Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer
Abstract
1. Introduction

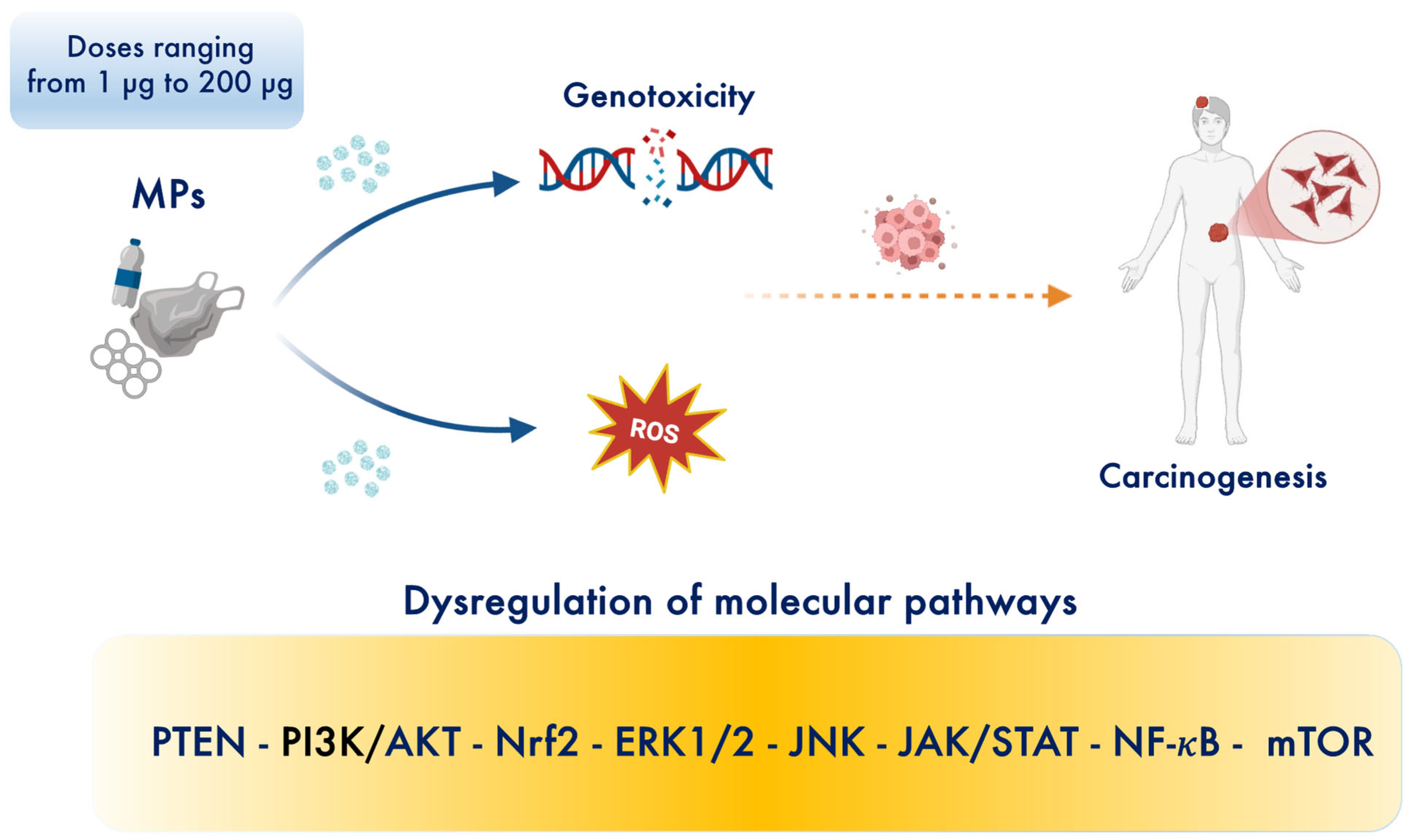
2. Microplastics as Emerging Contaminants for Cancer Risk in Gastric–Colon–Brain Axis
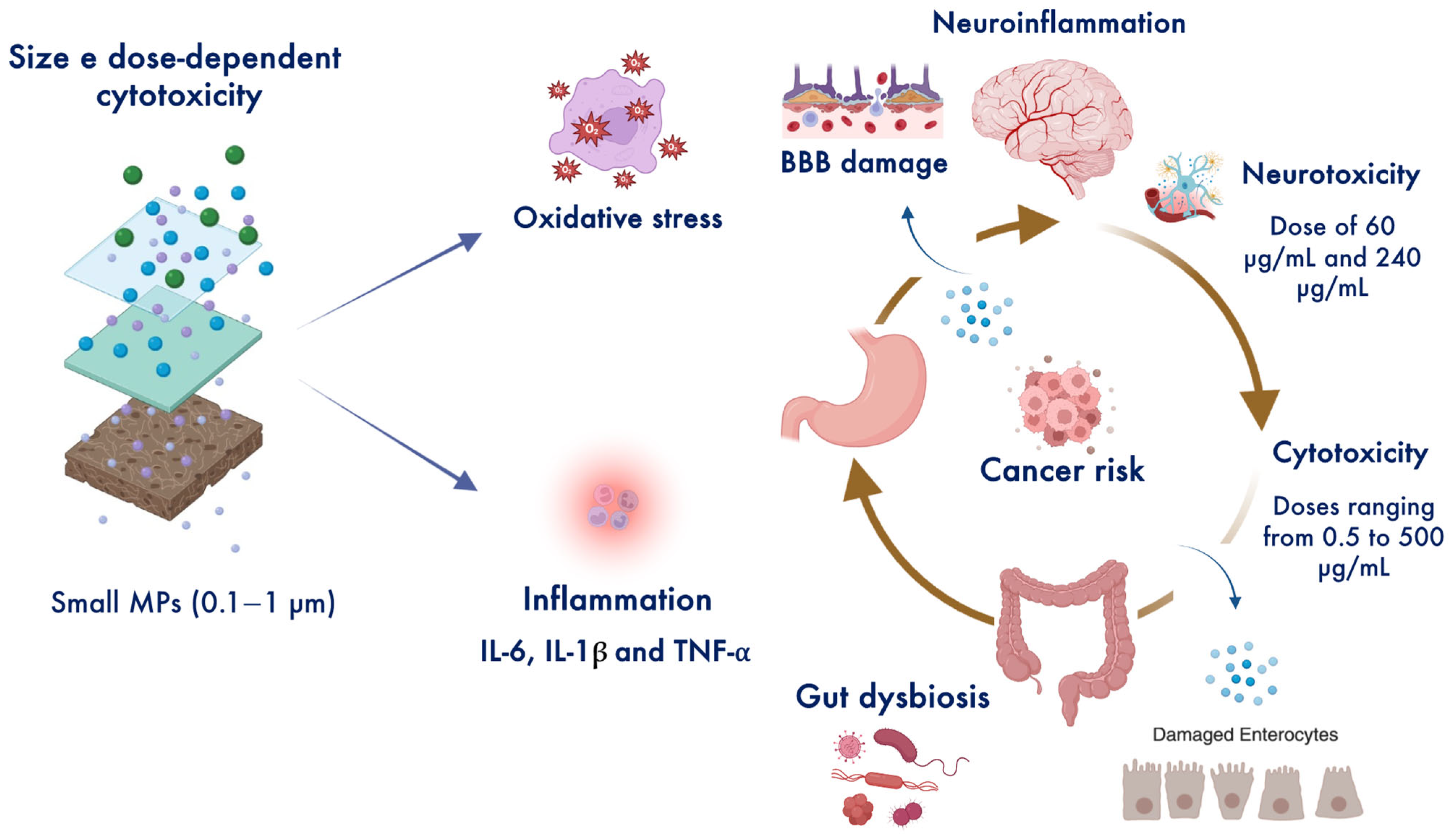
| Models/Biological Matrix | Pathways | MPs Sizes and Doses | Outcomes | Ref. |
|---|---|---|---|---|
| Human renal tubular epithelial cells and human testis Cancer cells | ↑ TNF-α, TNF-α-R, ↑ RTK, RAS, JNK, ↑ ERK, P38, ↑ PI3K-AKT ↑ NRF2 ↑ MAPK | 50 nm 200 μg/mL for 24 h | PS-NPs cross into cells through endocytotic vesicles and affect cellular micro-structures and pathways related to cancer progression and metastasis. | [42] |
| Human gastric cancer cells | ↑ ASGR2 ↑ CD44 ↑ N-cadherin ↑ PD-L1 | 10 μm PS-MPs, 8.61 × 105 particles/mL | PS-MPs exposure induce invasion, migration and multidrug resistance. | [43,44,45,46,47] |
| Mice | 1.72 × 104 particles/mL orally administered for 4 weeks | PS-MPs accumulated in gastric tissue induced resistance to chemo- and monoclonal antibody-therapy. | ||
| Gastric cancer cells | Not specified | 60 nm PS-NPs and 500 nm PS-MPs at a dose of 200, 400, 600 mg/L | Induce intracellular ROS and genotoxicity. | [48] |
| Drinking water | Not specified | 0.125–0.15 mm, dose of 10 mg | MP-sorbed PHE and their derivatives increase gastrointestinal toxicity and human cancer risk particularly to higher levels of 10−4 in both adults and children. | [49] |
| Human colorectal cancer cells and spheroid cells | Not specified | 0.25 and 1 μm and dose of 0.1, 1, and 10 μg mL | PS-MPs smaller than 1 μm enhance cell migration, potentially promoting metastasis. | [50,51] |
| Resistant HCT-116 and colorectal SW480 cancer | ↑ mTOR/ULK1 | 60 to 80 nm dose 25 µg/mL | Enhance drug resistance and CRC cancer progression by promoting mTOR-mediated protective autophagy. | [52] |
| Human colorectal adenocarcinoma caco-2 and HT-29 cells | ↑ ROS | 0.45 μm dose 0.25–1.0 mg/ml for 48 h | Decrease cell viability and increase oxidative stress, particularly mitochondrial superoxide production dose-dependently. | [53] |
| Mice | ↑ VLA4-VCAM1 ↑ IL-10 ↑ TNF-α ↑ IFN-γ | 0.1, 5, and 50 μm 10 mg/L | Long-term oral ingestion of the smallest MPs (0.1 μm) promotes gut epithelium damage and colitis related to depressive-like behaviors. | [54,55,56,57] |
| Mouse brain neuroblastoma NEURO-2A cells and human choriocarcinoma HLA-G-positive cells | ↓ GABA ↓ Claudin 3 ↑ ROS | Size PS-MPs 1000 nm, PS-NPs 100 nm, PS-NP-COOH 100 nm dose 60 μg/mL and 240 μg/mL for 48 h | Promote neurotoxic effects by inducing oxidative stress and apoptosis with GABA depletion. | [7] |
| Mice | ↓ GABA ↑ ROS | Size 100 nm, 1 mg/day via intragastric gavage for 17 consecutive days | Maternal administration of PS-MPs during gestation cross maternal blood-placental barrier and lead to anxiety-like behavior of the progenies and GABA reduction in the prefrontal cortex and amygdala after 8 weeks. | |
| Human SH-SY5Y neuroblastoma cells | ↑ AMPK/ULK1 | Size 50 nm, 0.5–500 μg/mL for 28 days | Induce neurotoxicity and mitochondrial dysfunction causing dopaminergic neuron death in a dose-dependent manner. | [58,59,60] |
| Mice | ↑ AMPK/ULK1 | 250 mg/kg/day by oral gavage | ||
| Human U87 glioblastoma cells | Not specified | Size range of 37–75 μm 0.005 g for 26 days | Long-term exposure to MPs increases the proliferative and migratory capacities with a tendency to aggregate into a cluster of cells (spheroids). | [61] |
2.1. Gastric Cancer
2.2. Colorectal Cancer
2.3. Brain Cancer
2.3.1. Nutritional Medicine Mitigates MP Toxicity and Colon Cancer Risk
2.3.2. The Nrf2 Pathway in Brain Cancer: The Role of Functional Nutrients
3. Functional Nutrition Targeting Stress Resilience Signaling Exerts Anticancer Effects to Mitigate MP Damage
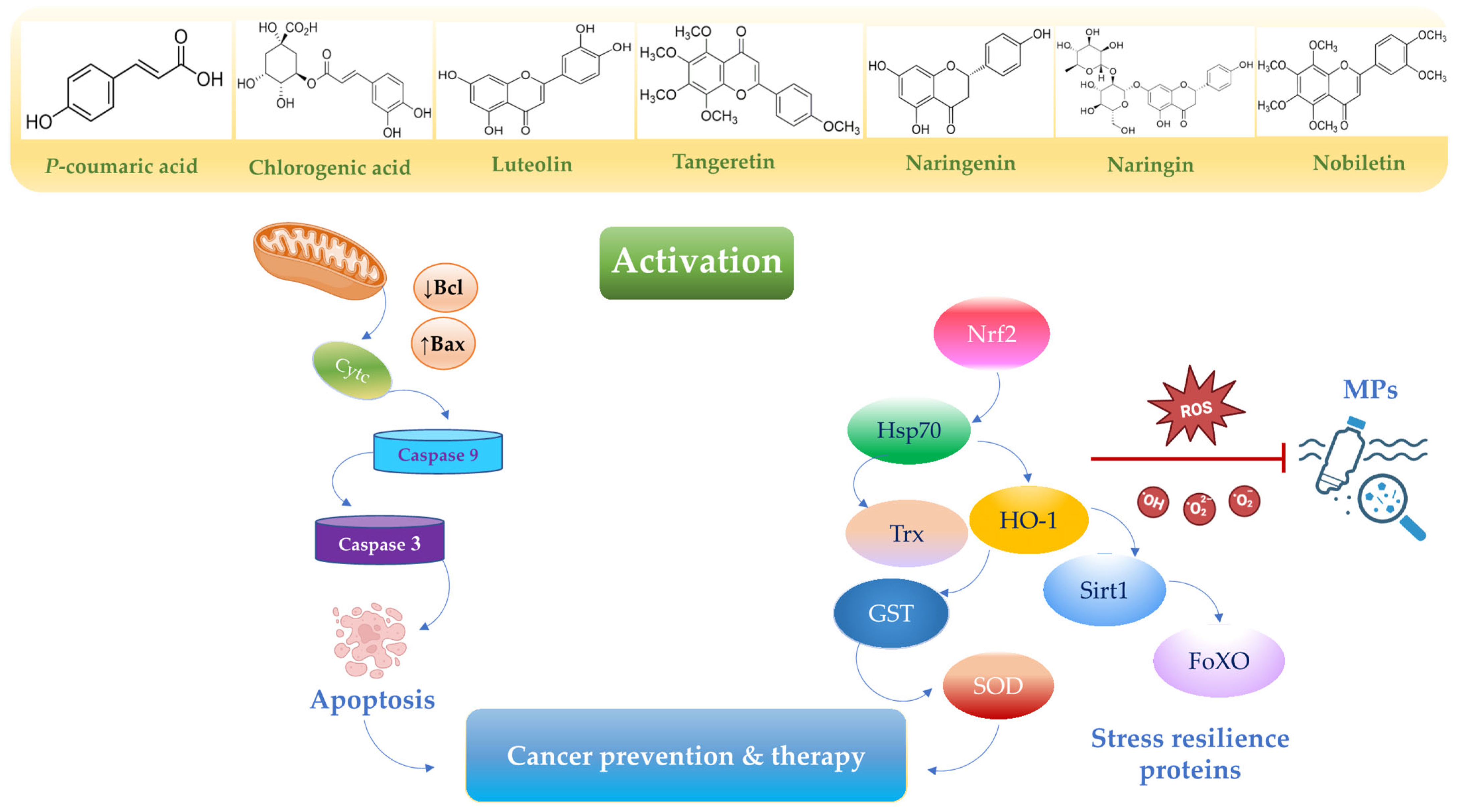
3.1. Chlorogenic Acid
| Nutrients | Pathways Upregulated | Pathways Downregulated | Outcomes | Ref. |
|---|---|---|---|---|
| Chlorogenic acid | Nrf2, GST, NQO1 | NF-κB, MAPK, AP-1 | Protect against environmental carcinogen-induced carcinogenesis | [75] |
| Bax, caspasi-3 | Bcl-2, c-myc, survivin, VEGFA and cyclin D | Induces apoptosis in gastric cancer. | [76,77] | |
| Cyclin D, Wnt/β-catenin | Inhibits proliferation of A549 human cancer cells and colon cancer. | [78,79,80,81,82] | ||
| TAMs | Reduces tumor growth in a G422 glioma xenograft model. | [83,84,85,86] | ||
| mTOR/TFEB TPK1-PDH | ACAT1 | Promotes autophagic flux in neuroblastoma cells | [87,88] | |
| P-coumaric acid | Nrf2, HO-1, TRXN, GPX2, GST | NF-κB | Inhibits cell proliferation in gastric adenocarcinoma cells. | [89] |
| Inhibits cell proliferation in colon adenocarcinoma and induces apoptosis. | [90,91] | |||
| Cyclin B1, cdc2, mdm2, c-fos, c-jun, c-myc, Bax/Bcl-2 ratio | ||||
| Reduces cell viability by inducing G2/M cell cycle arrest and apoptosis in neuroblastoma N2a and in U87MG glioblastoma cells. | [92,93] | |||
| Luteolin | Nrf2 NQO1 | IL-1, IL-6 JAK/STAT3 | Suppresses cell proliferation in colon adenocarcinoma. | [94,95,96,97] |
| PI3K/Akt | Inhibits cell proliferation in gastric cancer. | [98,99] | ||
| HDAC | Inhibits cell proliferation in colorectal cancer. | [100,101] | ||
| IL-6/STAT3 | Promotes apoptosis in glioblastoma. | [102,103,104,105] | ||
| Tangeretin | Bax, caspasis-3, caspasis-9 | Inhibits cell proliferation in gastric cancer. | [106,107,108,109,110] | |
| ROS, Bax | JNK | Reduces mitochondrial membrane potential and ATPase activity in colorectal cancer cells. | [111,112] | |
| PTEN | Cyclin-D, cdc-2 | Chemopreventive agent in glioblastoma cells. | [113] | |
| JAK2-STAT3-BCL-2/BCL-xL | Inhibits glioblastoma multiforme cells and induces pro-apoptotic effects. | [114,115] | ||
| Nobiletin | Nrf2 | SREBP1 PI3K/Akt/mTOR | Inhibits gastric cancer cells in a dose-dependent manner. | [116,117,118,119] |
| iNOS, HO-1, NQO1 | Inhibits colitis-associated colon carcinogenesis in (AOM)/(DSS)-treated mice. | [120] | ||
| AKT/GSK3β/β-catenin cyclin D1 and CDK4 NF-κB | Induces apoptosis in glioblastoma. | [121,122] | ||
| Naringin | MAPK | PI3K-AKT/Zeb1 | Promotes apoptosis in gastric cancer. | [123,124,125] |
| p53, caspase-3 | PI3K/Akt/mTOR | Induces autophagy in adenocarcinoma cells | [126,127,128,129] | |
| Bcl-2, PI3K–Akt | Promotes apoptosis in glioblastoma cells. | [130,131] | ||
| Naringenin | MAPK, Bax, caspase-3, p53, ASK1 | Bcl-2, PI3K–Akt, TGF-β/Smad-3, PRDX1, MMP2 and MMP9 | Inhibits cell proliferation in gastric and pancreatic cells. | [132,133,134,135,136,137,138] |
| IL-6/STAT3 | Attenuates colorectal cancer progression in vitro and in vivo. | [139,140] | ||
| AMPK SOD, CAT, GSH, and GSH-Px | ||||
| Hedgehog MMP/ERK/p38 | Reduces glioblastoma cell migration and invasion. | [141,142,143,144] |

3.1.1. The Potential Effects of Chlorogenic Acid in Gastric Cancer
3.1.2. The Potential Effects of Chlorogenic Acid in Colorectal Cancer
3.1.3. The Potential Effects of Chlorogenic Acid in Brain Cancer
3.2. P-Coumaric Acid
3.2.1. The Potential Effects of P-Coumaric Acid in Gastric Cancer
3.2.2. The Potential Effects of P-Coumaric Acid in Colorectal Cancer
3.2.3. The Potential Effects of P-Coumaric Acid in Brain Cancer
3.3. Luteolin
3.3.1. The Potential Effects of Luteolin in Gastric Cancer
3.3.2. The Potential Effects of Luteolin in Colorectal Cancer
3.3.3. The Potential Effects of Luteolin in Brain Cancer
3.4. Tangeretin
3.4.1. The Potential Effects of Tangeretin in Gastric Cancer
3.4.2. The Potential Effects of Tangeretin in Colorectal Cancer
3.4.3. The Potential Effects of Tangeretin in Brain Cancer
3.5. Nobiletin
3.5.1. The Potential Effects of Nobiletin in Gastric Cancer
3.5.2. The Potential Effects of Nobiletin in Colorectal Cancer
3.5.3. The Potential Effects of Nobiletin in Brain Cancer
3.6. Naringin
3.6.1. The Potential Effects of Naringin in Gastric Cancer
3.6.2. The Potential Effects of Naringin in Colorectal Cancer
3.6.3. The Potential Effects of Naringin in Brain Cancer
3.7. Naringenin
3.7.1. The Potential Effects of Naringenin in Gastric Cancer
3.7.2. The Potential Effects of Naringenin in Colorectal Cancer
3.7.3. The Potential Effects of Naringenin in Brain Cancer
4. Polyphenol-Based Nanomedicine Platforms Inhibit Gastric–Colon–Brain Axis Cancer
4.1. Polyphenol-Based Nanocarriers in Gastric Cancer
4.2. Polyphenol-Based Nanocarriers in Colorectal Cancer
4.3. Polyphenol-Based Nanocarriers in Brain Cancer
4.4. Polyphenol-Based Nanocarriers Inhibit MP-Induced Damage
5. Other Emerging Contaminants: Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs)
5.1. PFAS and Cancer Risk
5.2. PFASs and Brain Disorders
5.3. PFAS Toxicity: Focus on Functional Nutrients
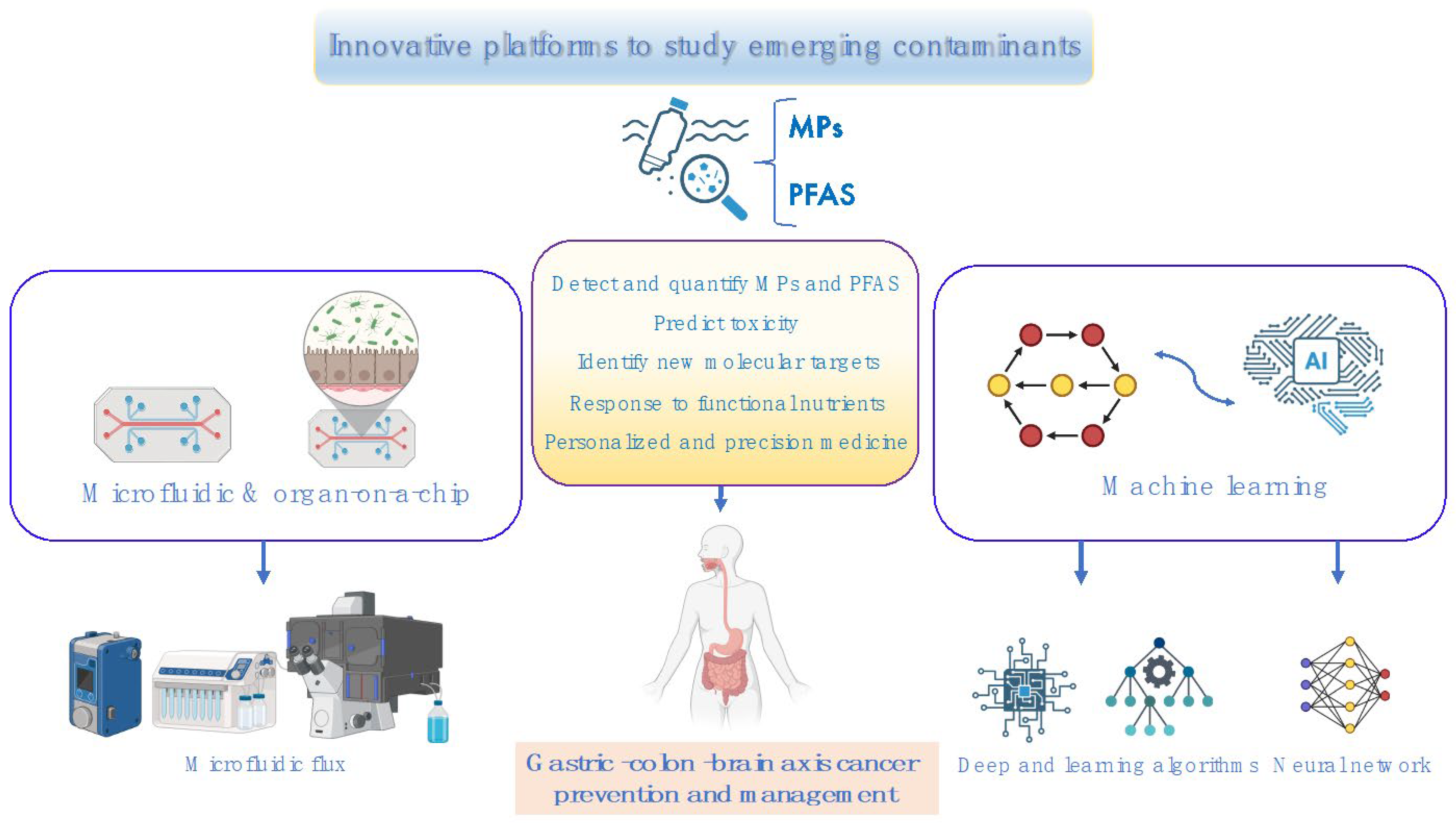
6. Innovative Technologies to Predict Toxicity and Cancer Risk of Emerging Contaminants: Therapeutic Nutritional Strategies for Future Medicine
6.1. Microfluidic Platforms
6.2. Organ-on-a-Chip Modeling
6.3. Machine Learning Techniques
6.3.1. Machine Learning Devices Predict MP Toxicity
6.3.2. Machine Learning Devices Predict Cancer Risk Along the Gastric–Colon–Brain Axis
6.3.3. Machine Learning Devices Detect Novel Nutritional Drugs and Targets
| Innovative Platforms | MPs Size | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Microfluidic platforms (KTP) | 50 nm | Simulate the morphology and function of the organs in vitro. Analyze the molecular mechanisms and the damage to cellular micro-structures. | Low ability to capture and identify very small sizes of MPs. | [42] |
| Surface-nanodroplet-decorated microfluidic device | 10 μm | Predicts the adsorption mechanisms and removal of contaminants | Contamination during sample preparation and reduction in capture performance <0.2 μm | [179] |
| Microfluidic device | PS-MPs, 1 μm PS-NPs, 100 nm | Predicts toxicological and translocation mechanisms of plastic particles and mediate particle–cell interactions. | Does not simulate the shear stress that cells would experience in other physiological microenvironments such as capillary blood flow. | [181] |
| Microfluidic chip | 80 nm | Automatically generates gradient concentrations and rapidly analyzes the interactions between cells and a series of different concentrations of MPs. | Difficult to standardize and scale up. External pumps, tubing, and connectors are required to operate, reducing accessibility and reproducibility and increasing system costs. | [182] |
| Microfluidic electric parallel egg-laying assay | PS-MPs 1 μm, dose of 100 and 1000 mg/L | Captures and detects different sizes and shapes of MPs using Raman spectroscopy and identifies phenotypical heterogeneity in response to MPs. | Data preprocessing remains complex, making it difficult to detect very small or irregularly shaped particles. Requires extensive sample pre-treatment. | [183] |
| Microfluidic chip | 1 μm | Endothelializes and regionalizes optical irradiation and dynamic blood flow manipulation; realistically reproduces the invasion of MPs. | Requires standardization efforts; reproducibility of data is challenging and trained cell biologists are needed. | [184] |
| Artificial neural network | 0.1–5 mm | Predicts and identifies MP abundance accurately on 67 surface soil samples. | Performance varies depending on dataset quality and spectral noise; preprocessing methods are complex; not suitable for very small or diverse microplastic samples. | [188] |
| Deep learning algorithms (1D-CNN, SVM AND PLS-DA) | 50 µm | Predict the toxicity of MPs and do not require preprocessing of experimental data; can directly analyze and classify spectra with high accuracy. | Difficulty in data collection and lack of standardized criteria. Limited data volume. | [189] |
| Machine learning device | ≤0.1 μm | Enhances predictive accuracy to cytotoxicity in Caco-2 cells. | Diversified datasets and standardized experimental protocols are needed to refine these predictive models. | [190] |
| Machine learning algorithms (MLR, RF, KNN, SVM, GBDT, AND XGB) | 1–25 μm | Improve prediction of MP cytotoxicity with high accuracy; expand the range of MP types that can be detected. | Insufficiency of the number of samples that not adequately represent all possible types of MPs and their diversity. Standardized MPs does not fully capture the complexity of the real environment. | [191] |
7. Environmental Contaminants, Autism, and Potential Cancer Risk: The Role of Nutrients
7.1. MPs and PFASs Enhance the Risk of Developing Autism
7.2. Potential Signaling Pathways Related to Environmental Pollutants, Autism, and Cancer Risk
7.3. Personalized Nutritional Medicine Restores MP and PFAS Damage and Improves Autism
8. Conclusions and Future Research Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Zu, D.; Liu, H.; He, H.; Bao, Q.; He, Y.; Liang, C.; Luo, G.; Teng, Y.; et al. PMMA nanoplastics induce gastric epithelial cellular senescence and cGAS-STING-mediated inflammation via ROS overproduction and NHEJ suppression. Ecotoxicol. Environ. Saf. 2024, 287, 117284. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Choi, J.; Ryu, K.Y. Recent Progress and Future Directions of the Research on Nanoplastic-Induced Neurotoxicity. Neural Regen. Res. 2024, 19, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Ontario, M.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J.; et al. Polystyrene micro- and nano-particle co-exposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ. Int. 2022, 166, 107362. [Google Scholar] [CrossRef]
- Manuguerra, S.; Espinosa Ruiz, C.; Santulli, A.; Messina, C.M. Sub-lethal doses of polybrominated diphenyl ethers, in vitro, promote oxidative stress and modulate molecular markers related to cell cycle, antioxidant balance and cellular energy management. Int. J. Environ. Res. Public Health 2019, 16, 588. [Google Scholar] [CrossRef]
- Schnee, M.; Sieler, M.; Dörnen, J.; Dittmar, T. Effects of polystyrene nano- and microplastics on human breast epithelial cells and human breast cancer cells. Heliyon 2024, 10, e38686. [Google Scholar] [CrossRef]
- Chen, G.; Shan, H.; Xiong, S.; Zhao, Y.; van Gestel, C.A.M.; Qiu, H.; Wang, Y. Polystyrene nanoparticle exposure accelerates ovarian cancer development in mice by altering the tumor microenvironment. Sci. Total Environ. 2024, 906, 167592. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jermal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, L.; Tian, H.; Yang, Q.; Wu, J.; Ji, Z.; Cai, J.; Chen, Y.; Li, Z. A scientometric analysis of research trends on emerging contaminants in the field of cancer in 2012–2021. Front. Public Health 2022, 10, 1034585. [Google Scholar] [CrossRef] [PubMed]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Hu, L.; Feng, X.; Wang, M.; Yuan, H.; Xu, H. Synergistic effect of PS-MPs and Cd on male reproductive toxicity: Ferroptosis via Keap1-Nrf2 pathway. J. Hazard. Mater. 2024, 461, 132584. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Yu, C.; Wei, F.; Xi, B. Microplastics exacerbate tissue damage and promote carcinogenesis following liver infection in mice. Ecotoxicol. Environ. Saf. 2024, 286, 117217. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species During Alzheimer’s Disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Trovato-Salinaro, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato-Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef]
- D’Amico, R.; Trovato-Salinaro, A.; Cordaro, M.; Fusco, R.; Impellizzeri, D.; Interdonato, L.; Scuto, M.L.; Ontario, M.; Crea, R.; Siracusa, R.; et al. Hidrox® and chronic cystitis: Biochemical evaluation of inflammation, oxidative stress, and pain. Antioxidants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato-Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Trovato-Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Trovato-Salinaro, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. NeuroToxicology 2016, 53, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, A.; Agafonova, A.; Modafferi, S.; Trovato-Salinaro, A.; Scuto, M.; Maiolino, L.; Fritsch, T.; Calabrese, E.J.; Lupo, G.; Anfuso, C.D.; et al. Blood-Labyrinth Barrier in Health and Diseases: Effect of Hormetic Nutrients. Antioxid. Redox Signal. 2024, 40, 542–563. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato-Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Trovato-Salinaro, A.; Cornelius, C.; Koverech, G.; Koverech, A.; Scuto, M.; Lodato, F.; Fronte, V.; Muccilli, V.; Reibaldi, M.; Longo, A.; et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder lin-ked to Alzheimer’s disease. Front. Pharmacol. 2014, 5, 129. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Scuto, M.; Ontario, M.; Trovato-Salinaro, A.; Calabrese, E.J.; Calabrese, V.; Stefani, M. Xenohormesis underlyes the anti-aging and healthy properties of olive polyphenols. Mech. Ageing Dev. 2022, 202, 111620. [Google Scholar] [CrossRef]
- Trovato-Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef]
- Amara, I.; Ontario, M.L.; Scuto, M.; Lo Dico, G.M.; Sciuto, S.; Greco, V.; Abid-Essefi, S.; Signorile, A.; Trovato-Salinaro, A.; Calabrese, V. Moringa oleifera Protects SH-SY5YCells from DEHP-Induced Endoplasmic Reticulum Stress and Apoptosis. Antioxidants 2021, 10, 532. [Google Scholar] [CrossRef]
- Fusco, R.; Trovato-Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S.; et al. Hidrox® Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Scuto, M.; Zappalà, A.; Ontario, M.L.; Petralia, A.; Abid-Essefi, S.; Maiolino, L.; Signorile, A.; Trovato-Salinaro, A.; Calabrese, V. Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2138. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Trovato -Salinaro, A.; Modafferi, S.; Polimeni, A.; Pfeffer, T.; Weigand, T.; Calabrese, V.; Schmitt, C.P.; Peters, V. Carnosine Activates Cellular Stress Response in Podocytes and Reduces Glycative and Lipoperoxidative Stress. Biomedicines 2020, 8, 177. [Google Scholar] [CrossRef]
- Cordaro, M.; Scuto, M.; Siracusa, R.; D’amico, R.; Peritore, A.F.; Gugliandolo, E.; Fusco, R.; Crupi, R.; Impellizzeri, D.; Pozzebon, M.; et al. Effect of N-palmitoylethanolamine-oxazoline on comorbid neuropsychiatric disturbance associated with inflammatory bowel disease. FASEB J. 2020, 34, 4085–4106. [Google Scholar] [CrossRef]
- Fusco, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. N-Palmitoylethanolamide-Oxazoline Protects Against Middle Cerebral Artery Occlusion Injury in Diabetic Rats by Regulating the SIRT1 Pathway. Int. J. Mol. Sci. 2019, 20, 4845. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Rafi, Z.; Hamza, A.; Sayed, A.A.; Albadrani, G.M.; Al-Ghadi, M.Q.; Abdel-Daim, M.M. Mitigative potential of kaempferide against polyethylene microplastics induced testicular damage by activating Nrf-2/Keap-1 pathway. Ecotoxicol. Environ. Saf. 2024, 269, 115746. [Google Scholar] [CrossRef]
- Tang, Y.C.; Chuang, Y.J.; Chang, H.H.; Juang, S.H.; Yen, G.C.; Chang, J.Y.; Kuo, C.C. How to deal with frenemy NRF2: Targeting NRF2 for chemoprevention and cancer therapy. J. Food Drug Anal. 2023, 31, 387–407. [Google Scholar] [CrossRef]
- Scuto, M.; Ontario, M.L.; Trovato-Salinaro, A.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free. Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef]
- Scuto, M.; Trovato-Salinaro, A.; Caligiuri, I.; Ontario, M.L.; Greco, V.; Sciuto, N.; Crea, R.; Calabrese, E.J.; Rizzolio, F.; Canzonieri, V.; et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021, 199, 111551. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, X.; Zhang, X.; Duan, X.; Lin, H.; Liu, S.; Sui, G. Assessment of cancer-related signaling pathways in responses to polystyrene nanoplastics via a kidney-testis microfluidic platform (KTP). Sci. Total Environ. 2023, 857, 159306. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dovizio, M.; Milillo, C.; Aruffo, E.; Pesce, M.; Gatta, M.; Chiacchiaretta, P.; Di Carlo, P.; Ballerini, P. Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 3079. [Google Scholar] [CrossRef] [PubMed]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Shi, L.; Jia, Y.; Sheng, H. Detection and quantification of microplastics in various types of human tumor tissues. Ecotoxicol. Environ. Saf. 2024, 283, 116818. [Google Scholar] [CrossRef]
- Kim, H.; Zaheer, J.; Choi, E.J.; Kim, J.S. Enhanced ASGR2 by microplastic exposure leads to resistance to therapy in gastric cancer. Theranostics 2022, 12, 3217–3236. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Lu, Y.; He, L.; Qu, J.; Zhou, C.; Hong, P.; Sun, S.; Zhao, H.; Liang, Y.; et al. The Complex Toxicity of Tetracycline with Polystyrene Spheres on Gastric Cancer Cells. Int. J. Environ. Res. Public Health 2020, 17, 2808. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Gatheru-Waigi, M.; Ling, W.; Qin, C.; Wang, J.; Gao, Y. Microplastics-sorbed phenanthrene and its derivatives are highly bioaccessible and may induce human cancer risks. Environ. Int. 2022, 168, 107459. [Google Scholar] [CrossRef]
- Eng, C.; Jacome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022, 23, E116–E128. [Google Scholar] [CrossRef]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; Del Favero, G.; et al. Microplastics role in cell migration and distribution during cancer cell division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Han, Y.; Zhang, M.; Zhu, K.; Yang, Z.; Qiu, M.; Guo, Y.; Dong, Z.; Hao, J.; Zhang, X.; et al. Effects of microplastics on chemo-resistance and tumorigenesis of colorectal cancer. Apoptosis 2025, 30, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Herrala, M.; Huovinen, M.; Järvel, E.; Hellman, J.; Tolonen, P.; Lahtela-Kakkonen, M.; Rysä, J. Micro-sized polyethylene particles affect cell viability and oxidative stress responses in human colorectal adenocarcinoma Caco-2 and HT-29 cells. Sci. Total Environ. 2023, 867, 161512. [Google Scholar] [CrossRef] [PubMed]
- Kuai, Y.; Chen, Z.; Xie, K.; Chen, J.; He, J.; Gao, J.; Yu, C. Long-term exposure to polystyrene microplastics reduces macrophages and affects the microbiota-gut-brain axis in mice. Toxicology 2024, 509, 153951. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan-Mohd-Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2020, 5, 116–121. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ratliff, M.; Karimian-Jazi, K.; Hoffmann, D.C.; Rauschenbach, L.; Simon, M.; Hai, L.; Mandelbaum, H.; Schubert, M.C.; Kessler, T.; Uhlig, S.; et al. Individual glioblastoma cells harbor both proliferative and invasive capabilities during tumor progression. Neuro-Oncol. 2023, 25, 2150–2162. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef]
- Almeida Lima, K.; Osawa, I.Y.A.; Ramalho, M.C.C.; de Souza, I.; Guedes, C.B.; Souza Filho, C.H.D.; Monteiro, L.K.S.; Latancia, M.T.; Rocha, C.R.R. Temozolomide Resistance in Glioblastoma by NRF2: Protecting the Evil. Biomedicines 2023, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Rafazi, P.; Bagheri, Z.; Haghi-Aminjan, H.; Rahimifard, M.; Ahvaraki, A. Long-term exposure of human U87 glioblastoma cells to polyethylene microplastics: Investigating the potential cancer progression. Toxicol. Rep. 2024, 13, 101757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zheng, J.; Gu, Y.; Xu, X.; Yu, J.; Li, J.; Yang, S.; Chen, B.; Du, J.; Dong, R. Quercetin intervention mitigates small intestinal damage and immunologic derangement induced by polystyrene nanoplastics: Insights from multi-omics analysis in mice. Environ. Pollut. 2024, 361, 124862. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, X.; Yan, F.; Xu, L.; Ye, X. Modulation of Gut Microbial Metabolism by Cyanidin-3-O-Glucoside in Mitigating Polystyrene-Induced Colonic Inflammation: Insights from 16S rRNA Sequencing and Metabolomics. J. Agric. Food Chem. 2024, 72, 7140–7154. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhu, J.; Ding, K.; Xu, J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumor Biol. 2014, 35, 10707–10714. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. FTY720 inhibits the Nrf2/ARE pathway in human glioblastoma cell lines and sensitizes glioblastoma cells to temozolomide. Pharmacol. Rep. 2017, 69, 1186–1193. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, Z.; Liu, J.; Chen, L.; Tian, Y.; Xu, W.; Zeng, T.; Wu, W.; Lu, L. Chlorogenic Acid Alleviates LPS-Induced Inflammation and Oxidative Stress by Modulating CD36/AMPK/PGC-1α in RAW264.7 Macrophages. Int. J. Mol. Sci. 2023, 24, 13516. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.D.; Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Sanjay; Sood, R.; Jaiswal, V.; Kang, S.U.; Park, M.; Lee, H.J. Nobiletin regulates intracellular Ca2+ levels via IP3R and ameliorates neuroinflammation in Aβ42-induced astrocytes. Redox Biol. 2024, 73, 103197. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Wu, H.; Fan, W.; Zhang, J.; Su, W.; Wang, Y.; Li, P. Naringenin attenuates inflammation, apoptosis, and ferroptosis in silver nanoparticle-induced lung injury through a mechanism associated with Nrf2/HO-1 axis: In vitro and in vivo studies. Life Sci. 2022, 311, 121127. [Google Scholar] [CrossRef] [PubMed]
- Matić, I.Z.; Mraković, A.; Rakočević, Z.; Stoiljković, M.; Pavlović, V.B.; Momić, T. Anticancer effect of novel luteolin capped gold nanoparticles selectively cytotoxic towards human cervical adenocarcinoma HeLa cells: An in vitro approach. J. Trace Elem. Med. Biol. 2023, 80, 127286. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, X.; Gan, Q.; Lu, Z.; Du, Y.; Noor, I.; Wang, L.; Liu, S.; Jin, B. Flavonoids Mitigate Nanoplastics Stress in Ginkgo biloba. Plant Cell Environ. 2025, 48, 1790–1811. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.; Villegas, C.; Burgos, V.; Ortiz, L.; Cabrera-Pardo, J.R.; Paz, C. Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5189. [Google Scholar] [CrossRef]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef]
- Sánchez-Quezada, V.; Velázquez-Guadarrama, N.; Mendoza-Elizalde, S.; Hernández-Iturriaga, M.; Landaverdem, P.V.; Loarca-Piña, G. Bioaccessibility of bioactive compounds present in Persea americana Mill. seed ingredient during oral-gastric digestion with antibacterial capacity against Helicobacter pylori. J. Ethnopharmacol. 2024, 331, 118259. [Google Scholar] [CrossRef]
- Jafari, N.; Zargar, S.J.; Delnavazi, M.R.; Yassa, N. Cell Cycle Arrest and Apoptosis Induction of Phloroacetophenone Glycosides and Caffeoylquinic Acid Derivatives in Gastric Adenocarcinoma (AGS) Cells. Anti-Cancer Agents Med. Chem. 2018, 18, 610–616. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int. J. Mol. Sci. 2020, 21, 3108. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Castrejón, J.V.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer potential of dihydrocaffeic acid: A chlorogenic acid metabolite. CyTA J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef]
- Villota, H.; Santa-González, G.A.; Uribe, D.; Henao, I.C.; Arroyave-Ospina, J.C.; Barrera-Causil, C.J.; Pedroza-Díaz, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef]
- Vélez, M.D.; Pedroza-Díaz, J.; Santa-González, G.A. Data on the cytotoxicity of chlorogenic acid in 3D cultures of HT-29 cells. Data Brief 2023, 50, 109527. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.; Sulaiman, M.K.; Sudhandiran, G. Caffeic acid phenethyl ester mediates apoptosis in serum-starved HT29 colon cancer cells through modulation of heat shock proteins and MAPK pathways. Cell Biochem. Funct. 2024, 42, 3942. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef]
- Xue, N.; Zhou, Q.; Ji, M.; Jin, J.; Lai, F.; Chen, J.; Zhang, M.; Jia, J.; Yang, H.; Zhang, J.; et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017, 7, 39011. [Google Scholar] [CrossRef]
- Daisy Precilla, S.; Kuduvalli, S.S.; Biswas, I.; Bhavani, K.; Pillai, A.B.; Thomas, J.M.; Anitha, T.S. Repurposing synthetic and natural derivatives induces apoptosis in an orthotopic glioma-induced xenograft model by modulating WNT/β-catenin signaling. Fundam. Clin. Pharmacol. 2023, 37, 1179–1197. [Google Scholar] [CrossRef]
- You, S.; Wang, M.J.; Hou, Z.Y.; Wang, W.D.; Du, T.T.; Xue, N.N.; Ji, M.; Chen, X.G. Chlorogenic Acid Induced Neuroblastoma Cells Differentiation via the ACAT1-TPK1-PDH Pathway. Pharmaceuticals 2023, 16, 877. [Google Scholar] [CrossRef]
- Adeyemo-Salami, O.A.; Afolabi, D.A.; Amuzat, A.A.; Adekanye, J.O.; Oladokun, O.O. Effect of Acute Exposure of Swiss Mice to Chlorogenic Acid. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70017. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Sharma, S.H.; Chellappan, D.R.; Chinnaswamy, P.; Nagarajan, S. Protective effect of p-coumaric acid against 1,2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017, 94, 577–588. [Google Scholar] [CrossRef]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Supplementation of p-coumaric acid exhibits chemopreventive effect via induction of Nrf2 in a short-term preclinical model of colon cancer. Eur. J. Cancer Prev. 2019, 28, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.A.; Castaldo, S.; Rotondo, R.; Staffieri, S.; Sanchez, M.; Arcella, A. Inhibiting effect of p-Coumaric acid on U87MG human glioblastoma cell growth. J. Chemother. 2022, 34, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Shailasree, S.; Venkataramana, M.; Niranjana, S.R.; Prakash, H.S. Cytotoxic effect of p-Coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol. Neurobiol. 2015, 51, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Anson, D.M.; Wilcox, R.M.; Huseman, E.D.; Stump, T.A.; Paris, R.L.; Darkwah, B.O.; Lin, S.; Adegoke, A.O.; Gryka, R.J.; Jean-Louis, D.S.; et al. Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2018, 123, 678–686. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Butt, G.; El-Zahaby, S.A.; Attar, R.; Sabitaliyevich, U.Y.; Jovic, J.J.; Tang, K.F.; Naureen, H.; Xu, B. Luteolin mediated targeting of protein network and microRNAs in different cancers: Focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways. Pharmacol. Res. 2020, 160, 105188. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, F.; Zhang, H.; Sun, T.; Fu, F.; Chen, X.; Zhang, Y. Luteolin suppresses the growth of colon cancer cells by inhibiting the IL-6/STAT3 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 1722–1732. [Google Scholar] [CrossRef]
- Yajie, D.; Feng, L.; Zhaoyan, L.I.; Yan, X.; Nida, C.; Guangao, Z.; Rui, W.; Aiguang, Z. Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism. J. Tradit. Chin. Med. 2023, 43, 34–41. [Google Scholar]
- Ma, J.; Pan, Z.; Du, H.; Chen, X.; Zhu, X.; Hao, W.; Zheng, Q.; Tang, X. Luteolin induces apoptosis by impairing mitochondrial function and targeting the intrinsic apoptosis pathway in gastric cancer cells. Oncol. Lett. 2023, 26, 327. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, R.; Xiao, X.; Yang, C.; Yang, Y.; Wang, C.; Lin, L.; Kong, A.N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell. Biochem. 2018, 119, 9573–9582. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Li, X.; Zhang, G.; Hu, J.; Li, H.; Guo, Z.; Zhao, X.; Chen, J.; Wang, Y.; Jing, Z. Effect of luteolin on glioblastoma’s immune microenvironment and tumor growth suppression. Phytomedicine 2024, 130, 155611. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ouyang, J.; Long, C. Effects and Mechanism of Luteolin on Proliferation and Apoptosis of Glioma. Altern. Ther. Health Med. 2024, 30, 284–288. [Google Scholar] [PubMed]
- Lee, H.S.; Park, B.S.; Kang, H.M.; Kim, J.H.; Shin, S.H.; Kim, I.R. Role of Luteolin-Induced Apoptosis and Autophagy in Human Glioblastoma Cell Lines. Medicina 2021, 57, 879. [Google Scholar] [CrossRef]
- Pradeep, S.; Sai Chakith, M.R.; Sindhushree, S.R.; Reddy, P.; Sushmitha, E.; Purohit, M.N.; Suresh, D.; Swamy Shivananju, N.; Silina, E.; Manturova, N.; et al. Exploring shared therapeutic targets for Alzheimer’s disease and glioblastoma using network pharmacology and protein-protein interaction approach. Front Chem. 2025, 13, 1549186. [Google Scholar] [CrossRef]
- Raza, W.; Luqman, S.; Meena, A. Prospects of tangeretin as a modulator of cancer targets/pathways. Pharmacol. Res. 2020, 161, 105202. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Mdkhana, B.; Zaher, D.M.; Abdin, S.M.; Omar, H.A. Tangeretin boosts the anticancer activity of metformin in breast cancer cells via curbing the energy production. Phytomedicine 2021, 83, 153470. [Google Scholar] [CrossRef]
- Pereira, C.V.; Duarte, M.; Silva, P.; Bento da Silva, A.; Duarte, C.M.M.; Cifuentes, A.; García-Cañas, V.; Bronze, M.R.; Albuquerque, C.; Serra, A.T. Polymethoxylated Flavones Target Cancer Stemness and Improve the Antiproliferative Effect of 5-Fluorouracil in a 3D Cell Model of Colorectal Cancer. Nutrients 2019, 11, 326. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, Y.U.; Huang, H.; Duan, Y.; Yuan, Z.; Cao, L.; Ying, J.; Zhou, Y.; Feng, S. The superiority of PMFs on reversing drug resistance of colon cancer and the effect on aerobic glycolysis-ROS-autophagy signaling axis. Oncol. Res. 2024, 32, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem. Toxicol. 2020, 143, 111529. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, D.W.; Yu, X.D.; Zhou, Y.L. Tangeretin induces cell cycle arrest and apoptosis through upregulation of PTEN expression in glioma cells. Biomed. Pharmacother. 2016, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Li, S.; Chuang, W.L.; Li, C.H.; Chen, G.J.; Chang, C.C.; Or, C.R.; Lin, P.Y.; Chang, C.C. Blockade of STAT3 Signaling Contributes to Anticancer Effect of 5-Acetyloxy-6,7,8,4′-Tetra-Methoxyflavone, a Tangeretin Derivative, on Human Glioblastoma Multiforme Cells. Int. J. Mol. Sci. 2019, 20, 3366. [Google Scholar] [CrossRef]
- Ting, Y.; Chiou, Y.S.; Jiang, Y.; Pan, M.H.; Lin, Z.; Huang, Q. Safety evaluation of tangeretin and the effect of using emulsion-based delivery system: Oral acute and 28-day sub-acute toxicity study using mice. Food Res. Int. 2015, 74, 140–150. [Google Scholar] [CrossRef]
- Peng, Z.; Song, L.; Chen, M.; Liu, Z.; Yuan, Z.; Wen, H.; Zhang, H.; Huang, Y.; Peng, Z.; Yang, H.; et al. Neofunctionalization of an OMT cluster dominates polymethoxyflavone biosynthesis associated with the domestication of citrus. Proc. Natl. Acad. Sci. USA 2024, 121, e2321615121. [Google Scholar] [CrossRef]
- Rooprai, H.K.; Kandanearatchi, A.; Maidment, S.L.; Christidou, M.; Trillo-Pazos, G.; Dexter, D.T.; Rucklidge, G.J.; Widmer, W.; Pilkington, G.J. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol. Appl. Neurobiol. 2001, 27, 29–39. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Zheng, S.; Shen, J.; Chen, Y.; Li, Y.; Yuan, M.; Wu, J.; Sun, Q. Nobiletin targets SREBP1/ACLY to induce autophagy-dependent cell death of gastric cancer cells through PI3K/Akt/mTOR signaling pathway. Phytomedicine 2024, 128, 155360. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chou, Y.C.; Hung, W.L.; Cheng, A.C.; Yu, R.C.; Ho, C.T.; Pan, M.H. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, H.; Jin, C.; Mo, J.; Wang, H. Nobiletin flavone inhibits the growth and metastasis of human pancreatic cancer cells via induction of autophagy, G0/G1 cell cycle arrest and inhibition of NF-kB signalling pathway. J. BUON 2020, 25, 1070–1075. [Google Scholar] [PubMed]
- Zhang, X.; Zheng, K.; Li, C.; Zhao, Y.; Li, H.; Liu, X.; Long, Y.; Yao, J. Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells. Oncol. Rep. 2017, 37, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Sarkaki, A.; Dianat, M.; Mard, S.A.; Ahangarpour, A.; Badavi, M. Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacol. Rep. 2019, 71, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.; Mu, M.; Chen, Z.; Zhao, C.; Li, X.; Qu, C.; Ye, C.; Zhao, W.; Sun, X.; et al. Naringin Inhibits the Proliferation, Migration, Invasion and Epithelial-to-Mesenchymal Transition of Gastric Cancer Cells via the PI3K/AKT Signaling Pathway. Altern. Ther. Health Med. 2023, 29, 191–197. [Google Scholar]
- Raha, S.; Yumnam, S.; Hong, G.E.; Lee, H.J.; Saralamma, V.V.; Park, H.S.; Heo, J.D.; Lee, S.J.; Kim, E.H.; Kim, J.A.; et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. Int. J. Oncol. 2015, 47, 1061–1069. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, X.; Zhang, Q.; Ma, J.; Cheng, R.; Yong, H.; Shi, H.; Zhou, X.; Ge, L.; Gao, G. Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 19, 3798–3804. [Google Scholar] [CrossRef]
- Zeng, J.N.; Tan, J.Y.; Mo, L. Naringin Inhibits Colorectal Carcinogenesis by Inhibiting Viability of Colorectal Cancer Cells. Chin. J. Integr. Med. 2023, 29, 707–713. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Wang, F.; Cui, S.X.; Qu, X.J. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol. Ther. 2018, 19, 735–744. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Hao, G.; Wang, B.; Wang, J.; Liang, Y.; Liu, Y.; Zhen, E.; Feng, D.; Liang, G. Naringin suppresses the development of glioblastoma by inhibiting FAK activity. J. Drug Target. 2017, 25, 41–48. [Google Scholar] [CrossRef]
- Bisht, P.; Prasad, S.R.; Choudhary, K.; Pandey, R.; Aishwarya, D.; Aravind, V.; Ramalingam, P.; Velayutham, R.; Kumar, N. Naringin and temozolomide combination suppressed the growth of glioblastoma cells by promoting cell apoptosis: Network pharmacology, in-vitro assays and metabolomics based study. Front. Pharmacol. 2024, 15, 1431085. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, F.; Guo, H.B.; Li, Y.; Tan, B.B.; Zhang, W.X.; Peng, Y.H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumor Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, X.; Zhang, X.; Shang, D.; Zhou, Y.I.; Zhang, C. Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Exp. Ther. Med. 2016, 11, 669–673. [Google Scholar] [CrossRef]
- Song, S.; Huang, W.; Lu, X.; Liu, J.; Zhou, J.; Li, Y.; Shu, P. A Network Pharmacology Study Based on the Mechanism of Citri Reticulatae Pericarpium-Pinelliae Rhizoma in the Treatment of Gastric Cancer. Evid.-Based Complement. Altern. Med. 2021, 2021, 6667560. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, F.; Yang, M.; Zhao, J.; Zeng, W.; Fang, X.; Zhang, Y.; Zhang, C.; Liang, W. Naringenin decreases invasiveness and metastasis by inhibiting TGF beta-induced epithelial to mesenchymal transition in pancreatic cancer cells. PLoS ONE 2012, 7, e50956. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H.; Kim, J.H. Combined administration of naringenin and hesperetin with optimal ratio maximizes the anti-cancer effect in human pancreatic cancer via down regulation of FAK and p38 signaling pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, Y.J.; Lee, J.H.; Nam, M.J. Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem. Toxicol. 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Hua, L.; Hu, M.; Zhu, N.; Liu, Y.; Zhou, Y. The role of the natural compound naringenin in AMPK-mitochondria modulation and colorectal cancer inhibition. Phytomedicine 2024, 131, 155786. [Google Scholar] [CrossRef]
- Sun, J.; Shi, L.; Xu, F.; Sun, H.; Liu, Y.; Sun, J.; Zhou, Q. Naringenin Inhibits Colorectal Cancer associated with a High-Fat Diet through Modulation of Gut Microbiota and IL-6/STAT3 Pathway. J. Microbiol. Biotechnol. 2025, 35, e2412029. [Google Scholar] [CrossRef]
- Gautam, M.; Gabrani, R. Combinatorial Effect of Temozolomide and Naringenin in Human Glioblastoma Multiforme Cell Lines. Nutr. Cancer 2022, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Zaim, Ö.; Doğanlar, O.; Banu Doğanlar, Z.; Özcan, H.; Zreigh, M.M.; Kurtdere, K. Novel synthesis naringenin-benzyl piperazine derivatives prevent glioblastoma invasion by inhibiting the hypoxia-induced IL6/JAK2/STAT3 axis and activating caspase-dependent apoptosis. Bioorganic Chem. 2022, 129, 106209. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, M.L.; Juybari, K.B.; Tarzi, M.E.; Amirkhosravi, A.; Nematollahi, M.H.; Mirzamohammdi, S.; Mehrbani, M.; Mehrabani, M.; Mehrabani, M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: A possible role of hedgehog signaling pathway. Mol. Biol. Rep. 2021, 48, 6413–6421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chang, Y.M.; Wang, K.Y.; Chen, P.N.; Hseu, Y.C.; Chen, K.M.; Yeh, K.T.; Chen, C.J.; Hsu, L.S. Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Environ. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef]
- Hou, Y.; Tu, S.; Zhao, X.; Li, G.; Li, N.; Zou, A. An integrative method for evaluating the biological effects of nanoparticle-protein corona. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130300. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Yin, W.; Liu, Z.; Shi, L.; Tang, M. A Novel Drug Delivery System: The Encapsulation of Naringenin in Metal-Organic Frameworks into Liposomes. AAPS PharmSciTech 2021, 22, 61. [Google Scholar] [CrossRef]
- Morais, R.P.; Novais, G.B.; Sangenito, L.S.; Santos, A.L.S.; Priefer, R.; Morsink, M.; Mendonça, M.C.; Souto, E.B.; Severino, P.; Cardoso, J.C. Naringenin-Functionalized Multi-Walled Carbon Nanotubes: A Potential Approach for Site-Specific Remote-Controlled Anticancer Delivery for the Treatment of Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4557. [Google Scholar] [CrossRef]
- Ronka, S.; Kowalczyk, A.; Baczynska, D.; Zółnierczyk, A.K. Pluronics-Based Drug Delivery Systems for Flavonoids Anticancer Treatment. Gels 2023, 9, 143. [Google Scholar] [CrossRef]
- Luque-Badillo, A.C.; Hernandez-Tapia, G.; Ramirez-Castillo, D.A.; Espinoza-Serrano, D.; Cortes-Limon, A.M.; Cortes-Gallardo, J.P.; Jacobo-Velázquez, D.A.; Martinez-Fierro, M.L.; Rios-Ibarra, C.P. Gold nanoparticles enhance microRNA 31 detection in colon cancer cells after inhibition with chlorogenic acid. Oncol. Lett. 2021, 22, 742. [Google Scholar] [CrossRef]
- Bao, H.; Zheng, N.; Li, Z.; Zhi, Y. Synergistic Effect of Tangeretin and Atorvastatin for Colon Cancer Combination Therapy: Targeted Delivery of These Dual Drugs Using RGD Peptide Decorated Nanocarriers. Drug Des. Dev. Ther. 2020, 14, 3057–3068. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted delivery of chlorogenic acid by mannosylated liposomes to effectively promote the polarization of TAMs for the treatment of glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019, 4, 7515–7531. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Tian, Y.; Liu, R.; Wang, Y.; Guo, F.; Gong, Y.; Yan, M. Development, Characterization, and Investigation of In Vivo Targeted Delivery Efficacy of Luteolin-Loaded, Eudragit S100-Coated mPEG-PLGA Nanoparticles. AAPS PharmSciTech 2022, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Murugan, R.; Almutairi, B.O.; Arokiyaraj, S.; Arockiaraj, J. Brain targeted luteolin-graphene oxide nanoparticle abrogates polyethylene terephthalate induced altered neurological response in zebrafish. Mol. Biol. Rep. 2023, 51, 27. [Google Scholar] [CrossRef]
- Lei, S.; Hu, Z.; Liu, H. Treatment with quercetin mitigates polystyrene nanoparticle-induced reduction in neuron capacity by inhibiting dopaminergic neurodegeneration and facilitating dopamine metabolism in Caenorhabditis elegans. Chemosphere 2024, 364, 143303. [Google Scholar] [CrossRef]
- Liu, D.; Tang, B.; Nie, S.; Zhao, N.; He, L.; Cui, J.; Mao, W.; Jin, H. Distribution of per- and poly-fluoroalkyl substances and their precursors in human blood. J. Hazard. Mater. 2023, 441, 129908. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.Y.; Liu, Y.J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.Y.; Prins, G.S.; Madak Erdogan, Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; et al. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, 6223. [Google Scholar]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 2008, 28, 251–269. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Li, S.; Oliva, P.; Zhang, L.; Goodrich, J.A.; McConnell, R.; Conti, D.V.; Chatzi, L.; Aung, M. Associations between per-and polyfluoroalkyl substances (PFAS) and county-level cancer incidence between 2016 and 2021 and incident cancer burden attributable to PFAS in drinking water in the United States. J. Expo. Sci. Environ. Epidemiol. 2025, 35, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kappil, E.M.; Zheng, T.; Boffetta, P.; Seyyedsalehi, M.S. Per- and poly-fluoroalkyl substances exposure and risk of gastrointestinal cancers: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.Y.; Lin, Z.Y.; Sun, X.F.; Feng, J.J.; Mai, L.; Wu, C.C.; Huang, G.L.; Wang, P.; Liu, Y.W.; Liu, L.Y.; et al. Per- and polyfluoroalkyl substances (PFAS) exposure in plasma and their blood-brain barrier transmission efficiency—A pilot study. Environ. Int. 2024, 187, 108719. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; MacKillop, E.A.; Melnick, R.L.; Thayer, K.A.; Seidler, F.J. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ. Health Perspect. 2008, 116, 716–722. [Google Scholar] [CrossRef]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect. 2020, 128, 47005. [Google Scholar] [CrossRef]
- Xie, M.Y.; Sun, X.F.; Wu, C.C.; Huang, G.L.; Wang, P.; Lin, Z.Y.; Liu, Y.W.; Liu, L.Y.; Zeng, E.Y. Glioma is associated with exposure to legacy and alternative per- and polyfluoroalkyl substances. J. Hazard. Mater. 2023, 441, 129819. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, T.; Zhan, X.; Hong, S.; Lin, L.; Tan, P.; Xiong, Y.; Zhao, H.; Zheng, Z.; Bi, R.; et al. Legacy and novel perfluoroalkyl substances in raw and cooked squids: Perspective from health risks and nutrient benefits. Environ. Int. 2023, 177, 108024. [Google Scholar] [CrossRef]
- Pietrini, F.; Wyrwicka-Drewniak, A.; Passatore, L.; Nogués, I.; Zacchini, M.; Donati, E. PFOA accumulation in the leaves of basil (Ocimum basilicum L.) and its effects on plant growth, oxidative status, and photosynthetic performance. BMC Plant Biol. 2024, 24, 556. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Wang, Y.X.; Sun, Y.; Agudelo, J.; Bibi, Z.; Torres, N.; Oulhote, Y.; Slitt, A.; Messerlian, C. Folate concentrations and serum perfluoroalkyl and polyfluoroalkyl substance concentrations in adolescents and adults in the USA (National Health and Nutrition Examination Study 2003–2016): An observational study. Lancet Planet. Health 2023, 7, e449–e458. [Google Scholar] [CrossRef]
- Li, P.; Oyang, X.; Xie, X.; Guo, Y.; Li, Z.; Xi, J.; Zhu, D.; Ma, X.; Liu, B.; Li, J.; et al. Perfluorooctanoic acid and perfluorooctane sulfonate co-exposure induced changes of metabolites and defense pathways in lettuce leaves. Environ. Pollut. 2020, 256, 113512. [Google Scholar] [CrossRef]
- Karbassiyazdi, E.; Fattahi, F.; Yousefi, N.; Tahmassebi, A.; Taromi, A.A.; Manzari, J.Z.; Gandomi, A.H.; Altaee, A.; Razmjou, A. XGBoost model as an efficient machine learning approach for PFAS removal: Effects of material characteristics and operation conditions. Environ. Res. 2022, 215, 114286. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kong, F.; Li, X.; Shen, J. Artificial intelligence in microplastic detection and pollution control. Environ. Res. 2024, 262, 119812. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hardy, T.J.; Yoon, J.Y. Receptor-based detection of microplastics and nanoplastics: Current and future. Biosens. Bioelectron. 2023, 234, 115361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Jiao, S.; Li, Y.; Zhou, Y.; Zhang, X.; Maryam, B.; Liu, X. Microfluidic sensors for the detection of emerging contaminants in water: A review. Sci. Total Environ. 2024, 929, 172734. [Google Scholar] [CrossRef]
- Xu, N.; Lin, H.; Lin, J.M.; Cheng, J.; Wang, P.; Lin, L. Microfluidic Chip-Based Modeling of Three-Dimensional Intestine-Vessel-Liver Interactions in Fluorotelomer Alcohol Biotransformation. Anal. Chem. 2023, 95, 17064–17072. [Google Scholar] [CrossRef]
- Huang, Y.N.; Hsu, C.N.; Hou, C.Y.; Chen, S.Y.; Tain, Y.L. Resveratrol Butyrate Esters Reduce Hypertension in a Juvenile Rat Model of Chronic Kidney Disease Exacerbated by Microplastics. Nutrients 2024, 16, 4076. [Google Scholar] [CrossRef]
- Chen, J.; Spoljaric, S.; Calatayud-Sanchez, A.; Alvarez-Braña, Y.; Caruso, F. Engineering Metal-Phenolic Network Nanoparticles via Microfluidics. ACS Appl. Mater. Interfaces 2023, 15, 48050–48059. [Google Scholar] [CrossRef]
- Gu, Y.; Jin, L.; Wang, L.; Ma, X.; Tian, M.; Sohail, A.; Wang, J.; Wang, D. Preparation of Baicalin Liposomes Using Microfluidic Technology and Evaluation of Their Antitumor Activity by a Zebrafish Model. ACS Omega 2024, 9, 41289–41300. [Google Scholar] [CrossRef]
- Faramarzi, P.; Jang, W.; Oh, D.; Kim, B.; Kim, J.H.; You, J.B. Microfluidic Detection and Analysis of Microplastics Using Surface Nanodroplets. ACS Sens. 2024, 9, 1489–1498. [Google Scholar] [CrossRef]
- Akiyama, Y.; Egawa, T.; Koyano, K.; Moriwaki, H. Acoustic Focusing of Microplastics in Microchannels: A Promising Continuous Collection Approach. Sens. Actuators B Chem. 2020, 304, 127328. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Shang, L.; Yin, J.; Qian, Z.; Chen, C.; Yang, Y. Size-dependent neurotoxicity of micro- and nanoplastics in flowing condition based on an in vitro microfluidic study. Chemosphere 2022, 303, 135280. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Dou, J.; Hou, Q.; Cheng, J.; Jiang, X. Bioeffects of Inhaled Nanoplastics on Neurons and Alteration of Animal Behaviors through Deposition in the Brain. Nano Lett. 2022, 22, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Archonta, D.; Kubiseski, T.J.; Tandon, A.; Rezai, P. Microfluidic Electric Parallel Egg-Laying Assay and Application to in-Vivo Toxicity Screening of Microplastics Using C. Elegans. Sci. Total Environ. 2021, 783, 147055. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, Y.; Liu, Y.; Tian, P.; Yu, L.; Bai, L.; Zhou, F.; Yang, Y.; Cheng, Y.; Wang, F.; et al. Microfluidic-Based in Vitro Thrombosis Model for Studying Microplastics Toxicity. Lab A Chip 2022, 22, 1344–1353. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, J.; Kamm, R.D.; Wang, Y.; Wang, C. Validating antimetastatic effects of natural products in an engineered microfluidic platform mimicking tumor microenvironment. Mol. Pharm. 2014, 11, 2022–2029. [Google Scholar] [CrossRef]
- Kpeli, G.W.; Conrad, K.M.; Bralower, W.; Byrne, C.E.; Boue, S.M.; Burow, M.E.; Mondrinos, M.J. Xenohormetic Phytochemicals Inhibit Neovascularization in Microphysiological Models of Vasculogenesis and Tumor Angiogenesis. Adv. Biol. 2024, 8, e2300480. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.S.; Ha, S.K.; Choi, I.; Lee, J.M.; Sung, J.H. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic-pharmacodynamic (PK-PD) model. Biotechnol. Bioeng. 2017, 114, 432–443. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Rahman, M.S.; Nice, M.S.; Netema, B.N.; Islam, K.R.; Debnath, P.C.; Chowdhury, P.; Halder, M.; Zaman, S.; Ghosh, G.C.; et al. Application of machine learning and multivariate approaches for assessing microplastic pollution and its associated risks in the urban outdoor environment of Bangladesh. J. Hazard. Mater. 2024, 472, 134359. [Google Scholar] [CrossRef]
- Xie, H.; Wei, C.; Wang, W.; Chen, R.; Cui, L.; Wang, L.; Chen, D.; Yu, Y.L.; Li, B.; Li, Y.F. Screening the phytotoxicity of micro/nanoplastics through non-targeted metallomics with synchrotron radiation X-ray fluorescence and deep learning: Taking micro/nano polyethylene terephthalate as an example. J. Hazard. Mater. 2024, 463, 132886. [Google Scholar] [CrossRef]
- Ferreira, R.O.G.; Nag, R.; Gowen, A.; Xu, J.L. Deciphering the cytotoxicity of micro- and nanoplastics in Caco-2 cells through meta-analysis and machine learning. Environ. Pollut. 2024, 362, 124971. [Google Scholar] [CrossRef]
- Liu, C.; Zong, C.; Chen, S.; Chu, J.; Yang, Y.; Pan, Y.; Yuan, B.; Zhang, H. Machine learning-driven QSAR models for predicting the cytotoxicity of five common microplastics. Toxicology 2024, 508, 153918. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A.; Khandelwal, R.; Tanwar, P.; Madhavi, M.; Sharma, D.; Thakur, G.; Speck-Planche, A.; Singh, S.K. Artificial Intelligence, Big Data and Machine Learning Approaches in Precision Medicine & Drug Discovery. Curr. Drug Targets 2021, 22, 631–655. [Google Scholar] [PubMed]
- Zothantluanga, J.H.; Chetia, D.; Rajkhowa, S.; Umar, A.K. Unsupervised machine learning, QSAR modelling and web tool development for streamlining the lead identification process of antimalarial flavonoids. SAR QSAR Environ. Res. 2023, 34, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, S.; Fang, C.; Jia, J.; Lin, L.; Yuan, T. Machine learning and SHAP value interpretation for predicting comorbidity of cardiovascular disease and cancer with dietary antioxidants. Redox Biol. 2025, 79, 103470. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Zhao, Y.; Shao, X.; Wang, M.; Ma, F.; Yang, L.; Nie, M.; Jin, P.; Yao, K.; et al. Metabolomic machine learning predictor for diagnosis and prognosis of gastric cancer. Nat. Commun. 2024, 15, 1657. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Cai, C.; Ding, Y.; Kim, R.S.; Lipchik, C.; Gavin, P.G.; Yothers, G.; Allegra, C.J.; Petrelli, N.J.; et al. Machine Learning Predicts Oxaliplatin Benefit in Early Colon Cancer. J. Clin. Oncol. 2024, 42, 1520–1530. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, F.; Zhang, W.; Guo, Y.; Jiang, X.; Yan, X.; Ke, Y. Machine learning-based identification of a cell death-related signature associated with prognosis and immune infiltration in glioma. J. Cell. Mol. Med. 2024, 28, e18463. [Google Scholar] [CrossRef]
- Guo, F.; Yang, X.; Hu, C.; Li, W.; Han, W. Network Pharmacology Combined with Machine Learning to Reveal the Action Mechanism of Licochalcone Intervention in Liver Cancer. Int. J. Mol. Sci. 2023, 24, 15935. [Google Scholar] [CrossRef]
- Lu, Y.; Shan, L.; Cheng, X.; Zhu, X.L. Exploring the mechanism underlying the therapeutic effects of butein in colorectal cancer using network pharmacology and single-cell RNA sequencing data. J. Gene Med. 2024, 26, e3628. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, C.; Chen, X.; Feng, Y.; Feng, J.; Zhang, R.; Ouyang, F.; Li, X.; Tan, Z.; Deng, L.; et al. Non invasive prediction of perineural invasion in intrahepatic cholangiocarcinoma by clinicoradiological features and computed tomography radiomics based on interpretable machine learning: A multicenter cohort study. Int. J. Surg. 2024, 110, 1039–1051. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Weyde, K.V.F.; Aase, H.; Engel, S.M.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Brantsæter, A.L.; Haug, L.S.; et al. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ. Res. 2021, 202, 111692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sokratian, A.; Duda, A.M.; Xu, E.; Stanhope, C.; Fu, A.; Strader, S.; Li, H.; Yuan, Y.; Bobay, B.G.; et al. Anionic nanoplastic contaminants promote Parkinson’s disease-associated α-synuclein aggregation. Sci. Adv. 2023, 9, eadi8716. [Google Scholar] [CrossRef]
- Marin, M.; Annunziato, K.M.; Tompach, M.C.; Liang, W.; Zahn, S.M.; Li, S.; Doherty, J.; Lee, J.; Clark, J.M.; Park, Y.; et al. Maternal PFOS exposure affects offspring development in Nrf2-dependent and independent ways in zebrafish (Danio rerio). Aquat. Toxicol. 2024, 271, 106923. [Google Scholar] [CrossRef]
- Sant, K.E.; Sinno, P.P.; Jacobs, H.M.; Timme-Laragy, A.R. Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio. Aquat. Toxicol. 2018, 198, 92–102. [Google Scholar] [CrossRef]
- Currie, S.D.; Benson, D.B.; Xie, Z.R.; Wang, J.S.; Tang, L. Utilization of Artificial Intelligence Coupled with a High-Throughput, High-Content Platform in the Exploration of Neurodevelopmental Toxicity of Individual and Combined PFAS. J. Xenobiotics 2025, 15, 24. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Li, M.; Wei, F.; Liao, Y.; Xi, B. Microplastics in the bloodstream can induce cerebral thrombosis by causing cell obstruction and lead to neurobehavioral abnormalities. Sci. Adv. 2025, 11, eadr8243. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Qian, Q.; Pu, Q.; Li, L.; Wu, J.; Cheng, G.; Cheng, Y.; Wang, X.; Wang, H. Polylactic acid microplastics before and after aging induced neurotoxicity in zebrafish by disrupting the microbiota-gut-brain axis. J. Hazard. Mater. 2025, 488, 137306. [Google Scholar] [CrossRef]
- Chwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, A.; Kumar, D.; Prajapati, K.B.; Mahajan, A.K.; Pant, D.; Yadav, A.; Giri, A.; Manda, S.; Bhandari, S.; et al. Microplastics influencing aquatic environment and human health: A review of source, determination, distribution, removal, degradation, management strategy and future perspective. J. Environ. Manag. 2025, 375, 124249. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, A.A.; van der Ent, W.; Lenters, V.; Iszatt, N.; Stigum, H.; Lyche, J.L.; Berg, V.; Kirstein-Smardzewska, K.J.; Esguerra, C.V.; Eggesbø, M. Perinatal exposure to potential endocrine disrupting chemicals and autism spectrum disorder: From Norwegian birth cohort to zebrafish studies. Environ. Int. 2023, 181, 108271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Ren, Q.; Song, C.; Yu, J.; Cai, Y.; Chen, D. Negative response to immunotherapy in dMMR or MSI-H gastric cancer with APC and PTEN mutations: A case report. Front. Oncol. 2024, 14, 1484802. [Google Scholar] [CrossRef]
- Shitara, K.; Muro, K.; Watanabe, J.; Yamazaki, K.; Ohori, H.; Shiozawa, M.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Baseline ctDNA gene alterations as a biomarker of survival after panitumumab and chemotherapy in metastatic colorectal cancer. Nat. Med. 2024, 30, 730–739. [Google Scholar] [CrossRef]
- Jang, H.; Chen, J.; Iakoucheva, L.M.; Nussinov, R. Cancer and Autism: How PTEN Mutations Degrade Function at the Membrane and Isoform Expression in the Human Brain. J. Mol. Biol. 2023, 435, 168354. [Google Scholar] [CrossRef]
- Cummings, K.; Watkins, A.; Jones, C.; Dias, R.; Welham, A. Behavioural and psychological features of PTEN mutations: A systematic review of the literature and meta-analysis of the prevalence of autism spectrum disorder characteristics. J. Neurodev. Disord. 2022, 14, 1. [Google Scholar] [CrossRef]
- Busch, R.M.; Srivastava, S.; Hogue, O.; Frazier, T.W.; Klaas, P.; Hardan, A.; Martinez-Agosto, J.A.; Sahin, M.; Eng, C.; on behalf of the Developmental Synaptopathies Consortium; et al. Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl. Psychiatry 2019, 9, 253. [Google Scholar] [CrossRef]
- Smith, I.N.; Dawson, J.E.; Eng, C. Comparative Protein Structural Network Analysis Reveals C-Terminal Tail Phosphorylation Structural Communication Fingerprint in PTEN-Associated Mutations in Autism and Cancer. J. Phys. Chem. B 2023, 127, 634–647. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Shi, X.; Wang, Y.; Xu, S. Polystyrene microplastics with different sizes induce the apoptosis and necroptosis in liver through the PTEN/PI3K/AKT/autophagy axis. Sci. Total Environ. 2023, 899, 165461. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Zhu, X.; Li, S.; Rihan, N.; Yao, Z.; Sun, Z.; Gao, P.; Zhao, Y.; Lai, Q. Polystyrene nanoplastics induce apoptosis, histopathological damage, and glutathione metabolism disorder in the intestine of juvenile East Asian river prawns (Macrobrachium nipponense). Sci. Total Environ. 2024, 954, 176718. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gong, X.; Zhou, Y.; Geng, Q.; Jiang, Y.; Yao, L.; Qu, M.; Tan, Z. Integrated evidence of transcriptional, metabolic, and intestinal microbiota changes in Ruditapes philippinarum due to perfluorooctanoic acid-induced immunotoxicity. Sci. Total Environ. 2024, 916, 170341. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Du, K.; Jin, H.; Chen, Y.; Jiang, Y.; Zhang, W.; Chen, D.; Zheng, S.; Cao, L. Evidence of promoting effects of 6:2 Cl-PFESA on hepatocellular carcinoma proliferation in humans: An ideal alternative for PFOS in terms of environmental health? Environ. Int. 2024, 186, 108582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, J.; Liu, A.; Liu, Q.Q.; Sun, T.; Li, X.; Du, Y.; Li, J.; Wang, B.; Yang, Q. Luteolin in the Qi Bi Anshen decoction improves propionic acid-induced autism-like behavior in rats by inhibiting LRP1/MMP9. Phytomedicine 2023, 118, 154965. [Google Scholar] [CrossRef]
- Alsubaiei, S.R.M.; Alfawaz, H.A.; Bhat, R.S.; El-Ansary, A. Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups. Metabolites 2023, 13, 738. [Google Scholar] [CrossRef]
- Gai, J.; Xing, J.; Wang, Y.; Lei, J.; Zhang, C.; Zhang, J.; Tang, J. Exploration of potential targets and mechanisms of Naringenin in treating autism spectrum disorder via network pharmacology and molecular docking. Medicine 2022, 101, e31787. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, J.; Wu, K.; Cao, B.; Li, X.; Jiang, F.; Hu, Z.; Xia, K.; Li, J.-D. Suppression of Akt-mTOR pathway rescued the social behavior in Cntnap2-deficient mice. Sci. Rep. 2019, 9, 3041. [Google Scholar] [CrossRef]
- Viana, C.E.; Bortolotto, V.C.; Araujo, S.M.; Dahleh, M.M.M.; Machado, F.R.; de Souza Pereira, A.; Moreira de Oliveira, B.P.; Leimann, F.V.; Gonçalves, O.H.; Prigol, M.; et al. Lutein-loaded nanoparticles reverse oxidative stress, apoptosis, and autism spectrum disorder-like behaviors induced by prenatal valproic acid exposure in female rats. Neurotoxicology 2023, 94, 223–234. [Google Scholar] [CrossRef]
- Kisku, A.; Nishad, A.; Agrawal, S.; Paliwal, R.; Datusalia, A.K.; Gupta, G.; Singh, S.K.; Dua, K.; Sulakhiya, K. Recent developments in intranasal drug delivery of nanomedicines for the treatment of neuropsychiatric disorders. Front. Med. 2024, 11, 1463976. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, X.; Zeng, P.; Hu, L.; Huang, Y.; Guo, X.; Chen, Q.; Wang, Y.; Lai, L.; Xue, A.; et al. Exposure to PFOA, PFOS, and PFHxS induces Alzheimer’s disease-like neuropathology in cerebral organoids. Environ. Pollut. 2024, 363, 125098. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuto, M.; Lombardo, C.M.G.; Lo Sasso, B.; Di Fatta, E.; Ferri, R.; Trovato Salinaro, A. Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer. Toxics 2025, 13, 438. https://doi.org/10.3390/toxics13060438
Scuto M, Lombardo CMG, Lo Sasso B, Di Fatta E, Ferri R, Trovato Salinaro A. Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer. Toxics. 2025; 13(6):438. https://doi.org/10.3390/toxics13060438
Chicago/Turabian StyleScuto, Maria, Cinzia Maria Grazia Lombardo, Bruna Lo Sasso, Eleonora Di Fatta, Raffaele Ferri, and Angela Trovato Salinaro. 2025. "Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer" Toxics 13, no. 6: 438. https://doi.org/10.3390/toxics13060438
APA StyleScuto, M., Lombardo, C. M. G., Lo Sasso, B., Di Fatta, E., Ferri, R., & Trovato Salinaro, A. (2025). Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer. Toxics, 13(6), 438. https://doi.org/10.3390/toxics13060438








