The Impact of Seasonally Varying Dissolved Organic Matter in Natural Aquatic Environments on the Photodegradation of Pharmaceutical Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagents and Equipment
2.2. Sample Collection and Chemical Analysis
2.3. Photochemical Experiments
2.4. Toxicity Testing and Toxicity Prediction
2.5. Methods of Analysis
3. Results
3.1. Photophysical and Photochemical Properties of Isolated DOM
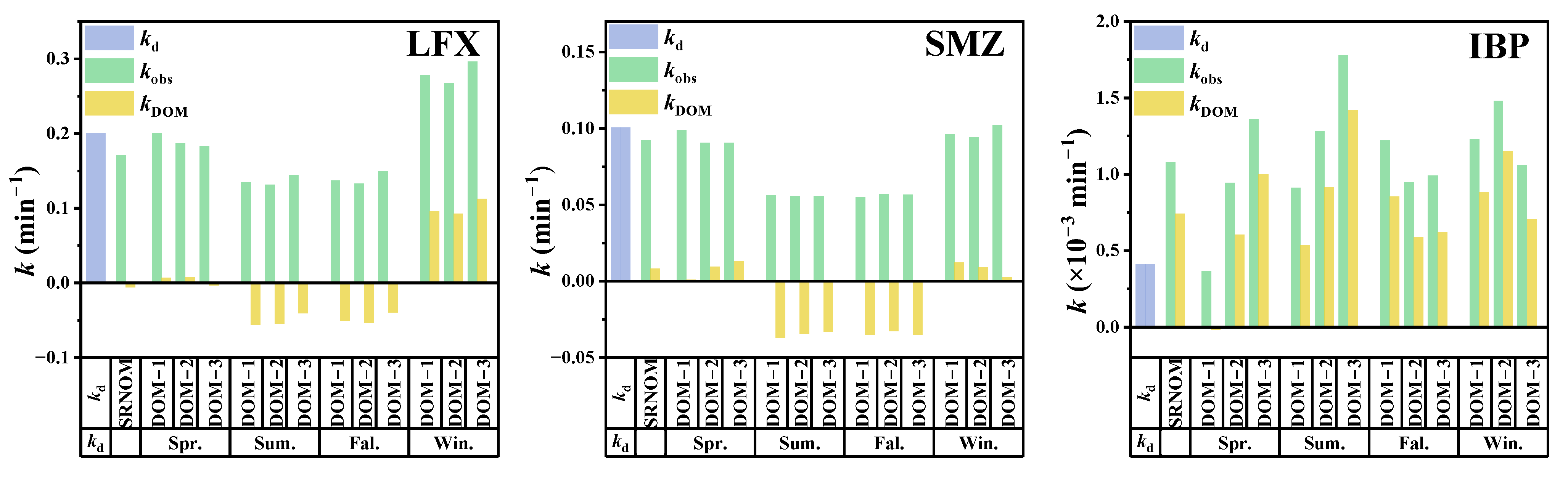
3.2. Effect of DOM on Photodegradation of Pharmaceutical Pollutants
3.3. Effects of DOM at Different Concentrations on Photodegradation of Pharmaceutical Pollutants
3.4. Different Roles of PPRIs on Photodegradation of Pharmaceutical Pollutants
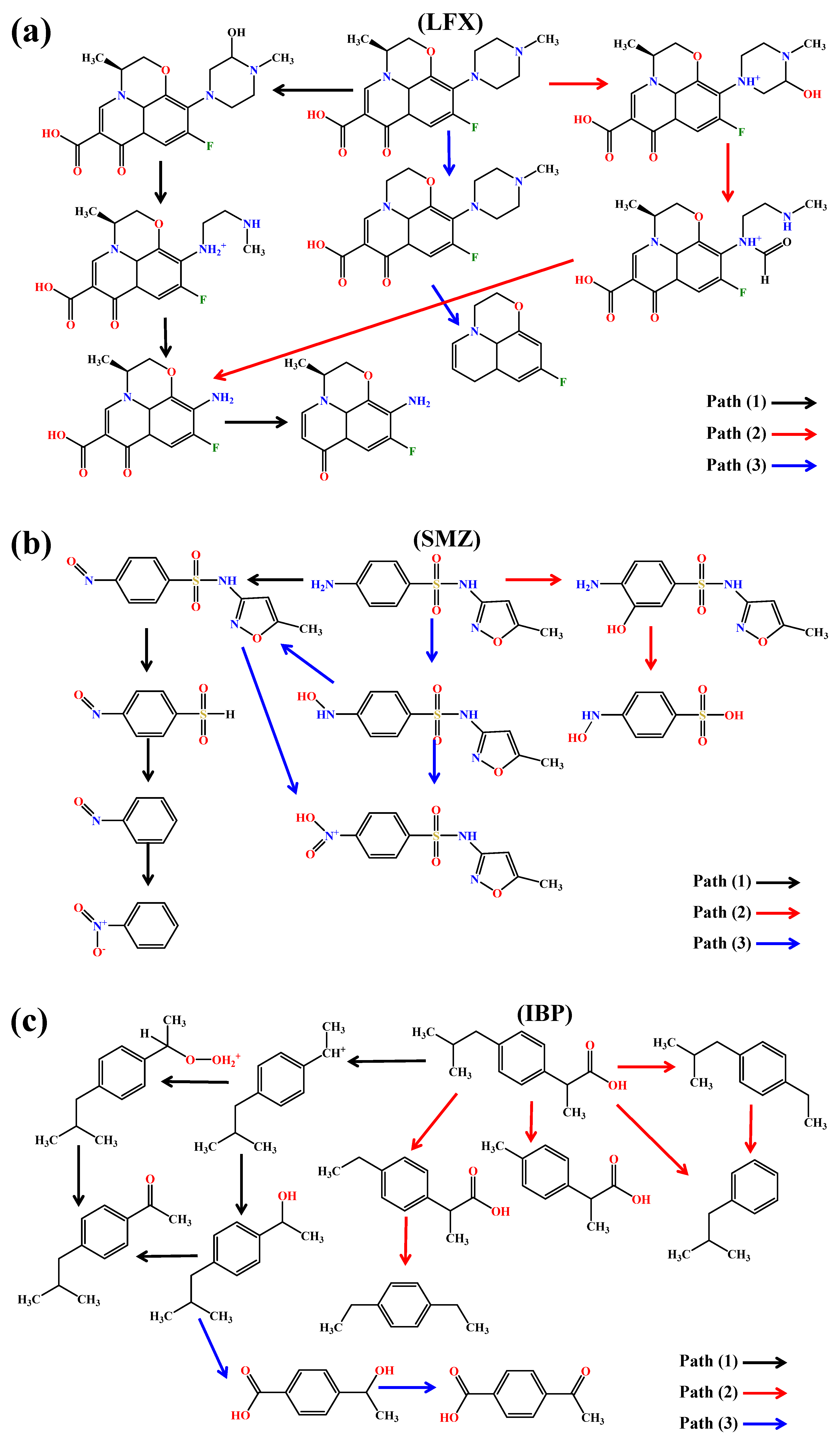
3.5. Photodegradation Pathways
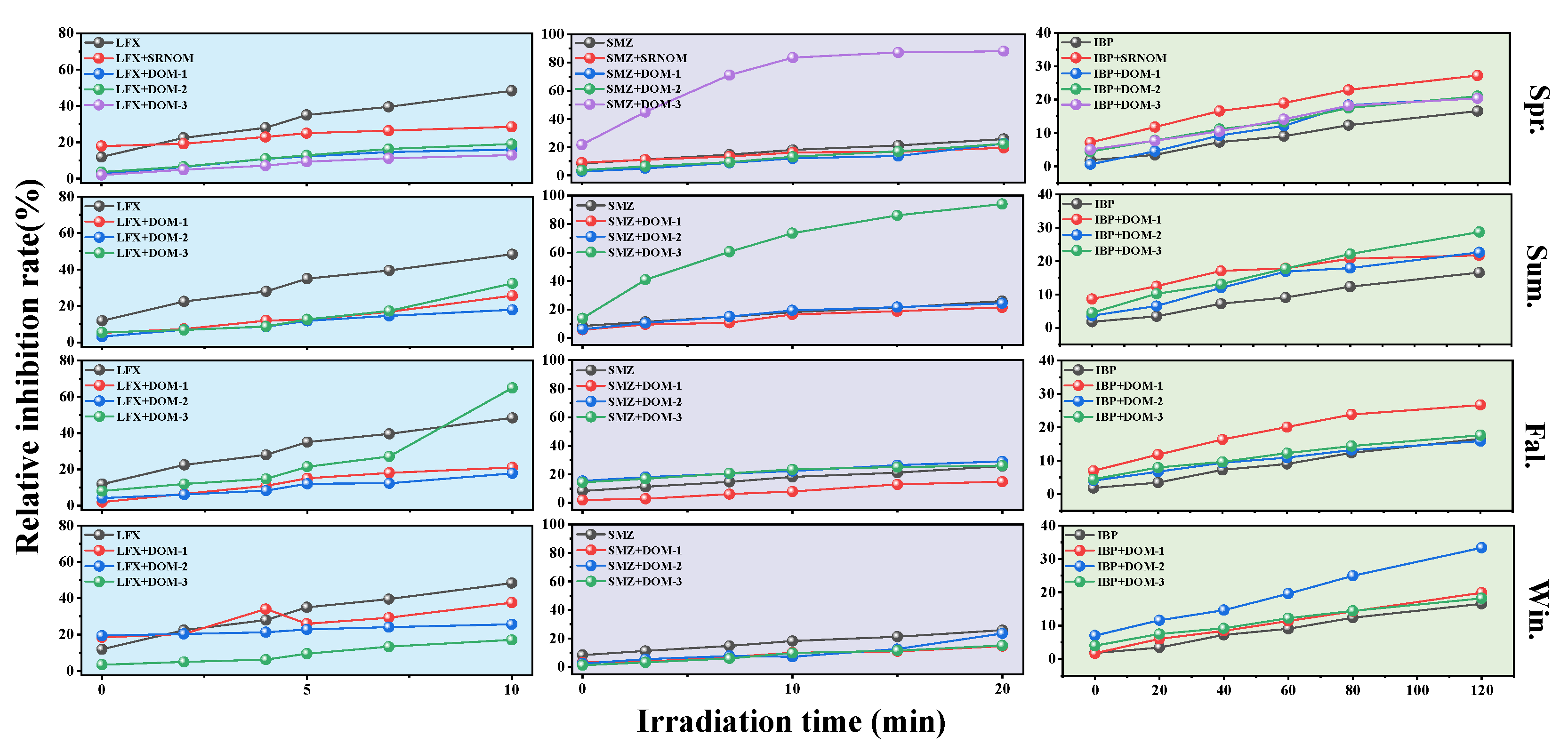
3.6. Influence of Dissolved Organic Matter on Contaminant Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Lemus, N.; López-Serna, R.; Perez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004, 39, 158–183. [Google Scholar] [CrossRef]
- MacManus-Spencer, L.A.; Tse, M.L.; Klein, J.L.; Kracunas, A.E. Aqueous Photolysis of the Organic Ultraviolet Filter Chemical Octyl Methoxycinnamate. Environ. Sci. Technol. 2011, 45, 3931–3937. [Google Scholar] [CrossRef]
- De Laurentiis, E.; Minella, M.; Sarakha, M.; Marrese, A.; Minero, C.; Mailhot, G.; Brigante, M.; Vione, D. Photochemical processes involving the UV absorber benzophenone-4 (2-hydroxy-4-methoxybenzophenone-5-sulphonic acid) in aqueous solution: Reaction pathways and implications for surface waters. Water Res. 2013, 47, 5943–5953. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Minella, M.; Maurino, V.; Minero, C. Indirect Photochemistry in Sunlit Surface Waters: Photoinduced Production of Reactive Transient Species. Chem. Eur. J. 2014, 20, 10590–10606. [Google Scholar] [CrossRef]
- Zhou, H.X.; Yan, S.W.; Lian, L.S.; Song, W.H. Triplet-State Photochemistry of Dissolved Organic Matter: Triplet-State Energy Distribution and Surface Electric Charge Conditions. Environ. Sci. Technol. 2019, 53, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Black, F.J.; Poulin, B.A.; Flegal, A.R. Factors controlling the abiotic photo-degradation of monomethylmercury in surface waters. Geochim. Cosmochim. Acta 2012, 84, 492–507. [Google Scholar] [CrossRef]
- Erickson, P.R.; Moor, K.J.; Werner, J.J.; Latch, D.E.; Arnold, W.A.; McNeill, K. Singlet Oxygen Phosphorescence as a Probe for Triplet-State Dissolved Organic Matter Reactivity. Environ. Sci. Technol. 2018, 52, 9170–9178. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Zhao, H.X.; Xia, D.M.; Li, X.T.; Qu, B.C. Formation of free radicals by direct photolysis of halogenated phenols (HPs) and effects of DOM: A case study on monobromophenols. J. Hazard. Mater. 2020, 391, 122220. [Google Scholar] [CrossRef]
- Guerard, J.J.; Miller, P.L.; Trouts, T.D.; Chin, Y.P. The role of fulvic acid composition in the photosensitized degradation of aquatic contaminants. Aquat. Sci. 2009, 71, 160–169. [Google Scholar] [CrossRef]
- Xu, H.C.; Guan, D.X.; Zou, L.; Lin, H.; Guo, L.D. Contrasting effects of photochemical and microbial degradation on Cu(II) binding with fluorescent DOM from different origins. Environ. Pollut. 2018, 239, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Chen, J.W.; Qiao, X.L.; Zhang, Y.N.; Uddin, M.; Guo, Z.Y. Disparate effects of DOM extracted from coastal seawaters and freshwaters on photodegradation of 2,4-Dihydroxybenzophenone. Water Res. 2019, 151, 280–287. [Google Scholar] [CrossRef]
- Wang, J.Q.; Chen, J.W.; Qiao, X.L.; Wang, Y.; Cai, X.Y.; Zhou, C.Z.; Zhang, Y.L.; Ding, G.H. DOM from mariculture ponds exhibits higher reactivity on photodegradation of sulfonamide antibiotics than from offshore seawaters. Water Res. 2018, 144, 365–372. [Google Scholar] [CrossRef]
- Jia, N.; Shi, Y.; Qi, J.; Yang, W.; Bu, Q.; Zhao, R.; Yang, L.; Tang, J. Effects of dissolved organic matter from different sources on ritonavir photolysis. Chemosphere 2024, 367, 143685. [Google Scholar] [CrossRef]
- Wenk, J.; Canonica, S. Phenolic Antioxidants Inhibit the Triplet-Induced Transformation of Anilines and Sulfonamide Antibiotics in Aqueous Solution. Environ. Sci. Technol. 2012, 46, 5455–5462. [Google Scholar] [CrossRef]
- Xu, H.M.; Cooper, W.J.; Jung, J.; Song, W.H. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Vidal, L.O.; Lambert, T.; Cotovicz, L.C., Jr.; Bernardes, M.C.; Sobrinho, R.; Thompson, F.; Garcia, G.D.; Knoppers, B.A.; Gatts, P.; Régis, C.R.; et al. Seasonal and diel modulation of DOM in a mangrove-dominated estuary. Sci. Total Environ. 2023, 857, 159045. [Google Scholar] [CrossRef] [PubMed]
- He, S.N.; Dong, D.M.; Zhang, X.; Sun, C.; Wang, C.Q.; Hua, X.Y.; Zhang, L.W.; Guo, Z.Y. Occurrence and ecological risk assessment of 22 emerging contaminants in the Jilin Songhua River (Northeast China). Environ. Sci. Pollut. Res. 2018, 25, 24003–24012. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Q.; Liu, H.Y.; Li, Y.J.; Cui, H.S.; Zhang, B.J.; Tian, S.L. Halide-specific enhancement of photodegradation for sulfadiazine in estuarine waters: Roles of halogen radicals and main water constituents. Water Res. 2019, 160, 209–216. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Sorbic acid as a quantitative probe for the formation, scavenging and steady-state concentrations of the triplet-excited state of organic compounds. Water Res. 2011, 45, 6535–6544. [Google Scholar] [CrossRef] [PubMed]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Latch, D.E.; Stender, B.L.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine. Environ. Sci. Technol. 2003, 37, 3342–3350. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Jaffrain, J.; Gérard, F.; Meyer, M.; Ranger, J. Assessing the quality of dissolved organic matter in forest soils using ultraviolet absorption spectrophotometry. Soil Sci. Soc. Am. J. 2007, 71, 1851–1858. [Google Scholar] [CrossRef]
- Shao, S.C.; Hu, Y.Y.; Cheng, J.H.; Chen, Y.C. Degradation of oxytetracycline (OTC) and nitrogen conversion characteristics using a novel strain. Chem. Eng. J. 2018, 354, 758–766. [Google Scholar] [CrossRef]
- Hosen, J.D.; McDonough, O.T.; Febria, C.M.; Palmer, M.A. Dissolved Organic Matter Quality and Bioavailability Changes Across an Urbanization Gradient in Headwater Streams. Environ. Sci. Technol. 2014, 48, 7817–7824. [Google Scholar] [CrossRef]
- Grasset, C.; Einarsdottir, K.; Catalán, N.; Tranvik, L.J.; Groeneveld, M.; Hawkes, J.A.; Attermeyer, K. Decreasing Photoreactivity and Concurrent Change in Dissolved Organic Matter Composition with Increasing Inland Water Residence Time. Glob. Biogeochem. Cycles 2024, 38, e2023GB007989. [Google Scholar] [CrossRef]
- Wu, X.W.; Liu, P.; Gong, Z.M.; Wang, H.Y.; Huang, H.X.Y.; Shi, Y.Q.; Zhao, X.L.; Gao, S.X. Humic Acid and Fulvic Acid Hinder Long-Term Weathering of Microplastics in Lake Water. Environ. Sci. Technol. 2021, 55, 15810–15820. [Google Scholar] [CrossRef]
- Yin, G.G.; Zhang, P.; Wang, Y.H.; Aftab, B.; Du, P.H.; Zhang, Q.; Chen, G.P.; Wang, M.K.; Yang, B.W.; Wang, S.H.; et al. Photochemical transformation of terrestrial dissolved organic matter derived from multiple sources in tropical plantations. Geochim. Cosmochim. Acta 2023, 358, 162–173. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.A.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Methods 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Tremblay, L.B.; Dittmar, T.; Marshall, A.G.; Cooper, W.J.; Cooper, W.T. Molecular characterization of dissolved organic matter in a North Brazilian mangrove porewater and mangrove-fringed estuaries by ultrahigh resolution Fourier Transform-Ion Cyclotron Resonance mass spectrometry and excitation/emission spectroscopy. Mar. Chem. 2007, 105, 15–29. [Google Scholar] [CrossRef]
- Czyrski, A.; Anusiak, K.; Tezyk, A. The degradation of levofloxacin in infusions exposed to daylight with an identification of a degradation product with HPLC-MS. Sci. Rep. 2019, 9, 3621. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, K.; Zhang, J.W.; Li, Y.H.; Li, Y.X.; Yang, N.; Guo, X. Study on Fe-doped BiOBr with enhanced visible photocatalytic activity for the degradation of levofloxacin. Mater. Lett. 2024, 373, 137122. [Google Scholar] [CrossRef]
- Wang, X.O.; Li, J.Y.; Zhang, C.P.; Xue, M.; Xie, H.J. Degradation products and transformation pathways of sulfamethoxazole chlorination disinfection by-products in constructed wetlands. Environ. Res. 2024, 249, 118343. [Google Scholar] [CrossRef]
- Li, H.X.; Li, Z.Y.; Zhang, X.; Sun, W.J.; Ao, X.W.; Li, Z.F. Nitrate Enhanced Sulfamethoxazole Degradation by 222 nm Far-UVC Irradiation: Role of Reactive Nitrogen Species. Environ. Sci. Technol. 2024, 58, 17510–17519. [Google Scholar] [CrossRef]
- Ferens, T.F.; Visioli, L.J.; Paulino, A.T.; Enzweiler, H. Photodegradation of ibuprofen by Pd-TiO2/ZSM-5 catalyst. Int. J. Environ. Sci. Technol. 2024, 22, 6759–6768. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Gelao, V.; Briguglio, S.; Mattiussi, M.; Baratta, W.; Zuccaccia, D.; Trovarelli, A.; Goi, D. Potential Residual Toxicity of the Ibuprofen Oxidative Degradation Products by HPLC-MS and Principal Component Analysis. ACS EST Water 2024, 4, 2057–2063. [Google Scholar] [CrossRef]
- Upender, I.; Yoshida, O.; Schrecengost, A.; Ranson, H.; Wu, Q.H.; Rowley, D.C.; Kishore, S.; Cywes, C.; Miller, E.L.; Whalen, K.E. A marine-derived fatty acid targets the cell membrane of Gram-positive bacteria. J. Bacteriol. 2023, 205, e0031023. [Google Scholar] [CrossRef]
- Odani, K.; Kobayashi, T.; Ogawa, Y.; Yoshida, S.; Seguchi, H. ML-7 inhibits exocytosis of superoxide-producing intracellular compartments in human neutrophils stimulated with phorbol myristate acetate in a myosin light chain kinase-independent manner. Histochem. Cell Biol. 2003, 119, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Canonica, S.; Freiburghaus, M. Electron-Rich Phenols for Probing the Photochemical Reactivity of Freshwaters. Environ. Sci. Technol. 2001, 35, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Al Housari, F.; Vione, D.; Chiron, S.; Barbati, S. Reactive photoinduced species in estuarine waters. Characterization of hydroxyl radical, singlet oxygen and dissolved organic matter triplet state in natural oxidation processes. Photochem. Photobiol. Sci. 2010, 9, 78–86. [Google Scholar] [CrossRef]
- Marchisio, A.; Minella, M.; Maurino, V.; Minero, C.; Vione, D. Photogeneration of reactive transient species upon irradiation of natural water samples: Formation quantum yields in different spectral intervals, and implications for the photochemistry of surface waters. Water Res. 2015, 73, 145–156. [Google Scholar] [CrossRef]
- Appiani, E.; Ossola, R.; Latch, D.E.; Erickson, P.R.; McNeill, K. Aqueous singlet oxygen reaction kinetics of furfuryl alcohol: Effect of temperature, pH, and salt content. Environ. Sci. Process. Impacts 2017, 19, 507–516. [Google Scholar] [CrossRef]
- Dong, M.M.; Rosario-Ortiz, F.L. Photochemical formation of hydroxyl radical from effluent organic matter. Environ. Sci. Technol. 2012, 46, 3788–3794. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
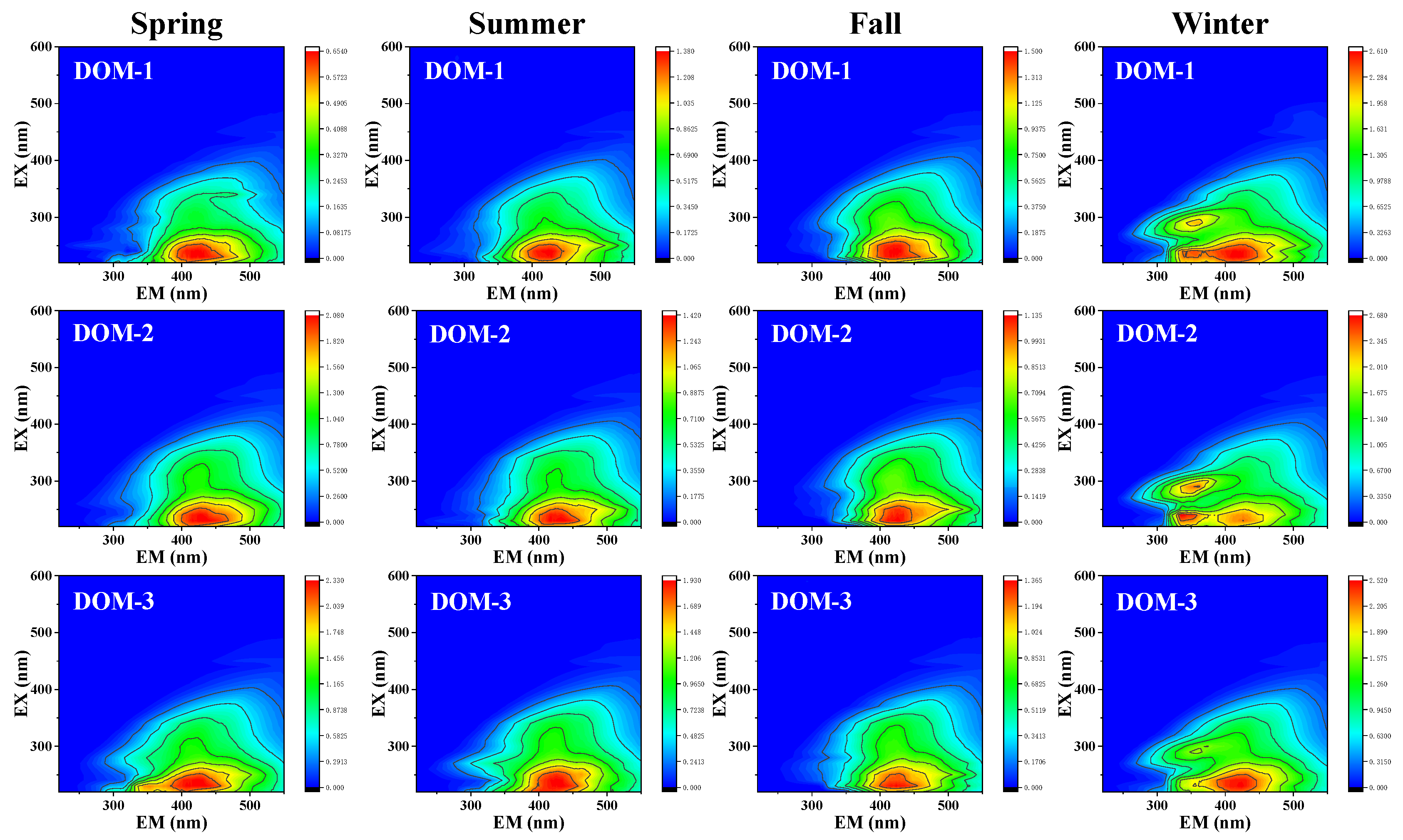


| Site | SUVA254 | SUVA260 | S275–295 | S350–400 | SR | E2/E3 | |
|---|---|---|---|---|---|---|---|
| Spr. | DOM-1 | 2.58 | 2.35 | 0.021 | 0.020 | 1.32 | 14.75 |
| DOM-2 | 7.69 | 7.14 | 0.018 | 0.019 | 0.87 | 7.29 | |
| DOM-3 | 5.76 | 5.34 | 0.020 | 0.022 | 1.24 | 10.15 | |
| Sum. | DOM-1 | 4.01 | 3.68 | 0.020 | 0.016 | 0.99 | 7.58 |
| DOM-2 | 5.11 | 4.74 | 0.018 | 0.016 | 0.81 | 6.16 | |
| DOM-3 | 6.22 | 5.62 | 0.016 | 0.016 | 0.85 | 7.00 | |
| Fal. | DOM-1 | 5.34 | 4.88 | 0.016 | 0.011 | 0.82 | 8.13 |
| DOM-2 | 5.53 | 5.11 | 0.016 | 0.016 | 0.77 | 7.01 | |
| DOM-3 | 4.51 | 4.15 | 0.014 | 0.022 | 0.74 | 7.43 | |
| Win. | DOM-1 | 7.12 | 6.56 | 0.017 | 0.012 | 0.88 | 6.81 |
| DOM-2 | 9.49 | 8.84 | 0.016 | 0.016 | 0.76 | 6.29 | |
| DOM-3 | 6.43 | 5.87 | 0.011 | 0.005 | 0.71 | 7.05 | |
| SRNOM | 8.64 | 6.77 | 0.012 | 0.017 | 0.77 | 5.55 | |
| Site | Φ3DOM* (×10−2) | [3DOM*]ss (×10−13 M) | Φ1O2 (×10−2) | [1O2]ss (×10−12 M) | Φ•OH (×10−5) | [•OH]ss (×10−17 M) | |
|---|---|---|---|---|---|---|---|
| Spr. | DOM-1 | 6.03 | 0.96 | 10.10 | 0.51 | 7.07 | 0.38 |
| DOM-2 | 2.03 | 1.32 | 4.07 | 0.83 | 2.51 | 0.55 | |
| DOM-3 | 4.14 | 1.79 | 6.31 | 0.85 | 1.66 | 0.12 | |
| Sum. | DOM-1 | 4.94 | 1.57 | 6.90 | 0.69 | 10.01 | 1.08 |
| DOM-2 | 2.77 | 1.31 | 4.71 | 0.70 | 9.31 | 1.49 | |
| DOM-3 | 4.25 | 2.29 | 6.26 | 1.06 | 5.09 | 0.93 | |
| Fal. | DOM-1 | 4.76 | 1.86 | 6.13 | 0.75 | 7.64 | 1.01 |
| DOM-2 | 2.72 | 1.26 | 5.38 | 0.64 | 7.90 | 1.24 | |
| DOM-3 | 4.11 | 1.48 | 7.73 | 0.83 | 9.03 | 1.10 | |
| Win. | DOM-1 | 0.76 | 1.05 | 0.88 | 0.38 | 2.50 | 1.17 |
| DOM-2 | 0.55 | 1.02 | 1.32 | 0.78 | 1.65 | 1.04 | |
| DOM-3 | 0.87 | 1.04 | 2.83 | 1.06 | 3.46 | 1.40 | |
| SRNOM | 0.41 | 0.34 | 1.88 | 0.31 | 4.19 | 5.36 | |
| Samples | Concentration (mgC L−1) | Φ3DOM* (×10−2) | [3DOM*]ss (×10−13 M) | Φ1O2 (×10−2) | [1O2]ss (×10−12 M) | Φ•OH (×10−5) | [•OH]ss (×10−17 M) |
|---|---|---|---|---|---|---|---|
| DOM-1 | 2.5 | 7.92 | 1.49 | 7.89 | 1.48 | 4.13 | 1.00 |
| 5 | 4.36 | 1.64 | 2.49 | 1.49 | 1.76 | 1.14 | |
| 10 | 2.63 | 6.94 | 1.41 | 3.73 | 0.91 | 3.50 | |
| 20 | 1.16 | 10.62 | 0.59 | 5.44 | 0.44 | 4.70 | |
| 25 | 0.93 | 13.14 | 0.42 | 5.86 | 0.27 | 5.00 | |
| DOM-2 | 2.5 | 8.38 | 1.45 | 6.73 | 1.17 | 3.11 | 0.90 |
| 5 | 3.70 | 1.69 | 1.84 | 1.34 | 1.16 | 1.17 | |
| 10 | 1.59 | 4.72 | 1.21 | 3.61 | 0.40 | 2.10 | |
| 20 | 0.60 | 6.45 | 0.48 | 5.18 | 0.13 | 4.10 | |
| 25 | 0.50 | 7.97 | 0.37 | 5.98 | 0.11 | 4.90 | |
| DOM-3 | 2.5 | 12.09 | 1.35 | 10.25 | 1.15 | 3.86 | 0.80 |
| 5 | 5.30 | 1.78 | 1.80 | 0.97 | 1.58 | 0.85 | |
| 10 | 2.41 | 5.25 | 1.45 | 3.16 | 0.56 | 2.50 | |
| 20 | 0.85 | 6.88 | 0.60 | 4.88 | 0.16 | 4.20 | |
| 25 | 0.85 | 10.25 | 0.45 | 5.40 | 0.15 | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cui, J.; Cheng, F.; Qu, J.; Zhang, Y.-N. The Impact of Seasonally Varying Dissolved Organic Matter in Natural Aquatic Environments on the Photodegradation of Pharmaceutical Pollutants. Toxics 2025, 13, 450. https://doi.org/10.3390/toxics13060450
Chen Y, Cui J, Cheng F, Qu J, Zhang Y-N. The Impact of Seasonally Varying Dissolved Organic Matter in Natural Aquatic Environments on the Photodegradation of Pharmaceutical Pollutants. Toxics. 2025; 13(6):450. https://doi.org/10.3390/toxics13060450
Chicago/Turabian StyleChen, Yue, Jingshuang Cui, Fangyuan Cheng, Jiao Qu, and Ya-Nan Zhang. 2025. "The Impact of Seasonally Varying Dissolved Organic Matter in Natural Aquatic Environments on the Photodegradation of Pharmaceutical Pollutants" Toxics 13, no. 6: 450. https://doi.org/10.3390/toxics13060450
APA StyleChen, Y., Cui, J., Cheng, F., Qu, J., & Zhang, Y.-N. (2025). The Impact of Seasonally Varying Dissolved Organic Matter in Natural Aquatic Environments on the Photodegradation of Pharmaceutical Pollutants. Toxics, 13(6), 450. https://doi.org/10.3390/toxics13060450






