Ozone Aggravated the Toxicity of Fine Particulate Matter by Impairing Membrane Stability and Facilitating Particle Internalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Size Distribution and Colloidal Stability of PM2.5 Particles
2.3. Environmental Persistent Free Radicals (EPFRs) Detection
2.4. Cell Treatment and Exposure
2.5. Cytotoxicity Testing
2.6. Western Blotting
2.7. Membrane Potential Assay
2.8. Membrane Rupture Assay
2.9. Ca2+ Flux Assay
2.10. Observation of the Morphology of PM2.5 and the Localization of PM2.5 in Cells by TEM
2.11. Animal Experimentation
2.12. Histopathology Detection
2.13. Determination of Inflammatory Cytokines
2.14. Statistical Analysis
3. Results and Discussion
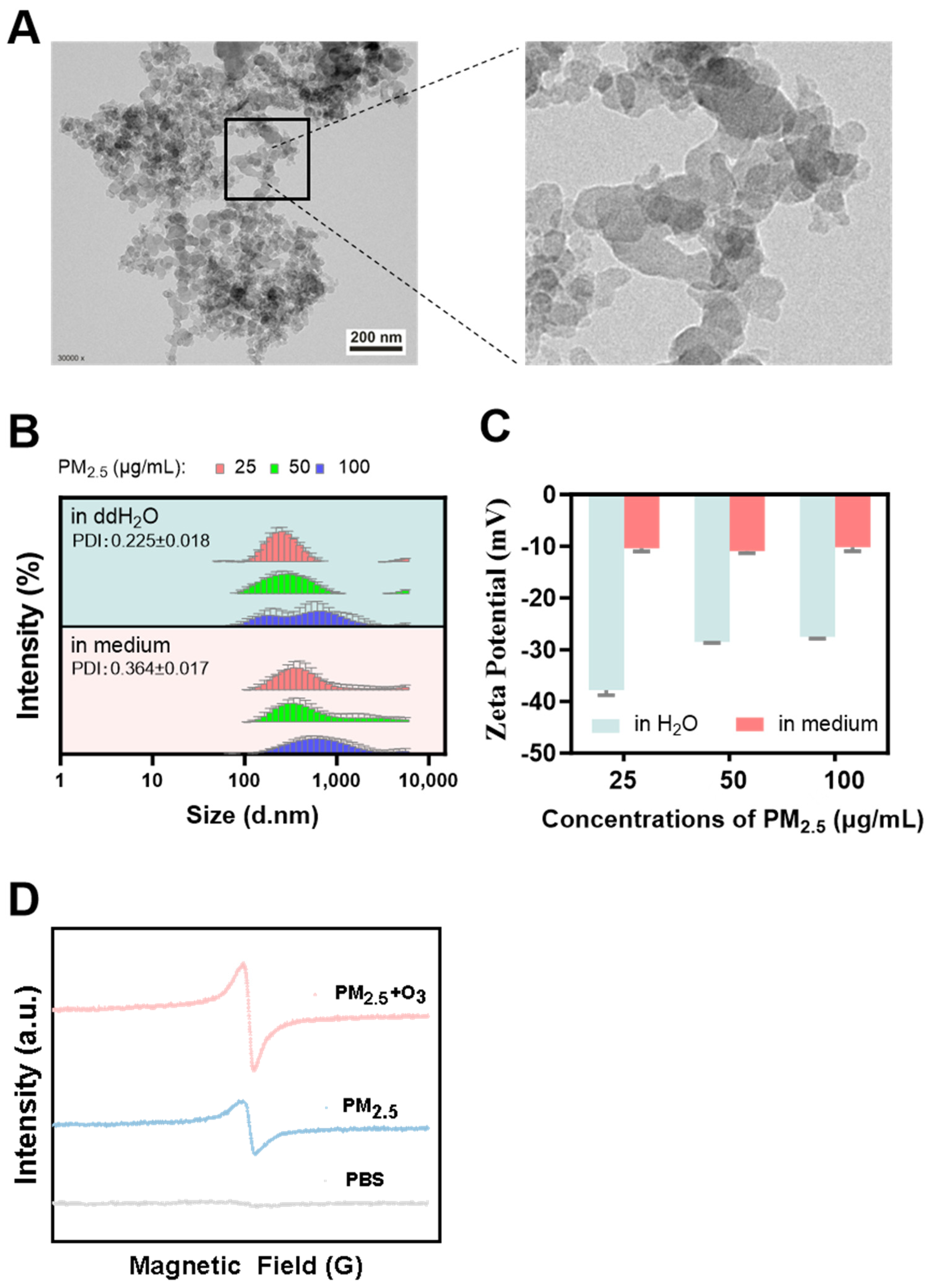
3.1. Characterization of PM2.5
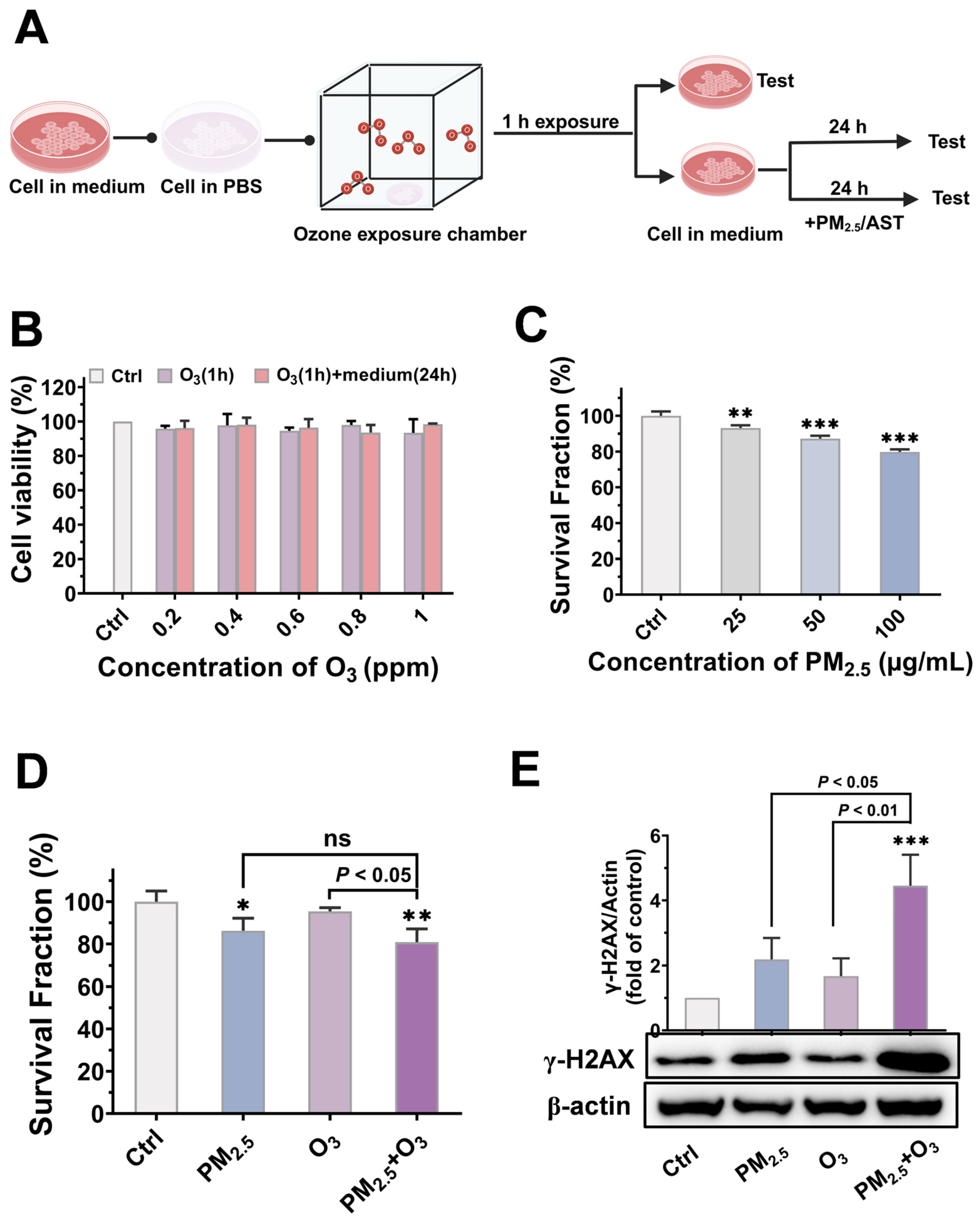
3.2. Cytotoxicity and Genotoxicity Induced by PM2.5 and O3
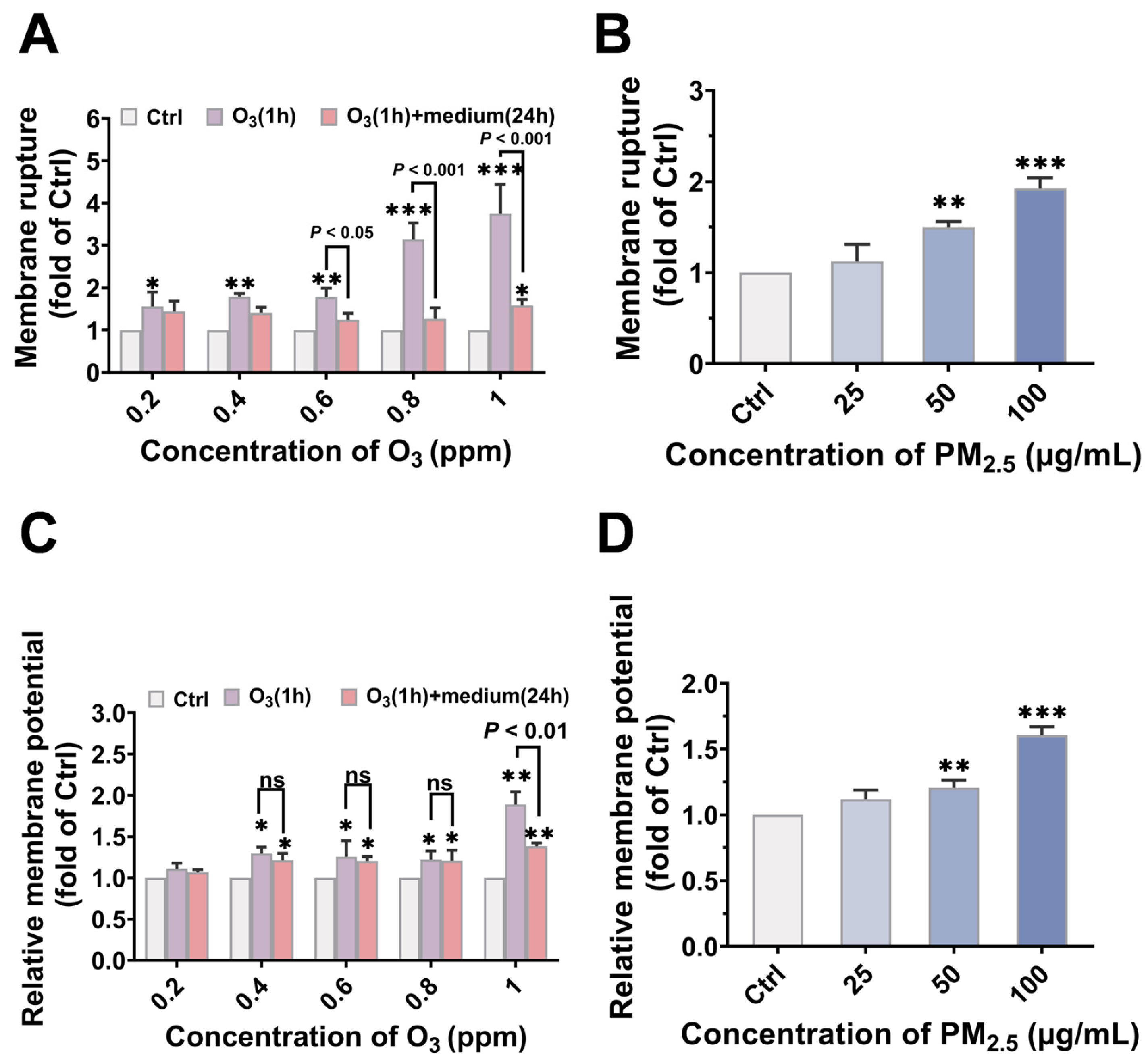
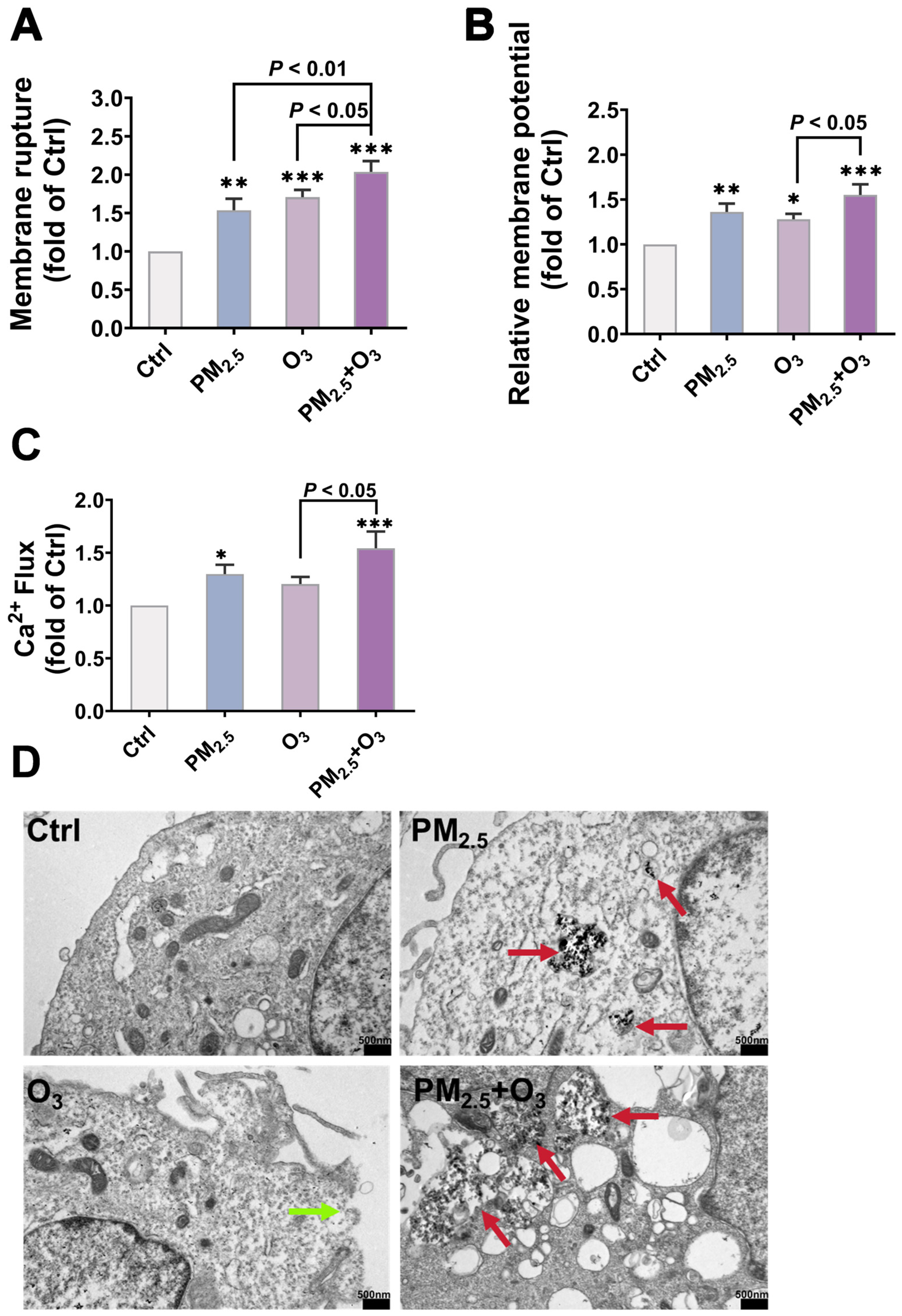
3.3. O3 Pre-Treatment Enhanced the Toxicity of PM2.5 via Destroying the Plasma Membrane Integrity and Facilitating the Bioaccumulation of PM2.5
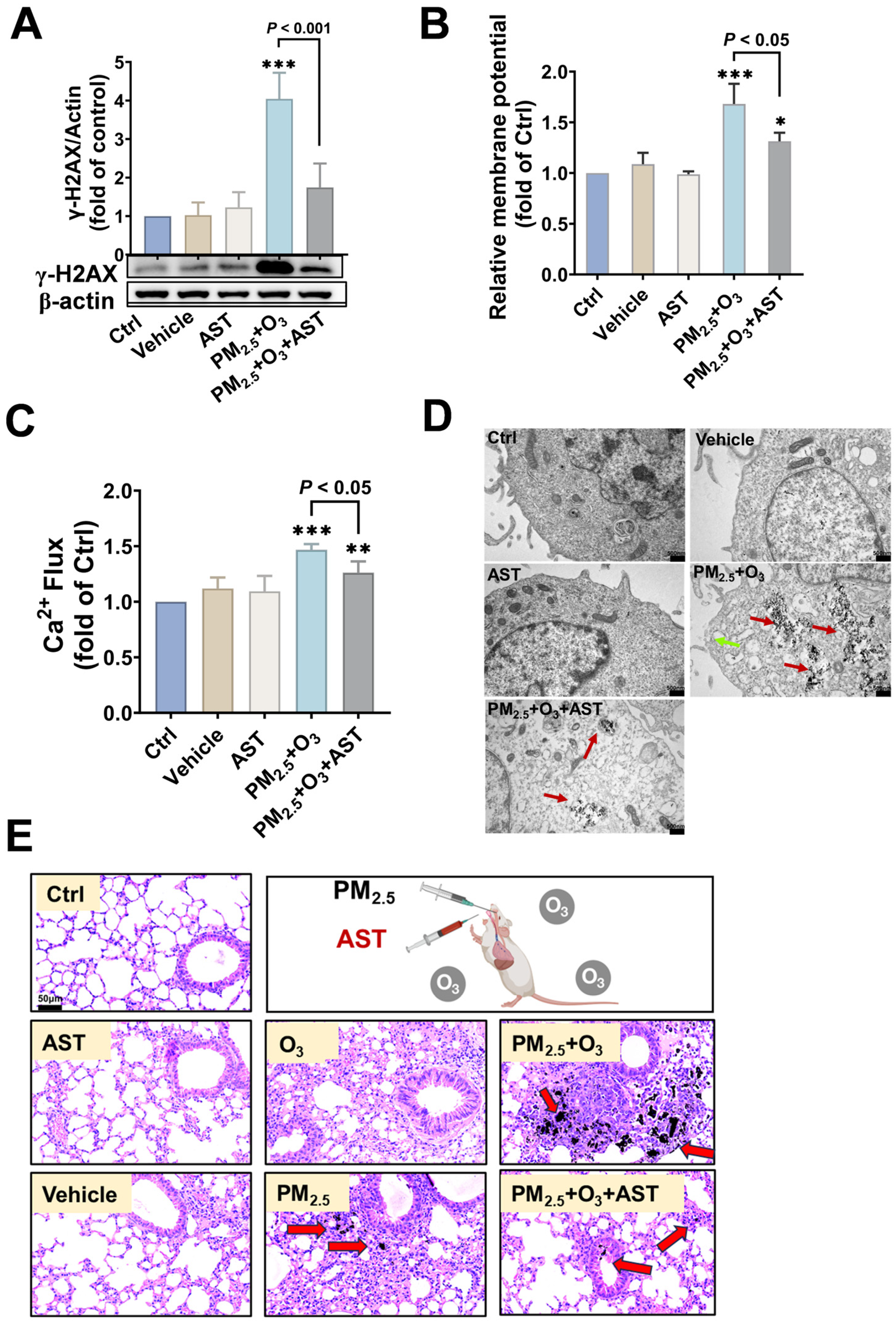
3.4. AST Mitigated the Adverse Effects Caused by PM2.5 and O3 Co-Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ambient (Outdoor) Air Pollution; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 16 November 2024).
- Santiago, J.V.; Hata, H.; Martinez-Noriega, E.J.; Inoue, K. Ozone trends and their sensitivity in global megacities under the warming climate. Nat. Commun. 2024, 15, 10236. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Geng, G.; Xue, T.; Liu, S.; Cai, C.; He, K.; Zhang, Q. Tracking PM2.5 and O3 Pollution and the Related Health Burden in China 2013–2020. Environ. Sci. Technol. 2022, 56, 6922–6932. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Song, M.; Li, X.; Liu, Y.; Lu, S.; Zeng, L.; Zhang, Y. Analysis of China’s PM2.5 and ozone coordinated control strategy based on the observation data from 2015 to 2020. J. Environ. Sci. 2024, 138, 385–394. [Google Scholar] [CrossRef]
- Wang, B.; Sun, M.; Si, L.; Niu, Z. Spatio-temporal variation of O3 concentration and exposure risk assessment in key regions of China, 2015–2021. Atmos. Pollut. Res. 2024, 15, 101941. [Google Scholar] [CrossRef]
- He, C.; Liu, J.; Zhou, Y.; Zhou, J.; Zhang, L.; Wang, Y.; Liu, L.; Peng, S. Synergistic PM2.5 and O3 control to address the emerging global PM2.5-O3 compound pollution challenges. Eco-Environ. Health 2024, 3, 325–337. [Google Scholar] [CrossRef]
- Holm, S.M.; Balmes, J.R. Systematic Review of Ozone Effects on Human Lung Function, 2013 Through 2020. Chest 2022, 161, 190–201. [Google Scholar] [CrossRef]
- He, C.; Liu, J.; Zhou, Y.; Zhou, J.; Zhang, L.; Wang, Y.; Liu, L.; Peng, S. PM2.5 promotes lung cancer progression through activation of the AhR-TMPRSS2-IL18 pathway. EMBO Mol. Med. 2023, 15, e17014. [Google Scholar]
- Tu, H.; Hu, Y.; Hu, K.; Dong, P.; Wen, Y.; Jiang, J.; Xu, X.; Huang, J.; Zhu, J.; He, C.; et al. Assessment of the Respiratory Disease Mortality Risk from Single and Composite Exposures to PM2.5 and Ozone—Guangzhou City, Guangdong Province, China, 2018–2021. China CDC Wkly. 2024, 6, 857–861. [Google Scholar] [CrossRef]
- Xu, C.; Yin, P.; Jiang, Y.; Lin, X.; Shi, S.; Li, X.; Chen, J.; Jiang, Y.; Meng, X.; Zhou, M. Interactive effects of ambient fine particulate matter and ozone on daily mortality in 372 cities: Two stage time series analysis. BMJ-Brit. Med. J. 2023, 383, e075203. [Google Scholar]
- Xu, C.; Yin, P.; Jiang, Y.; Lin, X.; Shi, S.; Li, X.; Chen, J.; Jiang, Y.; Meng, X.; Zhou, M. Joint Effect of Short-Term Exposure to Fine Particulate Matter and Ozone on Mortality: A Time Series Study in 272 Chinese Cities. Environ. Sci. Technol. 2024, 58, 12865–12874. [Google Scholar] [CrossRef]
- Farraj, A.K.; Walsh, L.; Haykal-Coates, N.; Malik, F.; McGee, J.; Winsett, D.; Duvall, R.; Kovalcik, K.; Cascio, W.E.; Higuchi, M.; et al. Cardiac effects of seasonal ambient particulate matter and ozone co-exposure in rats. Part. Fibre Toxicol. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Han, Y.; Qiu, X.; Wang, Y.; Liu, J.; Cheng, Z.; Tian, Y.; Xu, Y.; Chen, X.; Fan, Y.; et al. Ozone Enhances the Inflammation Effects of PM2.5 Components Based on an Exposomic Approach in a Panel Study. Environ. Sci. Technol. Lett. 2024, 11, 818–824. [Google Scholar] [CrossRef]
- Wong, E.M.; Walby, W.F.; Wilson, D.W.; Tablin, F.; Schelegle, E.S. Ultrafine Particulate Matter Combined With Ozone Exacerbates Lung Injury in Mature Adult Rats With Cardiovascular Disease. Toxicol. Sci. 2018, 163, 140–151. [Google Scholar] [CrossRef]
- An, J.; Zhou, Q.; Wu, M.; Wang, L.; Zhong, Y.; Feng, J.; Shang, Y.; Chen, Y. Interactions between oxidative stress, autophagy and apoptosis in A549 cells treated with aged black carbon. Toxicol. Vitro 2019, 54, 67–74. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Zhao, S.; Zeng, Y.; Wu, Q.; Zhang, L.; Shi, S.; Zhang, F.; Li, J.; An, Z.; et al. Airway microbiota dysbiosis and metabolic disorder in ozone and PM2.5 co-exposure induced lung inflammatory injury in mice. Ecotoxicol. Environ. Saf. 2025, 290, 117626. [Google Scholar] [CrossRef]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Toth, A.; Kannan, P.; Snowball, J.; Kofron, M.; Wayman, J.A.; Bridges, J.P.; Miraldi, E.R.; Swarr, D.; Zacharias, W.J. Alveolar epithelial progenitor cells require Nkx2-1 to maintain progenitor-specific epigenomic state during lung homeostasis and regeneration. Nat. Commun. 2023, 14, 8452. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302. [Google Scholar] [CrossRef]
- Juhl, P.; Breisnes, H.W.; Karsdal, M.A.; Sand, J.M.B. The basement membrane and its role in pulmonary disease. In Biochemistry of Collagens, Laminins and Elastin, 3rd ed.; Karsdal, M.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2024; pp. 473–482. [Google Scholar]
- Chen, C.; Arjomandi, M.; Balmes, J.; Tager, I.; Holland, N. Effects of Chronic and Acute Ozone Exposure on Lipid Peroxidation and Antioxidant Capacity in Healthy Young Adults. Environ. Health Perspect. 2007, 115, 1732–1737. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Chánová, E.; Syková, E.; Dejneka, A.; Kubinová, Š. Cell death induced by ozone and various non-thermal plasmas: Therapeutic perspectives and limitations. Sci. Rep. 2014, 4, 7129. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Diksha; Kumari, A.; Panwar, A. A super antioxidant from microalgae and its therapeutic potential. J. Basic. Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Du, H.-H.; Liang, R.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Astaxanthin Protecting Membrane Integrity against Photosensitized Oxidation through Synergism with Other Carotenoids. J. Agric. Food Chem. 2015, 63, 9124–9130. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kim, R.-E.; Shin, C.Y.; Han, S.-H.; Kwon, K.J. Astaxanthin Suppresses PM2.5-Induced Neuroinflammation by Regulating Akt Phosphorylation in BV-2 Microglial Cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef]
- Yin, B.; Ren, J.; Cui, Q.; Liu, X.; Wang, Z.; Pei, H.; Zuo, J.; Zhang, Y.; Wen, R.; Sun, X.; et al. Astaxanthin alleviates fine particulate matter (PM2.5)-induced lung injury in rats by suppressing ferroptosis and apoptosis. Food Funct. 2023, 14, 10841–10854. [Google Scholar] [CrossRef]
- Çelik, D.A.; Toğay, V.A. In vivo protective efficacy of astaxanthin against ionizing radiation-induced DNA damage. Chem. Biol. Drug Des. 2023, 102, 882–888. [Google Scholar]
- Dai, S.; Wang, B.; Song, Y.; Xie, Z.; Li, C.; Li, S.; Huang, Y.; Jiang, M. Astaxanthin and its gold nanoparticles mitigate cadmium toxicity in rice by inhibiting cadmium translocation and uptake. Sci. Total Environ. 2021, 786, 147496. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.; Xu, X.; Zhuang, H.; Shen, W. Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 2008, 109, 264–268. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, X.; Yu, M.; Yuan, Y.; Li, B. PM2.5 reduces the daytime/nighttime urban heat island intensity over mainland China. Sustain. Cities Soc. 2025, 118, 106001. [Google Scholar] [CrossRef]
- Deng, J.; Qiu, S.; Zhang, Y.; Cui, H.; Li, K.; Cheng, H.; Liu, Z.; Dou, X.; Qian, Y. Estimating Nighttime PM2.5 Concentration in Beijing Based on NPP/VIIRS Day/Night Band. Remote Sens. 2023, 15, 349. [Google Scholar] [CrossRef]
- Ding, Y.; Li, S.; Xing, J.; Li, X.; Ma, X.; Song, G.; Teng, M.; Yang, J.; Dong, J.; Meng, S. Retrieving hourly seamless PM2.5 concentration across China with physically informed spatiotemporal connection. Remote Sens. Environ. 2024, 301, 113901. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Ji, Y.; Chen, T.; Li, H.; Gao, R.; Xue, L.; Wang, Y.; Zhao, Y.; Yang, X. Comparison of the ozone formation mechanisms and VOCs apportionment in different ozone pollution episodes in urban Beijing in 2019 and 2020: Insights for ozone pollution control strategies. Sci. Total Environ. 2024, 908, 168332. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Bao, G.; Li, S.; Yang, Z.; Cheng, C.; Le, W. PM2.5 exposure triggers cell death through lysosomal membrane permeabilization and leads to ferroptosis insensitivity via the autophagy dysfunction/p62-KEAP1-NRF2 activation in neuronal cells. Ecotox. Environ. Saf. 2022, 248, 114333. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, P.; Wu, X.; Gong, Z.; Yang, X.; Zhu, H.; Zhang, J.; Hu, Y.; Li, G.; Sang, N.; et al. Lung injuries induced by ozone exposure in female mice: Potential roles of the gut and lung microbes. Environ. Int. 2024, 183, 108422. [Google Scholar] [CrossRef]
- Hatch, G.E.; Slade, R.; Harris, L.P.; McDonnell, W.F.; Devlin, R.B.; Koren, H.S.; Costa, D.L.; McKee, J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Resp. Crit Care 1994, 150, 676–683. [Google Scholar] [CrossRef]
- Greve, H.J.; Dunbar, A.L.; Lombo, C.G.; Ahmed, C.; Thang, M.; Messenger, E.J.; Mumaw, C.L.; Johnson, J.A.; Kodavanti, U.P.; Oblak, A.L.; et al. The bidirectional lung brain-axis of amyloid-β pathology: Ozone dysregulates the peri-plaque microenvironment. Brain 2023, 146, 991–1005. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Wang, T.; Li, H.; He, J.; Xu, A. Role of iron homeostasis in the mutagenicity of disinfection by-products in mammalian cells. Ecotox. Environ. Saf. 2024, 285, 117122. [Google Scholar] [CrossRef]
- Ji, Z.; Dai, R.; Zhang, Z. Characterization of fine particulate matter in ambient air by combining TEM and multiple spectroscopic techniques--NMR, FTIR and Raman spectroscopy. Environ. Sci. Process Impacts 2015, 17, 552–560. [Google Scholar] [CrossRef]
- Liu, Q.; Weng, J.; Li, C.; Feng, Y.; Xie, M.; Wang, X.; Chang, Q.; Li, M.; Chung, K.F.; Adcock, I.M.; et al. Attenuation of PM2.5-induced alveolar epithelial cells and lung injury through regulation of mitochondrial fission and fusion. Part. Fibre Toxicol. 2023, 20, 28. [Google Scholar] [CrossRef]
- GB 14925-2023; Laboratory Animals—Environment and Housing Facilitie. Standards Press of China: Beijing, China, 2023.
- Slaoui, M.; Fiette, L. Histopathology Procedures: From Tissue Sampling to Histopathological Evaluation. In Drug Safety Evaluation. Methods in Molecular Biology; Gautier, J.C., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 691. [Google Scholar]
- Wong, P.K.; Ghadikolaei, M.A.; Chen, S.H.; Fadairo, A.A.; Ng, K.W.; Lee, S.M.; Xu, J.C.; Lian, Z.D.; Li, L.; Wong, H.C.; et al. Physical, chemical, and cell toxicity properties of mature/aged particulate matter (PM) trapped in a diesel particulate filter (DPF) along with the results from freshly produced PM of a diesel engine. J. Hazard. Mater. 2022, 434, 128855. [Google Scholar] [CrossRef]
- Wong, P.K.; Chen, S.H.; Ghadikolaei, M.A.; Ng, K.W.; Lee, S.M.; Xu, J.C.; Lian, Z.D.; Li, L.; Li, S.; Ning, Z.; et al. Chemical and cell toxicity properties of particulate matter emitted from a diesel engine fueled with biodiesel and ethanol blends. Environ. Pollut. 2025, 379, 126484. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Loya, J.; Lerma, M.; Gardea-Torresdey, J.L. Dynamic Light Scattering and Its Application to Control Nanoparticle Aggregation in Colloidal Systems: A Review. Micromachines 2024, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Hu, C.; Wang, K.; Chang, Q.; Liu, L.; Han, Z.; Shao, Y.; Zhai, Y.; Zuo, Z.; et al. Protein corona of airborne nanoscale PM2.5 induces aberrant proliferation of human lung fibroblasts based on a 3D organotypic culture. Sci. Rep. 2018, 8, 1939. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally persistent free radicals: Occurrence, formation mechanisms and implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Z.; Zhang, P.; Wan, M.; Lei, J.; Pan, B.; Xing, B. Environmental persistent free radicals in diesel engine exhaust particles at different altitudes and engine speeds. Sci. Total Environ. 2021, 796, 148963. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Wei, Y.; Fang, G.; Jiang, Q.; Pang, Y.; Huang, W.; Tang, M.; Jing, Y.; Feng, X.; et al. Airborne environmentally persistent free radicals (EPFRs) in PM2.5 from combustion sources: Abundance, cytotoxicity and potential exposure risks. Sci. Total Environ. 2024, 927, 172202. [Google Scholar] [CrossRef]
- Borrowman, C.K.; Zhou, S.; Burrow, T.E.; Abbatt, J.P.D. Formation of environmentally persistent free radicals from the heterogeneous reaction of ozone and polycyclic aromatic compounds. Phys. Chem. Chem. Phys. 2016, 18, 205–212. [Google Scholar] [CrossRef]
- Singh, S.A.; Suresh, S.; Vellapandian, C. Ozone-induced neurotoxicity: In vitro and in vivo evidence. Ageing Res. Rev. 2023, 91, 102045. [Google Scholar] [CrossRef]
- Stewart, E.J.; Dye, J.A.; Schladweiler, M.C.; Phillips, P.M.; McDaniel, K.L.; Richards, J.H.; Grindstaff, R.D.; Padgett, W.T. Prenatal ozone exposure programs a sexually dimorphic susceptibility to high-fat diet in adolescent Long Evans rats. FASEB J. 2022, 36, e22664. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.; Sun, E.G.; Fedkenheuer, M.; Fu, H.; Jung, S.; Thakur, B.L.; Redon, C.E.; Pegoraro, G.; Tran, A.D.; Gross, J.M.; et al. Mechanism for local attenuation of DNA replication at double-strand breaks. Nature 2025, 639, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Diesel and Gasoline Engine Exhausts and Some Nitroarenes; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Cataldo, F. DNA degradation with ozone. Int. J. Biol. Macromol. 2006, 38, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wu, Q.P.; Zhang, J.M.; Yang, X.H. Effects of ozone on membrane permeability and ultrastructure in Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 111, 1006–1015. [Google Scholar] [CrossRef]
- Wang, Y. Ambient fine particulate matter provokes multiple modalities of cell death via perturbation of subcellular structures. Environ. Int. 2025, 195, 109193. [Google Scholar] [CrossRef]
- Tang, S.K.Y.; Marshall, W.F. Self-Repairing Cells. Science 2017, 356, 1022–1025. [Google Scholar] [CrossRef]
- Jairaman, A.; Prakriya, M. Calcium Signaling in Airway Epithelial Cells: Current Understanding and Implications for Inflammatory Airway Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 772–783. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef]
- Seriani, R.; Junqueira, M.S.; Carvalho-Sousa, C.E.; Arruda, A.C.; Martinez, D.; Alencar, A.M.; Garippo, A.L.; Brito, J.M.; Martins, M.A.; Saldiva, P.H.; et al. Enriched inorganic compounds in diesel exhaust particles induce mitogen-activated protein kinase activation, cytoskeleton instability, and cytotoxicity in human bronchial epithelial cells. Exp. Toxicol. Pathol. 2015, 67, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, C.H.; Li, F.; Ryffel, B.; Togbe, D.; Chung, K.F. Oxidative Stress in Ozone-Induced Chronic Lung Inflammation and Emphysema: A Facet of Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 1957. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, R.; Zhao, Z.; Song, W. Effects of ozone and fine particulate matter (PM2.5) on rat system inflammation and cardiac function. Toxicol. Lett. 2013, 217, 23–33. [Google Scholar] [CrossRef]
- Yan, Z.; Jin, Y.; An, Z.; Liu, Y.; Samet, J.M.; Wu, W. Inflammatory cell signaling following exposures to particulate matter and ozone. Biochim. Biophys. Acta 2016, 1860, 2826–2834. [Google Scholar] [CrossRef]
- He, L.; Weschler, C.J.; Morrison, G.; Li, F.; Zhang, Y.; Bergin, M.H.; Black, M.; Zhang, J.J. Synergistic Effects of Ozone Reaction Products and Fine Particulate Matter on Respiratory Pathophysiology in Children with Asthma. ACS EST Air 2024, 1, 918–926. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Zhou, Y.; Liu, Q.; Zhang, Q.; Sun, M.; Sun, M.; Li, H.; Xu, A.; Liu, Y. Astaxanthin protected against the adverse effects induced by diesel exhaust particulate matter via improving membrane stability and anti-oxidative property. J. Hazard. Mater. 2023, 456, 131684. [Google Scholar] [CrossRef]
- Rackley, C.R.; Stripp, B.R. Building and maintaining the epithelium of the lung. J. Clin. Investig. 2012, 122, 2724–2730. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wei, S.; Xin, T.; Huang, C.; Pu, Y.; Ma, J.; Zhang, C.; Liu, Y.; Lynch, I.; Liu, S. Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc. Natl. Acad. Sci. USA 2022, 119, e2117083119. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, J.; Jiang, R.; Song, W. Rat lung response to ozone and fine particulate matter (PM2.5) exposures. Environ. Toxicol. 2015, 30, 343–356. [Google Scholar] [CrossRef]
- Wu, Y.; Bashir, M.A.; Shao, C.; Wang, H.; Zhu, J.; Huang, Q. Astaxanthin targets IL-6 and alleviates the LPS-induced adverse inflammatory response of macrophages. Food Funct. 2024, 15, 4207–4222. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, T.; Li, H.; Zhou, Y.; Liu, Y.; Xu, A. Ozone Aggravated the Toxicity of Fine Particulate Matter by Impairing Membrane Stability and Facilitating Particle Internalization. Toxics 2025, 13, 446. https://doi.org/10.3390/toxics13060446
He J, Wang T, Li H, Zhou Y, Liu Y, Xu A. Ozone Aggravated the Toxicity of Fine Particulate Matter by Impairing Membrane Stability and Facilitating Particle Internalization. Toxics. 2025; 13(6):446. https://doi.org/10.3390/toxics13060446
Chicago/Turabian StyleHe, Jing, Tong Wang, Han Li, Yemian Zhou, Yun Liu, and An Xu. 2025. "Ozone Aggravated the Toxicity of Fine Particulate Matter by Impairing Membrane Stability and Facilitating Particle Internalization" Toxics 13, no. 6: 446. https://doi.org/10.3390/toxics13060446
APA StyleHe, J., Wang, T., Li, H., Zhou, Y., Liu, Y., & Xu, A. (2025). Ozone Aggravated the Toxicity of Fine Particulate Matter by Impairing Membrane Stability and Facilitating Particle Internalization. Toxics, 13(6), 446. https://doi.org/10.3390/toxics13060446






