Assessment of Unintentional Acute Pesticide Poisoning (UAPP) Amongst Cotton Farmers in Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Population, Sample Size Determination, and Sampling
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Social Demographic Characteristics of Respondents
3.2. Conditions of Use of Pesticides in Cotton Production
3.3. Pesticide Poisoning
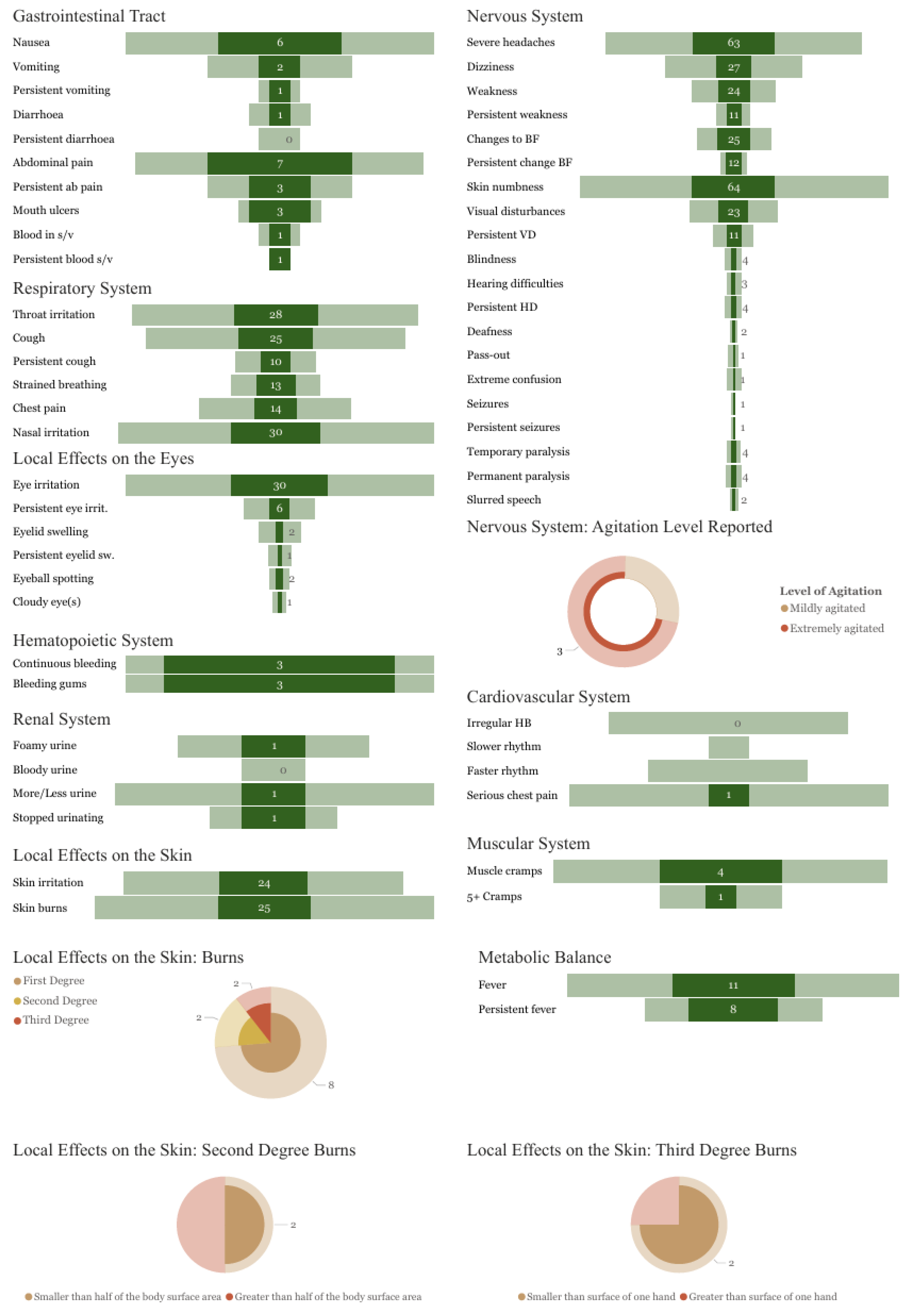
3.4. Symptoms Reported
4. Discussion
4.1. Pesticides of Concern
4.2. Health and Economic Consequences of UAPP
4.3. Conditions of Use
4.4. Pests Targeted by HHPs and Available Alternatives
| Method | Example | Country | Reference |
|---|---|---|---|
| Cultural control | Cotton intercropped with maize had significantly less damage (0.5%) than monocropped cotton (5–9%). In the cotton–maize intercropping system, H. armigera preferred to lay eggs on the maize plants. | China | Chi, Zhang and Dong [88] |
| Cotton–basil intercropping significantly reduced total pest infestation and led to a 50% reduced abundance of the pink bollworm (Pectinophora gossypiella) in comparison with non-intercropped plots. | Egypt | Schader, et al. [102] | |
| Intercropping cotton with sesame and the release of Trichogramma. chilonis adults alternated with neem provided significantly better control of spotted bollworm, Earias vittella, and pink bollworm, Pectinophora gossypiella, compared to the control. | India | Devi, et al. [103] | |
| Field experiments conducted over a 6-year period showed that the densities of H. armigera, Earias spp., and Sudan bollworm, Diparopsis watersi, were significantly lower in topped compared with non-topped cotton plots. | Mali | Renou, et al. [104] | |
| Biological control | Application of a supplementary food spray product attracted beneficial insects and significantly reduced the number of pests (including Helicoverpa spp.) and increased cotton yields and profitability. | Ethiopia/Benin | Mensah, Vodouhe, Sanfillippo, Assogba and Monday [83]; Amera, Mensah and Belay [84] |
| Biopesticdes | Application of neem oil in the laboratory and the field resulted in a considerable reduction in the hatching of the eggs of H. armigera. | India | Patel, et al. [105] |
| 100% mortality of red cotton stainers was reported following treatment with B. bassiana isolates. | India | Moorthi, et al. [106] | |
| B. bassiana and Metarhizium rileyi were as effective as lambda-cyhalothrin or chlorpyrifos against cotton stainers, with no significant difference in seed cotton yield. | South Africa | Malinga and Laing [97] | |
| Treatment with Nomuraea rileyi led to 87% mortality of H. armigera. | South Africa | Hatting [107] | |
| Treatment with B. thuringiensis led to a 95–100% and 76% H. armigera mortality under laboratory and field conditions, respectively. | South Africa | Malinga and Laing [96] | |
| Integrated package | CABI Plantwise guidance for H. armigera management in cotton in Tanzania recommends regular scouting for pests and using neem-based products a max. of 3 times (usually 2.5–3 litres/ha or 50–60 mL/20 litres of water, or 20–50 g of neem seed cake or powder/litre water) to control small 1st–2nd instars of larvae if the ratio is less than 1:2 (bollworm: beneficial organisms). Field sanitation involving the removal of cotton plant debris and ratoon cotton as soon as harvesting is over is also recommended. | Tanzania | Ndomba and Kitandu [90] |
| CABI Plantwise guidance for D. cingulatus management in Tanzania includes regular monitoring, hand-picking, and destroying the bugs in small plots at the beginning of infestations or spraying fresh custard apple leaf extract or pyrethrum powder. Field sanitation involving the removal of cotton plant debris and ratoon cotton as soon as harvesting is over is also recommended. | Tanzania | Mussa [77] |
4.5. Limitations
5. Conclusions and Recommendations
- Phase out highly hazardous pesticides and prioritise the elimination of the four pesticides most commonly involved in poisoning incidents in this study—namely profenofos, cypermethrin, chlorpyrifos, and lambda-cyhalothrin.
- Make less hazardous alternatives available and support farmers to access them.
- Enforce regulatory control measures to prevent exposure to banned or restricted pesticides.
- Provide information and training to farmers and farm workers on pesticide hazards and on ways to reduce pesticide-related risks, including PPE and, more importantly, on alternative pest management strategies that are based on agroecological or IPM principles.
- Scale up community self-surveillance to complement poison centre/hospital-based pesticide poisoning surveillance.
- More research into the health effects of co-formulations is needed, particularly those containing pyrethroids and organophosphates.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eddleston, M.; Karalliedde, L.; Buckley, N.; Fernando, R.; Hutchinson, G.; Isbister, G.; Konradsen, F.; Murray, D.; Piola, J.C.; Senanayake, N.; et al. Pesticide poisoning in the developing world--a minimum pesticides list. Lancet 2002, 360, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Thundiyil, J.G.; Stober, J.; Besbelli, N.; Pronczuk, J. Acute pesticide poisoning: A proposed classification tool. Bull. World Health Organ. 2008, 86, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Jors, E.; Neupane, D.; London, L. Pesticide Poisonings in Low- and Middle-Income Countries. Environ. Health Insights 2018, 12, 1178630217750876. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Response to Jors et al, Environmental Health Insights. Environ. Health Insights 2018, 12, 1178630218788554. [Google Scholar] [CrossRef]
- Robinson, D.E.; Stuart, A.M.; Willis, S.; Salmon, J.P.; Ramjattan, J.; Ganpat, W.; Williamson, S.; Tyrell, K.F.; Saravanakumar, D. Assessment of unintentional acute pesticide poisoning among smallholder vegetable farmers in Trinidad and Jamaica. Front. Public Health 2024, 12, 1470276. [Google Scholar] [CrossRef]
- Tomenson, J.A.; Matthews, G.A. Causes and types of health effects during the use of crop protection chemicals: Data from a survey of over 6,300 smallholder applicators in 24 different countries. Int. Arch. Occup. Environ. Health 2009, 82, 935–949. [Google Scholar] [CrossRef]
- Kousse, J.N.D.; Ilboudo, S.; Ouedraogo, J.; Hunsmann, M.; Ouedraogo, G.G.; Ouedraogo, M.; Kini, F.B.; Ouedraogo, S. Self-reported health effects of pesticides among cotton farmers from the Central-West region in Burkina Faso. Toxicol. Rep. 2023, 11, 273–282. [Google Scholar] [CrossRef]
- Mew, E.J.; Padmanathan, P.; Konradsen, F.; Eddleston, M.; Chang, S.S.; Phillips, M.R.; Gunnell, D. The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review. J. Affect. Disord. 2017, 219, 93–104. [Google Scholar] [CrossRef]
- Ntzani, E.E.; Ntritsos, G.C.M.; Evangelou, E.; Tzoulaki, I. Literature review on epidemiological studies linking exposure to pesticides and health effects. EFSA Support. Publ. 2013, 10, 497E. [Google Scholar] [CrossRef]
- Koutros, S.; Silverman, D.T.; Alavanja, M.C.; Andreotti, G.; Lerro, C.C.; Heltshe, S.; Lynch, C.F.; Sandler, D.P.; Blair, A.; Beane Freeman, L.E. Occupational exposure to pesticides and bladder cancer risk. Int. J. Epidemiol. 2016, 45, 792–805. [Google Scholar] [CrossRef]
- Shekhar, C.; Khosya, R.; Thakur, K.; Mahajan, D.; Kumar, R.; Kumar, S.; Sharma, A.K. A systematic review of pesticide exposure, associated risks, and long-term human health impacts. Toxicol. Rep. 2024, 13, 101840. [Google Scholar] [CrossRef] [PubMed]
- UNEP; WHO; FAO (Eds.) Chapter 6/12: Current Pesticide Risk Reduction and Risk Management. In Environmental and Health Impacts of Pesticides and Fertilizers and Ways of Minimizing Them; United Nations Environment Programme: Geneva, Switzerland; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; p. 20. [Google Scholar]
- UNEP. Report on the Costs of Inaction on the Sound Management of Chemicals, Job Number: DTI/1551/GE.; United Nations Environment Programme: Geneva, Switzerland, 2013. [Google Scholar]
- Sherwood, S.; Cole, D.; Crissman, C.; Paredes, M. From pesticides to people: Improving ecosystem health in the northern Andes. In The Pesticide Detox: Towards a More Sustainable Agriculture; Pretty, J., Ed.; Earthscan: London, UK, 2005; pp. 147–164. [Google Scholar]
- Stephanie, W. Understanding the Full Costs of Pesticides: Experience from the Field, with a Focus on Africa. In Pesticides—The Impacts of Pesticides Exposure; Margarita, S., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 25–48. [Google Scholar]
- London, L.; Bailie, R. Challenges for improving surveillance for pesticide poisoning: Policy implications for developing countries. Int. J. Epidemiol. 2001, 30, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Laborde, A.; Tomasina, F.; Bianchi, F.; Bruné, M.N.; Buka, I.; Comba, P.; Corra, L.; Cori, L.; Duffert, C.M.; Harari, R.; et al. Children’s health in Latin America: The influence of environmental exposures. Environ. Health Perspect. 2015, 123, 201–209. [Google Scholar] [CrossRef]
- Murray, D.; Wesseling, C.; Keifer, M.; Corriols, M.; Henao, S. Surveillance of pesticide-related illness in the developing world: Putting the data to work. Int. J. Occup. Environ. Health 2002, 8, 243–248. [Google Scholar] [CrossRef]
- Lekei, E.E.; Ngowi, A.V.; London, L. Undereporting of acute pesticide poisoning in Tanzania: Modelling results from two cross-sectional studies. Environ. Health 2016, 15, 118. [Google Scholar] [CrossRef]
- Lekei, E.E.; Ngowi, A.V.; London, L. Farmers’ knowledge, practices and injuries associated with pesticide exposure in rural farming villages in Tanzania. BMC Public Health 2014, 14, 389. [Google Scholar] [CrossRef]
- UiO. DHIS2. Available online: https://dhis2.org/ (accessed on 10 February 2025).
- Kishi, M. The health impacts of pesticides: What do we now know? In The Pesticide Detox; Routledge: London, UK, 2012; pp. 23–38. [Google Scholar]
- Murphy, H.H.; Hoan, N.P.; Matteson, P.; Abubakar, A.L. Farmers’ self-surveillance of pesticide poisoning: A 12-month pilot in northern Vietnam. Int. J. Occup. Environ. Health 2002, 8, 201–211. [Google Scholar] [CrossRef]
- Mancini, F.; Van Bruggen, A.H.; Jiggins, J.L.; Ambatipudi, A.C.; Murphy, H. Acute pesticide poisoning among female and male cotton growers in India. Int. J. Occup. Environ. Health 2005, 11, 221–232. [Google Scholar] [CrossRef]
- Dasgupta, S.; Meisner, C.; Wheeler, D.; Xuyen, K.; Thi Lam, N. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int. J. Hyg. Environ. Health 2007, 210, 121–132. [Google Scholar] [CrossRef]
- Ngowi, A.; Semali, I. Controlling Pesticide Poisoning at Community Level in Lake Eyasi Basin, Karatu District, Tanzania. Eur. J. Oncol. 2011, 16, 139–148. [Google Scholar]
- PAN. Communities in Peril: Global Report on Health Impacts of Pesticide Use in Agriculture; Pesticide Action Network International: Penang, Malaysia, 2010. [Google Scholar]
- FAO; WHO. International Code of Conduct on Pesticide Management: Guidelines on Developing a Reporting System for Health and Environmental Incidents Resulting from Exposure to Pesticide; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- FAO. The SHPF Toolkit: A Toolkit to Help You to Monitor and Report Incidents of Pesticide Poisoning Caused by Severely Hazardous Pesticide Formulations in Your Country Under Article 6 of the Rotterdam Convention; Food and Agriculture Organization: Rome, Italy, 2017. [Google Scholar]
- Anderson, K.A.; Seck, D.; Hobbie, K.A.; Traore, A.N.; McCartney, M.A.; Ndaye, A.; Forsberg, N.D.; Haigh, T.A.; Sower, G.J. Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2014, 369, 20130110. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.E.; Scott, R.P.; Blaustein, K.L.; Halbleib, M.L.; Sarr, M.; Jepson, P.C.; Anderson, K.A. Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci. 2016, 3, 160433. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Lewandowski, P.; Brumby, S. Cholinesterase Research Outreach Project (CROP): Measuring cholinesterase activity and pesticide use in an agricultural community. BMC Public Health 2015, 15, 748. [Google Scholar] [CrossRef] [PubMed]
- Hamis, M. Tanzania’s Cotton Revolution: Doubling Production for Economic Growth. Available online: https://www.digest.tz/tanzanias-cotton-revolution-doubling-production-for-economic-growth/ (accessed on 10 February 2025).
- UNCTAD. Cotton and Its By-Products in Tanzania: Analysis of Cotton By-Products Survey; UNCTAD: Geneva, Switzerland, 2019. [Google Scholar]
- Fishel, F.; Andre, P. Pesticide Poisoning Symptoms and First Aid. Available online: https://extension.missouri.edu/publications/g1915 (accessed on 19 June 2024).
- PAN. PAN International List of Highly Hazardous Pesticides; Pesticide Action Network International: Hamburg, Germany, 2024. [Google Scholar]
- FAO; WHO. International Code of Conduct on Pesticide Management: Guidelines on Highly Hazardous Pesticides; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Rome, Italy, 2016. [Google Scholar]
- PAN. PAN International Consolidated List of Banned Pesticides, 6th ed.; Pesticide Action Network International: Penang, Malaysia, 2022. [Google Scholar]
- Manjunath, R.; Hemavathi; Reddy, N.T.; Chaithra, C.; Mishra, P. Pesticides and its toxicity. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 419–428. [Google Scholar]
- Ma, M.; Dong, S.; Jin, W.; Zhang, C.; Zhou, W. Fate of the organophosphorus pesticide profenofos in cotton fiber. J. Environ. Sci. Health Part. B 2019, 54, 70–75. [Google Scholar] [CrossRef]
- Robb, E.; Baker, M. Organophosphate Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470430 (accessed on 10 February 2025).
- Eddleston, M.; Worek, F.; Eyer, P.; Thiermann, H.; Von Meyer, L.; Jeganathan, K.; Sheriff, M.H.; Dawson, A.H.; Buckley, N.A. Poisoning with the S-Alkyl organophosphorus insecticides profenofos and prothiofos. QJM 2009, 102, 785–792. [Google Scholar] [CrossRef]
- Raj, A.; Kumar, A.; Khare, P.K. The looming threat of profenofos organophosphate and microbes in action for their sustainable degradation. Environ. Sci. Pollut. Res. 2024, 31, 14367–14387. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Saber, H. Recent advances in the treatment of organophosphorous poisonings. Iran. J. Med. Sci. 2012, 37, 74–91. [Google Scholar]
- Ranjan, A.; Jindal, T. Toxicology of Organophosphate Poisoning in Human. In Toxicology of Organophosphate Poisoning: New Insights; Ranjan, A., Jindal, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 27–43. [Google Scholar]
- Stockholm_Convention. Chemicals proposed for listing Under the Convention. Available online: https://pops.int/TheConvention/ThePOPs/ChemicalsProposedforListing/tabid/2510/Default.aspx (accessed on 10 February 2025).
- Fuhrimann, S.; Wan, C.; Blouzard, E.; Veludo, A.; Holtman, Z.; Chetty-Mhlanga, S.; Dalvie, M.A.; Atuhaire, A.; Kromhout, H.; Röösli, M.; et al. Pesticide Research on Environmental and Human Exposure and Risks in Sub-Saharan Africa: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 259. [Google Scholar] [CrossRef]
- Sun, X. Compound Preparation of Efficient Cypermethrin and Chlorpyrifos. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20110720&DB=EPODOC&locale=&CC=CN&NR=102125050A&KC=A&ND=1# (accessed on 10 February 2025).
- Ray, D.E.; Forshaw, P.J. Pyrethroid insecticides: Poisoning syndromes, synergies, and therapy. J. Toxicol. Clin. Toxicol. 2000, 38, 95–101. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- Ramchandra, A.M.; Chacko, B.; Victor, P.J. Pyrethroid Poisoning. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2019, 23, S267–S271. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Chang, S.-S.; Chen, H.-Y.; Tsai, K.-F.; Lee, W.-C.; Wang, I.-K.; Lee, C.-H.; Chen, C.-Y.; Liu, S.-H.; Weng, C.-H. Human poisoning with chlorpyrifos and cypermethrin pesticide mixture: Assessment of clinical outcome of cases admitted in a tertiary care hospital in Taiwan. Int. J. Gen. Med. 2023, 16, 4795–4804. [Google Scholar] [CrossRef] [PubMed]
- Iyyadurai, R.; Peter, J.V.; Immanuel, S.; Begum, A.; Zachariah, A.; Jasmine, S.; Abhilash, K.P.P. Organophosphate-pyrethroid combination pesticides may be associated with increased toxicity in human poisoning compared to either pesticide alone. Clin. Toxicol. 2014, 52, 538–541. [Google Scholar] [CrossRef]
- Scheepers, L.D.; Freercks, R.; Merwe, E.V. Acute cypermethrin and other pyrethroid poisoning—An organophosphate-like poisoning: A case report and review. Toxicol. Rep. 2023, 11, 107–110. [Google Scholar] [CrossRef]
- Shilpakar, O.; Karki, B. Cypermethrin poisoning manifesting with prolonged bradycardia: A case report. Toxicol. Rep. 2021, 8, 10–12. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, B.; Rohatgi, S.; Yadav, B. Cypermethrin toxicity: A review. J. Forensic. Sci. Crim. Investig. 2018, 9, 555767. [Google Scholar]
- NPIC. Lambda-Cyhalothrin (General Fact Sheet); National Pesticide Information Center (NPIC): Corvallis, OR, USA, 2001. [Google Scholar]
- Wang, Q.; Xia, X.; Deng, X.; Li, N.; Wu, D.; Zhang, L.; Yang, C.; Tao, F.; Zhou, J. Lambda-cyhalothrin disrupts the up-regulation effect of 17beta-estradiol on post-synaptic density 95 protein expression via estrogen receptor alpha-dependent Akt pathway. J Environ. Sci. 2016, 41, 252–260. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Liu, W.; Xu, C.; Wang, L.; Gan, J. Estrogenic activity of lambda-cyhalothrin in the MCF-7 human breast carcinoma cell line. Environ. Toxicol. Chem. 2008, 27, 1194–1200. [Google Scholar] [CrossRef]
- EPA. Incident Data System; United States Environmental Protection Agency (EPA): Washington, DC, USA, 2024. [Google Scholar]
- Health_Canada. Proposed Re-Evaluation Decision Lambda-Cyhalothrin; Pest Management Regulatory Agency: Ottawa, ON, Canada, 2017. [Google Scholar]
- Mukherjee, S. Retrospective Designs in Sports Injury Surveillance Studies: All is not Lost. Sports Exerc. Med.-Open J. 2015, 1, 164–166. [Google Scholar] [CrossRef]
- Ajayi, O.C.; Akinnifesi, F.K. Farmers’ understanding of pesticide safety labels and field spraying practices: A case study of cotton farmers in northern Côte d’Ivoire. Sci. Res. Essays 2007, 2, 204–210. [Google Scholar]
- Utyasheva, L.; Rother, H.-A.; London, L.; Eddleston, M. Stop blaming the farmer: Dispelling the myths of ‘misuse’ and ‘safe’ use of pesticides to protect health and human rights. J. Hum. Rights 2024, 23, 231–252. [Google Scholar] [CrossRef]
- Ngowi, A.; Mrema, E.; Kishinhi, S. Pesticide Health and Safety Challenges Facing Informal Sector Workers: A Case of Small-scale Agricultural Workers in Tanzania. New Solut. J. Environ. Occup. Health Policy NS 2016, 26, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Maumbe, B.M.; Swinton, S.M. Hidden health costs of pesticide use in Zimbabwe’s smallholder cotton growers. Soc. Sci. Med. 2003, 57, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In Integrated Pest Management: Innovation-Development Process: Volume 1; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Nederland, 2009; pp. 89–111. [Google Scholar]
- Antle, J.M.; Pingali, P.L. Pesticides, Productivity, and Farmer Health: A Philippine Case Study. Am. J. Agric. Econ. 1994, 76, 418–430. [Google Scholar] [CrossRef]
- Mwakalasya, W.N.; Mamuya, S.H.; Moen, B.E.; Ngowi, A.V. Self-Reported Pesticide Exposure During Pregnancy and Pesticide-Handling Knowledge Among Small-Scale Horticulture Women Workers in Tanzania, a Descriptive Cross-Sectional Study. Int. J. Environ. Res. Public Health 2024, 22, 40. [Google Scholar] [CrossRef]
- Mrema, E.J.; Ngowi, A.V.; Kishinhi, S.S.; Mamuya, S.H. Pesticide Exposure and Health Problems Among Female Horticulture Workers in Tanzania. Environ. Health Insights 2017, 11, 1178630217715237. [Google Scholar] [CrossRef]
- Asmare, B.A.; Freyer, B.; Bingen, J. Women in agriculture: Pathways of pesticide exposure, potential health risks and vulnerability in sub-Saharan Africa. Environ. Sci. Eur. 2022, 34, 89. [Google Scholar] [CrossRef]
- Jain, D.; Kumar Verma, R.; Sharma, V.; Kaur, A.; Rai, A.R.; Kumari, P.; Nagar, V.; Singh Sankhla, M.; Parihar, K. Associations between high levels pesticide and adverse reproductive outcomes in females: A comprehensive review. Mater. Today Proc. 2023, 95, 50–60. [Google Scholar] [CrossRef]
- Garrigou, A.; Laurent, C.; Berthet, A.; Colosio, C.; Jas, N.; Daubas-Letourneux, V.; Jackson Filho, J.M.; Jouzel, J.N.; Samuel, O.; Baldi, I.; et al. Critical review of the role of PPE in the prevention of risks related to agricultural pesticide use. Saf. Sci. 2020, 123, 104527. [Google Scholar] [CrossRef]
- Kishi, M.; Hirschhorn, N.; Djajadisastra, M.; Satterlee, L.N.; Strowman, S.; Dilts, R. Relationship of pesticide spraying to signs and symptoms in Indonesian farmers. Scand. J. Work. Environ. Health 1995, 21, 124–133. [Google Scholar] [CrossRef]
- Laughlin, J.; Newburn, K.; Gold, R.E. Pyrethroid insecticides and formulations as factors in residues remaining in apparel fabrics after laundering. Bull. Environ. Contam. Toxicol. 1991, 47, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Surgan, M. Unidentified Inert Ingredients in Pesticides: Implications for Human and Environmental Health. Environ. Health Perspect. 2006, 114, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Mussa, P. Red Cotton Stainer Bug on Cotton; CABI: Wallingford, UK, 2015. [Google Scholar]
- Mgomba, H.; Nthenga, I.; Nyirenda, A.; Bwalya, C.; Mung’ambata, M.M.; Kakumbi, C.; Kasunga, K.J.; Luangala, M.S.A.; Chipabika, G.; Chisunka, B.; et al. Bollworm in Cotton—Zambia; CABI: Wallingford, UK, 2024. [Google Scholar]
- Janssen, A.; van Rijn, P.C.J. Pesticides do not significantly reduce arthropod pest densities in the presence of natural enemies. Ecol. Lett. 2021, 24, 2010–2024. [Google Scholar] [CrossRef] [PubMed]
- Hardin, M.R.; Benrey, B.; Coll, M.; Lamp, W.O.; Roderick, G.K.; Barbosa, P. Arthropod pest resurgence: An overview of potential mechanisms. Crop Prot. 1995, 14, 3–18. [Google Scholar] [CrossRef]
- Guedes, R.N.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-Induced Stress in Arthropod Pests for Optimized Integrated Pest Management Programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- CRDC. 2024-25 Cotton Pest Management Guide; Cotton Research and Development Corporation (CRDC): Narrabri, Australia, 2024. [Google Scholar]
- Mensah, R.K.; Vodouhe, D.S.; Sanfillippo, D.; Assogba, G.; Monday, P. Increasing organic cotton production in Benin West Africa with a supplementary food spray product to manage pests and beneficial insects. Int. J. Pest Manag. 2012, 58, 53–64. [Google Scholar] [CrossRef]
- Amera, T.; Mensah, R.K.; Belay, A. Integrated pest management in a cotton-growing area in the Southern Rift Valley region of Ethiopia: Development and application of a supplementary food spray product to manage pests and beneficial insects. Int. J. Pest Manag. 2017, 63, 185–204. [Google Scholar] [CrossRef]
- PANGermany. Field Guide to Non-Chemical Pest Management in Cotton Production. Available online: www.oisat.org/downloads/field_guide_cotton.pdf (accessed on 10 February 2025).
- Eyhorn, F.; Ratter, S.G.; Ramakrishnan, M. Organic Cotton Crop Guide; FiBL Research Institute of Organic Agriculture: Frick, Switzerland, 2005. [Google Scholar]
- Watts, M.; Williamson, S. Replacing Chemicals with Biology: Phasing Out Highly Hazardous Pesticides with Agroecology; PAN Asia and the Pacific: Penang, Malaysia, 2015. [Google Scholar]
- Chi, B.-J.; Zhang, D.-M.; Dong, H.-Z. Control of cotton pests and diseases by intercropping: A review. J. Integr. Agric. 2021, 20, 3089–3100. [Google Scholar] [CrossRef]
- Mensah, R.K. Habitat diversity: Implications for the conservation and use of predatory insects of Helicoverpa spp. in cotton systems in Australia. Int. J. Pest Manag. 1999, 45, 91–100. [Google Scholar] [CrossRef]
- Ndomba, O.; Kitandu, L. American Bollworm in Cotton—Tanzania; CABI: Wallingford, UK, 2024. [Google Scholar]
- Llandres, A.L.; Almohamad, R.; Brévault, T.; Renou, A.; Téréta, I.; Jean, J.; Goebel, F.-R. Plant training for induced defense against insect pests: A promising tool for integrated pest management in cotton. Pest Manag. Sci. 2018, 74, 2004–2012. [Google Scholar] [CrossRef]
- Cherif, A.; Mansour, R.; Grissa-Lebdi, K. The egg parasitoidsTrichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 2021, 31, 661–693. [Google Scholar] [CrossRef]
- Costa, M.A.; Farias, E.S.; Passos, L.C.; Carvalho, V.C.; Carvalho, G.A. Side effects of insecticides applied to cotton on adult Trichogramma pretiosum by three exposure routes. Pest Manag. Sci. 2022, 78, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Mensah, R.K. Development of an integrated pest management programme for cotton. Part 1: Establishing and utililizing natural enemies. Int. J. Pest Manag. 2002, 48, 87–94. [Google Scholar] [CrossRef]
- Mensah, R.K. Development of an integrated pest management programme for cotton. Part 2: Integration of a lucerne/cotton interplant system, food supplement sprays with biological and synthetic insecticides. Int. J. Pest Manag. 2002, 48, 95–105. [Google Scholar] [CrossRef]
- Malinga, L.N.; Laing, M.D. Role of Microbial Biopesticides as an Alternative to Insecticides in Integrated Pest Management of Cotton Pests. In Insecticides—Impact and Benefits of Its Use for Humanity; IntechOpen: London, UK, 2022. [Google Scholar]
- Malinga, L.N.; Laing, M.D. Efficacy of three biopesticides against cotton pests under field conditions in South Africa. Crop Prot. 2021, 145, 105578. [Google Scholar] [CrossRef]
- Textile_Exchange. Organic Cotton Market Report; Textile Exchange: Burbank, CA, USA, 2022. [Google Scholar]
- Williamson, S. Natural Enemies and Farmer Decision-making. Biocontrol News Inf. 2001, 22, 91N–94N. [Google Scholar]
- Williamson, S.; Belay, A.; Zemenu, G.; Gemeda, K.; Asaminew, N.; Amera, T.; Horgan, F.G.; Stuart, A.M.; Willis, S.; Salmon, J.; et al. Transitioning from harmful insecticides to agroecological IPM with vegetable smallholders in Ethiopia’s Central Rift Valley. J. Integr. Pest Manag. 2025; in review. [Google Scholar]
- Williamson, S.F.J. Understanding natural enemies: A review of training and information in the practical use of biological control. Biocontrol News Inf. 1998, 19, 117N–125N. [Google Scholar]
- Schader, C.; Zaller, J.G.; Köpke, U. Cotton-Basil Intercropping: Effects on Pests, Yields and Economical Parameters in an Organic Field in Fayoum, Egypt. Biol. Agric. Hortic. 2005, 23, 59–72. [Google Scholar] [CrossRef]
- Devi, S.; Ram, P.; Rolania, K.; Kumar, A. Evaluation of management practices against bollworms in cotton. Indian. J. Agric. Sci. 2021, 91, 132–136. [Google Scholar] [CrossRef]
- Renou, A.; Téréta, I.; Togola, M. Manual topping decreases bollworm infestations in cotton cultivation in Mali. Crop Prot. 2011, 30, 1370–1375. [Google Scholar] [CrossRef]
- Patel, N.C.; Valand, V.M.; Patel, S.N. Ovicidal action of some new insecticide s against eggs of Helicoverpa armigera (Hubner). Indian. J. Plant Prot. 1995, 23, 99–100. [Google Scholar]
- Moorthi, P.V.; Balasubramanian, C.; Avery, P.B.; Najitha Banu, A.; Kubendran, T. Efficacy of Fungal Entomopathogens against Red Cotton Stainer, Dysdercus Cingulatus Fabricius (Hemiptera: Pyrrhocoridae). J. Biopestic. 2011, 05, 140–143. [Google Scholar] [CrossRef]
- Hatting, J.L. Comparison of Three Entomopathogenic Fungi Against the Bollworm, Helicoverpa armigera(Hübner) (Lepidoptera: Noctuidae), Employing Topicalvs per osInoculation Techniques. Afr. Entomol. 2012, 20, 91–100. [Google Scholar] [CrossRef]
- Zuniga-Venegas, L.A.; Hyland, C.; Munoz-Quezada, M.T.; Quiros-Alcala, L.; Butinof, M.; Buralli, R.; Cardenas, A.; Fernandez, R.A.; Foerster, C.; Gouveia, N.; et al. Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ. Health Perspect. 2022, 130, 96002. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Finch, C.F.; Bennell, K.L.; Wajswelner, H. How valid is a self reported 12 month sports injury history? Br. J. Sports Med. 2003, 37, 545–547. [Google Scholar] [CrossRef]
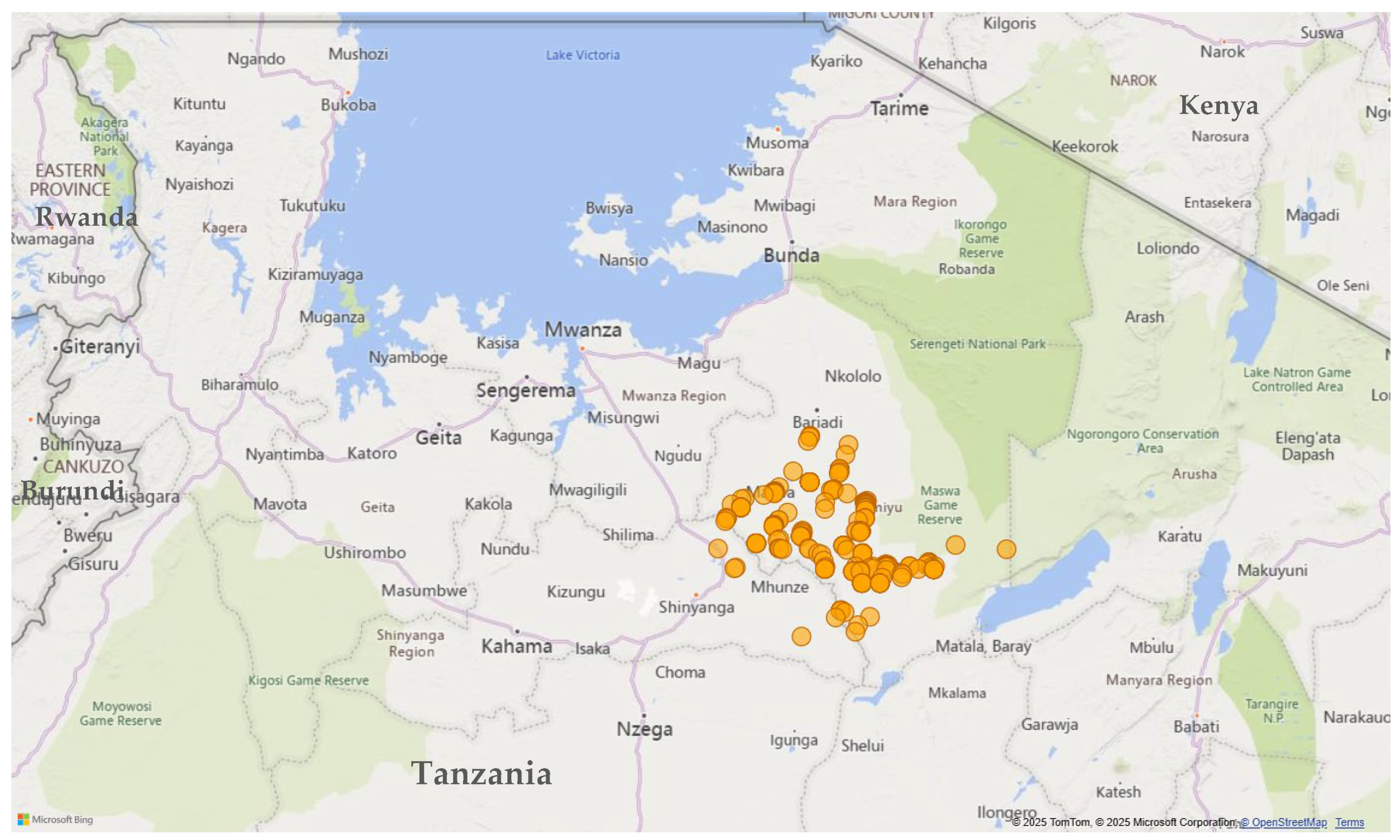
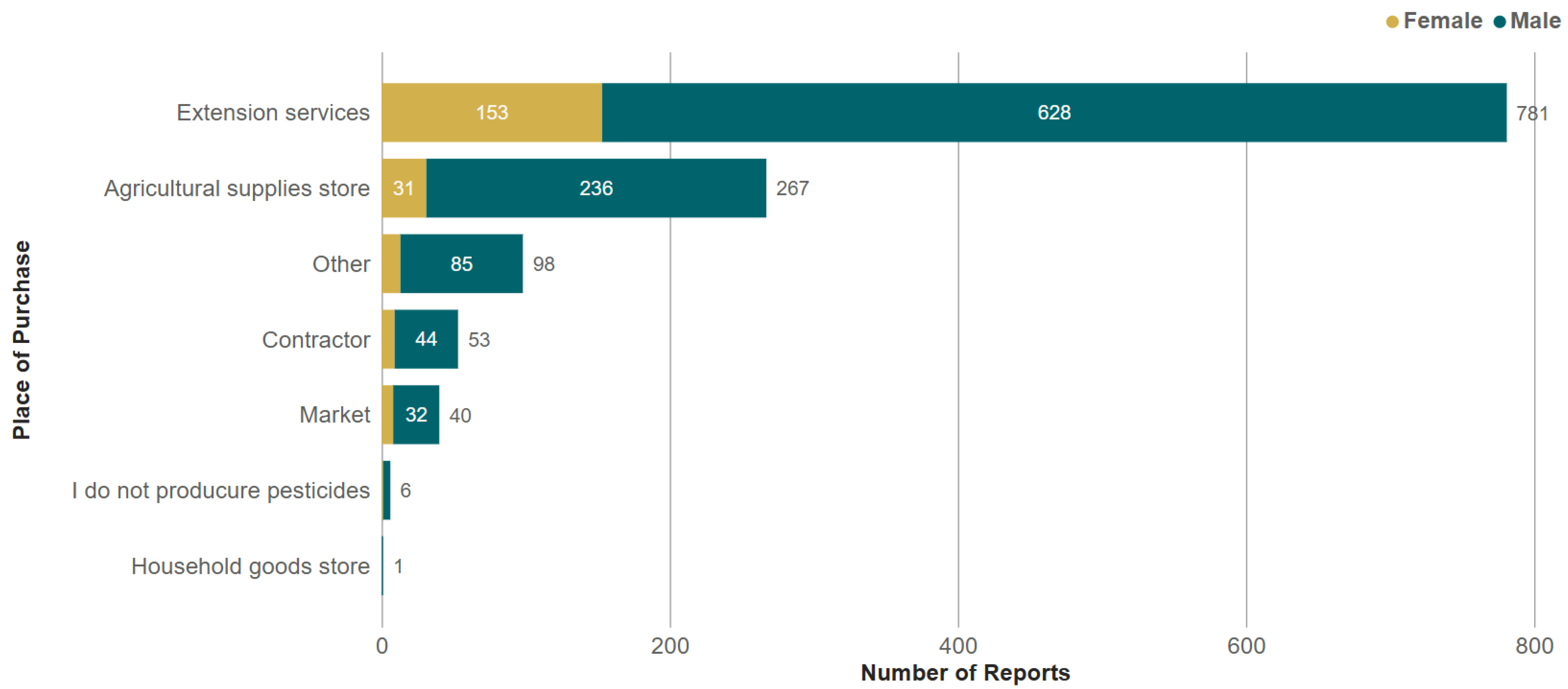


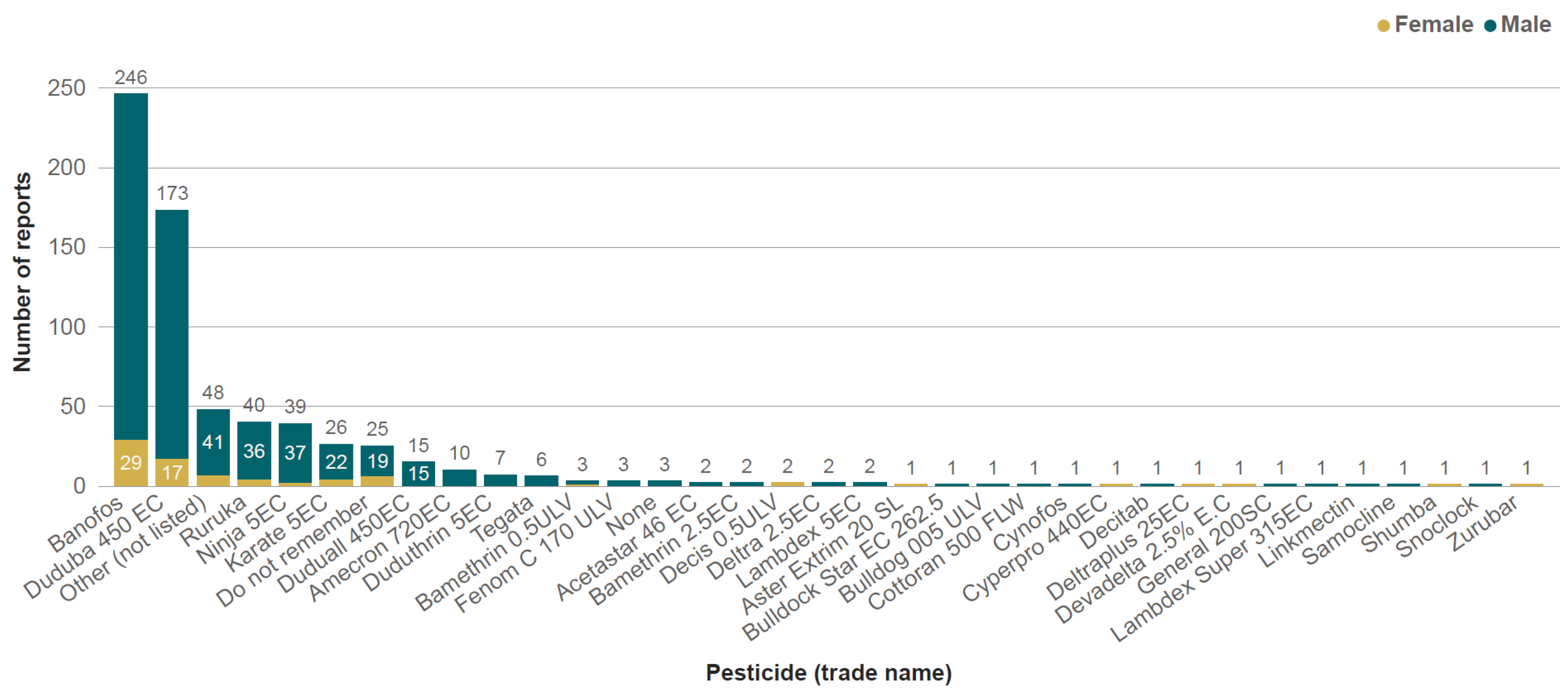
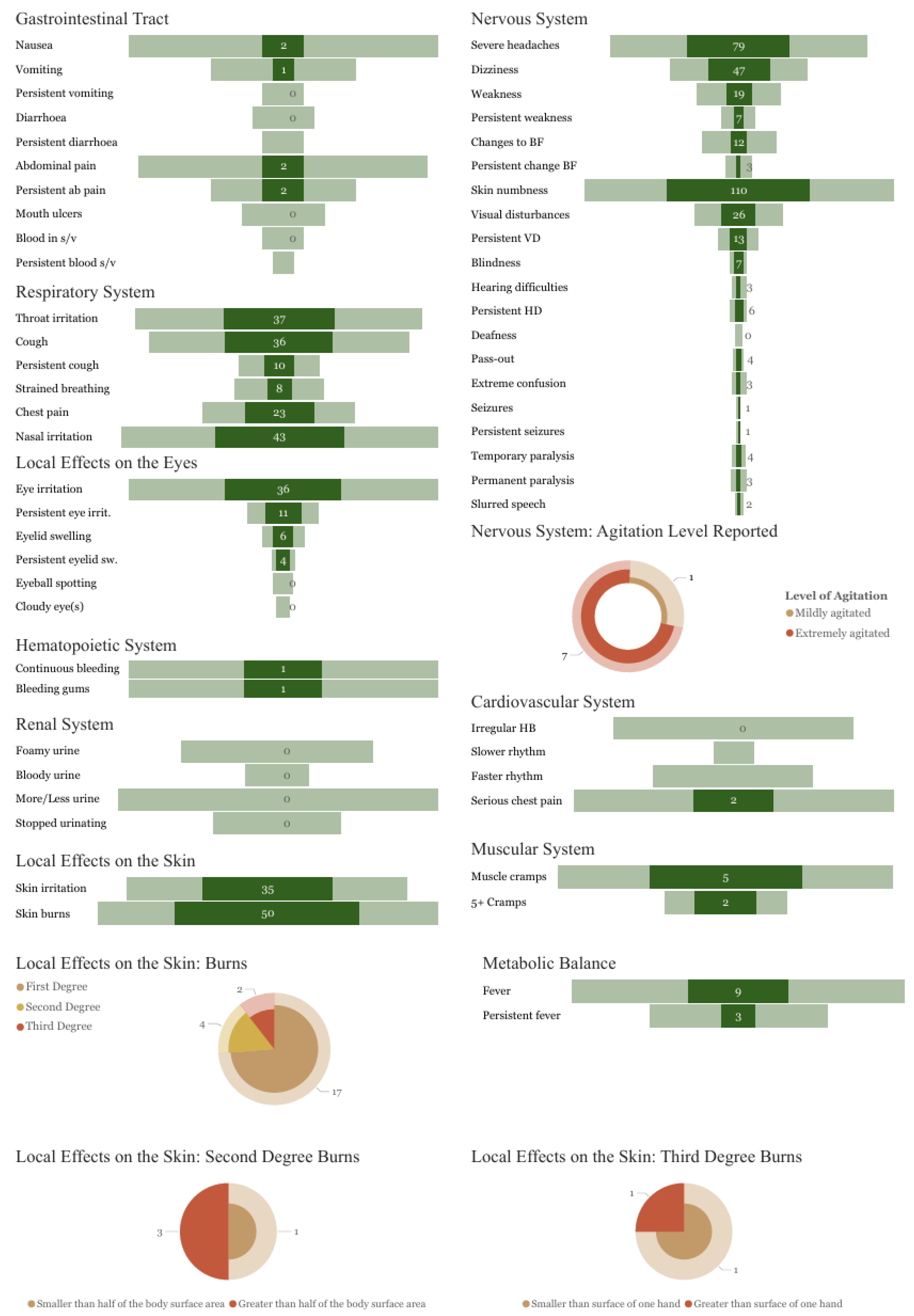
| Gender | ||||
|---|---|---|---|---|
| Total n (%) | Male n (%) | Female n (%) | ||
| Gender | 1074 (100) | 881 (82) | 193 (18) | |
| Age | 14–18 | 8 (1) | 7 (1) | 1 (1) |
| 18–40 | 567 (53) | 464 (53) | 103 (53) | |
| 40–60 | 407 (38) | 331 (38) | 76 (39) | |
| 60+ | 84 (8) | 71 (8) | 13 (7) | |
| Missing information | 8 (1) | 8 (1) | 0 | |
| Occupation Type | Work on family farm * | 903 (85) | 746 (85) | 157 (81) |
| Work on family farm and do hired work | 54 (5) | 48 (5) | 6 (3) | |
| Only do hired work | 111 (10) | 81 (9) | 30 (16) | |
| Missing information | 6 (1) | 6 (1) | 0 | |
| Activity When Poisoned (n = 501) | Total n (%) | Male n (%) | Female n (%) |
|---|---|---|---|
| Applying pesticides to a crop | 464 (93) | 418 (95) | 46 (73) |
| Mixing/loading the pesticide ready to use | 23 (5) | 17 (4) | 6 (10) |
| Entering a field treated with pesticides | 6 (1) | 0 | 6 (10) |
| Other (please specify) | 5 (1) | 0 | 5 (8) |
| Using pesticides in the home | 3 (1) | 3 (1) | 0 |
| Variable | Category | B | S.E. | Wald | df | Sig. | Exp(B) | 95.0% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| PPE | 0 items | 2.594 | 3 | 0.459 | |||||
| 1 item | 0.442 | 0.345 | 1.639 | 1 | 0.200 | 1.555 | 0.791 | 3.058 | |
| 2–3 items | 0.284 | 0.350 | 0.657 | 1 | 0.418 | 1.328 | 0.668 | 2.640 | |
| >3 items | 0.297 | 0.362 | 0.672 | 1 | 0.412 | 1.345 | 0.662 | 2.735 | |
| Occupation type | Work on family farm * | 1.363 | 2 | 0.506 | |||||
| Work on family farm and do hired work | −0.191 | 0.206 | 0.862 | 1 | 0.353 | 0.826 | 0.551 | 1.237 | |
| Only do hired work | 0.032 | 0.339 | 0.009 | 1 | 0.925 | 1.032 | 0.532 | 2.005 | |
| Gender | 0.503 | 0.166 | 9.179 | 1 | 0.002 | 1.654 | 1.194 | 2.290 | |
| Access to training | 0.265 | 0.147 | 3.267 | 1 | 0.041 | 1.303 | 0.978 | 1.737 | |
| Age | −0.060 | 0.096 | 0.385 | 1 | 0.535 | 0.942 | 0.780 | 1.138 | |
| Farm size | <1 ha | 3.785 | 3 | 0.286 | |||||
| 1–5 ha | 0.336 | 0.363 | 0.860 | 1 | 0.354 | 1.400 | 0.687 | 2.850 | |
| 5–15 ha | 0.453 | 0.256 | 3.149 | 1 | 0.076 | 1.574 | 0.954 | 2.597 | |
| 15 ha | 0.296 | 0.269 | 1.217 | 1 | 0.270 | 1.345 | 0.794 | 2.277 | |
| Pesticides sold in original container | −0.276 | 0.321 | 0.738 | 1 | 0.390 | 0.759 | 0.405 | 1.424 | |
| Constant | −1.590 | 0.729 | 4.763 | 1 | 0.029 | 0.204 | |||
| Body System | Total n (%) | Male n (%) | Female n (%) |
|---|---|---|---|
| Nervous system | 476 (100) | 414 (100) | 62 (100) |
| Hematopoietic system | 476 (100) | 414 (100) | 62 (100) |
| Local effects on the skin | 367 (77) | 327 (79) | 40 (65) |
| Respiratory system | 132 (28) | 109 (26) | 23 (37) |
| Local effects on the eyes | 109 (23) | 100 (24) | 9 (15) |
| Metabolic balance | 52 (11) | 44 (11) | 8 (13) |
| Muscular system | 21 (4) | 21 (5) | 0 (0) |
| Gastrointestinal system | 20 (4) | 16 (4) | 4 (6) |
| Cardiovascular system | 18 (4) | 15 (4) | 3 (5) |
| Renal system | 10 (2) | 9 (2) | 1 (2) |
| Number of Occurrences | Total n (%) | Male n (%) | Female n (%) |
|---|---|---|---|
| 1 | 236 (50) | 200 (48) | 36 (58) |
| 2 | 125 (26) | 108 (26) | 17 (27) |
| 3 | 73 (15) | 68 (16) | 5 (8) |
| 4 | 22 (5) | 19 (5) | 3 (4) |
| 5 | 10 (2) | 10 (2) | 0 |
| 6 | 8 (2) | 8 (2) | 0 |
| 8 | 1 (0.2) | 1 (0.2) | 0 |
| 11 | 1 (0.2) | 0 | 1 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapeleka, J.A.; Ngowi, A.V.; Mng’anya, S.; Willis, S.E.; Salmon, J.P.; Tyrell, K.F.; Williamson, S.; Eddleston, M.; Stuart, A.M. Assessment of Unintentional Acute Pesticide Poisoning (UAPP) Amongst Cotton Farmers in Tanzania. Toxics 2025, 13, 300. https://doi.org/10.3390/toxics13040300
Kapeleka JA, Ngowi AV, Mng’anya S, Willis SE, Salmon JP, Tyrell KF, Williamson S, Eddleston M, Stuart AM. Assessment of Unintentional Acute Pesticide Poisoning (UAPP) Amongst Cotton Farmers in Tanzania. Toxics. 2025; 13(4):300. https://doi.org/10.3390/toxics13040300
Chicago/Turabian StyleKapeleka, Jones Ackson, Aiwerasia Vera Ngowi, Silvani Mng’anya, Sheila E. Willis, Joey P. Salmon, Keith F. Tyrell, Stephanie Williamson, Michael Eddleston, and Alexander M. Stuart. 2025. "Assessment of Unintentional Acute Pesticide Poisoning (UAPP) Amongst Cotton Farmers in Tanzania" Toxics 13, no. 4: 300. https://doi.org/10.3390/toxics13040300
APA StyleKapeleka, J. A., Ngowi, A. V., Mng’anya, S., Willis, S. E., Salmon, J. P., Tyrell, K. F., Williamson, S., Eddleston, M., & Stuart, A. M. (2025). Assessment of Unintentional Acute Pesticide Poisoning (UAPP) Amongst Cotton Farmers in Tanzania. Toxics, 13(4), 300. https://doi.org/10.3390/toxics13040300









