Comprehensive In Vitro Safety Assessment of Acorus calamus Rhizome Oil Using OECD-Compliant New Approach Methods: Classification as a GHS Category 1B Sensitiser and Category 2 Irritant
Abstract
1. Introduction
Test Guidelines and GLP Compliance
2. Materials and Methods
2.1. Plant Material and Extraction of Rhizome Oil
2.2. Chemicals and Reagents
2.3. Determination and Quantification of α-, β-, and γ-Asarone
2.3.1. Chemicals and Reference Standards
2.3.2. Sample Preparation for HPLC Analysis
2.3.3. HPLC Quantification
2.3.4. LC-MS/MS Confirmation
2.3.5. Quantification and Data Analysis
2.3.6. Solubility Test
2.3.7. In Vitro Skin Irritation and Corrosion Tests
2.3.8. Skin Irritation Test
2.3.9. Skin Corrosion Test
2.3.10. The Direct Peptide Reactivity Assay (DPRA)
2.3.11. KeratinoSens™ Assay
2.3.12. Human Cell Line Activation Test (h-CLAT)
3. Results
3.1. The A. calamus Rhizome Oil Is Dominated by Beta-Asarone
3.2. Quantitative Determination of Asarone Isomers
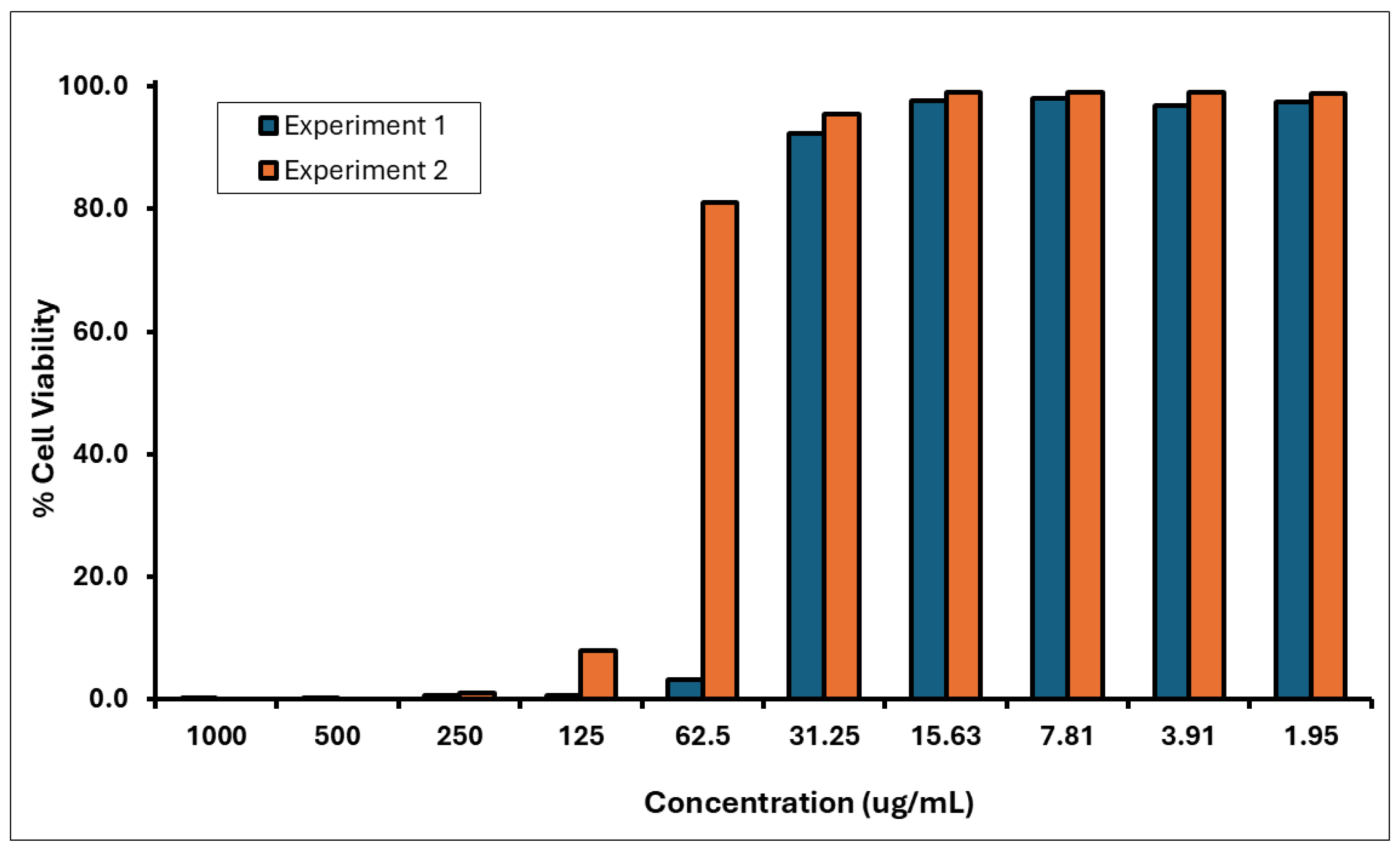
3.3. The Skin Irritation Test Results Show That A. calamus Rhizome Oil Is an Irritant
3.4. The Skin Corrosion Test Identified A. calamus Rhizome Oil as Non-Corrosive
3.5. A. calamus Rhizome Oil Is Assigned to GHS Category 2, i.e., Skin Irritant
3.6. A. calamus Rhizome Oil Showed Moderate Reactivity in the DPRA
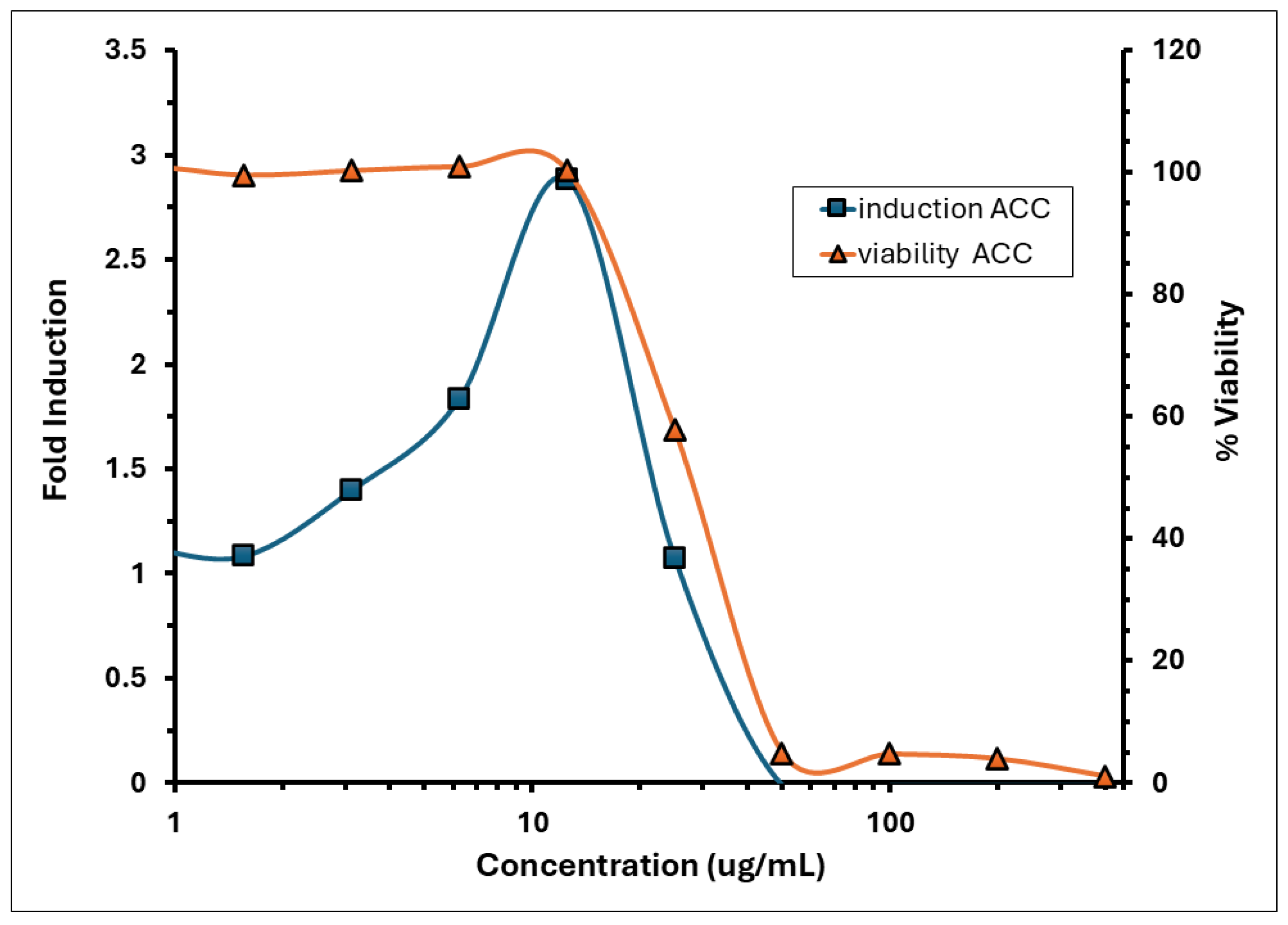
3.7. The KeratinoSens™ Assay Identified A. calamus Rhizome Oil as a Sensitiser
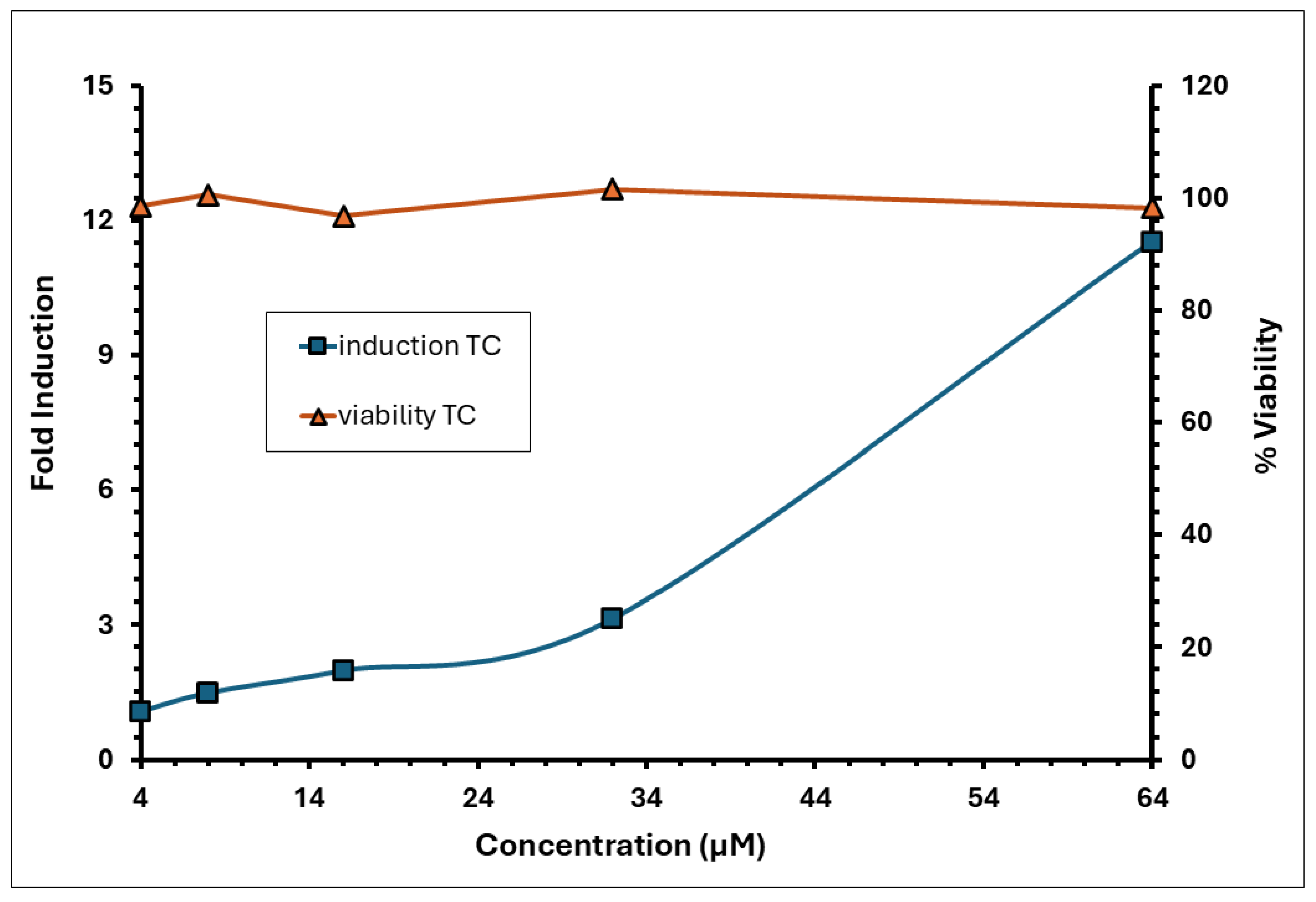
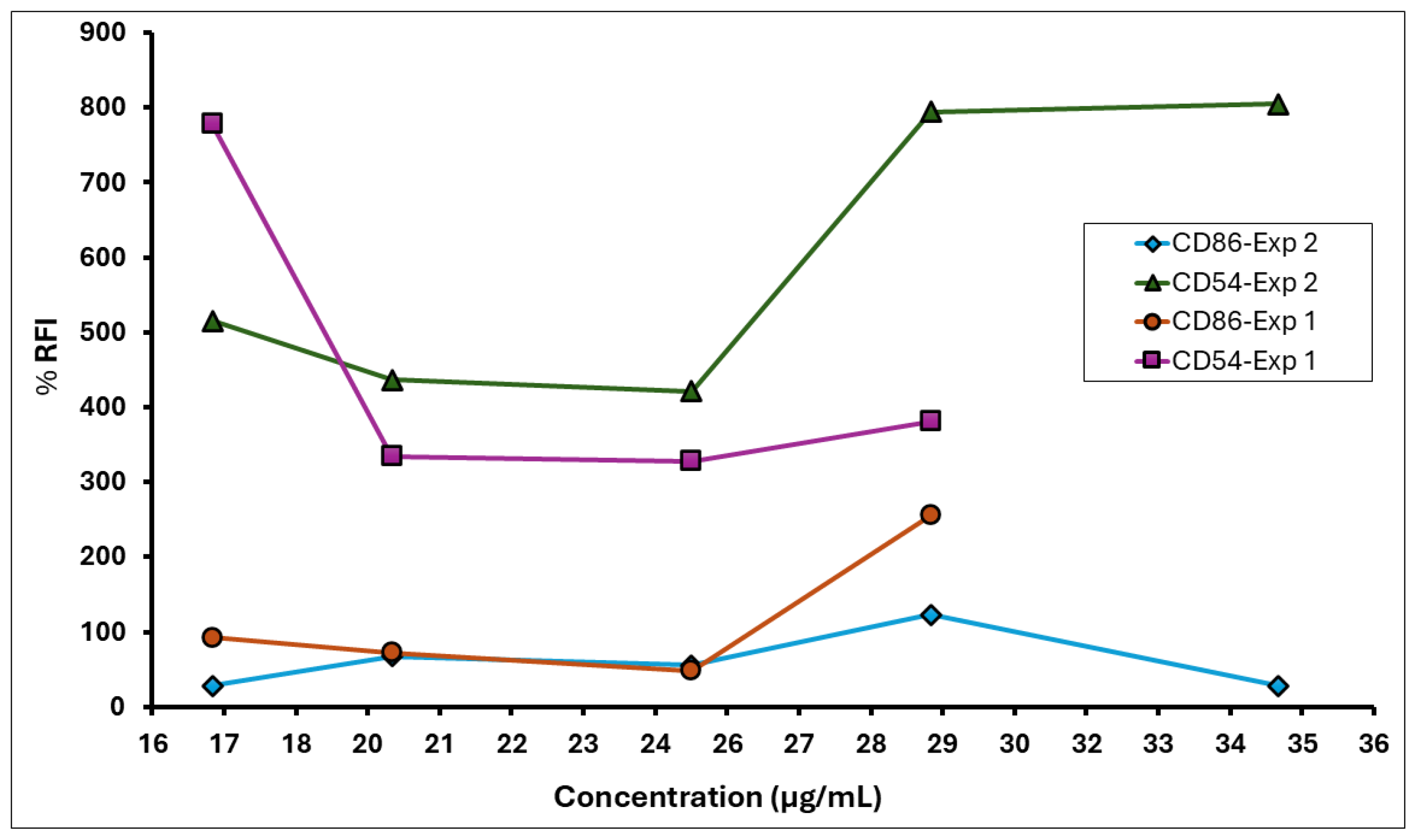
3.8. A. calamus Rhizome Oil Met the Positive Response Criteria of RFI ≥ 150% for CD86 and RFI ≥ 200% for CD54, Indicating Its Sensitising Potential
3.9. A. calamus Rhizome Oil Was Predicted to Be a GHS Category 1B Sensitiser
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakumbahan, R.; Rajamani, K.; Kumanan, K. Acorus calamus: An overview. J. Med. Plants Res. 2010, 4, 2740–2745. [Google Scholar]
- Sharma, V.; Singh, I.; Chaudhary, P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014, 28, 1454–1466. [Google Scholar] [CrossRef]
- Sharma, P.K.; Chauhan, N.S.; Lal, B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J. Ethnopharmacol. 2004, 92, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Mahendra Pal Sing Publishers: Dehradun, India, 1987; Volume IV, pp. 1229–1230. [Google Scholar]
- Raj, R.K. Screening of some indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J. Physiol. Pharmacol. 1974, 18, 129–131. [Google Scholar] [PubMed]
- Kapur, S.K. Ethno-medico plants of Kangra valley (Himachal Pradesh). J. Econ. Tax. Bot. 1993, 17, 395–408. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acorus calamus: Scientific Validation of Ayurvedic Tradition from Natural Resources. Pharm. Biol. 2007, 45, 651–666. [Google Scholar] [CrossRef]
- Du, Z.; Clery, R.A.; Hammond, C.J. Volatiles from leaves and rhizomes of fragrant Acorus spp. (Acoraceae). Chem. Biodivers. 2008, 5, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bains, J.S.; Dhuna, V.; Singh, J.; Kamboj, S.S.; Nijjar, K.K.; Agrewala, J.N. Novel lectins from rhizomes of two Acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int. Immunopharmacol. 2005, 5, 1470–1478. [Google Scholar] [CrossRef]
- Arseculeratne, S.N.; Gunatilaka, A.A.L.; Panabokke, R.G. Studies on medicinal plants of Sri Lanka. Part 14: Toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 1985, 13, 323–335. [Google Scholar] [CrossRef]
- Tariq, R.M.; Naqvi, N.H.; Choudhary, M.I.; Abbas, A. Importance and implementation of essential oil of Pakistanian Acorus calamus Linn., as a biopesticide. Pak. J. Bot. 2010, 42, 2043–2050. [Google Scholar]
- Gilani, A.U.H.; Shah, A.J.; Ahmad, M.; Shaheen, F. Antispasmodic effect of Acorus calamus Linn. is mediated through calcium channel blockade. Phytother. Res. 2006, 20, 1080–1084. [Google Scholar] [CrossRef]
- Glara, D.A.; Sivaranjani, D.K.; Ashmi, R.; Shymala, D.K. Vasambu kaapu in prevention of common paediatric ailments—An overview. World J. Pharm. Pharm. Sci. 2017, 360–367. [Google Scholar] [CrossRef]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef]
- Gilissen, L.; Huygens, S.; Goossens, A. Allergic contact dermatitis caused by topical herbal remedies: Importance of patch testing with the patients’ own products. Contact Dermat. 2018, 78, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.-J.; Robles, M.; Fernández-Castañer, A.; López-Ortega, S.; López-Vega, M.-C.; Lucena, M.-I. Assessment of drug-induced hepatotoxicity in clinical practice: A challenge for gastroenterologists. World J. Gastroenterol. 2007, 13, 329–340. [Google Scholar] [CrossRef]
- Andrade, T.; Aguiar, A.; Guedes, F.; Leite, M.; Caetano, G.; Coelho, E.; Das, P.; Frade, M. Ex vivo model of human skin (hOSEC) as alternative to animal use for cosmetic tests. Procedia Eng. 2015, 110, 67–73. [Google Scholar] [CrossRef]
- Maffè, S.; Paffoni, P.; Colombo, M.L.; Davanzo, F.; Dellavesa, P.; Cucchi, L.; Zenone, F.; Paino, A.M.; Pardo, N.F.; Bergamasco, L.; et al. Cardiotossicità da erbe selvatiche. G. Ital. Cardiol. 2013, 14, 445–455. [Google Scholar]
- Parez, B.E.; Rodriquez, O.R.; Sanchez, V.M.C. Toxic plants: Brugmansia (floripondio) neurotoxicity. Arch. Med. Urg. Mex. 2012, 4, 119–124. [Google Scholar]
- Strickland, J.A.; Maldonado, H.; Farley-Dawson, E.A.; LaPratt, T.; To, K.T.; Truax, J.F.; Reinke, E.; Allen, D.G.; Germolec, D.; Kleinstreuer, N. Evaluation of Substances of Regulatory Interest Using Non-Animal Skin Sensitization Test Methods; NICEATM Report 05; Division of Translational Toxicology: Durham, NC, USA, 2025. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, G.; Isola, S.; Minciullo, P.L.; Gangemi, S. Rosmarinus officinalis L. as cause of contact dermatitis. Allergol. Immunopathol. 2014, 42, 616–619. [Google Scholar] [CrossRef]
- Monroe, J. Toxicodendron Contact Dermatitis: A Case Report and Brief Review. J. Clin. Aesthet. Dermatol. 2020, 13 (Suppl. S1), S29–S34. [Google Scholar]
- Jack, A.R.; Norris, P.L.; Storrs, F.J. Allergic contact dermatitis to plant extracts in cosmetics. Semin. Cutan. Med. Surg. 2013, 32, 140–146. [Google Scholar] [CrossRef]
- Collett, M.G. Photosensitisation diseases of animals: Classification and a weight of evidence approach to primary causes. Toxicon X 2019, 3, 100012. [Google Scholar] [CrossRef]
- Walling, A.L.; Walling, H.W. Phytophotodermatitis induced by wild parsnip. Dermatol. Online J. 2018, 24, 19. [Google Scholar] [CrossRef]
- Sugimoto, N.; Kiuchi, F.; Mikage, M.; Mori, M.; Mizukami, H.; Tsuda, Y. DNA profiling of Acorus calamus chemotypes differing in essential oil composition. Biol. Pharm. Bull. 1999, 22, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Deshmukh, P.; Joshi, S.; Ghag, M.; Kulkarni, Y.; Vyas, B.; Shah, D. Toxicity study of ethanolic extract of Acorus calamus rhizome. Int. J. Green Pharm. 2012, 6, 29–35. [Google Scholar] [CrossRef]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- Calamus and Its Derivatives. 21 CFR 189.110. Available online: https://www.ecfr.gov/current/title-21/section-189.110 (accessed on 12 June 2025).
- Sharma, V.; Sharma, R.; Gautam, D.S.; Kuca, K.; Nepovimova, E.; Martins, N. Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 2020, 9, 1176. [Google Scholar] [CrossRef]
- International Fragrance Association (IFRA). IFRA Standard: Cis- and Trans-Asarone (Amendment 40); IFRA: Geneva, Switzerland, 2006. [Google Scholar]
- Singh, C.; Jamwal, U.S.P. Acorus calamus (sweet flag), an overview of oil decomposition, biological activity and usage. Int. J. Med. Arom Plants 2001, 23, 687–708. [Google Scholar]
- Maibach, H.I. Principles and methods of toxicology. J. Am. Acad. Dermatol. 1984, 10, 152. [Google Scholar] [CrossRef]
- Talalay, P.; Talalay, P. The importance of using scientific principles in the development of medicinal agents from plants. Acad. Med. 2001, 76, 238–247. [Google Scholar] [CrossRef]
- Barceloux, D.G. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–1157. [Google Scholar]
- Kingston, C.; Jeeva, S.; Jeeva, G.M.; Kiruba, S.; Mishra, B.P.; Kannan, D. Indigenous knowledge of using medicinal plants in treating skin diseases in Kanyakumari district, Southern India. Indian J. Tradit. Knowl. 2009, 8, 196–200. [Google Scholar]
- Haupenthal, S.; Berg, K.; Gründken, M.; Vallicotti, S.; Hemgesberg, M.; Sak, K.; Schrenk, D.; Esselen, M. In vitro genotoxicity of carcinogenic asarone isomers. Food Funct. 2017, 8, 1227–1234. [Google Scholar] [CrossRef]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Keratinocyte Activation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Souza, T.T.; Leite, B.S.; Lessa, N.M.; Bonjardim, L.R.; Santos, M.R.; Alves, P.B.; Blank, A.F.; Antoniolli, A.R. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 2008, 15, 619–624. [Google Scholar] [CrossRef]
- Nukada, Y.; Miyazawa, M.; Kazutoshi, S.; Sakaguchi, H.; Nishiyama, N. Data integration of non-animal tests for the development of a test battery to predict the skin sensitizing potential and potency of chemicals. Toxicol. Vitr. 2013, 27, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, O.; Fukui, S.; Okamoto, K.; Kurotani, S.; Imai, N.; Fujishiro, M.; Kyotani, D.; Kato, Y.; Kasahara, T.; Fujita, M.; et al. Test battery with the human cell line activation test, direct peptide reactivity assay and DEREK based on a 139 chemical data set for predicting skin sensitizing potential and potency of chemicals. J. Appl. Toxicol. 2015, 35, 1318–1332. [Google Scholar] [CrossRef]
- Strickland, J.; Truax, J.; Corvaro, M.; Settivari, R.; Henriquez, J.; McFadden, J.; Gulledge, T.; Johnson, V.; Gehen, S.; Germolec, D.; et al. Application of Defined Approaches for Skin Sensitization to Agrochemical Products. Front.Toxicol. 2022, 4, 852856. [Google Scholar] [CrossRef] [PubMed]
- Mohoric, T.; Wilm, A.; Onken, S.; Milovich, A.; Logavoch, A.; Ankli, P.; Tagorti, G.; Kirchmair, J.; Schepky, A.; Kühnl, J.; et al. Increasing Accessibility of Bayesian Network-Based Defined Approaches for Skin Sensitisation Potency Assessment. Toxics 2024, 12, 666. [Google Scholar] [CrossRef]
- To, K.T.; Strickland, J.; Reinke, E.; Borrel, A.; Truax, J.; Maldonado, H.; Allen, D.; Kleinstreuer, N. Computational application of internationally harmonized defined approaches to skin sensitization: DASS App. BMC Bioinform. 2024, 25, 4. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Panteri, E.; Rogiers, V.; et al. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation: 12th Revision; Publications Office of the European Union: Luxembourg, 2024; Available online: https://hal.science/hal-04100663/ (accessed on 12 June 2025).
- Reinke, E.N.; Reynolds, J.; Gilmour, N.; Reynolds, G.; Strickland, J.; Germolec, D.; Allen, D.G.; Maxwell, G.; Kleinstreuer, N.C. The skin allergy risk assessment-integrated chemical environment (SARA-ICE) defined approach to derive points of departure for skin sensitization. Curr. Res. Toxicol. 2025, 8, 100205. [Google Scholar] [CrossRef]
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E.; et al. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef]
- Haber, L.T.; Bradley, M.A.; Buerger, A.N.; Behrsing, H.; Burla, S.; Clapp, P.W.; Dotson, S.; Fisher, C.; Genco, K.R.; Kruszewski, F.H.; et al. New approach methodologies (NAMs) for the in vitro assessment of cleaning products for respiratory irritation: Workshop report. Front. Toxicol. 2024, 6, 1431790. [Google Scholar] [CrossRef]
- Kluxen, F.M.; Roper, C.S.; Jensen, S.M.; Koenig, C.M. Characterizing local acute irritation properties of captan and folpet with new approach methods. Appl. Vitr. Toxicol. 2022, 8, 83–101. [Google Scholar] [CrossRef]
- Roseli, R.B.; Keto, A.B.; Krenske, E.H. Mechanistic Aspects of Thiol Additions to Michael Acceptors: Insights from Computations. WIREs Comput. Mol. Sci. 2023, 13, e1636. [Google Scholar] [CrossRef]
- Gao, S.; Tzeng, T.; Sastry, M.; Chu, C.-M.; Liu, J.-T.; Lin, C.; Yao, C.-F. Iodine catalyzed conjugate addition of mercaptans to α,β-unsaturated carboxylic acids under solvent-free condition. Tetrahedron Lett. 2006, 47, 1889–1893. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Tirelli, N.; Cerritelli, S.; Cavalli, L.; Hubbell, J.A. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug. Chem. 2001, 12, 1051–1056. [Google Scholar] [CrossRef]
- Little, R.D.; Masjedizadeh, M.R.; Wallquist, O.; McLoughlin, J.I. The Intramolecular Michael Reaction. Org. React. 1995, 47, 315–552. [Google Scholar] [CrossRef]
- Omeragic, E.; Dedic, M.; Elezovic, A.; Becic, E.; Imamovic, B.; Kladar, N.; Niksic, H. Application of direct peptide reactivity assay for assessing the skin sensitization potential of essential oils. Sci. Rep. 2022, 12, 7470. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.S.; Ellingson, K.; Gao, Y.; Krutz, N.L.; Krivos, K.; Quijano, M.; Xu, Y.; Ryan, C.A. Development of a peptide reactivity assay for screening botanicals and natural substances: Proof of concept studies. Toxicol. Vitr. 2023, 90, 105591. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef]
- van Endert, P. Editorial: Insights in antigen presenting cell biology: 2021. Front. Immunol. 2022, 13, 1079913. [Google Scholar] [CrossRef]
- Mitachi, T.; Kouzui, M.; Maruyama, R.; Yamashita, K.; Ogata, S.; Kojima, H.; Itagaki, H. Some non-sensitizers upregulate CD54 expression by activation of the NLRP3 inflammasome in THP-1 cells. J. Toxicol. Sci. 2019, 44, 213–224. [Google Scholar] [CrossRef]
- Correa, M.C.M.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef]
- Darmstadt, G.L.; Mao-Qiang, M.; Chi, E.; Saha, S.K.; Ziboh, V.A.; Black, R.E.; Santosham, M.; Elias, P.M. Impact of topical oils on the skin barrier: Possible implications for neonatal health in developing countries. Acta Paediatr. 2002, 91, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M.; Andersson, A.C. Effect of topically applied lipids on surfactant-irritated skin. Br. J. Dermatol. 1996, 134, 215–220. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. [Google Scholar] [CrossRef]
- Robinson, M.K.; Cohen, C.; de Fraissinette Ade, B.; Ponec, M.; Whittle, E.; Fentem, J.H. Non-animal testing strategies for assessment of the skin corrosion and skin irritation potential of ingredients and finished products. Food Chem. Toxicol. 2002, 40, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Bao, H.; Kang, L. Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.B.; Kishore, A.S.; Surekha, P. Assessment of in vitro skin irritation potential of nanoparticles: RHE model. Methods Mol. Biol. 2012, 926, 219–234. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [PubMed]
- Danilenko, D.M.; Phillips, G.D.; Diaz, D. In Vitro Skin Models and Their Predictability in Defining Normal and Disease Biology, Pharmacology, and Toxicity. Toxicol. Pathol. 2016, 44, 555–563. [Google Scholar] [CrossRef]
- do Nascimento Pedrosa, T.; Catarino, C.M.; Pennacchi, P.C.; de Moraes Barros, S.B.; Maria-Engler, S.S. Skin Equivalent Models: Protocols for In Vitro Reconstruction for Dermal Toxicity Evaluation. In Toxicity Assessment. Methods in Molecular Biology; Palmeira, C.M.M., de Oliveira, D.P., Dorta, D.J., Eds.; Humana: New York, NY, USA, 2021; Volume 2240. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 5684–5696. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Chemical Composition of Root Essential Oil of Acorus calamus L. Natl. Acad. Sci. Lett. 2015, 38, 121–125. [Google Scholar] [CrossRef]
- Chandra, D.; Prasad, K. Phytochemicals of Acorus calamus (Sweet flag). J. Med. Plants Stud. 2017, 5, 277–281. [Google Scholar]
- Timilsina, R.; Tandukar, P.; Pathak, I. Biological and Chemical Studies of Essential Oil and Extracts of Rhizome of Acorus calamus Linn. J. Nepal Chem. Soc. 2022, 43, 35–42. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils—Natural Products Not Necessarily Safe. Int. J. Women’s Dermatol. 2020, 7, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Chiang, S.Y.; Lee, P.Y.; Lai, M.T.; Shen, L.C.; Chung, W.S.; Huang, H.F.; Wu, K.Y.; Wu, H.C. Safrole-2′,3′-oxide induces cytotoxic and genotoxic effects in HepG2 cells and in mice. Mutat. Res. 2011, 726, 234–241. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; He, J.; Zhong, L.; Song, Y.; Shao, H. Cytotoxicity of safrole in HepaRG cells: Studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 2019, 49, 1504–1515. [Google Scholar] [CrossRef]
- Ahn, J.; Avonto, C.; Pandey, P.; Khan, S.I.; Khan, I.A.; Roberts, D.W.; Chittiboyina, A.G. Chemistry of Isoeugenol and Its Oxidation Products: Mechanism and Kinetics of Isoeugenol as a Skin Sensitizer. Chem. Res. Toxicol. 2023, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Port-Lougarre, Y.; Gourlaouen, C.; Vileno, B.; Giménez-Arnau, E. Antioxidant Activity and Skin. Sensitization of Eugenol and Isoeugenol: Two Sides of the Same Coin? Chem. Res. Toxicol. 2023, 36, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Haupt, T. Utility of rat liver S9 fractions to study skin-sensitizing prohaptens in a modified KeratinoSens assay. Toxicol. Sci. 2013, 135, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Harada, K.; Ohmori, S.; Minamoto, K.; Wei, C.; Ueda, A. Toxicity study of the volatile constituents of Myoga utilizing acute dermal irritation assays and the Guinea-pig Maximization test. J. Occup. Health 2006, 48, 480–486. [Google Scholar] [CrossRef]
- Wei, Q.J.; Wei, C.N.; Harada, K.; Minamoto, K.; Okamoto, Y.; Otsuka, M.; Ueda, A. Evaluation of allergenicity of constituents of myoga using the murine local lymph node assay. Int. J. Immunopathol. Pharmacol. 2010, 23, 463–470. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, J.Y.; Liu, Y.; Ru, Q.G.; Wang, Y.F.; Yu, J.X.; Wu, Q. Effect of terpene penetration enhancer and its mechanisms on membrane fluidity and potential of HaCaT keratinocytes. China J. Chin. Mater. Med. 2015, 40, 643–648. (In Chinese) [Google Scholar]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Lee, C.L.; Lo, P.T.; Jhan, Y.L.; Chen, C.J.; Chang, Y.S. New ent-kauran diterpene and antioxidant components from the seed of Ipomoea nil. Nat. Prod. Res. 2021, 35, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Raabe, H.A.; Costin, G.E.; Allen, D.G.; Lowit, A.; Corvaro, M.; O’Dell, L.; Sullivan, K. Human relevance of in vivo and in vitro skin irritation tests for hazard classification of pesticides. Cutan. Ocul. Toxicol. 2024, 44, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S.; Deng, W. Dual responsive linalool capsules with high loading ratio for excellent antioxidant and antibacterial efficiency. Colloids Surf. B Biointerfaces 2020, 190, 110978. [Google Scholar] [CrossRef] [PubMed]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Api, A.M.; Vey, M. Implementation of the dermal sensitization Quantitative Risk Assessment (QRA) for fragrance ingredients. Regul. Toxicol. Pharmacol. 2008, 52, 53–61. [Google Scholar] [CrossRef]
- Api, A.M.; Basketter, D.; Bridges, J.; Cadby, P.; Ellis, G.; Gilmour, N.; Greim, H.; Griem, P.; Kern, P.; Khaiat, A.; et al. Updating exposure assessment for skin sensitization quantitative risk assessment for fragrance materials. Regul. Toxicol. Pharmacol. 2020, 118, 104805. [Google Scholar] [CrossRef]
- Tourneix, F.; Carron, L.; Jouffe, L.; Hoffmann, S.; Alépée, N. Deriving a Continuous Point of Departure for Skin Sensitization Risk Assessment Using a Bayesian Network Model. Toxics 2024, 12, 536. [Google Scholar] [CrossRef]
- Ebmeyer, J.; Najjar, A.; Lange, D.; Boettcher, M.; Voß, S.; Brandmair, K.; Meinhardt, J.; Kuehnl, J.; Hewitt, N.J.; Krueger, C.T.; et al. Next generation risk assessment: An ab initio case study to assess the systemic safety of the cosmetic ingredient, benzyl salicylate, after dermal exposure. Front. Pharmacol. 2024, 15, 1345992. [Google Scholar] [CrossRef]
- Na, M.; O’Brien, D.; Lavelle, M.; Lee, I.; Gerberick, G.F.; Api, A.M. Weight of Evidence Approach for Skin Sensitization Potency Categorization of Fragrance Ingredients. Dermatitis 2022, 33, 161–175. [Google Scholar] [CrossRef]
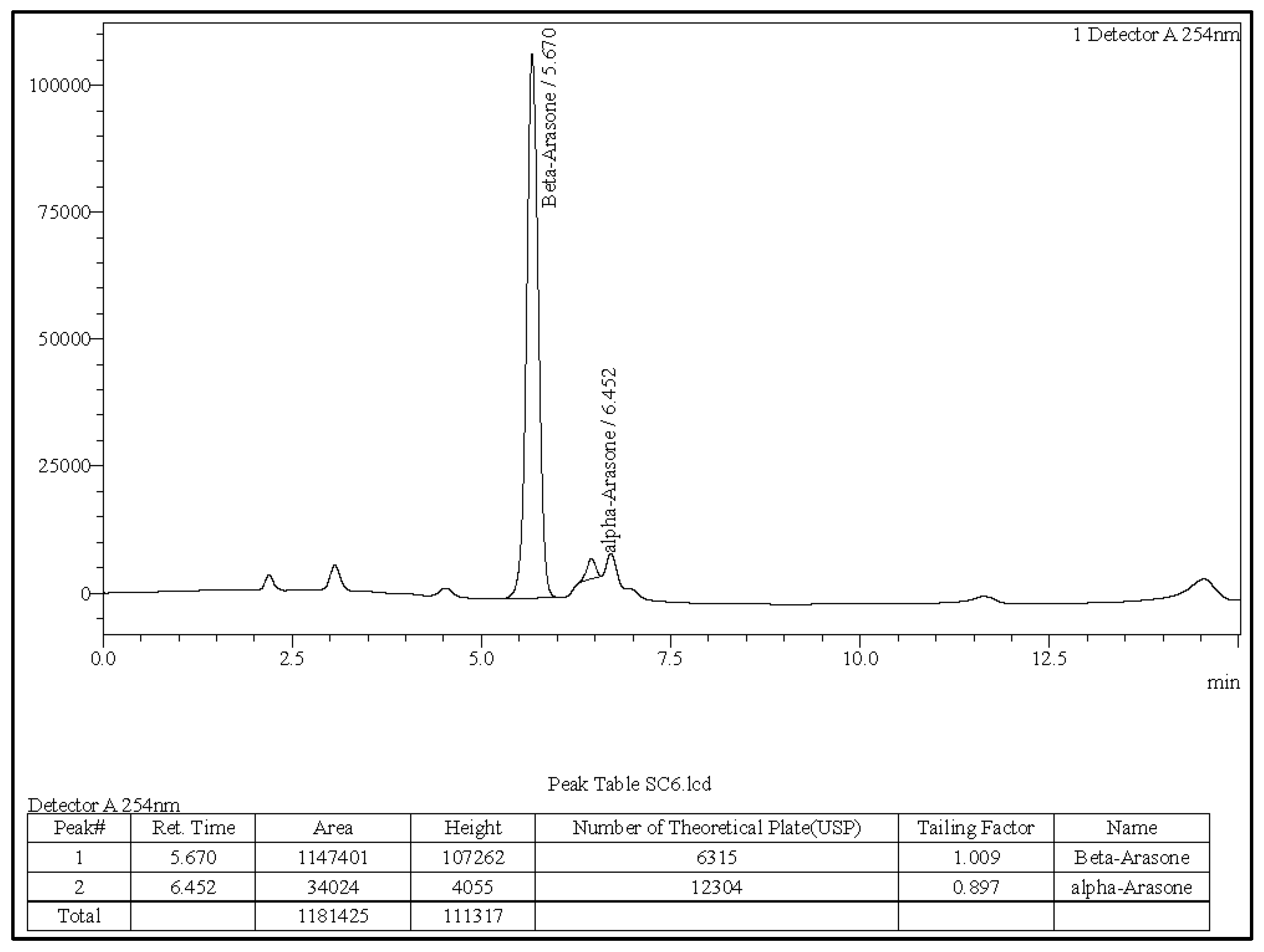
| Asarone | Retention Time (Reference) | Retention Time (Sample) | Reference Standard Peak Area | Sample Peak Area | % Content |
|---|---|---|---|---|---|
| α | 6.402 | 6.452 | 8,178,131 | 34,024 | 4.16 |
| β | 5.627 | 5.670 | 2,252,390 | 1,147,401 | 40.75 |
| γ | 5.988 | ND | 2236 | ND | ND |
| Sample Type | Mean % Viability | SD | CV (%) | NSMTT (%) |
|---|---|---|---|---|
| DPBS (Negative Control) | 100 | 0.037 | 1.91 | - |
| A. calamus Rhizome Oil | 23.5 | 1.50 | 6.39 | −11.2 |
| 5% SDS (Positive Control) | 1.5 | 0.058 | 3.87 | - |
| Exposure Time (min) | Sample Type | Mean % Viability | SD | CV (%) | NSMTT (%) |
|---|---|---|---|---|---|
| 3 | Distilled Water (Negative Control) | 100 | 0.04 | 1.87 | N/A |
| A. calamus Rhizome Oil | 92.72 | 2.13 | 2.30 | −0.7 | |
| 60 | Distilled Water (Negative Control) | 100 | 0.04 | 1.88 | N/A |
| A. calamus Rhizome Oil | 91.17 | 1.49 | 1.63 | 0.1 | |
| 8N Potassium Hydroxide (Positive Control) | 0.17 | 0.03 | 17.65 | −18.6 |
| Acceptance Criteria | Obtained Results |
|---|---|
| The standard calibration curve should have an R2 > 0.99. | >0.99 |
| The mean % peptide depletion value of the three replicates for the positive control cinnamic aldehyde should be between 60.8% and 100% for the cysteine peptide. | 81% |
| The mean % peptide depletion value of the three replicates for the positive control cinnamic aldehyde should be between 40.2% and 69.0% for the lysine peptide. | 67% |
| The maximum standard deviation (SD) for the positive control replicates should be <14.9% for cysteine depletion and <11.6% for lysine depletion. | 0.29% (Cys)/0.37% (Lys) |
| The mean peptide concentration of reference control A should be 0.50 ± 0.05 mM. | 0.5–0.51 mM |
| The coefficient of variation (CV) of peptide peak areas for the nine reference controls, B and C in acetonitrile should be <15.0%. | 0.38% (Ref B)/1.65% (Ref C) |
| Peptide | Sample | % Depletion | %Mean Peptide Depletion (Cysteine and Lysine Peptide) | ||
| A. calamus Rhizome Oil | Cinnamaldehyde | ||||
| Mean | SD | 23 | 74 | ||
| Cysteine | A. calamus Rhizome Oil | 46 | 0.52 | ||
| Cinnamaldehyde | 80.99 | 0.29 | |||
| Lysine | A. calamus Rhizome Oil | 0.47 | 0.18 | ||
| Cinnamaldehyde | 67 | 0.37 | |||
| Sample | IC30 | IC50 | Imax | EC1.5 |
|---|---|---|---|---|
| A. calamus Rhizome Oil | 21.48 | 28.51 | 2.88 ± 0.40 | 3.84 ± 0.21 * |
| Trans-cinnamaldehyde | N/A | N/A | 11.52 ± 10.14 | 8.84 ± 1.86 µM |
| MFI Ratios (CD86/CD54) | % Viability | % RFI (CD86/CD54) | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Experiment 1 | Experiment 2 | Criterion | Experiment 1 | Experiment 2 | Criterion | Experiment 1 | Experiment 2 |
| Medium Control | 131.33/ 111.45 | 122.56/ 115.24 | >105 | 96.50 | 97.90 | >90 | N/A | |
| Solvent (DMSO) Control | 149.04/ 120.38 | 124.29/ 110.73 | 95.10 | 96.70 | 148.08/ 168.42 | 116.22/ 76 | ||
| Positive Control | N/A | N/A | 87.70 | 67.10 | >50 | 476.62/ 1140.63 | 537.21/ 1342.11 | |
| CV75 Value (µg/mL) | Experiment 1 | Experiment 2 | EC150 (µg/mL) | * EC200 (µg/mL) | Final Prediction |
|---|---|---|---|---|---|
| 51 | P12 | P2 | 27 | 17 | Positive |
| PoD | ED01 5th | ED01 50th | ED01 95th |
|---|---|---|---|
| 170 | 2.0 | 180 | 13,000 |
| Product Category (SCCS Mapping) | SCCS-Derived Applied Mass per Application, M (mg/cm2) | SAF (QRA2) | Cmax (%, w/w) [ppm] |
|---|---|---|---|
| Face cream (leave-on) | 1.27 | 100 | 0.13% [1300 ppm] |
| Body lotion (leave-on, whole body) | 0.219 | 100 | 0.78% [7800 ppm] |
| Hand cream (leave-on) | 1.26 | 100 | 0.13% [1300 ppm] |
| Shower gel (rinse-off, whole body) | 0.0076 | 300 | 7.46% [74,600 ppm] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, K.R.; Ranade, J.R.; Nagane, R.M.; Patel, M.V.; Deshpande, A.D.; Roper, C.S.; Kantli, G.B. Comprehensive In Vitro Safety Assessment of Acorus calamus Rhizome Oil Using OECD-Compliant New Approach Methods: Classification as a GHS Category 1B Sensitiser and Category 2 Irritant. Toxics 2025, 13, 1006. https://doi.org/10.3390/toxics13121006
Desai KR, Ranade JR, Nagane RM, Patel MV, Deshpande AD, Roper CS, Kantli GB. Comprehensive In Vitro Safety Assessment of Acorus calamus Rhizome Oil Using OECD-Compliant New Approach Methods: Classification as a GHS Category 1B Sensitiser and Category 2 Irritant. Toxics. 2025; 13(12):1006. https://doi.org/10.3390/toxics13121006
Chicago/Turabian StyleDesai, Karishma R., Jay R. Ranade, Rajendra M. Nagane, Manish V. Patel, Abhay D. Deshpande, Clive S. Roper, and Gireesh Babu Kantli. 2025. "Comprehensive In Vitro Safety Assessment of Acorus calamus Rhizome Oil Using OECD-Compliant New Approach Methods: Classification as a GHS Category 1B Sensitiser and Category 2 Irritant" Toxics 13, no. 12: 1006. https://doi.org/10.3390/toxics13121006
APA StyleDesai, K. R., Ranade, J. R., Nagane, R. M., Patel, M. V., Deshpande, A. D., Roper, C. S., & Kantli, G. B. (2025). Comprehensive In Vitro Safety Assessment of Acorus calamus Rhizome Oil Using OECD-Compliant New Approach Methods: Classification as a GHS Category 1B Sensitiser and Category 2 Irritant. Toxics, 13(12), 1006. https://doi.org/10.3390/toxics13121006






