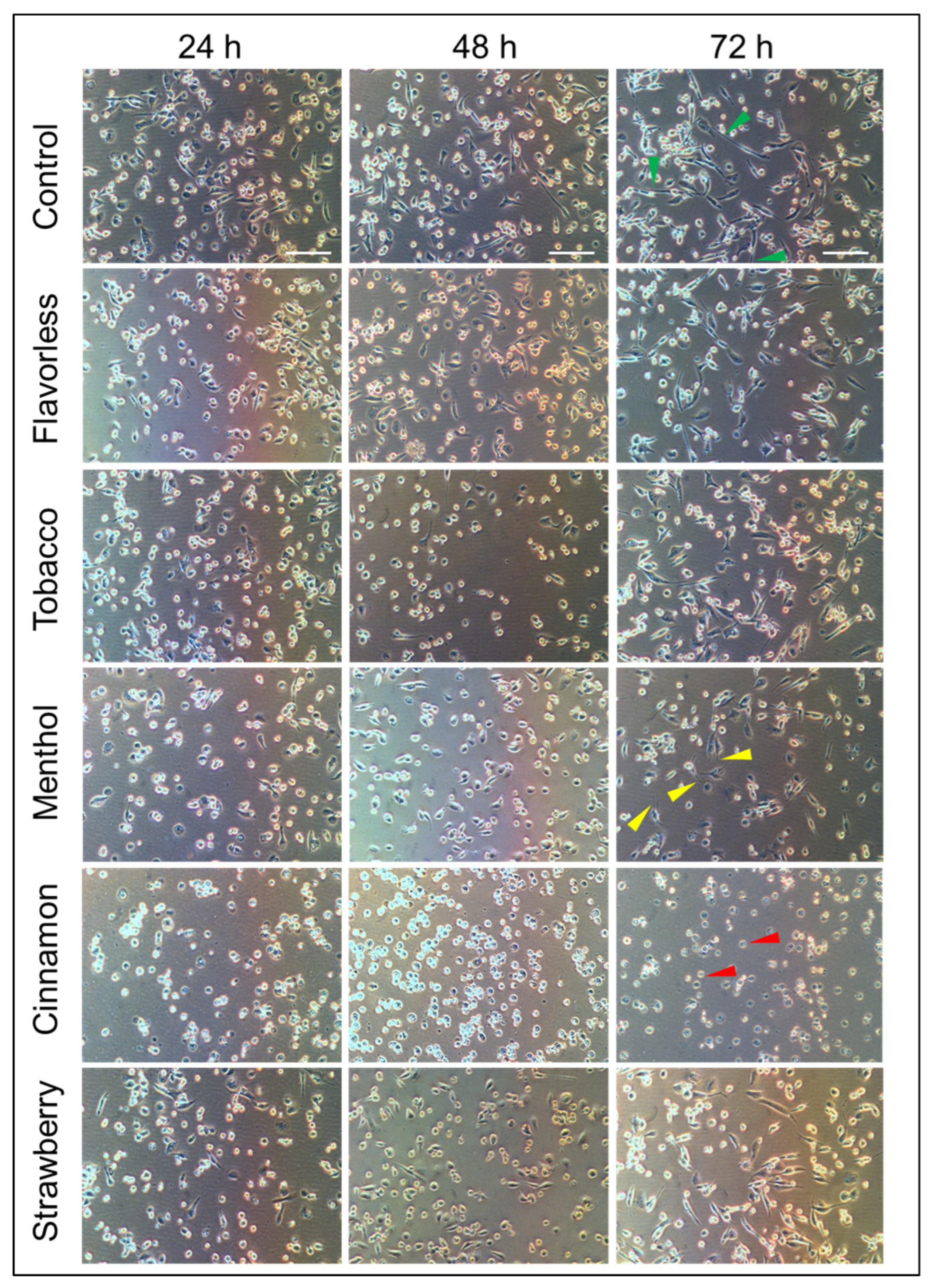
3.2. Cellular Morphology of M0 to M1 Macrophages in the Absence or Presence of 1% E-Liquids
3.3. LDH Cytotoxicity
3.4. Expression of HLA DR, CD80 and TLR-4
3.5. Cytokine Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Besaratinia, A.; Tommasi, S. Vaping Epidemic: Challenges and Opportunities. Cancer Causes Control 2020, 31, 663–667. [Google Scholar] [CrossRef]
- Pan, L.; Morton, J.; Mbulo, L.; Dean, A.; Ahluwalia, I.B. Electronic Cigarette Use among Adults in 14 Countries: A Cross-Sectional Study. eClinicalMedicine 2022, 47, 101401. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Rahimi, S.; Darvishi, N.; Abdolmaleki, A.; Mohammadi, M. The Global Prevalence of E-Cigarettes in Youth: A Comprehensive Systematic Review and Meta-Analysis. Public Health Pract. 2024, 7, 100506. [Google Scholar] [CrossRef] [PubMed]
- GVR-2-68038-433-8 E-Cigarette and Vape Market Size and Share Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/e-cigarette-vaping-market (accessed on 15 May 2025).
- Jerzyński, T.; Stimson, G.V.; Shapiro, H.; Król, G. Estimation of the Global Number of E-Cigarette Users in 2020. Harm Reduct. J. 2021, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.R. Tobacco Product Use Among High School Students—Youth Risk Behavior Survey, United States, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 56–63. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Vaping Devices (Electronic Cigarettes) DrugFacts. Available online: https://nida.nih.gov/publications/drugfacts/vaping-devices-electronic-cigarettes (accessed on 11 September 2022).
- Krüsemann, E.J.Z.; Boesveldt, S.; de Graaf, K.; Talhout, R. An E-Liquid Flavor Wheel: A Shared Vocabulary Based on Systematically Reviewing E-Liquid Flavor Classifications in Literature. Nicotine Tob. Res. 2019, 21, 1310–1319. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous Compounds in Tobacco Smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Palazzolo, D.L. Electronic Cigarettes and Vaping: A New Challenge in Clinical Medicine and Public Health. A Literature Review. Front. Public Health 2013, 1, 56. [Google Scholar] [CrossRef]
- Diaz, M.C.; Silver, N.A.; Bertrand, A.; Schillo, B.A. Bigger, Stronger and Cheaper: Growth in e-Cigarette Market Driven by Disposable Devices with More e-Liquid, Higher Nicotine Concentration and Declining Prices. Tob. Control 2025, 34, 65–70. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538680/ (accessed on 18 January 2023).
- Farsalinos, K.E.; Kistler, K.A.; Gillman, G.; Voudris, V. Evaluation of Electronic Cigarette Liquids and Aerosol for the Presence of Selected Inhalation Toxins. Nicotine Tob Res 2015, 17, 168–174. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Brinkman, M.C.; Granville, C.A.; Gordon, S.M.; Clark, P.I. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res. 2016, 18, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, D.L.; Crow, A.P.; Nelson, J.M.; Johnson, R.A. Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel. Front. Physiol. 2016, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.; Talbot, P. Comparison of Electronic Cigarette Refill Fluid Cytotoxicity Using Embryonic and Adult Models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar] [CrossRef]
- Behar, R.Z.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of Toxicants in Cinnamon-Flavored Electronic Cigarette Refill Fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Gerloff, J.; Sundar, I.K.; Freter, R.; Sekera, E.R.; Friedman, A.E.; Robinson, R.; Pagano, T.; Rahman, I. Inflammatory Response and Barrier Dysfunction by Different E-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. Vitr. Toxicol. 2017, 3, 28–40. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Muthumalage, T.; Prinz, M.; Ansah, K.O.; Gerloff, J.; Sundar, I.K.; Rahman, I. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used E-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front. Physiol. 2018, 8, 1130. [Google Scholar] [CrossRef]
- Shamim, A.; Herzog, H.; Shah, R.; Pecorelli, S.; Nisbet, V.; George, A.; Cuadra, G.A.; Palazzolo, D.L. Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids. Dent. J. 2025, 13, 60. [Google Scholar] [CrossRef]
- Wavreil, F.D.M.; Heggland, S.J. Cinnamon-Flavored Electronic Cigarette Liquids and Aerosols Induce Oxidative Stress in Human Osteoblast-like MG-63 Cells. Toxicol. Rep. 2020, 7, 23–29. [Google Scholar] [CrossRef]
- Willershausen, I.; Wolf, T.; Weyer, V.; Sader, R.; Ghanaati, S.; Willershausen, B. Influence of E-Smoking Liquids on Human Periodontal Ligament Fibroblasts. Head Face Med. 2014, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Moshensky, A.; Brand, C.S.; Alhaddad, H.; Shin, J.; Masso-Silva, J.A.; Advani, I.; Gunge, D.; Sharma, A.; Mehta, S.; Jahan, A.; et al. Effects of Mango and Mint Pod-Based e-Cigarette Aerosol Inhalation on Inflammatory States of the Brain, Lung, Heart, and Colon in Mice. eLife 2022, 11, e67621. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The Macrophage: Past, Present and Future. Eur. J. Immunol. 2007, 37, S9–S17. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kwon, H.-Y.; Sharma, A.; Lee, S.H.; Liu, X.; Miyamoto, N.; Kim, J.-J.; Im, S.-H.; Kang, N.-Y.; Chang, Y.-T. Visualizing Inflammation with an M1 Macrophage Selective Probe via GLUT1 as the Gating Target. Nat. Commun. 2022, 13, 5974. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Suleimanov, S.K.; Efremov, Y.M.; Klyucherev, T.O.; Salimov, E.L.; Ragimov, A.A.; Timashev, P.S.; Vlasova, I.I. Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages. Int. J. Mol. Sci. 2024, 25, 1860. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Bandola-Simon, J.; Roche, P.A. Regulation of MHC Class II and CD86 Expression by March-I in Immunity and Disease. Curr. Opin. Immunol. 2023, 82, 102325. [Google Scholar] [CrossRef] [PubMed]
- CD80—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/cd80 (accessed on 11 February 2025).
- Haile, S.T.; Dalal, S.P.; Clements, V.; Tamada, K.; Ostrand-Rosenberg, S. Soluble CD80 Restores T Cell Activation and Overcomes Tumor Cell Programmed Death Ligand 1–Mediated Immune Suppression. J. Immunol. 2013, 191, 2829–2836. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of Phagocytosis in Macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Available online: https://www.hindawi.com/journals/bmri/2017/9042851/ (accessed on 29 May 2019).
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the Maintenance of Homeostasis. Cell Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Kang, G.; Song, H.; Bo, L.; Liu, Q.; Li, Q.; Li, J.; Pan, P.; Wang, J.; Jia, Y.; Sun, H.; et al. Nicotine Promotes M2 Macrophage Polarization through A5-nAChR/SOX2/CSF-1 Axis in Lung Adenocarcinoma. Cancer Immunol. Immunother. 2024, 74, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Zou, C.; He, F.; Guo, F.; Liu, S.; Fan, Y.; Zhu, X.; Zhou, Q.; Shu, D. Nicotine’s Impact on Platelet Function: Insights into Hemostasis Mechanisms. Front. Pharmacol. 2025, 15, 1512142. [Google Scholar] [CrossRef]
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. [Google Scholar] [CrossRef]
- Bianchi, S.; Torge, D.; Rinaldi, F.; Piattelli, M.; Bernardi, S.; Varvara, G. Platelets’ Role in Dentistry: From Oral Pathology to Regenerative Potential. Biomedicines 2022, 10, 218. [Google Scholar] [CrossRef]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-Inflammatory Effects of e-Cigarette Vapour Condensate on Human Alveolar Macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored E-Cigarette Liquids and Cinnamaldehyde Impair Respiratory Innate Immune Cell Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.-G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N.; Whitlatch, A.; Mohammadi, L.; Guo, H.; Nadeau, K.C.; et al. Modeling Cardiovascular Risks of E-Cigarettes with Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.; Rahman, I. Pro-Inflammatory Effects of Aerosols from e-Cigarette-Derived Flavoring Chemicals on Murine Macrophages. Toxicol. Rep. 2023, 10, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Flora, J.W.; Meruva, N.; Huang, C.B.; Wilkinson, C.T.; Ballentine, R.; Smith, D.C.; Werley, M.S.; McKinney, W.J. Characterization of Potential Impurities and Degradation Products in Electronic Cigarette Formulations and Aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. [Google Scholar] [CrossRef]
- Werheim, E.R.; Senior, K.G.; Shaffer, C.A.; Cuadra, G.A. Oral Pathogen Porphyromonas Gingivalis Can Escape Phagocytosis of Mammalian Macrophages. Microorganisms 2020, 8, 1432. [Google Scholar] [CrossRef]
- Trypan Blue Exclusion-US. Available online: https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/cell-culture-protocols/trypan-blue-exclusion.html (accessed on 3 December 2022).
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of Pyroptosis by Measuring Released Lactate Dehydrogenase Activity. In The Inflammasome: Methods and Protocols; de Nardo, C.M., Latz, E., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 85–90. ISBN 978-1-62703-523-1. [Google Scholar]
- Invitrogen CyQUANT LDH Cytotoxicity Assay-Cell Analysis Products, Cell Based Assays. Available online: https://www.fishersci.com/shop/products/cyquant-ldh-cytotoxicity-assay-kit-2/C20300 (accessed on 6 February 2025).
- Quick-RNA 96 Kit. Available online: https://www.zymoresearch.com/products/quick-rna-96-kit (accessed on 6 February 2025).
- SuperScriptTM VILOTM cDNA Synthesis Kit. Available online: https://www.thermofisher.com/order/catalog/product/11754250 (accessed on 6 February 2025).
- Human IL-1 Beta Uncoated ELISA Kit-Invitrogen. Available online: https://www.thermofisher.com/elisa/product/Human-IL-1-beta-Uncoated-ELISA-Kit/88-7261-88 (accessed on 6 February 2025).
- Human IL-6 Uncoated ELISA Kit with Plates-Invitrogen. Available online: https://www.thermofisher.com/elisa/product/Human-IL-6-Uncoated-ELISA-Kit-with-Plates/88-7066-22 (accessed on 6 February 2025).
- Human IL-8 Uncoated ELISA Kit with Plates-Invitrogen. Available online: https://www.thermofisher.com/elisa/product/Human-IL-8-Uncoated-ELISA-Kit-with-Plates/88-8086-22 (accessed on 6 February 2025).
- Human TNF Alpha Uncoated ELISA Kit with Plates-Invitrogen. Available online: https://www.thermofisher.com/elisa/product/Human-TNF-alpha-Uncoated-ELISA-Kit-with-Plates/88-7346-86 (accessed on 6 February 2025).
- Morris, A.M.; Leonard, S.S.; Fowles, J.R.; Boots, T.E.; Mnatsakanova, A.; Attfield, K.R. Effects of E-Cigarette Flavoring Chemicals on Human Macrophages and Bronchial Epithelial Cells. Int. J. Environ. Res. Public Health 2021, 18, 11107. [Google Scholar] [CrossRef]
- Ghosh, A.; Beyazcicek, O.; Davis, E.S.; Onyenwoke, R.U.; Tarran, R. Cellular Effects of Nicotine Salt-Containing e-Liquids. J. Appl. Toxicol. 2021, 41, 493–505. [Google Scholar] [CrossRef]
- Ma, T.; Wang, X.; Li, L.; Sun, B.; Zhu, Y.; Xia, T. Electronic Cigarette Aerosols Induce Oxidative Stress-Dependent Cell Death and NF-κB Mediated Acute Lung Inflammation in Mice. Arch. Toxicol. 2021, 95, 195–205. [Google Scholar] [CrossRef]
- Forbes, J.D. Clinically Important Toxins in Bacterial Infection: Utility of Laboratory Detection. Clin. Microbiol. Newsl. 2020, 42, 163–170. [Google Scholar] [CrossRef]
- Henkel, J.S.; Baldwin, M.R.; Barbieri, J.T. Toxins from Bacteria. EXS 2010, 100, 1–29. [Google Scholar]
- He, F.; Umrath, F.; Reinert, S.; Alexander, D. Jaw Periosteum-Derived Mesenchymal Stem Cells Regulate THP-1-Derived Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 4310. [Google Scholar] [CrossRef]
- Kurynina, A.V.; Erokhina, M.V.; Makarevich, O.A.; Sysoeva, V.Y.; Lepekha, L.N.; Kuznetsov, S.A.; Onishchenko, G.E. Plasticity of Human THP–1 Cell Phagocytic Activity during Macrophagic Differentiation. Biochem. Mosc. 2018, 83, 200–214. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Ma, Z.; Iwashina, T.; Tredget, E.E. Alternatively Activated Macrophages Derived from THP-1 Cells Promote the Fibrogenic Activities of Human Dermal Fibroblasts. Wound Repair Regen. 2017, 25, 377–388. [Google Scholar] [CrossRef]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Davis, E.S.; Ghosh, A.; Coakley, R.D.; Wrennall, J.A.; Lubamba, B.A.; Rowell, T.R.; Dang, H.; Pawlak, E.A.; Li, Q.; Alexis, N.E.; et al. Chronic E-Cigarette Exposure Alters Human Alveolar Macrophage Morphology and Gene Expression. Nicotine Tob. Res. 2022, 24, 395–399. [Google Scholar] [CrossRef]
- Shields, P.G.; Ying, K.L.; Brasky, T.M.; Freudenheim, J.L.; Li, Z.; McElroy, J.P.; Reisinger, S.A.; Song, M.-A.; Weng, D.Y.; Wewers, M.D.; et al. A Pilot Cross-Sectional Study of Immunological and Microbiome Profiling Reveals Distinct Inflammatory Profiles for Smokers, Electronic Cigarette Users, and Never-Smokers. Microorganisms 2023, 11, 1405. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Acharya, S.; Deshmukh, S.K.; Khan, M.A.; Singh, S.; Singh, A.P. Nicotine Causes Alternative Polarization of Macrophages via Src-Mediated STAT3 Activation: Potential Pathobiological Implications. J. Cell. Physiol. 2022, 237, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Phuangbubpha, P.; Thara, S.; Sriboonaied, P.; Saetan, P.; Tumnoi, W.; Charoenpanich, A. Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model. Cells 2023, 12, 1427. [Google Scholar] [CrossRef]
- Association, A.L. E-Cigarette or Vaping Use-Associated Lung Injury (EVALI). Available online: https://www.lung.org/lung-health-diseases/lung-disease-lookup/evali (accessed on 29 April 2025).




| Abbreviations | Complete Terms |
|---|---|
| ECIG | Electronic cigarette |
| E-Liquid | ECIG liquid |
| PG | Propylene glycol |
| VG | Vegetable glycerin or glycerol |
| IL- | Interleukin-1β, 6, 8, 12, etc. |
| M1 | Pro-inflammatory activated macrophages derived from M0 macrophages |
| M2 | Alternatively activated macrophages associated with anti-inflammatory responses; derived from M0 macrophages |
| M0 | Naïve macrophages derived from blood stream monocytes (THP-1 cells) |
| LPS | Lipopolysaccharide |
| THP-1 | Tohoku Hospital Pediatrics-1; can differentiate into M0 macrophages |
| INF-γ | Interferon-gamma |
| TNF-α | Tumor necrosis factor-alpha |
| GM-CSF | Granulocyte-monocyte colony-stimulating factor |
| MHC | Major histocompatibility complex |
| CD80 | Cluster of differentiation 80 |
| TLR-4 | Toll-like receptor-4 |
| RPMI | Roswell Park Memorial Institute media |
| PMA | Phorbol 12-myristate 13-acetate |
| hrIFN-γ | Human recombinant interferon-gamma |
| LDH | Lactate dehydrogenase |
| HLA DR | Human Leukocyte Antigen DR isotype |
| SEM | Standard error of the mean |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| MALT | Mucosa-associated lymphoid tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.; Luo, E.D.; Shaffer, C.A.; Tabakha, M.; Tomov, S.; Minton, S.H.; Brown, M.K.; Palazzolo, D.L.; Cuadra, G.A. Polarization of THP-1-Derived Human M0 to M1 Macrophages Exposed to Flavored E-Liquids. Toxics 2025, 13, 451. https://doi.org/10.3390/toxics13060451
Shah R, Luo ED, Shaffer CA, Tabakha M, Tomov S, Minton SH, Brown MK, Palazzolo DL, Cuadra GA. Polarization of THP-1-Derived Human M0 to M1 Macrophages Exposed to Flavored E-Liquids. Toxics. 2025; 13(6):451. https://doi.org/10.3390/toxics13060451
Chicago/Turabian StyleShah, Raivat, Emily D. Luo, Carly A. Shaffer, Maya Tabakha, Sophie Tomov, Siara H. Minton, Mikaela K. Brown, Dominic L. Palazzolo, and Giancarlo A. Cuadra. 2025. "Polarization of THP-1-Derived Human M0 to M1 Macrophages Exposed to Flavored E-Liquids" Toxics 13, no. 6: 451. https://doi.org/10.3390/toxics13060451
APA StyleShah, R., Luo, E. D., Shaffer, C. A., Tabakha, M., Tomov, S., Minton, S. H., Brown, M. K., Palazzolo, D. L., & Cuadra, G. A. (2025). Polarization of THP-1-Derived Human M0 to M1 Macrophages Exposed to Flavored E-Liquids. Toxics, 13(6), 451. https://doi.org/10.3390/toxics13060451










