Quantitative Detection of Micro- and Nanoplastics (≥300 nm) in Human Urine Using Double-Shot Py-GC/MS with Internal Standard Calibration
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Preparation of MNP Calibration Curves
2.3. Sample Collection
2.4. Pre-Processing of Samples
2.5. Analysis by Py-GC/MS
2.6. Quality Assurance/Quality Control (QA/QC)
2.6.1. Recovery Experiment
2.6.2. Blank Control
2.6.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.7. Statistical Analysis
3. Results
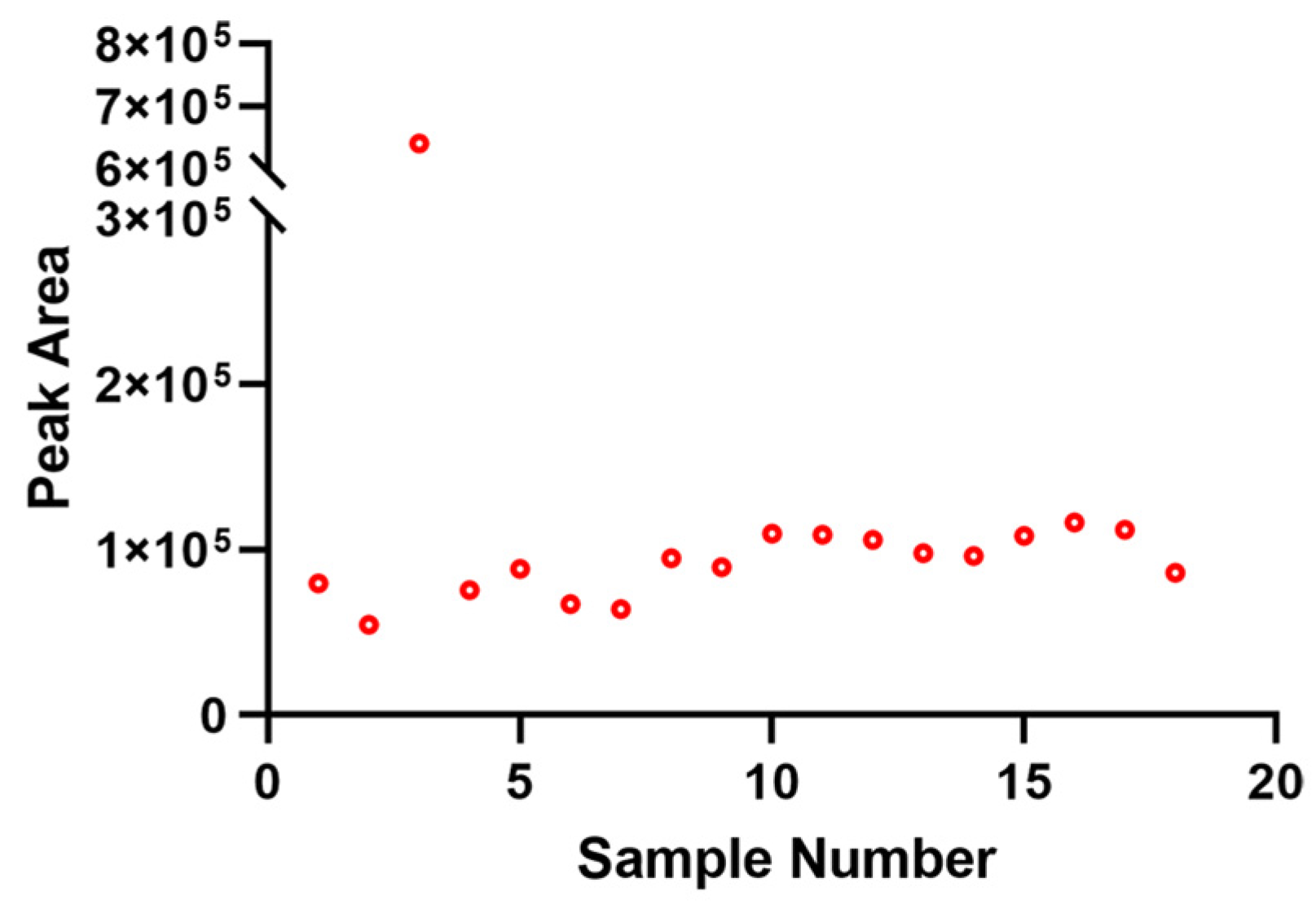
3.1. QA/QC
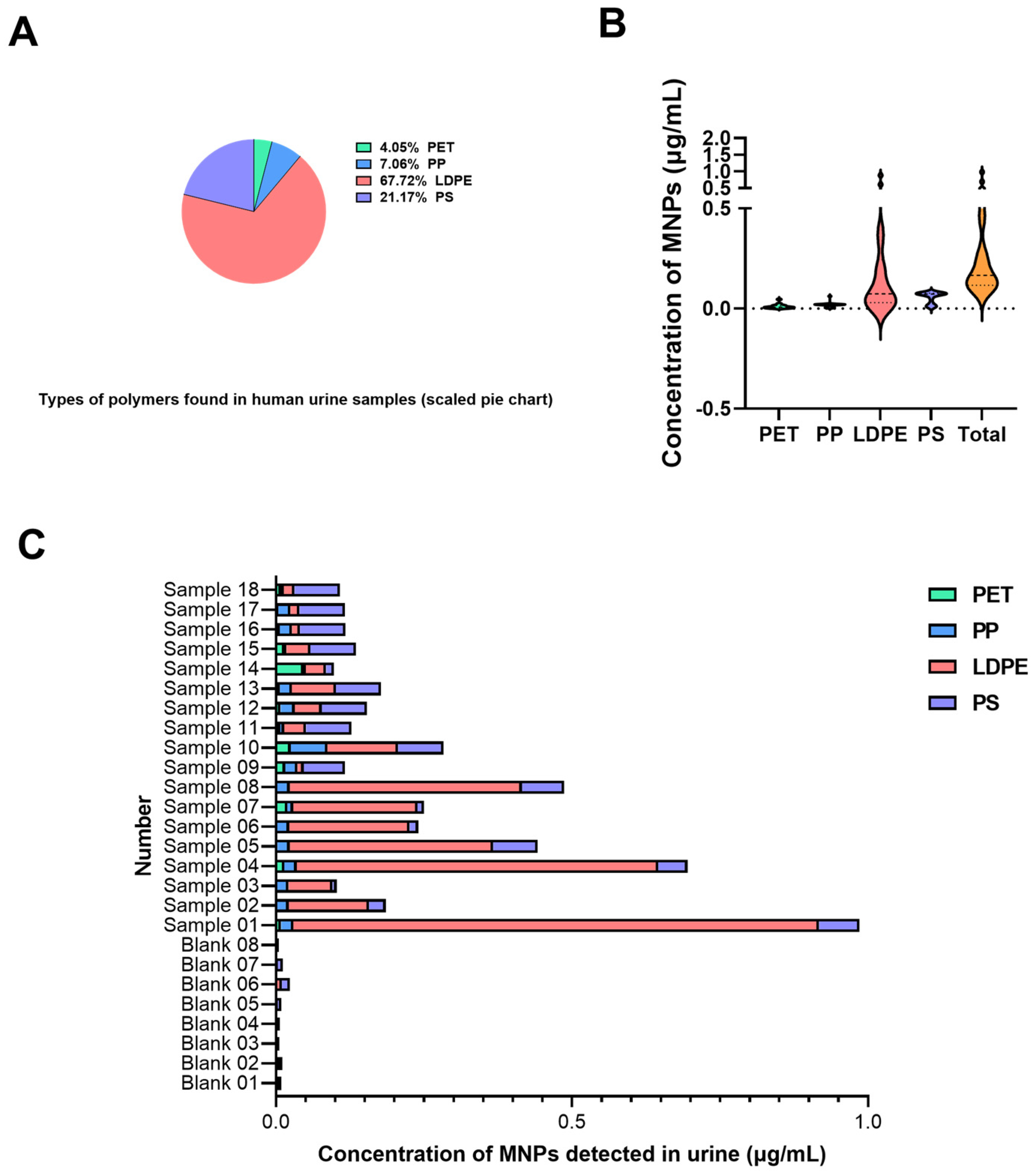
3.2. Measured Abundance of MNPs in Urine Samples
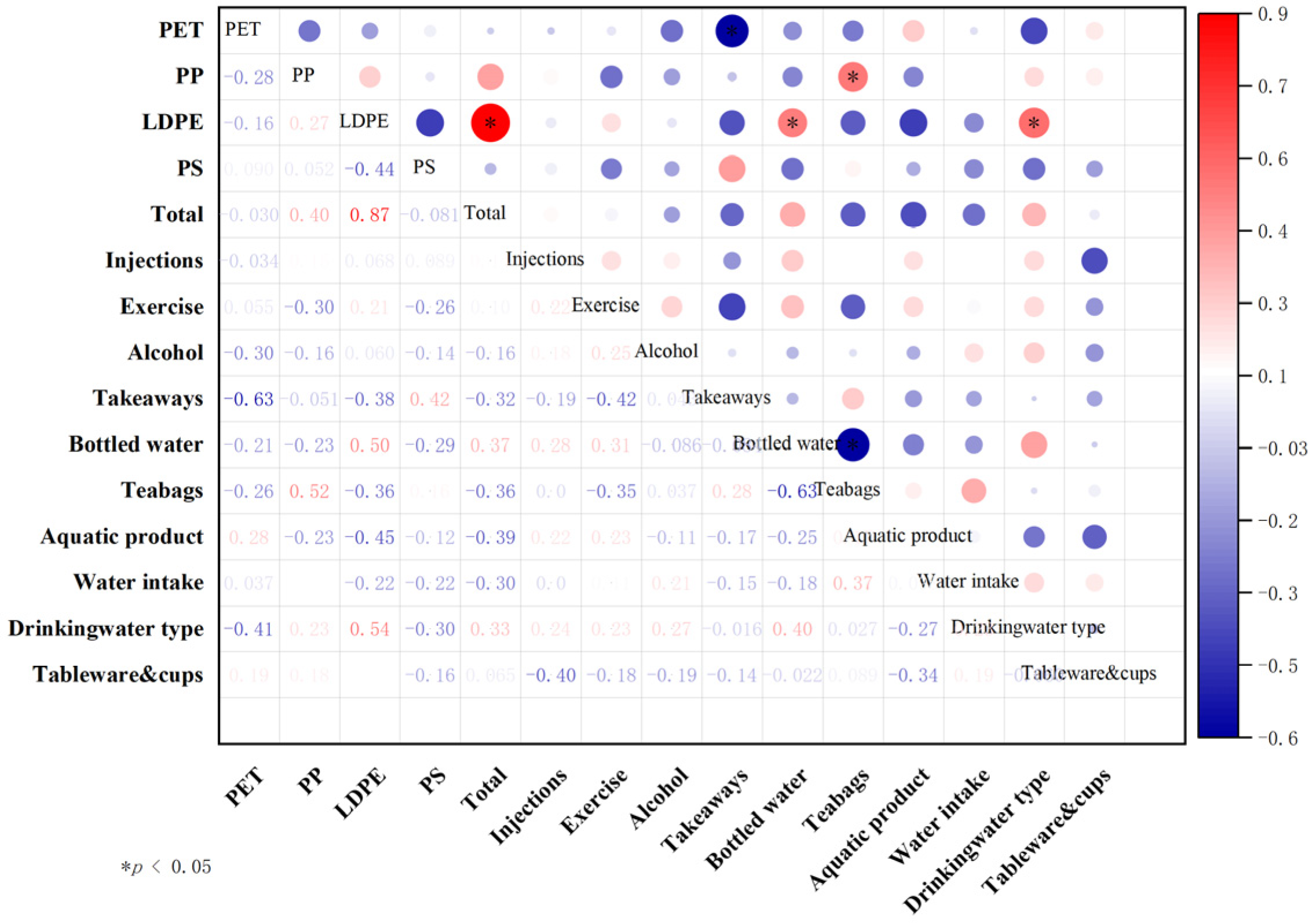
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilcox, C.; Van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 2016, 113, E491. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Kushwaha, M.; Shankar, S.; Goel, D.; Singh, S.; Rahul, J.; Rachna, K.; Singh, J. Microplastics pollution in the marine environment: A review of sources, impacts and mitigation. Mar. Pollut. Bull. 2024, 209, 100327. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.L.; Kang, S.C.; Wang, Z.Q.; Wu, C.X. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 801, 146546. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.P.; Du, F.N.; Cai, H.W.; Wang, G.H.; Shi, H.H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Astorga, D.; Caceres-Farías, L.; Bastidas, L.; Villegas, C.S.; Macay, K.C.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galapagos. Sci. Rep. 2022, 12, 6424. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, H.; Hou, J. Progress on the Effects of Microplastics on Aquatic Crustaceans: A Review. Int. J. Mol. Sci. 2023, 24, 5523. [Google Scholar] [CrossRef]
- da Silva Brito, W.A.; Mutter, F.; Wende, K.; Cecchini, A.L.; Schmidt, A.; Bekeschus, S. Consequences of nano and microplastic exposure in rodent models: The known and unknown. Part. Fibre Toxicol. 2022, 19, 28. [Google Scholar] [CrossRef]
- van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, R.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, A.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Zhang, X.Y.; He, Y.C.; Xie, Z.Y.; Peng, S.H.; Xie, C.G.; Wang, H.T.; Liu, L.; Kang, J.; Yuan, H.P.; Liu, Y. Effect of microplastics on nasal and gut microbiota of high-exposure population: Protocol for an observational cross-sectional study. Medicine 2022, 101, e30215. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Popp, J.; Bronsert, P.; Miernik, A. Raman Spectroscopic Imaging of Human Bladder Resectates towards Intraoperative Cancer Assessment. Cancers 2023, 15, 2162. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Wang, L.; Wang, X.Y.; Li, D.B.; Wang, H.; Xu, H.F.; Liu, Y.; Kang, R.H.; Chen, Q.; Zheng, L.Y.; et al. Discovery and analysis of microplastics in human bone marrow. J. Hazard. Mater. 2024, 477, 135266. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.Q.; Jiang, J.; Huang, Y.; Wang, Q.; Liu, Z.F.; Ma, X.; Yang, X.A.; Li, Y.; Wang, S.Q.; Cui, W.D.; et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mater. 2023, 442, 130138. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, G.; Sun, K.; Ren, J.; Zhou, J.; Liu, X.; Lin, F.; Yang, H.; Cao, J.; Nie, L.; et al. Association of mixed exposure to microplastics with sperm dysfunction: A multi-site study in China. eBioMedicine 2024, 108, 105369. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association Between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2023, 57, 17774–17785. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Malafaia, G.; Barceló, D. Microplastics in human samples: Recent advances, hot-spots, and analytical challenges. TrAC Trends Anal. Chem. 2023, 161, 117016. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Z.X.; Dong, J.; Zhao, S.Y.; Zhu, J.Y.; Wang, W.P.; Ma, F.J.; An, L.H. Unveiling Small-Sized Plastic Particles Hidden behind Large-Sized Ones in Human Excretion and Their Potential Sources. Environ. Sci. Technol. 2024, 58, 11901–11911. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Yang, L.; Huang, Y.; Zhang, N.; Ma, G. Internal and external microplastic exposure in young adults: A pilot study involving 26 college students in Changsha, China. Environ. Res. 2024, 263, 120250. [Google Scholar] [CrossRef]
- Song, X.; Chen, T.; Chen, Z.; Du, L.; Qiu, X.; Zhang, Y.; Li, Y.; Zhu, Y.; Tan, Z.; Mo, Y.; et al. Micro(nano)plastics in human urine: A surprising contrast between Chongqing’s urban and rural regions. Sci. Total Environ. 2024, 917, 170455. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Sun, W.; Jin, C.H.; Bai, Y.L.; Ma, R.X.; Deng, Y.; Gao, Y.; Pan, G.W.; Yang, Z.S.; Yan, L.J. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci. Total Environ. 2022, 848, 157639. [Google Scholar] [CrossRef]
- Exacoustos, O.; Artini, C.; Massardo, S.; Caboni, C.; Pastorino, A.; Chiarenza, S.; Zaza, G.; Stallone, G.; Ghiggeri, G.M.; Angeletti, A.; et al. First Identification and Characterization of Microplastics in Human Kidney and Urine. Nephrol. Dial. Transpl. 2023, 38, I31–I32. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in human urine: Characterisation using μFTIR and sampling challenges using healthy donors and endometriosis participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Jahedi, F.; Haghighi Fard, N.J.; Ahmadi, M.; Takdastan, A.; Shoushtari, M.H.; Dehbandi, R.; Turner, A. Microplastics in urine, sputum and lung lavage fluid from patients with respiratory illnesses. Environ. Res. 2025, 274, 121278. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Shim, W.J. A comparison of spectroscopic analysis methods for microplastics: Manual, semi-automated, and automated Fourier transform infrared and Raman techniques. Mar. Pollut. Bull. 2021, 173, 113101. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.E.; Lynch, J.M. Previous successes and untapped potential of pyrolysis-GC/MS for the analysis of plastic pollution. Anal. Bioanal. Chem. 2023, 415, 2873–2890. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Kontogiannis, A.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microplastics in the environment: Sampling, pretreatment, analysis and occurrence based on current and newly-exploited chromatographic approaches. Sci. Total Environ. 2021, 794, 148725. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis-Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef]
- Lauschke, T.; Dierkes, G.; Schweyen, P.; Ternes, T.A. Evaluation of poly(styrene-d5) and poly(4-fluorostyrene) as internal standards for microplastics quantification by thermoanalytical methods. J. Anal. Appl. Pyrolysis 2021, 159, 105310. [Google Scholar] [CrossRef]
- Rodland, E.S.; Samanipour, S.; Rauert, C.; Okoffo, E.D.; Reid, M.J.; Heier, L.S.; Lind, O.C.; Thomas, K.V.; Meland, S. A novel method for the quantification of tire and polymer-modified bitumen particles in environmental samples by pyrolysis gas chromatography mass spectroscopy. J. Hazard. Mater. 2022, 423, 127092. [Google Scholar] [CrossRef]
- ISO 11843-5:2008; Capability of Detection Part 5: Methodology in the Linear and Non-Linear Calibration Cases. International Organization for Standardization: Geneva, Switzerland, 2008.
- Ambiga, N.; Nagarajan, A. Possibilities of using nano particles in human urine for transient biometrics. Mater. Today Proc. 2023, 80, 2765–2768. [Google Scholar] [CrossRef]
- Saenen, N.D.; Bove, H.; Steuwe, C.; Roeffaers, M.B.J.; Provost, E.B.; Lefebvre, W.; Vanpoucke, C.; Ameloot, M.; Nawrot, T.S. Children’s Urinary Environmental Carbon Load. A Novel Marker Reflecting Residential Ambient Air Pollution Exposure? Am. J. Respir. Crit. Care Med. 2017, 196, 873–881. [Google Scholar] [CrossRef]
- Parra-Sánchez, M.; Marcuello López, A.; García-Rey, S.; Zakariya-Yousef Breval, I.; Bernal Martínez, S.; Pueyo Rodríguez, I.; Martín-Mazuelos, E.; Palomares Folía, J.C. Performance of the HSV OligoGen kit for the diagnosis of herpes simplex virus type 1 and 2. Diagn. Microbiol. Infect. Dis. 2016, 85, 315–317. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Schottstedt, V.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Montag-Lessing, T.; et al. Human Cytomegalovirus (HCMV)—Revised. Transfus. Med. Hemother. 2010, 37, 365–375. [Google Scholar] [CrossRef]
- Laine, R.F.; Albecka, A.; van de Linde, S.; Rees, E.J.; Crump, C.M.; Kaminski, C.F. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat. Commun. 2015, 6, 5980. [Google Scholar] [CrossRef]
- Henry, C.; Hartsock, R.J.; Kirk, Z.; Behrer, R. Detection of Viruria in Cytomegalovirus-infected Infants by Electron Microscopy. Am. J. Clin. Pathol. 1978, 69, 435–439. [Google Scholar] [CrossRef]
- Wyss, P.P.; Lamichhane, S.P.; Abed, A.; Vonwil, D.; Kretz, O.; Huber, T.B.; Sarem, M.; Shastri, V.P. Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials 2020, 230, 119643. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Naumenko, V.; Nikitin, A.; Kapitanova, K.; Melnikov, P.; Vodopyanov, S.; Garanina, A.; Valikhov, M.; Ilyasov, A.; Vishnevskiy, D.; Markov, A.; et al. Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. J. Control. Release 2019, 307, 368–378. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, M.; Zheng, J. Proximal tubules eliminate endocytosed gold nanoparticles through an organelle-extrusion-mediated self-renewal mechanism. Nat. Nanotechnol. 2023, 18, 637–646. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Altenburg, M.K.; Sanford, R.; Willett, J.D.; Bleasdale, B.; Ballou, B.; Wilder, J.; Li, F.; Miner, J.H.; Berg, U.B.; et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc. Natl. Acad. Sci. USA 2017, 114, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Cary, C.M.; DeLoid, G.M.; Yang, Z.; Bitounis, D.; Polunas, M.; Goedken, M.J.; Buckley, B.; Cheatham, B.; Stapleton, P.A.; Demokritou, P. Ingested Polystyrene Nanospheres Translocate to Placenta and Fetal Tissues in Pregnant Rats: Potential Health Implications. Nanomaterials 2023, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, G.E. Microplastics: An emerging threat to food security and human health. J. Food Sci. Technol. 2019, 57, 1601–1608. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Jalal, M.; Vignesh, G.; Ziauddin, M.; Sampath, S.; Bharat, G.K.; Nizzetto, L.; Chakraborty, P. Migration of Plasticizers from Polyethylene Terephthalate and Low-Density Polyethylene Casing into Bottled Water: A Case Study From India. Bull. Environ. Contam. Toxicol. 2022, 109, 949–955. [Google Scholar] [CrossRef]
- Xuejun, Z.; Jin, W.; Hongyan, L.; Huimin, Z.; Jiang, H.; Lei, Z.D. Microplastic pollution of bottled water in China. J. Water Process Eng. 2021, 40, 101884. [Google Scholar] [CrossRef]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef]
- Banaei, G.; Abass, D.; Tavakolpournegari, A.; Martín-Pérez, J.; Gutiérrez, J.; Peng, G.; Reemtsma, T.; Marcos, R.; Hernández, A.; García-Rodríguez, A. Teabag-derived micro/nanoplastics (true-to-life MNPLs) as a surrogate for real-life exposure scenarios. Chemosphere 2024, 368, 143736. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wick, P.; Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 2021, 16, 491–500. [Google Scholar] [CrossRef]
- Li, P.; Liu, J. Micro(nano)plastics in the Human Body: Sources, Occurrences, Fates, and Health Risks. Environ. Sci. Technol. 2024, 58, 3065–3078. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
| Polymers | Low-Dose Spiked (n = 3) | High-Dose Spiked (n = 3) | ||
|---|---|---|---|---|
| Recovery, % | RSD, % | Recovery, % | RSD, % | |
| PET | 117.64 ± 6.64 | 5.64 | 85.95 ± 13.43 | 15.62 |
| PP | 59.84 ± 3.09 | 5.16 | 56.73 ± 17.64 | 31.09 |
| LDPE | 96.16 ± 11.38 | 11.83 | 78.81± 0.68 | 0.86 |
| PS | 63.24 ± 9.91 | 15.67 | 69.51 ± 7.89 | 11.35 |
| Detection (μg) | PET | PP | LDPE | PS |
|---|---|---|---|---|
| Blank 1 | n.d. | 0.015 | 0.051 | 0.024 |
| Blank 2 | n.d. | n.d. | 0.065 | 0.047 |
| Blank 3 | n.d. | n.d. | 0.039 | 0.023 |
| Blank 4 | n.d. | 0.019 | n.d. | 0.047 |
| Blank 5 | n.d. | n.d. | 0.037 | 0.055 |
| Blank 6 | n.d. | 0.025 | 0.079 | 0.131 |
| Blank 7 | n.d. | 0.016 | 0.016 | 0.086 |
| Blank 8 | n.d. | 0.015 | n.d. | 0.039 |
| Mean | N/A | 0.011 | 0.036 | 0.057 |
| SD | N/A | 0.010 | 0.029 | 0.036 |
| Polymers | PET | PP | LDPE | PS |
|---|---|---|---|---|
| IDL | 0.66 | 0.10 | 6.94 | 0.52 |
| IQL | 2.19 | 0.32 | 23.13 | 1.73 |
| LOD | 0.66 | 29.23 | 87.36 | 108.49 |
| LOQ | 2.19 | 96.47 | 288.29 | 358.03 |
| Polymers | Bottled Water Consumption Frequency | Drinking Water Type | ||
|---|---|---|---|---|
| r | p | r | p | |
| LDPE | 0.382 | 0.144 | 0.533 | 0.033 |
| Polymers | Teabag usage frequency | |||
| r | p | |||
| PP | 0.307 | 0.248 | ||
| Study | Sample Pretreatment Method | Detection Method | Types of Polymers Detected | Detection Frequency % | Concentration |
|---|---|---|---|---|---|
| Zhu et al. [20] | Membrane filtration | TD-GC/MS | PVC PE PS PP PET Total | 100.0 | 6.49 ± 4.56 a |
| Song et al. [21] | Extraction with various organic solvents (chloroform, hexafluoroisopropanol, xylene) | Py-GC/MS | PS PE PP PMMA PVC PET PA66 Total | 92.3 88.5 57.7 3.8 96.2 42.3 26.9 100.0 | 0.10 (0.09–0.15) b 1.18 (1.01–1.52) 0.09 (0.00–0.14) 0.00 (0.00–0.00) 0.80 (0.65–1.34) 0.00 (0.00–4.80) 0.00 (0.00–1.67) 5.06 (2.21–8.61) |
| Song et al. [22] | Extraction with various organic solvents (chloroform, hexafluoroisopropanol, xylene) | Py-GC/MS | PE PVC PA66 Total | 75.0 58.3 16.7 75.0 | 1.44 (0.38–2.52) b 0.03 (0.00–0.15) 0.00 (0.00–0.00) 1.51 (0.40–2.71) |
| This Study | Membrane filtration | Py-GC/MS | LDPE PS PET PP Total | 100.0 55.6 100.0 44.4 100.0 | 0.268 ± 0.235 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Wang, W.; Wang, Y.; Bai, H.; Li, Z.; Huo, Z.; Luo, K. Quantitative Detection of Micro- and Nanoplastics (≥300 nm) in Human Urine Using Double-Shot Py-GC/MS with Internal Standard Calibration. Toxics 2025, 13, 452. https://doi.org/10.3390/toxics13060452
Ji S, Wang W, Wang Y, Bai H, Li Z, Huo Z, Luo K. Quantitative Detection of Micro- and Nanoplastics (≥300 nm) in Human Urine Using Double-Shot Py-GC/MS with Internal Standard Calibration. Toxics. 2025; 13(6):452. https://doi.org/10.3390/toxics13060452
Chicago/Turabian StyleJi, Shanshan, Wei Wang, Yong Wang, Hexiang Bai, Zhuo Li, Zongli Huo, and Kai Luo. 2025. "Quantitative Detection of Micro- and Nanoplastics (≥300 nm) in Human Urine Using Double-Shot Py-GC/MS with Internal Standard Calibration" Toxics 13, no. 6: 452. https://doi.org/10.3390/toxics13060452
APA StyleJi, S., Wang, W., Wang, Y., Bai, H., Li, Z., Huo, Z., & Luo, K. (2025). Quantitative Detection of Micro- and Nanoplastics (≥300 nm) in Human Urine Using Double-Shot Py-GC/MS with Internal Standard Calibration. Toxics, 13(6), 452. https://doi.org/10.3390/toxics13060452








