Role of Nanoplastics in Decreasing the Intestinal Microbiome Ratio: A Review of the Scope of Polystyrene
Abstract
1. Introduction
1.1. Micro- and Nanoplastic Pollution as an Emerging Health Concern
1.2. Gut Microbiome as a Target of Disruption
1.3. Previous Reviews and Current Gaps
1.4. Aim of This Scoping Review
2. The Intestinal Microbiome
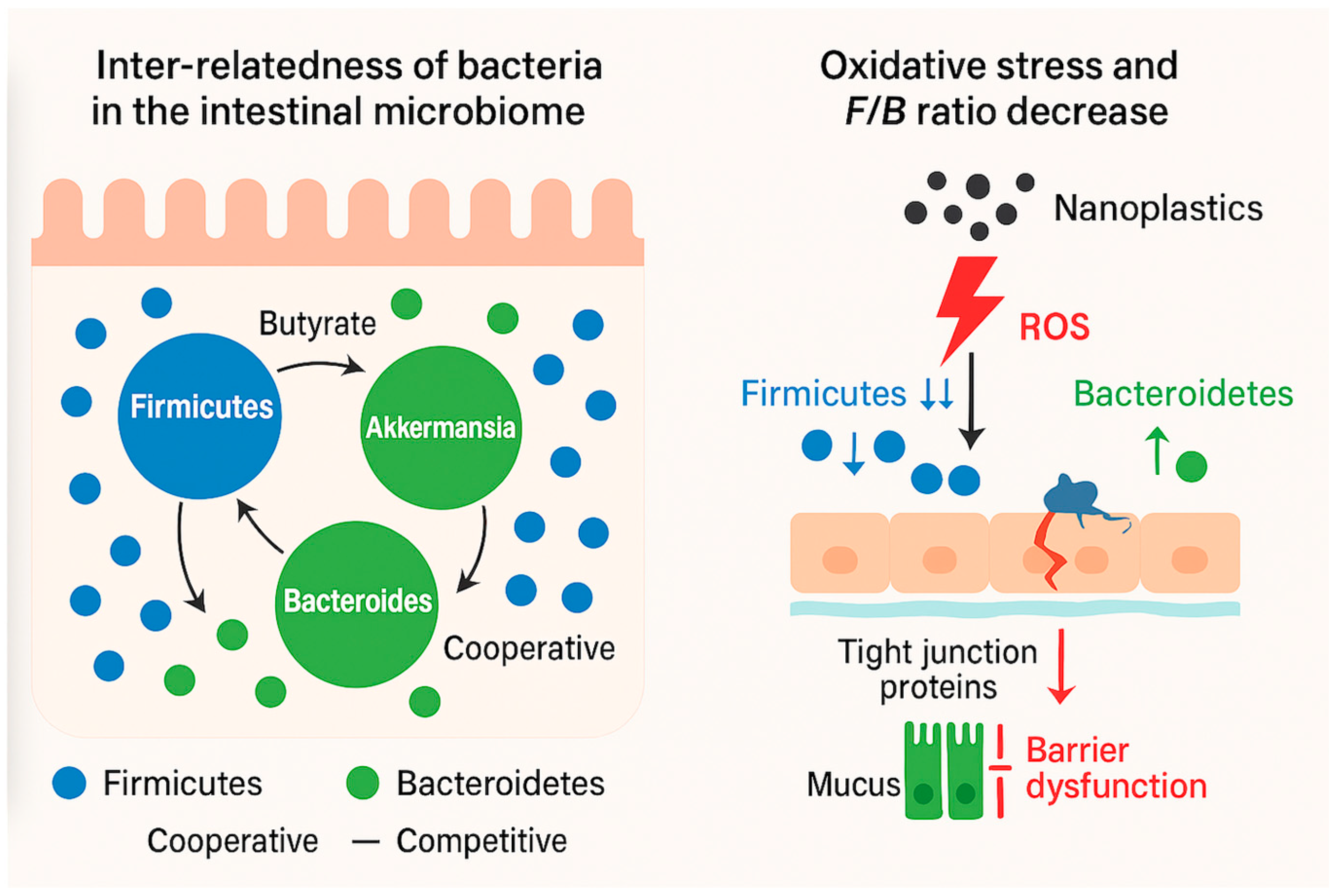
2.1. Microbiome Bacteria Inter-Relatedness
2.2. Niche Structuring
2.3. Keystone Species and Community Stability
2.4. Ecological Succession and Resilience
3. Interaction of MNP and Bacteria
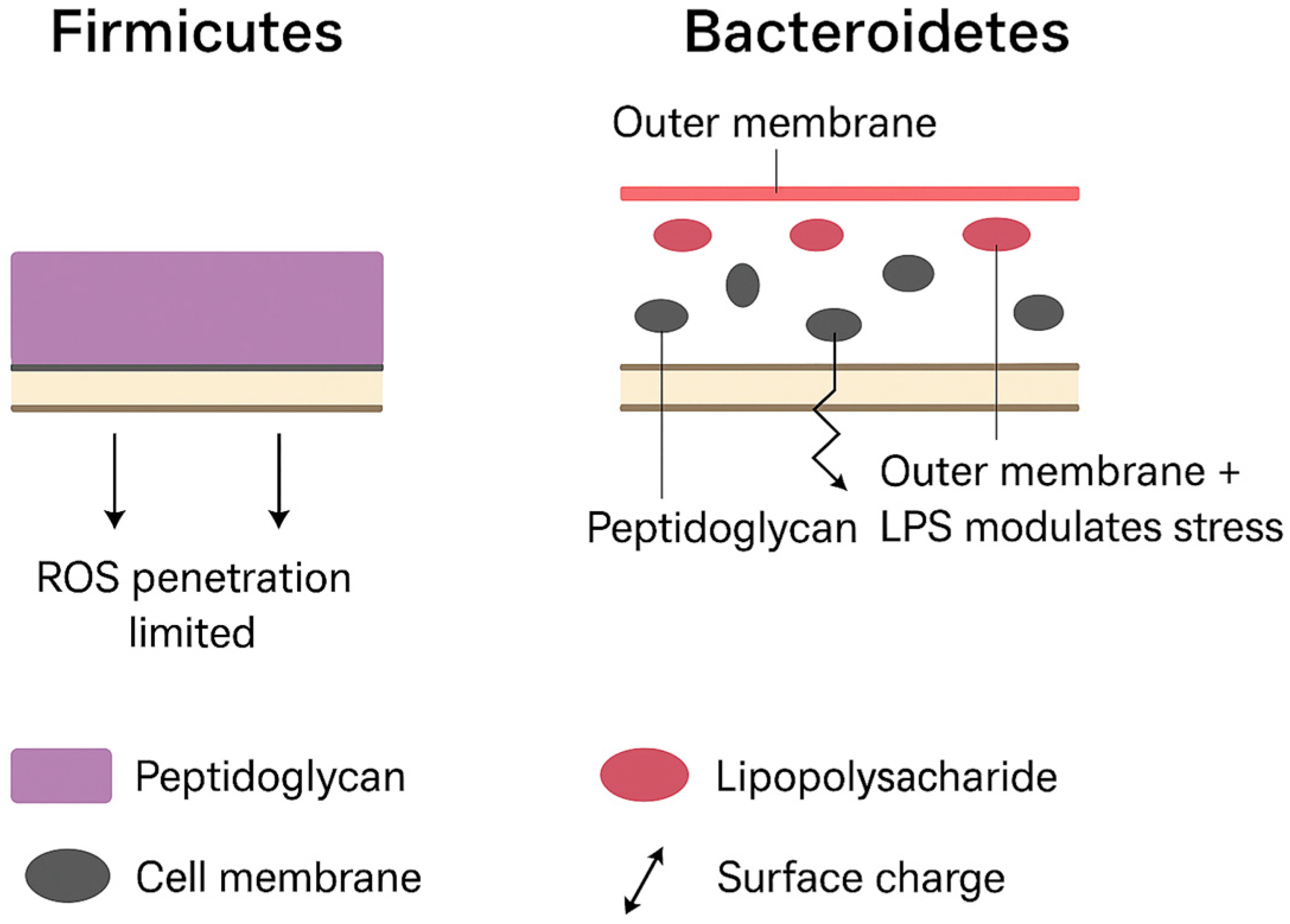
3.1. Binding and Physical Membrane Disruption
3.2. Electrostatic Interactions and Surface Charge Effects
3.3. Particle Size and Penetration
3.4. Oxidative Stress (Host and Bacterial Endpoints)
3.5. Biofilm Interactions
4. Materials and Methods
4.1. Eligibility Criteria
4.2. Information Sources and Search Strategy
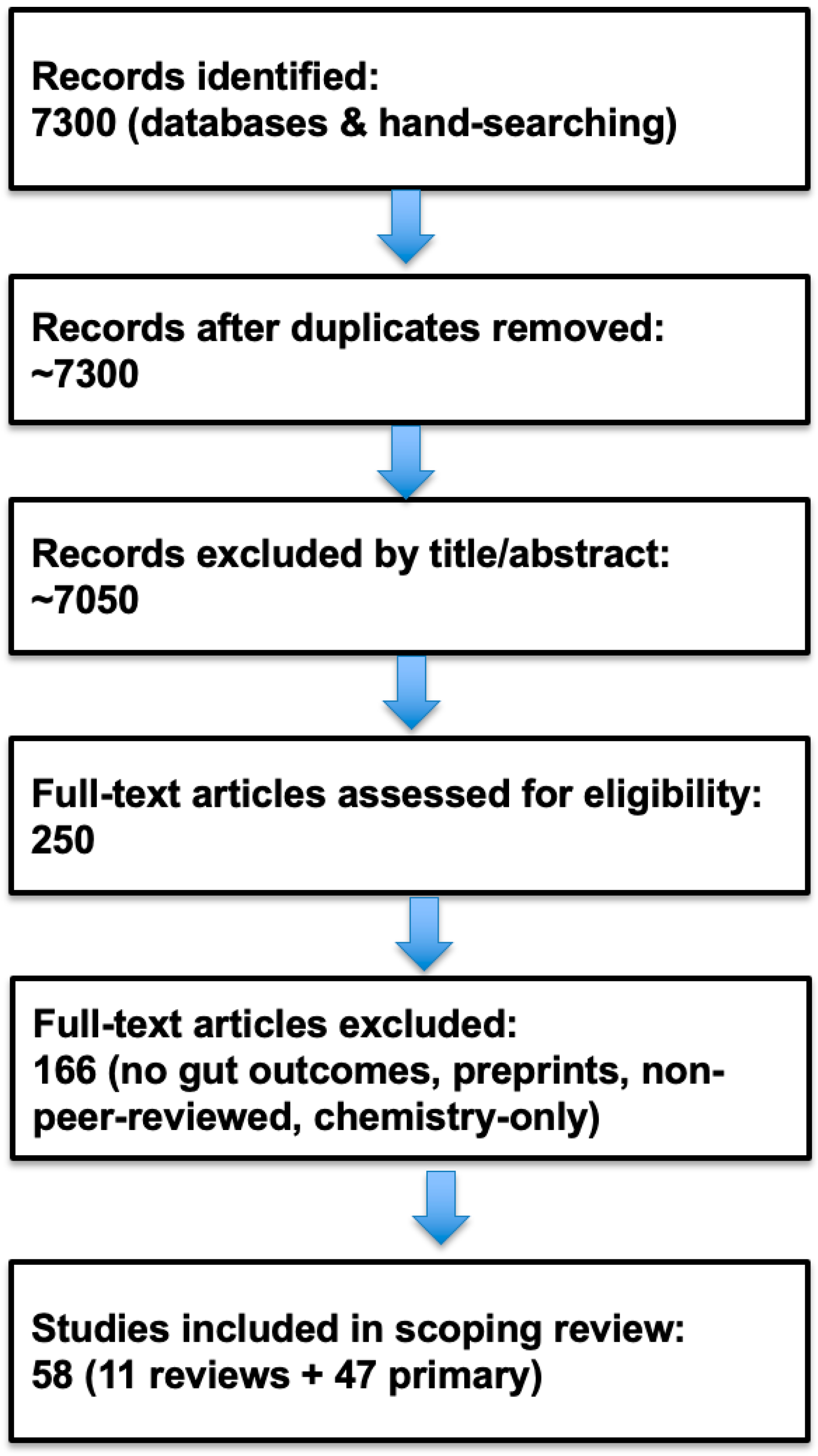
4.3. Flow of Evidence Selection
4.4. Data Charting Process
4.5. Data Items
- Bibliographic details: first author, year of publication, journal, DOI.
- Study design: in vivo (animal model), in vitro (bacterial or epithelial system), ex vivo (digestion or fermentation model), or review.
- Host/model: species (mouse, zebrafish, rabbit, chicken, turtle, bacterial culture, cell line).
- Particle characteristics: polymer type (polystyrene, polyethylene, polypropylene, PVC, PET), size classification (microplastic ≤ 5 mm, nanoplastic ≤ 100 nm), surface properties or modifications, and whether pristine or environmentally aged.
- Exposure details: route (oral gavage, diet, drinking water), duration of exposure, and dose/concentration.
- Microbiome outcomes: sequencing method, phylum/family/genus-level taxonomic changes, Firmicutes/Bacteroidetes ratio, alpha/beta diversity indices, presence of key functional taxa (e.g., SCFA producers, mucin degraders, endotoxin producers).
- Barrier and immune outcomes: expression of tight junction proteins (occludin, claudins, ZO-1), mucus secretion, epithelial apoptosis, reactive oxygen species (ROS), pro- and anti-inflammatory cytokine levels, immune gene expression.
- Systemic outcomes: host metabolic alterations (e.g., hepatic lipid metabolism, glucose regulation), extra-intestinal immune responses.
- Review-specific items: stated scope (humans, animals, in vitro), thematic focus (barrier, oxidative stress, dysbiosis), and main conclusions.
4.6. Gram-Positive/Gram-Negative Weighting Strategy
- When losses of Gram-positive commensals coincided with gains in Gram-negative taxa (Gram+ ↓ + Gram− ↑), the net was recorded as “↓ Gram+/Gram− (dominant Gram− expansion).”
- When increases in Gram-positive taxa coincided with losses in Gram-negative taxa (Gram+ ↑ + Gram− ↓), the net was recorded as “↑ Gram+/Gram− (commensal enrichment, pathogen reduction).”
- When tallies were balanced or shifts were nonsignificant, the net was recorded as “No net effect.”
4.7. Critical Appraisal of Sources of Evidence
- Dose levels: Many in vivo studies used high doses of microplastics (tens to hundreds of mg/day per mouse) that exceed environmentally relevant exposures, limiting translational inference.
- Particle characterization: Incomplete reporting of polymer identity, particle size distribution, or surface properties was common.
- Microbiome analysis: Several studies reported phylum-level changes (e.g., Firmicutes/Bacteroidetes ratio) without deeper taxonomic resolution or appropriate compositional analysis.
- Venue and integrity risk: Journals such as Bioengineered have been flagged for paper-mill infiltration, but none of the studies included here came from that venue. All included studies were screened against the “Aquarius checklist” to minimize the risk of incorporating unreliable evidence [72].
4.8. Selection of Sources of Evidence
5. Results
5.1. Characteristics of Sources of Evidence
- Host species and models included mice, zebrafish, chickens, rabbits, turtles, bacterial cultures, gut epithelial cell lines, and in vitro/ex vivo digestion models.
- Particle types most frequently investigated were polystyrene (PS), polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), and polyethylene terephthalate (PET).
- Particle sizes ranged from microplastics (10–5000 µm) to nanoplastics (≤100 nm), with some studies comparing different size fractions or surface modifications (carboxyl, amine).
- Exposure routes and durations included oral gavage, dietary supplementation, and drinking water administration, ranging from short-term (7 days) to chronic exposures (up to 12 weeks).
- Doses varied widely, from low environmentally relevant levels (100 µg/day) to high experimental doses (tens to hundreds of mg/days per mouse).
- Outcomes measured encompassed gut microbiome composition (phylum- to genus-level taxonomic shifts, diversity metrics, Firmicutes/Bacteroidetes ratio, presence of key functional taxa such as mucin degraders, SCFA producers, or endotoxin-associated groups), intestinal barrier integrity (tight junction proteins, mucin production, epithelial apoptosis, permeability), immune signaling and inflammatory markers (cytokine expression, ROS, apoptosis, immune gene regulation), and systemic outcomes (hepatic lipid metabolism, glucose homeostasis).
5.2. Results of Individual Sources of Evidence
- (a)
- Epithelial barrier injury;
- (b)
- Oxidative stress and immune activation;
- (c)
- Microbiome compositional and functional changes;
- (d)
- Direct bacteria–particle interactions.
5.2.1. Epithelial Barrier Injury
5.2.2. Oxidative Stress and Immune Activation
5.2.3. Microbiome Composition and Function
- Functional taxa: Depletion of SCFA-producing Firmicutes (e.g., Faecalibacterium, Roseburia) and mucin-degrading taxa (Akkermansia muciniphila) coincided with enrichment of Gram-negative groups such as Prevotellaceae and Proteobacteria.
- Dose dependence: High-dose PS or PE produced pronounced dysbiosis, whereas low-dose PET caused only minor shifts (Harusato 2023 [79]). Recent reviews (Bora 2024 [6]; Eichinger 2024 [9]) independently reached the same conclusion—that functional taxa provide stronger mechanistic insight than the phylum-level F/B ratio.
5.2.4. Direct Bacteria–Particle Interactions
5.3. Synthesis of Results
5.3.1. Oxidative Stress as the Initiating Event
5.3.2. Barrier Disruption and Inflammation
5.3.3. Microbiome Reconfiguration and Functional Loss
5.3.4. Integrative Model
- Oxidative stress—initiated by nanoparticle–cell interactions;
- Barrier disruption—loss of tight-junction integrity and mucosal protection;
- Microbiome shift—preferential loss of Gram-positive commensals and overgrowth of Gram-negative, stress-tolerant taxa.
5.4. Summary of Evidence
6. Limitations of the Review Process
7. Conclusions and Implications
7.1. Implications for Future Research
7.2. Broader Context
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | exopolysaccharide |
| F/B | Firmicutes and Bacteroidetes |
| GI | gastrointestinal |
| LPS | lipopolysaccharide |
| MP | microplastics |
| MNPs | Micro- and nano-plastics |
| NP | nanoparticle |
| PET | polyethylene terephthalate |
| PP | polypropylene |
| PE | polyethylene |
| PS | polystyrene |
| PS-NPs | Polystyrene nanoplastics |
| PVC | Polyvinyl chloride |
| ROS | Reactive Oxygen Species |
| SCFAs | short-chain fatty acids |
| SOD | superoxide dismutase |
References
- Eberhard, T.; Casillas, G.; Zarus, G.M.; Barr, D.B. Systematic review of microplastics and nanoplastics in indoor and outdoor air: Identifying a framework and data needs for quantifying human inhalation exposures. J. Expo. Sci. Env. Environ. Epidemiol. 2024, 34, 185–196. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef]
- Jyoti; Dey, P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. npj Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Sze, M.A.; Schloss, P.D. Looking for a signal in the noise: Revisiting obesity and the microbiome. mBio 2016, 7, e01018-16. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu; Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Front. Cell Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro(nano)plastics and their potential impact on human gut health: A narrative review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J. Microplastics and microbiota: Unraveling the hidden environmental challenge. World J. Gastroenterol. 2024, 30, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, J.; Marco, T.; Jana, S.; Brugger, D. Review: Interactions between microplastics and the gastrointestinal microbiome. Ital. J. Anim. Sci. 2024, 23, 1044–1056. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Khaledi, M.; Poureslamfar, B.; Alsaab, H.O.; Tafaghodi, S.; Hjazi, A.; Singh, R.; Alawadi, A.H.; Alsaalamy, A.; Qasim, Q.A.; Sameni, F. The role of gut microbiota in human metabolism and inflammatory diseases: A focus on elderly individuals. Ann. Microbiol. 2024, 74, 1. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Kaminski, P.; Tkaczenko, H. Role of gut microbiota in modulating oxidative stress induced by environmental factors. Cell Physiol. Biochem. 2025, 59, 2–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef] [PubMed]
- Popa, R.P.; Tabaran, A.F. A systematic review of the toxicokinetics of micro-and nanoplastics in mammals following digestive exposure. Appl. Sci. 2025, 15, 6135. [Google Scholar] [CrossRef]
- Souza-Silva, T.G.D.; Oliveira, I.A.; Silva, G.G.D.; Giusti, F.C.V.; Novaes, R.D.; Paula, H.A.D.A. Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence. Life Sci. 2022, 294, 120366. [Google Scholar] [CrossRef]
- Wang, X.; Deng, K.; Zhang, P.; Chen, Q.; Magnuson, J.T.; Qiu, W.; Zhou, Y. Microplastic-mediated new mechanism of liver damage: From the perspective of the gut-liver axis. Sci. Total Env. Environ. 2024, 919, 170962. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, Á.; McWilliam Leitch, E.C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Hols, P.; Bernard, E.; Rolain, T.; Zhou, M.; Siezen, R.J.; Bron, P.A. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 2010, 34, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Fala, A.K.; Alvarez-Ordonez, A.; Filloux, A.; Gahan, C.G.M.; Cotter, P.D. Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting. Front. Microbiol. 2022, 13, 1002185. [Google Scholar] [CrossRef]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Martens, E.C.; Koropatkin, N.M.; Smith, T.J.; Gordon, J.I. Complex glycan catabolism by the human gut microbiota: The Bacteroidetes sus-like paradigm. J. Biol. Chem. 2009, 284, 24673–24677. [Google Scholar] [CrossRef] [PubMed]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Stares, M.D.; Connor, T.R.; Raisen, C.; Goulding, D.; Rad, R.; Schreiber, F.; Brandt, C.; et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012, 8, e1002995. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermudez-Humaran, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chavez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best. Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. mBio 2017, 8, e00770-17. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Martens, E.C.; Chiang, H.C.; Gordon, J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 2008, 4, 447–457. [Google Scholar] [CrossRef]
- Reese, A.T.; Cho, E.H.; Klitzman, B.; Nichols, S.P.; Wisniewski, N.A.; Villa, M.M.; Durand, H.K.; Jiang, S.; Midani, F.S.; Nimmagadda, S.N.; et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. eLife 2018, 7, e35987. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal Erwin, G.; de Vos Willem, M.; Visser Caroline, E.; Kuijper Ed, J.; Bartelsman Joep, F.W.M.; Tijssen Jan, G.P.; et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Env. Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Rehman, A.; Pham, V.; Seifert, N.; Richard, N.; Sybesma, W.; Steinert, R.E. The polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid, and vitamin k1 modulate the gut microbiome: A study using an in vitro shime model. J. Diet. Suppl. 2024, 21, 135–153. [Google Scholar] [CrossRef]
- Fleury, J.B.; Baulin, V.A. Microplastics destabilize lipid membranes by mechanical stretching. Proc. Natl. Acad. Sci. USA 2021, 118, e2104610118. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Bai, L.; Cui, J. Microplastics: An often-overlooked issue in the transition from chronic inflammation to cancer. J. Transl. Med. 2024, 22, 959. [Google Scholar] [CrossRef]
- Zajac, M.; Kotynska, J.; Zambrowski, G.; Breczko, J.; Deptula, P.; Ciesluk, M.; Zambrzycka, M.; Swiecicka, I.; Bucki, R.; Naumowicz, M. Exposure to polystyrene nanoparticles leads to changes in the zeta potential of bacterial cells. Sci. Rep. 2023, 13, 9552. [Google Scholar] [CrossRef] [PubMed]
- Perini, D.A.; Parra-Ortiz, E.; Varó, I.; Queralt-Martín, M.; Malmsten, M.; Alcaraz, A. Surface-functionalized polystyrene nanoparticles alter the transmembrane potential via ion-selective pores maintaining global bilayer integrity. Langmuir 2022, 38, 14837–14849. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Chen, Y.-Z.; Chiang, Y.-T.; Chang, Y.-T.; Wang, Y.-W.; Hsu, K.-T.; Hsu, Y.-Y.; Wu, P.-T.; Lee, B.-H. Polystyrene nanoplastics disrupt the intestinal microenvironment by altering bacteria-host interactions through extracellular vesicle-delivered microRNAs. Nat. Commun. 2025, 16, 5026. [Google Scholar] [CrossRef]
- Dai, S.; Ye, R.; Huang, J.; Wang, B.; Xie, Z.; Ou, X.; Yu, N.; Huang, C.; Hua, Y.; Zhou, R.; et al. Distinct lipid membrane interaction and uptake of differentially charged nanoplastics in bacteria. J. Nanobiotechnol. 2022, 20, 191. [Google Scholar] [CrossRef]
- Perez, F.; Andoy, N.M.O.; Hua, U.T.T.; Yoshioka, K.; Sullan, R.M.A. Adaptive responses of Bacillus subtilis underlie differential nanoplastic toxicity with implications for root colonization. Environ. Sci. Nano 2025, 12, 1477–1486. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.J.; Lee, S.W.; Lee, E.H. Interactions between bacteria and nano (micro)-sized polystyrene particles by bacterial responses and microscopy. Chemosphere 2022, 306, 135584. [Google Scholar] [CrossRef]
- Zhao, L.; Dou, Q.; Chen, S.; Wang, Y.; Yang, Q.; Chen, W.; Zhang, H.; Du, Y.; Xie, M. Adsorption abilities and mechanisms of Lactobacillus on various nanoplastics. Chemosphere 2023, 320, 138038. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Li, J.; Dai, W.; Luan, Y. Bacterial interactions with nanoplastics and the environmental effects they cause. Fermentation 2023, 9, 939. [Google Scholar] [CrossRef]
- Yi, X.; Li, W.; Liu, Y.; Yang, K.; Wu, M.; Zhou, H. Effect of polystyrene microplastics of different sizes to Escherichia coli and Bacillus cereus. Bull. Environ. Contam. Toxicol. 2021, 107, 626–632. [Google Scholar] [CrossRef]
- Lu, Z.; Sethu, R.; Imlay, J.A. Endogenous superoxide is a key effector of the oxygen sensitivity of a model obligate anaerobe. Proc. Natl. Acad. Sci. USA 2018, 115, E3266–E3275. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Habumugisha, T.; Huang, F.; Zhang, Z.; Kiki, C.; Al, M.A.; Yan, C.; Shaheen, U.; Zhang, X. Impacts of polystyrene nanoplastics on zebrafish gut microbiota and mechanistic insights. Ecotoxicol. Environ. Saf. 2025, 299, 118332. [Google Scholar] [CrossRef]
- Djouina, M.; Loison, S.; Body-Malapel, M. Recent progress in intestinal toxicity of microplastics and nanoplastics: Systematic review of preclinical evidence. Microplastics 2024, 3, 217–233. [Google Scholar] [CrossRef]
- Su, Q.L.; Wu, J.; Tan, S.W.; Guo, X.Y.; Zou, D.Z.; Kang, K. The impact of microplastics polystyrene on the microscopic structure of mouse intestine, tight junction genes and gut microbiota. PLoS ONE 2024, 19, e0304686. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Kim, S.Y.; Woo, S.; Lee, S.W.; Jung, E.M.; Lee, E.H. Dose-dependent responses of Escherichia coli and Acinetobacter sp. to micron-sized polystyrene microplastics. J. Microbiol. Biotechnol. 2025, 35, e2410023. [Google Scholar] [CrossRef]
- Athulya, P.A.; Chandrasekaran, N.; Thomas, J. Polystyrene microplastics interaction and influence on the growth kinetics and metabolism of tilapia gut probiotic Bacillus tropicus ACS1. Environ. Sci. Process Impacts 2024, 26, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Zhang, J.; Liu, Y.; Li, X.; Zhao, Y. Combined effects of polystyrene microplastics and high-fat diet on gut health and microbiota in zebrafish. J. Fish. Biol. 2024, 104, 93–105. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Banares, M.A.; Fernandez, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Papp, P.P.; Hoffmann, O.I.; Libisch, B.; Kereszteny, T.; Gerocs, A.; Posta, K.; Hiripi, L.; Hegyi, A.; Gocza, E.; Szoke, Z.; et al. Effects of polyvinyl chloride (PVC) microplastic particles on gut microbiota composition and health status in rabbit livestock. Int. J. Mol. Sci. 2024, 25, 12646. [Google Scholar] [CrossRef] [PubMed]
- Galecka, I.; Rychlik, A.; Calka, J. Influence of selected dosages of plastic microparticles on the porcine fecal microbiome. Sci. Rep. 2025, 15, 1269. [Google Scholar] [CrossRef]
- Xie, S.; Ma, J.; Lu, Z. Bacteroides thetaiotaomicron enhances oxidative stress tolerance through rhamnose-dependent mechanisms. Front. Microbiol. 2024, 15, 1505218. [Google Scholar] [CrossRef]
- Aquarius, R.; Bik, E.M.; Bimler, D.; Oksvold, M.P.; Patrick, K. Tackling paper mills requires us to prevent future contamination and clean up the past—The case of the journal Bioengineered. Bioengineered 2025, 16, 2542668. [Google Scholar] [CrossRef] [PubMed]
- Abalkina, A.; Aquarius, R.; Bik, E.; Bimler, D.; Bishop, D.; Byrne, J.; Cabanac, G.; Day, A.; Labbé, C.; Wise, N. ‘Stamp out paper mills’—Science sleuths on how to fight fake research. Nature 2025, 637, 1047–1050. [Google Scholar] [CrossRef]
- Balthazar, D. Q&A: The Scientific Integrity Sleuth Taking on the Widespread Problem of Research Misconduct. Stat News, 28 February 2024. Available online: https://www.statnews.com/2024/02/28/elisabeth-bik-scientific-integrity-research-misconduct/ (accessed on 30 October 2025).
- COPE Council. COPE Discussion Document—Diversity and Inclusivity. 2021. Available online: https://publicationethics.org/guidance/discussion-document/diversity-and-inclusivity (accessed on 30 October 2025).
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Env. Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Harusato, A.; Seo, W.; Abo, H.; Nakanishi, Y.; Nishikawa, H.; Itoh, Y. Impact of particulate microplastics generated from polyethylene terephthalate on gut pathology and immune microenvironments. iScience 2023, 26, 106474. [Google Scholar] [CrossRef]
- Xie, L.; Chen, T.; Liu, J.; Hou, Y.; Tan, Q.; Zhang, X.; Li, Z.; Farooq, T.H.; Yan, W.; Li, Y. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice. Ecotoxicol. Environ. Saf. 2022, 246, 114194. [Google Scholar] [CrossRef]
- Li, P.; Liu, J. Micro(nano)plastics in the human body: Sources, occurrences, fates, and health risks. Environ. Sci. Technol. 2024, 58, 3065–3078. [Google Scholar] [CrossRef]
- Yang, J.Z.; Li, J.H.; Liu, J.L.; Zhou, A.D.; Wang, H.; Xie, X.L.; Zhang, K.K.; Wang, Q. Multiomics analysis revealed the effects of polystyrene nanoplastics at different environmentally relevant concentrations on intestinal homeostasis. Environ. Pollut. 2025, 372, 126050. [Google Scholar] [CrossRef] [PubMed]
- Li, V.S.W. Modelling intestinal inflammation and infection using ‘mini-gut’ organoids. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 89–90. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Review Title | Epithelial Barrier Effects | Oxidative Stress/ROS | Microbiome Changes (Dysbiosis) | Species Scope | Notes | Citation |
|---|---|---|---|---|---|---|---|---|
| Bora | 2024 | Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks | Weakened barrier integrity, ↑ gut permeability, disruption of tight junctions; systemic translocation of toxins and inflammatory mediators | Discusses oxidative stress, apoptosis, and fibrosis mechanisms in multiple organs (kidney, liver, brain) mediated by dysbiosis | ↓ beneficial taxa, ↑ opportunistic/pathogenic taxa; reduced SCFA producers; dysbiosis linked to gut–liver, gut–kidney, gut–brain axes | Human exposure evidence (urine MPs) + rodent models + mechanistic insights | Highlights FMT and probiotics (e.g., Akkermansia) as potential interventions; frames MPs as systemic disruptors | [6] |
| Covello | 2024 | Micro(nano)plastics and their potential impact on human gut health: A narrative review | ↓ tight junction integrity, ↓ mucus secretion, villus/crypt damage, ↑ proinflammatory cytokines; smaller NPs cross epithelium via endocytosis/transcytosis | ROS generation; mitochondrial dysfunction; activation of MAPK, TLR4, NF-κB cascades; apoptosis/necrosis at higher doses | Dysbiosis across Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria; ↓ Parabacteroides/SCFA-producers; ↑ Staphylococcus, Lactobacillus, Adlercreutzia, Bifidobacterium; inconsistent Proteobacteria outcomes | In vitro human cell lines; in vivo mammalian models; notes on aquatic models; human fecal microplastics linked to IBD | Plasticizers and metals may worsen toxicity; microbiota may contribute to plastic biodegradation; human evidence limited/conflicting | [7] |
| Demarquoy | 2024 | Microplastics and microbiota: Unraveling the hidden environmental challenge | Editorial mentions barrier dysfunction as a possible consequence of dysbiosis; no primary mechanistic data | Not a central focus; ROS implicated indirectly as part of pollutant–microbiota interactions | Consistently reported ↓ microbial diversity, compositional shifts (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia) in humans and models | Human observations + environmental/mammalian models (review-level evidence) | Editorial perspective; emphasizes MPs as dysbiosis inducers, possible microbial degradation but most polymers resistant; calls for long-term studies | [8] |
| Eichinger | 2024 | Review: Interactions between microplastics and the gastrointestinal microbiome | ↓ mucus secretion, tight junction disruption, impaired barrier integrity; translocation of smaller particles into tissues | ↑ ROS, mitochondrial dysfunction, oxidative stress linked to inflammation | ↑ Proteobacteria, Fusobacteria, Firmicutes (Staphylococcus, Lachnoclostridium); ↓ Bacteroides, Parabacteroides, Akkermansia, Bifidobacterium; dysbiosis linked to inflammation, lipid metabolism changes | Humans, rodents, poultry, fish, ruminants (review synthesis) | Highlights biofilm formation, pollutant and antibiotic resistance gene transfer; some microbial taxa (Actinobacteria, Acinetobacter) degrade plastics; calls for standardized methods and longitudinal animal studies | [9] |
| Hirt | 2020 | Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature | Reports barrier dysfunction and altered tolerance; immune disruption noted but not fully mechanistic | Mentions oxidative stress as part of immune-inflammatory cascade, but not the central focus | ↓ diversity; variable shifts across phyla (↑ Proteobacteria, ↓ Actinobacteria, ↓ Firmicutes); dysbiosis consistently observed | Animal models (mice, zebrafish, others); in vivo studies reviewed | Focus on interplay between microbiota and immunotoxicity; foundational synthesis highlighting host–microbiome imbalance due to MNPs | [10] |
| Khaledi | 2024 | The role of gut microbiota in human metabolism and inflammatory diseases: A focus on elderly individuals | Age-related decline in barrier integrity; MNPs may exacerbate permeability and barrier dysfunction in elderly populations | Links dysbiosis to ↑ oxidative stress; MPs/NPs can worsen ROS-related damage in aging gut | ↓ diversity, ↓ SCFA-producers (Faecalibacterium, Roseburia); ↑ opportunistic taxa; MNPs may compound dysbiosis | Human-focused review with emphasis on elderly; references to preclinical and environmental MNP evidence | Therapeutic suggestions include probiotics, prebiotics, dietary modulation, microbiome-targeted interventions | [11] |
| Kurhaluk | 2025 | Role of gut microbiota in modulating oxidative stress induced by environmental factors | ↓ Tight junction proteins (Claudin, Occludin, ZO-1) and gut vascular barrier disruption (via Wnt/β-catenin) mentioned in pollutant contexts | Emphasis on NF-κB, Nrf2/Keap1, PI3K/Akt, p38-MAPK, JAK/STAT, TLR4/MyD88 as central oxidative stress pathways | Pollutants (toxic metals, nanomaterials, micro/nanoplastics) induce dysbiosis, ↑ inflammation, ↑ resistance gene spread | Broad (rodents, humans, environmental models; review-based) | Identifies antioxidants, probiotics, and prebiotics as potential interventions | [12] |
| Li | 2024 | Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity | MPs cross barriers via endocytosis, paracellular leakage, persorption; NPs < 100 nm penetrate tissues; associated with inflammation and fibrosis | ROS generation, mitochondrial dysfunction, apoptosis, immune dysregulation; additives (phthalates, metals) may worsen toxicity | ↓ diversity, disruption of SCFA producers, dysbiosis linked to IBD and metabolic diseases | Human-focused review; integrates evidence from clinical findings, in vivo animal models, and in vitro studies | Comprehensive systemic review; highlights biodistribution to intestine, liver, kidney, placenta, brain; calls for standardized exposure models | [13] |
| Popa | 2025 | A systematic review of the toxicokinetics of micro- and nanoplastics in mammals following digestive exposure | ↓ mucus secretion, villus/crypt damage, barrier dysfunction, ↑ intestinal permeability, inflammation | ↑ ROS, hepatic lipid metabolism disruption, oxidative stress implicated in liver, kidney, brain, immune effects | Gut dysbiosis across mammalian models; compositional shifts linked to inflammation and metabolic disruption | Systematic review of 17 in vivo mammalian studies (mice, rats, pigs, guinea pigs) | MP/NP absorption via endocytosis, transcytosis, persorption; systemic distribution to liver, kidney, brain, placenta; chronic/transgenerational effects underexplored | [14] |
| Souza-Silva | 2022 | Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence | ↓ tight junction proteins (ZO-1, Claudin-1), ↓ mucins (Muc1/2/3), ↓ mucus secretion, villi/microvilli damage, ↑ permeability | ↑ ROS, CAT, SOD, GstD1, defensins; activation of oxidative stress and immune signaling pathways (TLR2/4, AP-1, IRF5) | ↑ Firmicutes, Proteobacteria, Verrucomicrobia, Chlamydiae; ↓ Bacteroidetes, Actinobacteria; ↑ Lactobacillus, Clostridium, Vibrio; ↓ Bifidobacterium, Ruminococcus | Systematic review of 28 in vivo studies (zeb rafish, mice, worms, insects, crustaceans, mollusks, soil organisms) | Functional changes in microbial metabolism; ↑ antibiotic resistance genes; high risk of bias noted in included studies | [15] |
| Wang | 2024 | Microplastic-mediated new mechanism of liver damage: From the perspective of the gut–liver axis | ↓ mucus secretion, goblet cell loss, ↑ permeability; barrier disruption allows LPS and MPs to translocate to liver | ↑ ROS, hepatic oxidative stress, steatosis, cholestasis, fibrosis; activation of TLR4 pathway | ↑ Proteobacteria, Actinobacteria; ↓ Bacteroidetes, Firmicutes; dysbiosis linked to liver injury | Animal models (mice, zebrafish, chickens, tilapia, medaka), liver organoids; limited emerging human data (cirrhosis patients) | Gut–liver axis central to MP toxicity; microbiome-targeted interventions (probiotics, prebiotics, FMT) suggested for mitigation | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutton, S.C.; Hills, R.D., Jr. Role of Nanoplastics in Decreasing the Intestinal Microbiome Ratio: A Review of the Scope of Polystyrene. Toxics 2025, 13, 1036. https://doi.org/10.3390/toxics13121036
Sutton SC, Hills RD Jr. Role of Nanoplastics in Decreasing the Intestinal Microbiome Ratio: A Review of the Scope of Polystyrene. Toxics. 2025; 13(12):1036. https://doi.org/10.3390/toxics13121036
Chicago/Turabian StyleSutton, Steven C., and Ronald D. Hills, Jr. 2025. "Role of Nanoplastics in Decreasing the Intestinal Microbiome Ratio: A Review of the Scope of Polystyrene" Toxics 13, no. 12: 1036. https://doi.org/10.3390/toxics13121036
APA StyleSutton, S. C., & Hills, R. D., Jr. (2025). Role of Nanoplastics in Decreasing the Intestinal Microbiome Ratio: A Review of the Scope of Polystyrene. Toxics, 13(12), 1036. https://doi.org/10.3390/toxics13121036








