Biomass Smoke Exposure Reduces DNA Methylation Levels in PRSS23 (cg23771366) in Women with Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| PRSS23 | Serine protease 23 |
| COPD | Chronic Obstructive Pulmonary Disease |
| TSCOPD | Smoking COPD |
| BSCOPD | Biomass-smoke-induced COPD |
| CO | Carbon monoxide |
| PAHs | Polycyclic aromatic hydrocarbons |
| PM | Particulate matter |
| COPD-BBS | Women with COPD by exposure to biomass smoke |
| BBES | Women with exposure to biomass smoke but without COPD |
| HW | Lung-healthy women without exposure to biomass smoke |
| BMI | Body mass index |
| FVC | Forced vital capacity |
| FEV1 | Forced expiratory volume in the first second |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| BEI | Biomass-burning smoke exposure index |
References
- Dutta, J.; Singh, S.; Greeshma, M.V.; Mahesh, P.A.; Mabalirajan, U. Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review. Diagnostics 2024, 14, 2154. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkikins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 5497–5511. [Google Scholar]
- Eriksson, A.; Abera, A.; Malmqvist, E.; Isaxon, C. Characterization of fine particulate matter from indoor cooking with solid biomass fuels. Indoor Air 2022, 32, 111–112. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S. The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef]
- Zhao, J.; Li, M.; Wang, Z.; Chen, J.; Zhao, J.; Xu, Y.; Wei, X.; Wang, J.; Xie, J. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir. Res. 2019, 20, 11–13. [Google Scholar] [CrossRef]
- Bourbeau, J.; Doiron, D.; Biswas, S.; Smith, B.M.; Benedetti, A.; Brook, J.R.; Aaron, S.D.; Chapman, K.R.; Hernandez, P.; Maltais, F.; et al. Ambient Air Pollution and Dysanapsis: Associations with Lung Function and Chronic Obstructive Pulmonary Disease in the Canadian Cohort Obstructive Lung Disease Study. Am. J. Respir. Crit. Care Med. 2022, 206, 144–155. [Google Scholar] [CrossRef]
- Qin, S.; Li, B.; Li, R.; Cai, Y.; Zheng, K.; Huang, H.; Xiao, F.; Zeng, M.; Xu, X. Proteomic characteristics and identification of PM2.5-induced differentially expressed proteins in hepatocytes and c-Myc silenced hepatocytes. Ecotoxicol. Environ. Saf. 2021, 209, 111838. [Google Scholar] [CrossRef]
- Chan, H.S.; Chang, S.J.; Wang, T.Y.; Ko, H.J.; Lin, Y.C.; Lin, K.T.; Chang, K.M.; Chuang, Y.J. Serine protease PRSS23 is upregulated by estrogen receptor α and associated with proliferation of breast cancer cells. PLoS ONE 2012, 7, e30397. [Google Scholar] [CrossRef]
- Brandsma, C.A.; Van den Berge, M.; Hackett, T.L.; Brusselle, G.; Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2020, 250, 5624–5635. [Google Scholar] [CrossRef]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K. Genetic landscape of chronic obstructive pulmonary disease. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef]
- Huang, B.Z.; Binder, A.M.; Quon, B.; Patel, Y.M.; Lum-Jones, A.; Tiirikainen, M.; Murphy, S.E.; Loo, L.; Maunakea, A.K.; Haiman, C.A.; et al. Epigenome-wide association study of total nicotine equivalents in multiethnic current smokers from three prospective cohorts. Am. J. Hum. Genet. 2024, 111, 3456–3472. [Google Scholar] [CrossRef] [PubMed]
- Tsaprouni, L.G.; Yang, T.P.; Bell, J.; Dick, K.J.; Kanoni, S.; Nisbet, J.; Viñuela, A.; Grundberg, E.; Nelson, C.P.; Meduri, E.; et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 2014, 9, 101382–101396. [Google Scholar] [CrossRef]
- Bravo-Gutiérrez, O.A.; Falfán-Valencia, R.; Ramírez-Venegas, A.; Sansores, R.H.; Hernández-Zenteno, R.J.; Hernández-Pérez, A.; García-Gómez, L.; Osio-Echánove, J.; Abarca-Rojano, E.; Pérez-Rubio, G.; et al. Hypomethylation of ahrr (Cg05575921) is related to smoking status in the mexican mestizo population. Genes 2021, 12, 1276. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Ramirez-Venegas, A.; Sansores-Martinez, R. Clinical Characteristics of Patients With Biomass Smoke-Associated COPD and Chronic Bronchitis, 2004–2014. Chronic. Obstr. Pulm. Dis. J. COPD Found. 2014, 1, 123–132. [Google Scholar] [CrossRef]
- Meneghini, A.C.; Koenigkam-Santos, M.; Pereira, M.C.; Tonidandel, P.R.; Terra-Filho, J.; Cunha, F.Q.; Menezes, M.B.; Vianna, E.O. Biomass smoke COPD has less tomographic abnormalities but worse hypoxemia compared with tobacco COPD. Brazilian J. Med. Biol. Res. 2019, 52, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Padilla, R.; Regalado, J.; Vedal, S.; Paré, P.; Chapela, R.; Sansores, R.; Selman, M. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am. J. Respir. Crit. Care Med. 1996, 154 Pt 1, 701–706. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Lefever, S.; Anckaert, J.; Hellemans, J.; Pfaffl, M.W.; Benes, V.; Bustin, S.A.; Vandesompele, J.; on behalf of the RDML Consortium. RDML-Ninja and RDMLdb for standardized exchange of qPCR data. BMC Bioinform. 2015, 16, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Rojas, L.; Blanco, S.; Serrano, P.; Cruz, J.; Angeles, F.; Tzintzun, G.; Armendariz, C.; Edwards, R.D.; Johnson, M.; et al. The impact of improved wood-burning stoves on fine particulate matter concentrations in rural Mexican homes. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Kyung, S.Y.; Jeong, S.H. Particulate-matter related respiratory diseases. Tuberc. Respir. Dis. 2020, 83, 2116–2121. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, M.; Guo, L.; Huang, H.; Sun, X.; Peng, Z.; Wu, D.; Chen, W. An Integrated Machine Learning Framework Identifies Prognostic Gene Pair Biomarkers Associated with Programmed Cell Death Modalities in Clear Cell Renal Cell Carcinoma. Front. Biosci. 2024, 29, 32024. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Z.; Huang, C.; Huang, P.; Li, D. Serine protease PRSS23 drives gastric cancer by enhancing tumor associated macrophage infiltration via FGF2. Front. Immunol. 2022, 13, 955841. [Google Scholar] [CrossRef]
- Morani, F.; Bisceglia, L.; Rosini, G.; Mutti, L.; Melaiu, O.; Landi, S.; Gemignani, F. Identification of overexpressed genes in malignant pleural mesothelioma. Int. J. Mol. Sci. 2021, 22, 2738. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.; Yang, Y.; Jiang, C.; Xingxing, H.; Nonghua, L.; Runwei, Y. PRSS23 knockdown inhibits gastric tumorigenesis through EIF2 signaling. Pharmacol. Res. 2019, 142, 50–57. [Google Scholar]
- Conn, E.; Hour, S.; Allegakoen, D.; Graham, G.; Petro, J.; Kouassi-Brou, M.; Hong, S.H.; Selvanathan, S.; Çelik, H.; Toretsky, J.; et al. Development of an Ewing sarcoma cell line with resistance to EWS–FLI1 inhibitor YK–4–279. Mol. Med. Rep. 2020, 21, 31667–31675. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Rottach, A.; Leonhardt, H.; Spada, F. DNA methylation-mediated epigenetic control. J. Cell Biochem. 2009, 108, 43–51. [Google Scholar] [CrossRef]
- Paul, S. Differential Effect of Active Smoking on Gene Expression in Male and Female Smokers. J. Carcinog. Mutagen. 2014, 5, 1000198. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, L.; Fu, J.; Shen, K.; Zhang, G. Integrated transcriptome sequencing and weighted gene co-expression network analysis reveals key genes of papillary thyroid carcinomas. Heliyon 2024, 10, e27928. [Google Scholar] [CrossRef]
- Ponce-Gallegos, M.A.; Pérez-Rubio, G.; García-Carmona, A.; García-Gómez, J.; Hernández-Zenteno, R.; Ramírez-Venegas, A.; Falfán-Valencia, R. Haplotype in SERPINA1 (AAT) is associated with reduced risk for COPD in a Mexican Mestizo population. Int. J. Mol. Sci. 2020, 21, 195. [Google Scholar] [CrossRef]
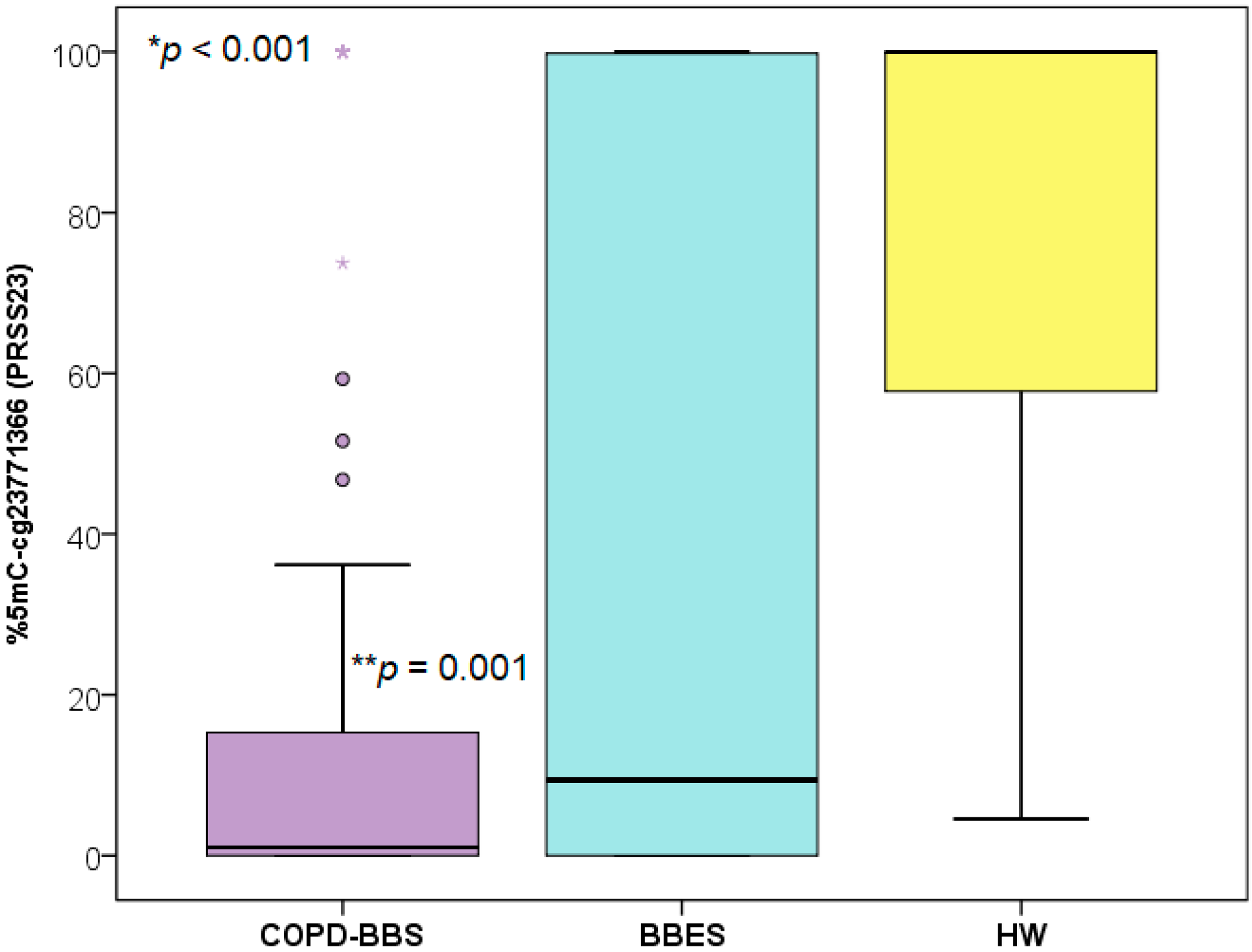
| Variable | COPD-BBS (n = 80) | BBES (n = 180) | HW (n = 79) | p-Value |
|---|---|---|---|---|
| Age (years) | 66 (58–74) * | 61 (56–69) | 50 (44–60) | <0.001 * |
| BMI (kg/m2) | 26.5 (24.1–30.4) | 28.4 (25.0–31.7) | 27.4 (24.6–28.9) | 0.080 * |
| BEI (h/year) | 300 (205–365) | 270 (200–365) | NA | 0.306 ** |
| FVC (%) | 87 (77–105) | 97 (88–110) | 88 (77–96) | 0.009 * |
| FEV1 (%) | 70 (59–83) | 104 (95–116) | 100 (82–106) | <0.001 * |
| FEV1/FVC | 61 (54–67) | 84 (80–88) | 86 (79–91) | <0.001 * |
| Variable | BEI (h/Year) | FVC % | FEV1 % | FEV1/FVC |
|---|---|---|---|---|
| Spearman’s rho | 0.015 | 0.096 | −0.077 | −0.150 |
| p-value | 0.897 | 0.408 | 0.504 | 0.183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rubio, G.; Falfán-Valencia, R.; Bravo-Gutiérrez, O.A.; Lozano-González, N.; Ramírez-Venegas, A.; Cruz-Vicente, F.; Ramírez-Díaz, M.E. Biomass Smoke Exposure Reduces DNA Methylation Levels in PRSS23 (cg23771366) in Women with Chronic Obstructive Pulmonary Disease. Toxics 2025, 13, 253. https://doi.org/10.3390/toxics13040253
Pérez-Rubio G, Falfán-Valencia R, Bravo-Gutiérrez OA, Lozano-González N, Ramírez-Venegas A, Cruz-Vicente F, Ramírez-Díaz ME. Biomass Smoke Exposure Reduces DNA Methylation Levels in PRSS23 (cg23771366) in Women with Chronic Obstructive Pulmonary Disease. Toxics. 2025; 13(4):253. https://doi.org/10.3390/toxics13040253
Chicago/Turabian StylePérez-Rubio, Gloria, Ramcés Falfán-Valencia, Omar Andrés Bravo-Gutiérrez, Nancy Lozano-González, Alejandra Ramírez-Venegas, Filiberto Cruz-Vicente, and María Elena Ramírez-Díaz. 2025. "Biomass Smoke Exposure Reduces DNA Methylation Levels in PRSS23 (cg23771366) in Women with Chronic Obstructive Pulmonary Disease" Toxics 13, no. 4: 253. https://doi.org/10.3390/toxics13040253
APA StylePérez-Rubio, G., Falfán-Valencia, R., Bravo-Gutiérrez, O. A., Lozano-González, N., Ramírez-Venegas, A., Cruz-Vicente, F., & Ramírez-Díaz, M. E. (2025). Biomass Smoke Exposure Reduces DNA Methylation Levels in PRSS23 (cg23771366) in Women with Chronic Obstructive Pulmonary Disease. Toxics, 13(4), 253. https://doi.org/10.3390/toxics13040253










