Effects of Biochar and Microbial Organic Fertilizers on Agricultural Productivity and Their Microbial Mechanisms Under Heavy Metal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Soil Sampling and Basic Analysis
- (1)
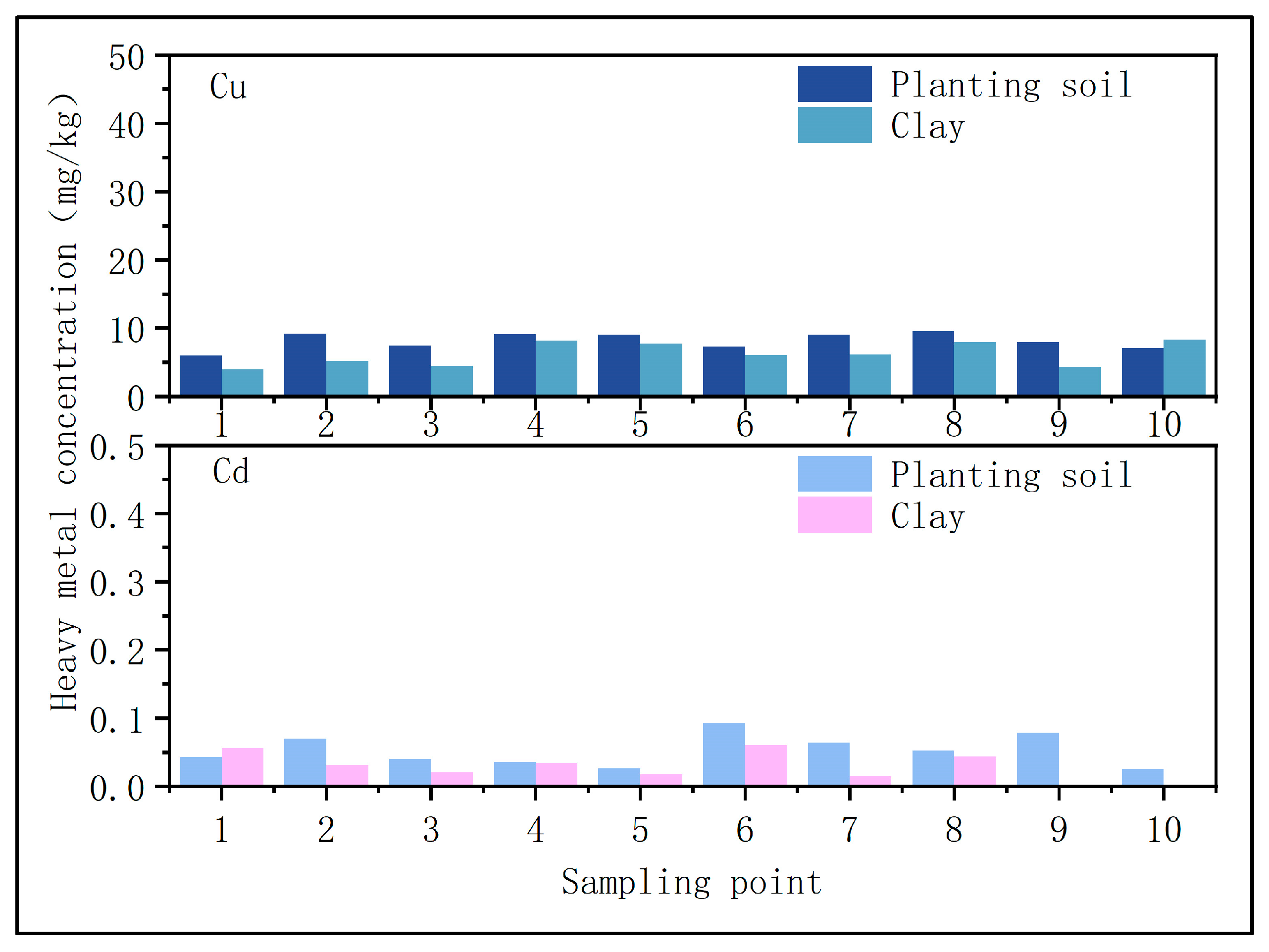
- Soil heavy metal content
- (2)
- Soil microbial community structure
- (3)
- Physical and chemical properties of microbial organic fertilizer
2.3. Experimental Design
3. Results
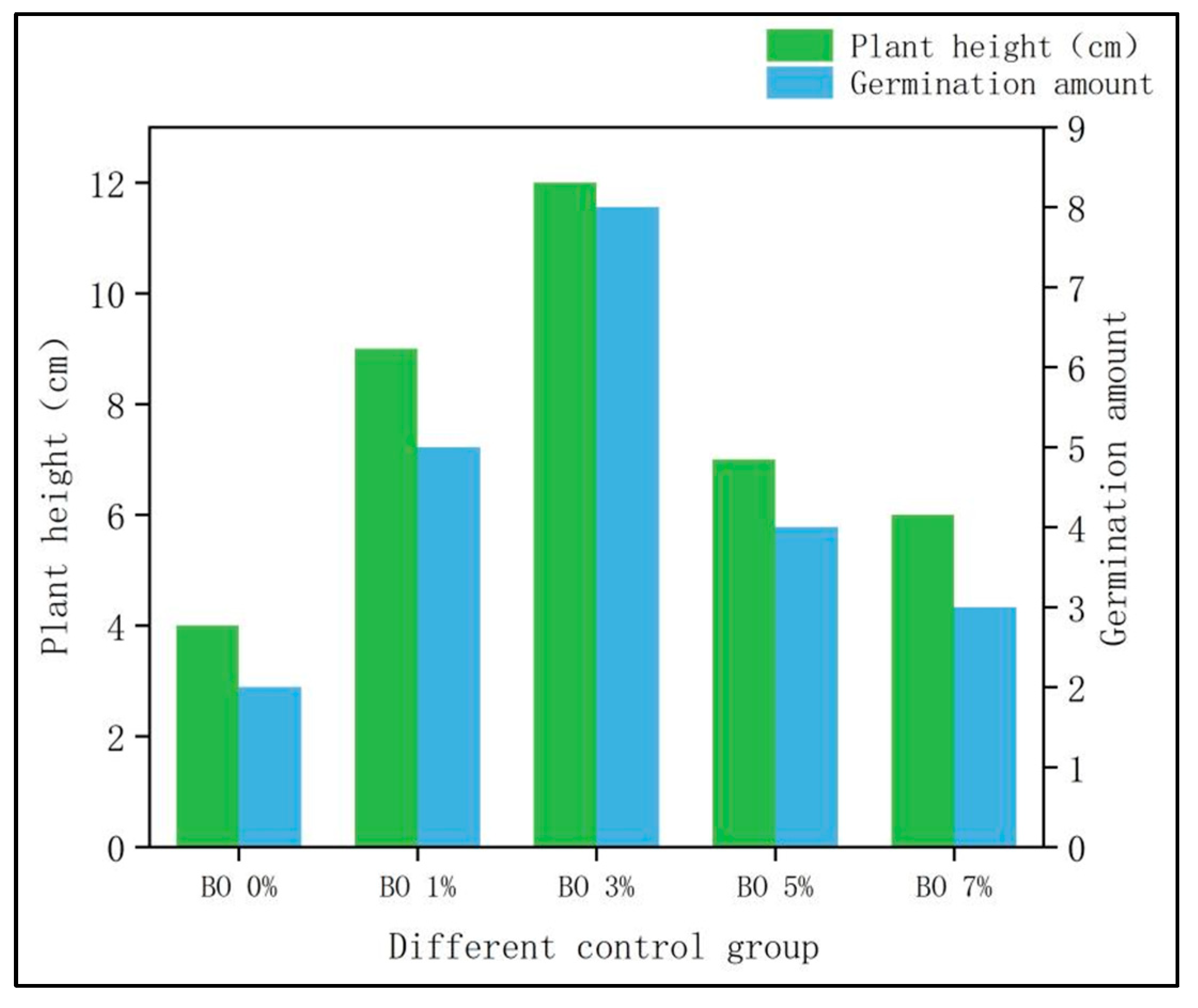
3.1. Growth of Potted Plants
- (1)
- Plant height: The plant height of Chinese cabbage in the BO 0% group (only microbial organic fertilizer) was 4 cm, while the heights in the biochar-amended groups (BO 1%, BO 3%, BO 5%, and BO 7%) were 9 cm, 12 cm, 7 cm, and 6 cm, respectively. The significant increase in plant height with biochar addition can be attributed to the combined effects of improved soil structure, enhanced nutrient availability, and reduced heavy metal toxicity. Biochar’s porous structure increases soil aeration and water retention, while its alkaline nature helps elevate soil pH, thereby decreasing the bioavailability of heavy metals such as Cd and Cu. Additionally, biochar facilitates the immobilization of heavy metals through adsorption and complexation, reducing their uptake by plants. The optimal plant growth observed in the BO 3% group suggests that this biochar concentration provides the most favorable conditions for root development and nutrient absorption, whereas higher biochar levels (e.g., BO 5% and BO 7%) may lead to nutrient imbalance or limited root space, thus inhibiting growth.
- (2)
- Percentage of germination: The germination rate in the BO 0% group was 9.09%, while the rates in the biochar-amended groups were 21.71%, 34.78%, 16.67%, and 13.04%, respectively. The notable enhancement in germination, particularly in the BO 3% group (34.78%), is likely due to the synergistic effects of biochar and microbial organic fertilizer in mitigating heavy metal stress. Biochar adsorbs heavy metals and reduces their exchangeable fractions, thereby lowering phytotoxicity. Moreover, the addition of biochar improves soil microbial activity and organic matter decomposition, which promotes nutrient release and creates a more favorable microenvironment for seed germination. The decline in germination at higher biochar concentrations (BO 5% and BO 7%) may be associated with excessive adsorption of essential nutrients or the release of inhibitory compounds, such as polycyclic aromatic hydrocarbons, which can adversely affect seed viability and early seedling growth.
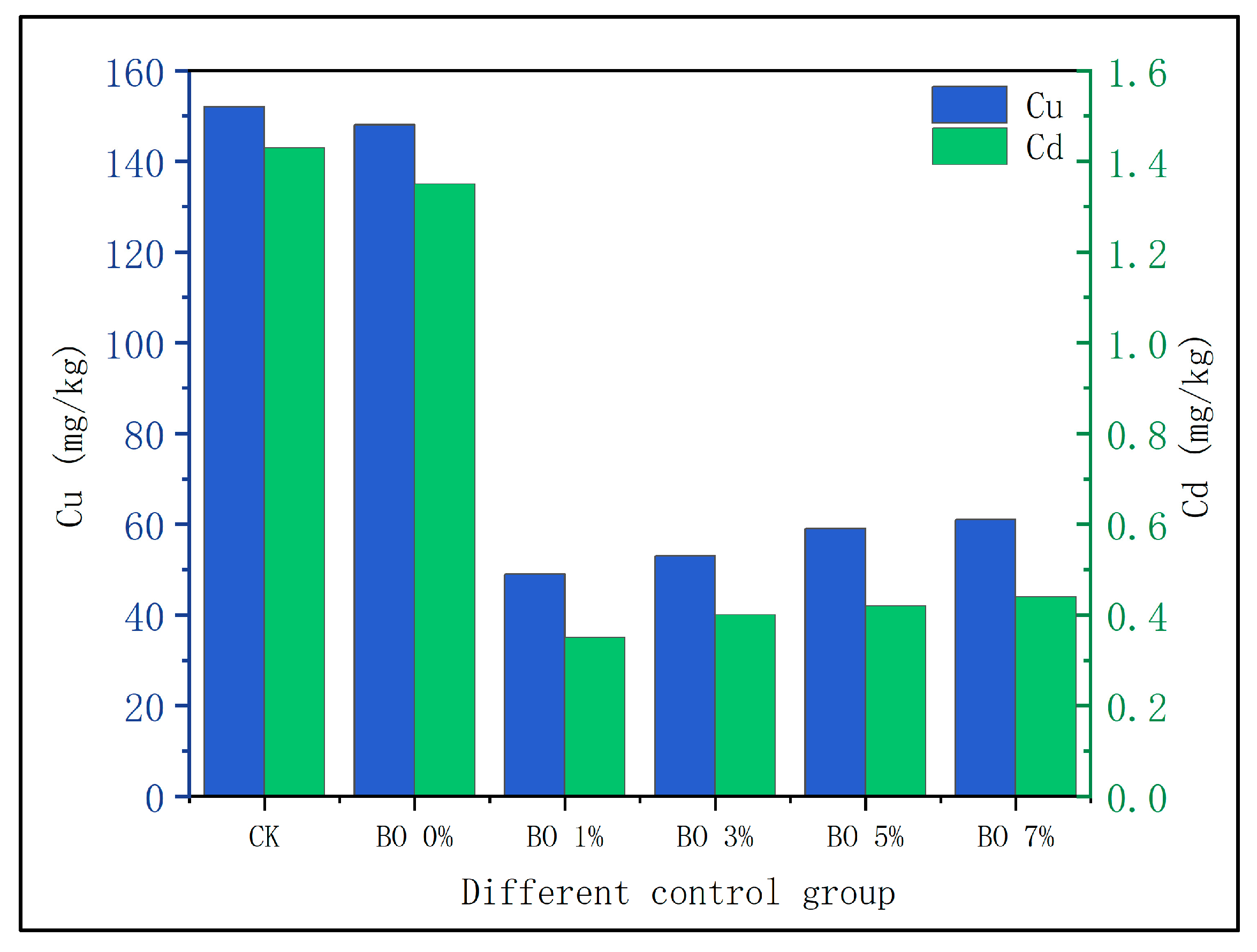
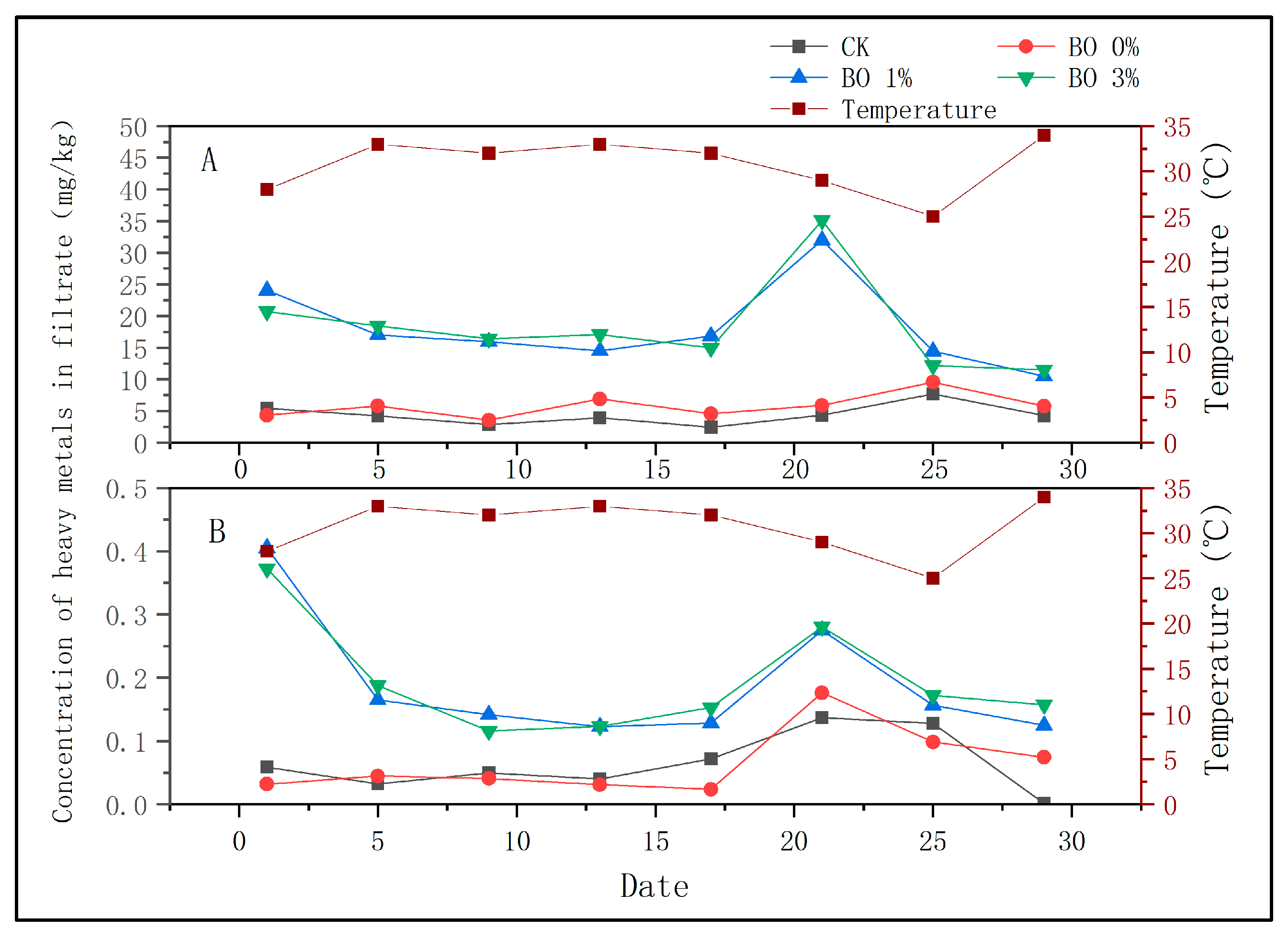
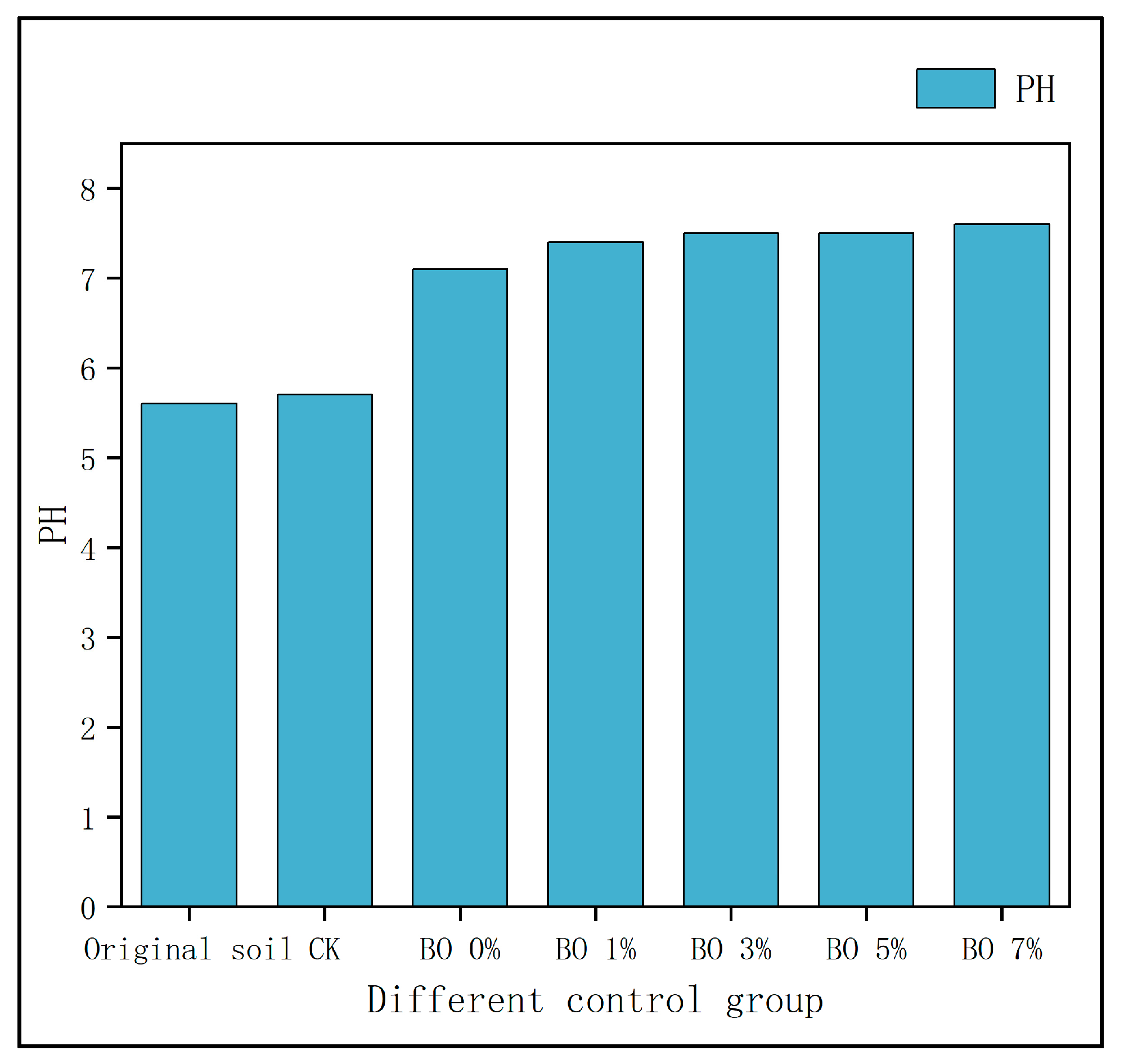
3.2. Changes in Heavy Metals in Soil
- (1)
- Migration of heavy metals into soil
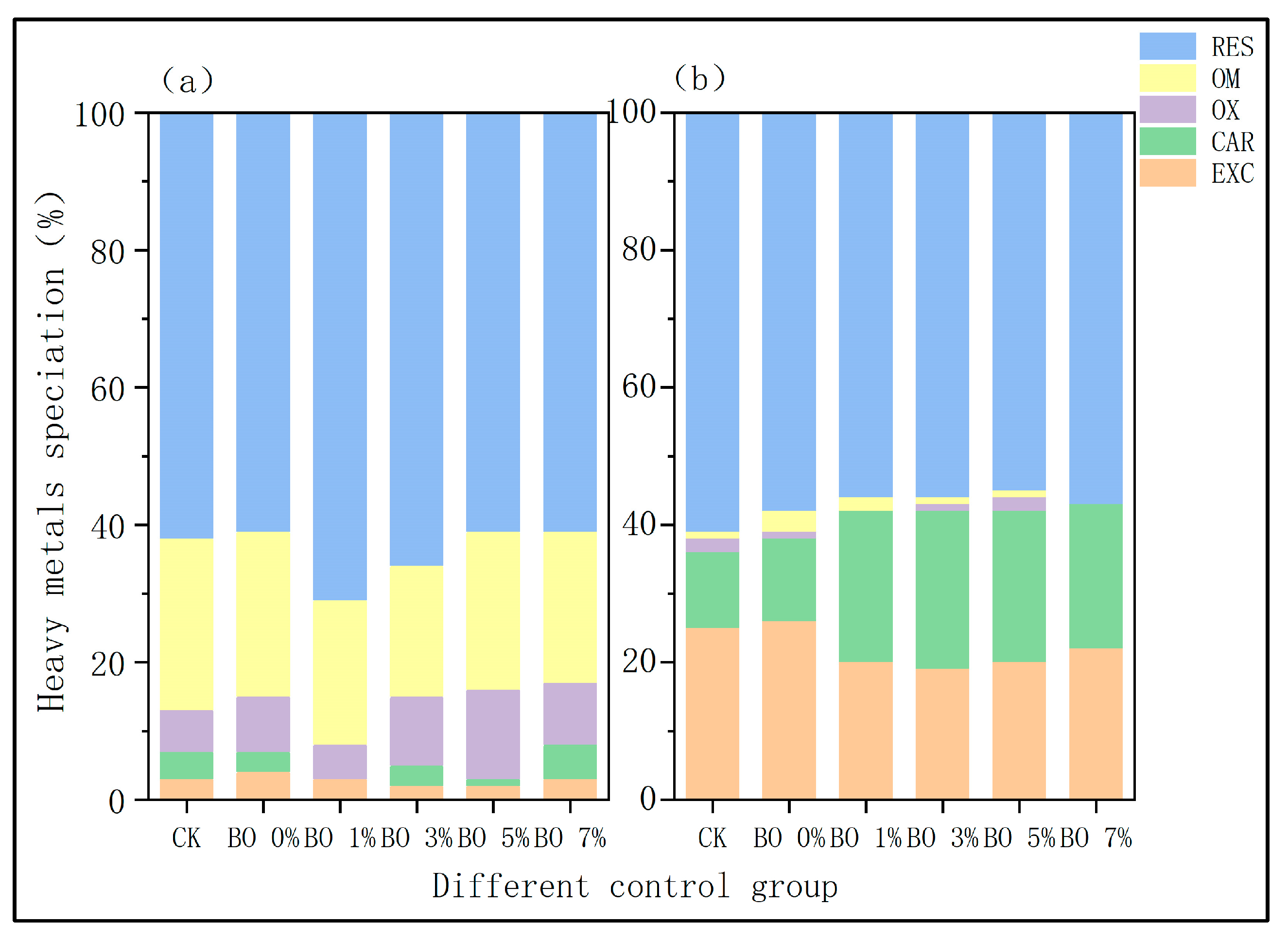
- (2)
- Morphological changes in soil heavy metals
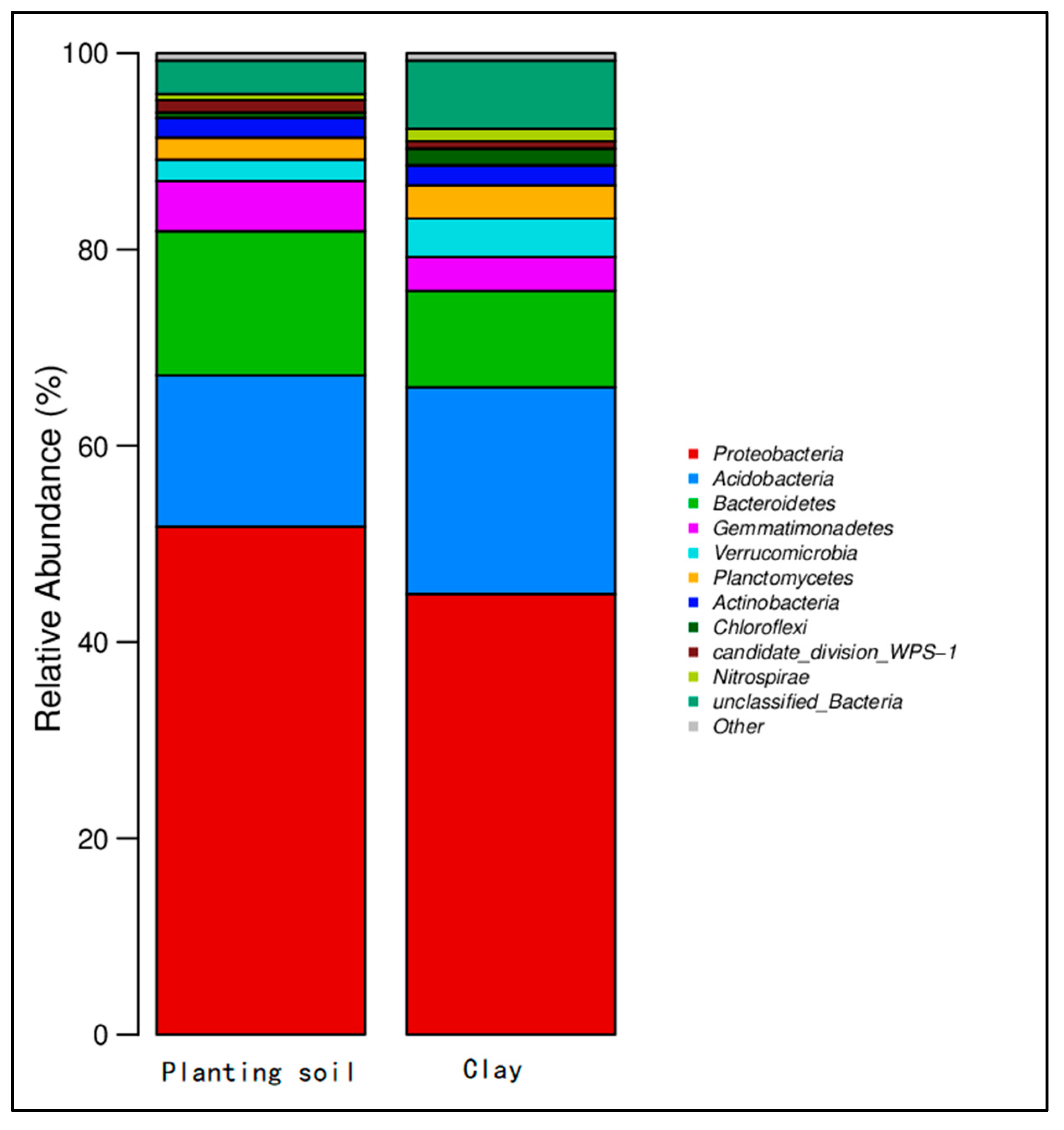
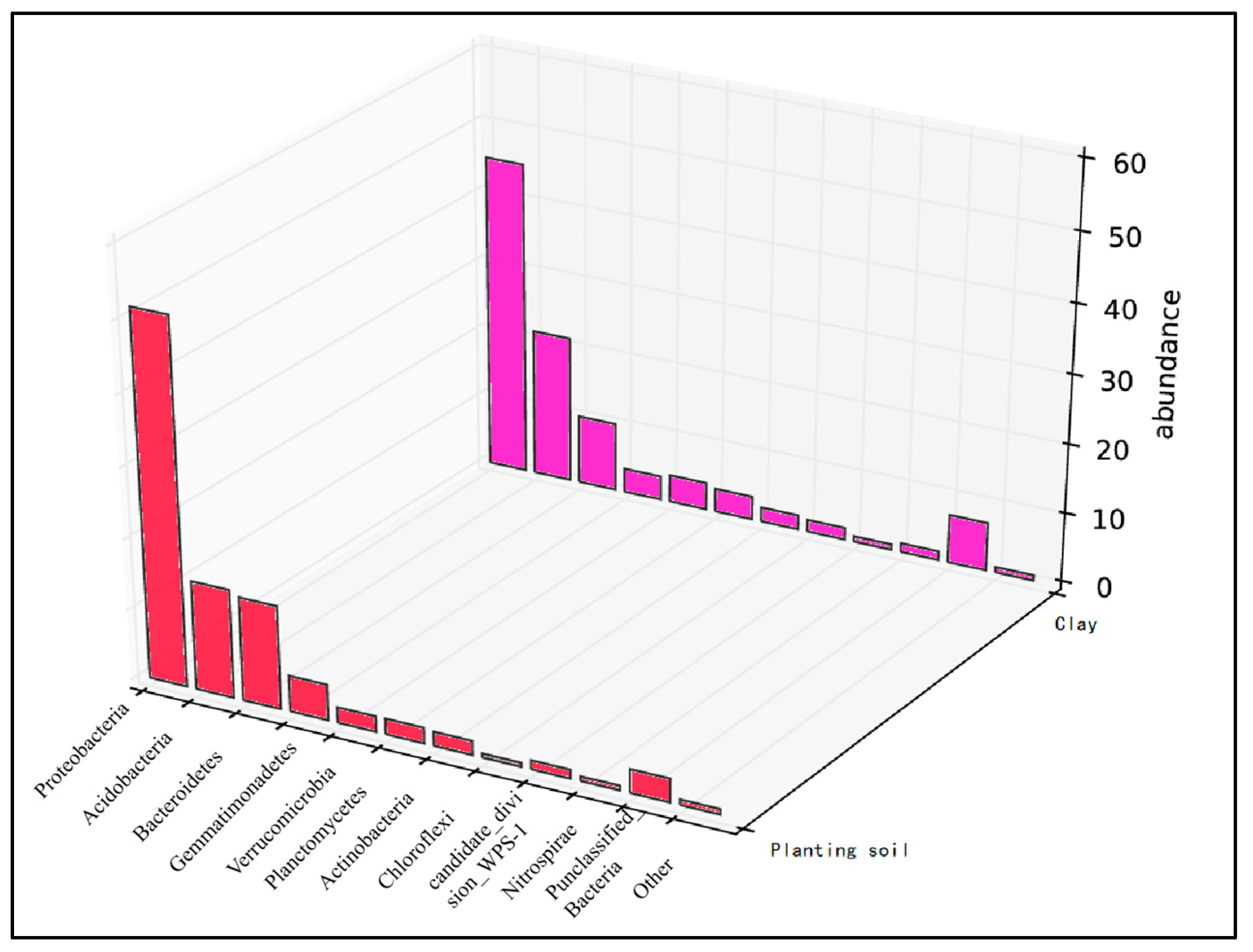
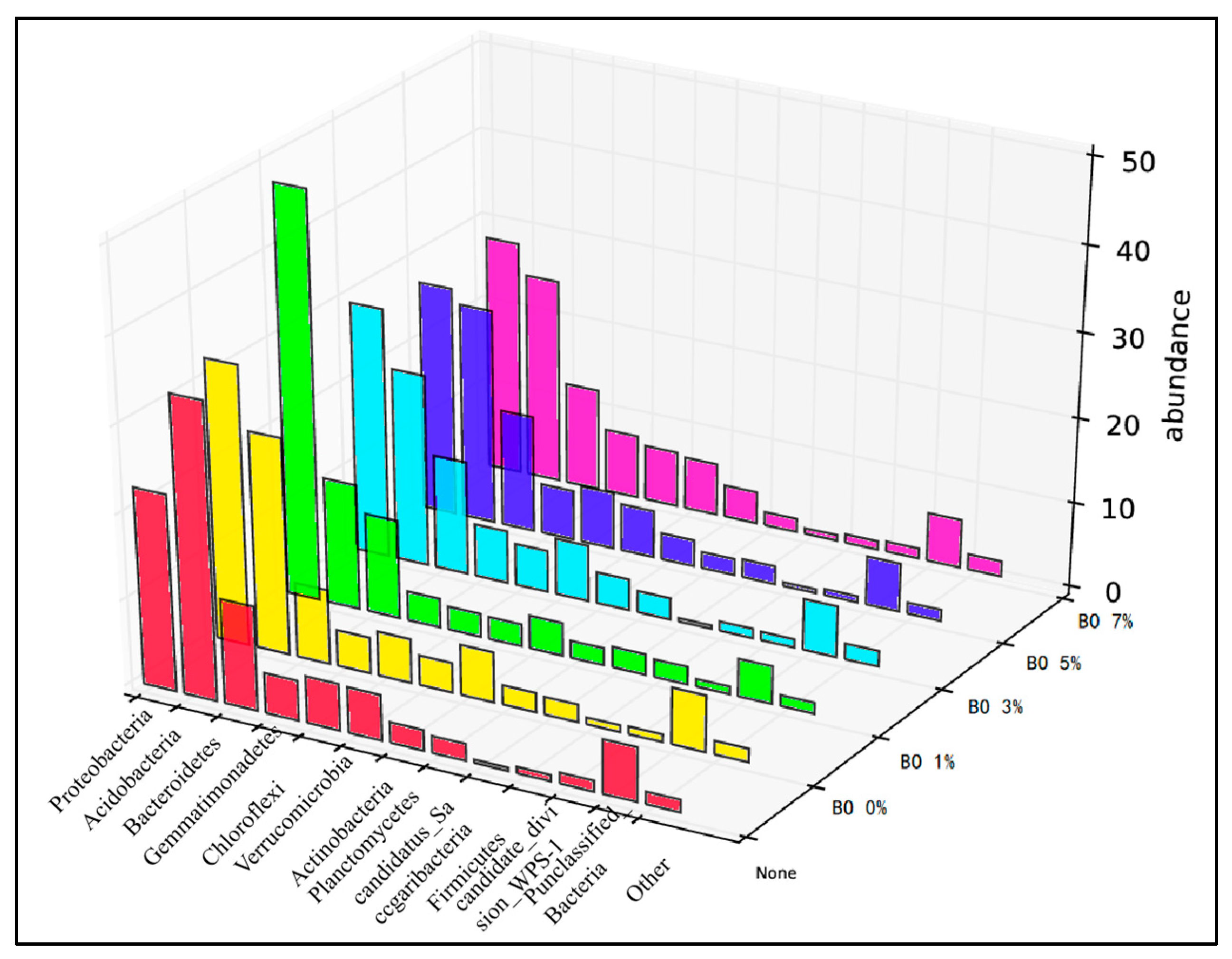
3.3. Change in Abundance of Microbial Community in Soil
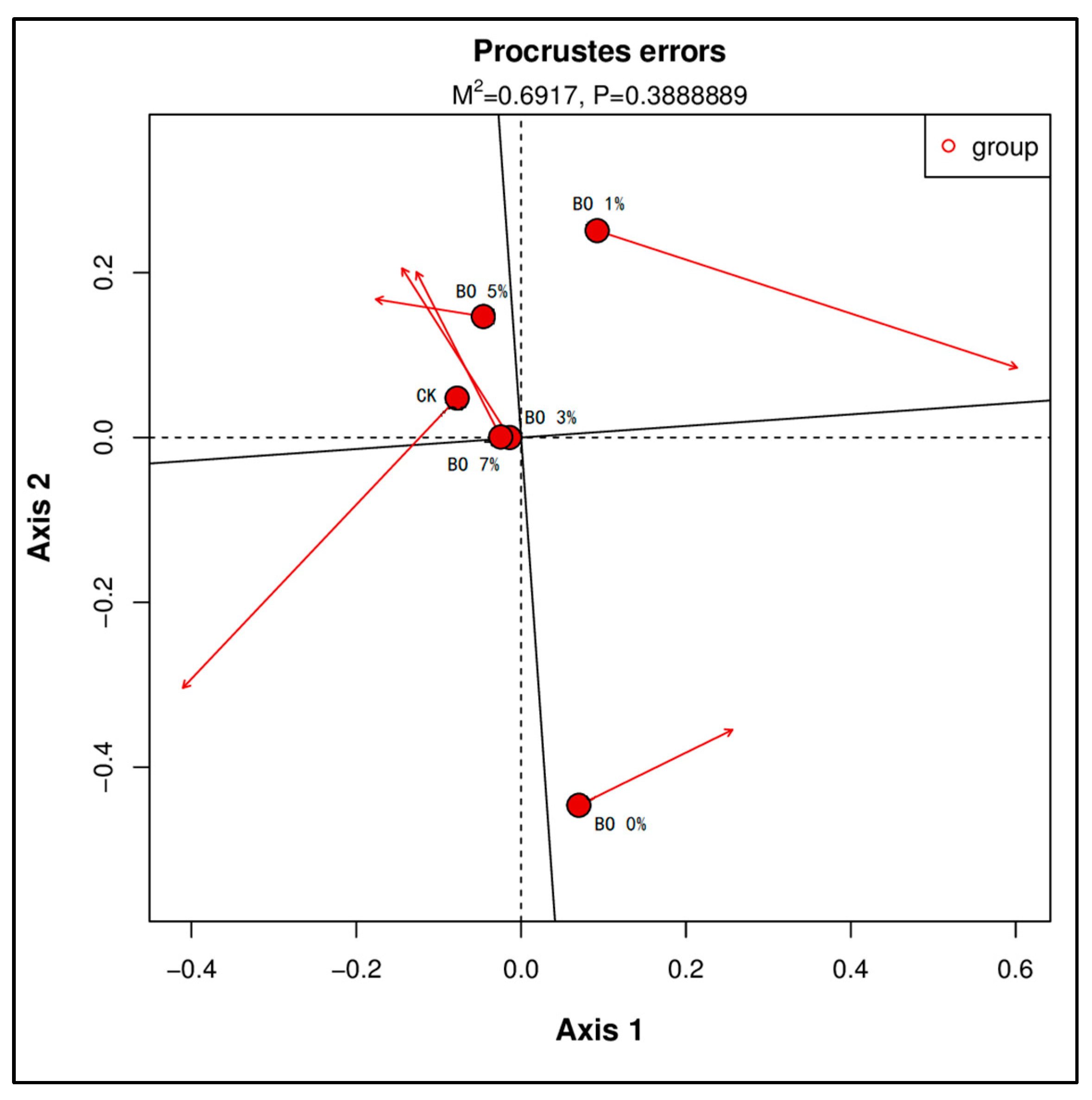
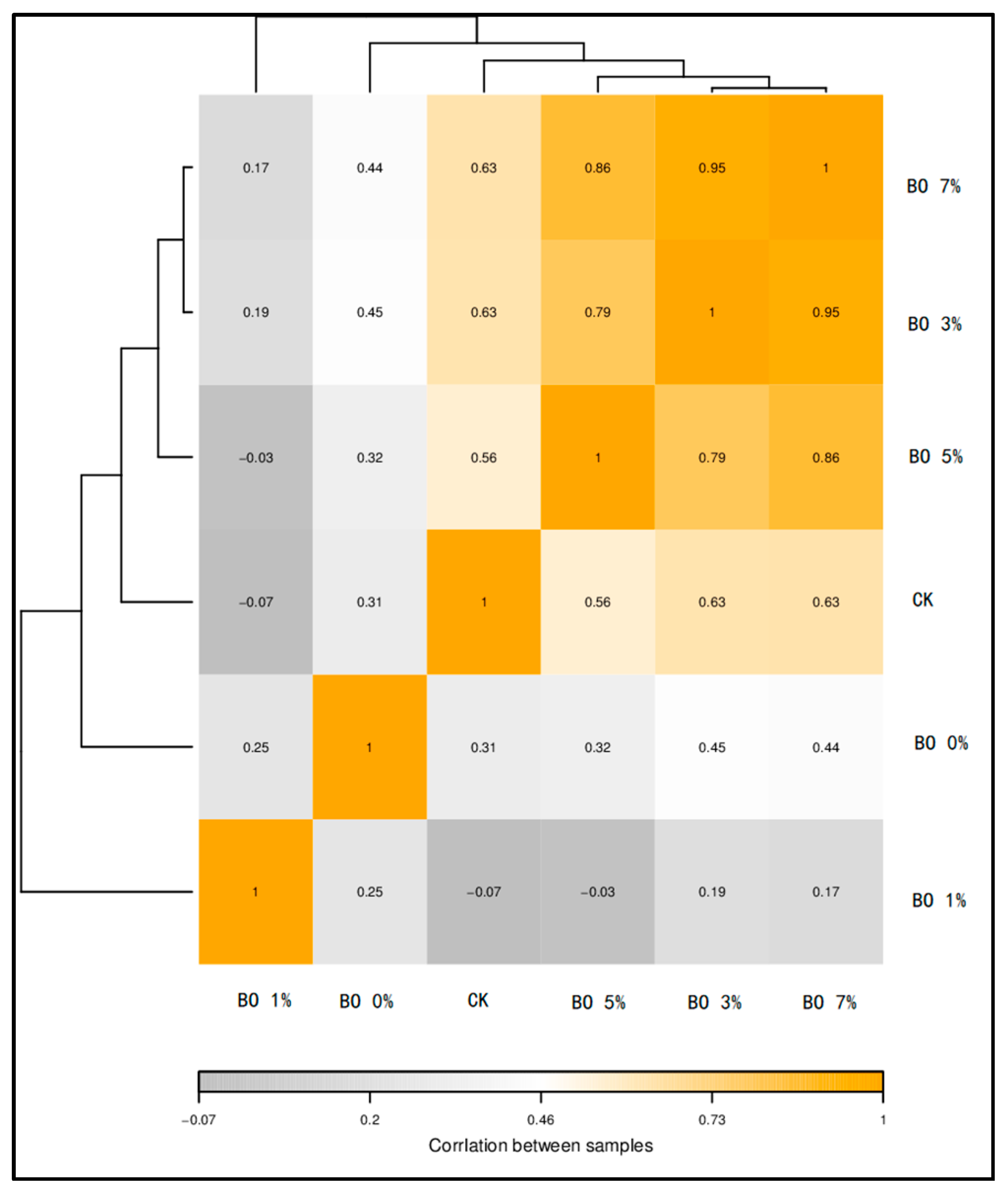
3.4. Soil Microbial and Metabolome Analysis
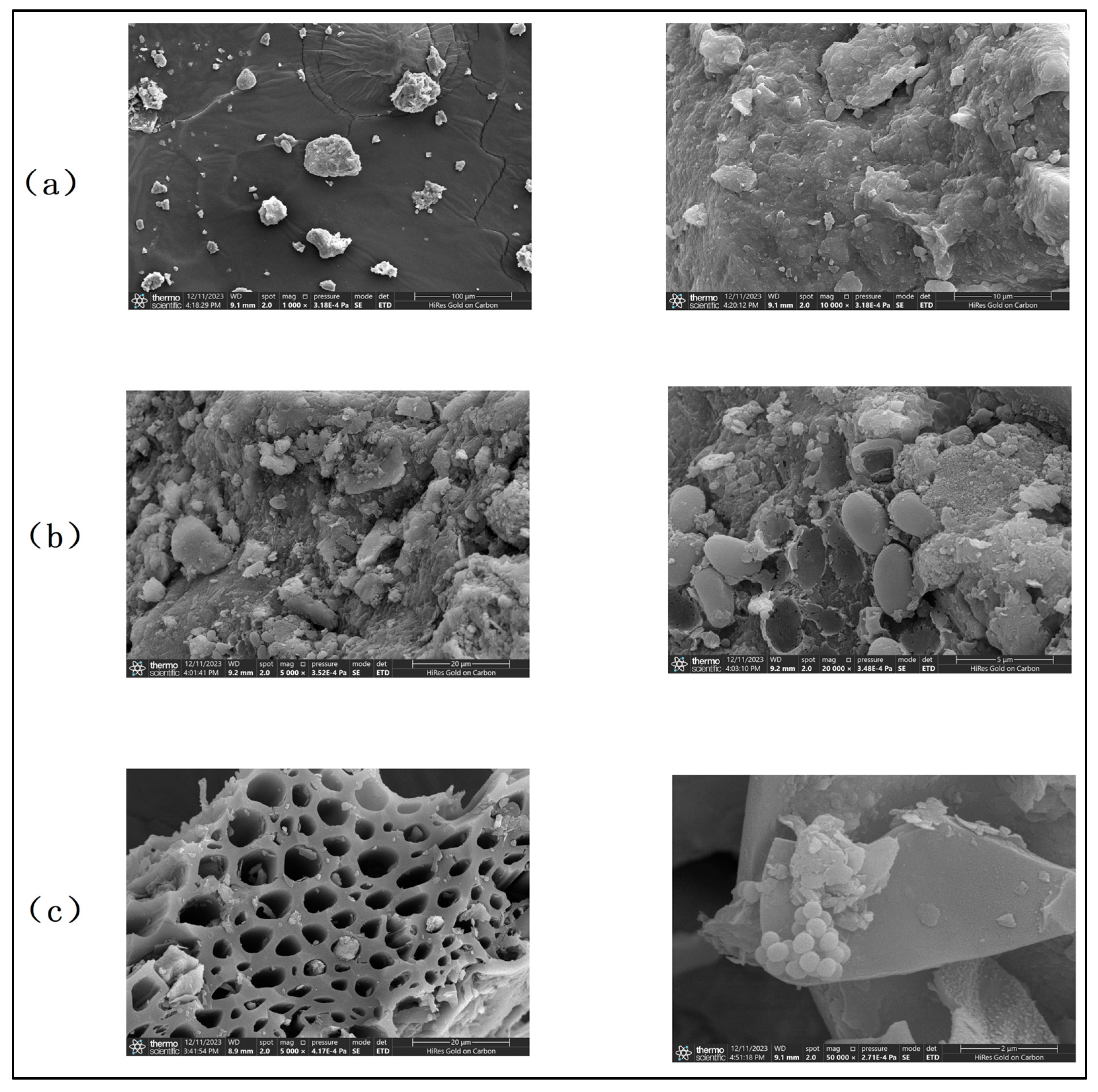
3.5. Soil Pore Structure of Different Treatment Groups Under Scanning Electron Microscopy
4. Discussion
4.1. Effects of Amendments on Biomass of Chinese Cabbage
4.2. Effects of Amendments on Soil Heavy Metal Concentration Changes
4.3. Effects of Amendments on the Heavy Metal Speciation in Soil
4.4. Effects of Amendments on Soil Microbial Community Structure
5. Conclusions
- Optimal ratio confirmation: Under the experimental conditions (200 mg/kg Cu and 2 mg/kg Cd contamination), the combination of 3% biochar (soil weight basis) and 10% microbial organic fertilizer (soil weight basis) is the optimal ratio. This combination achieved the highest germination rate (34.78%) and plant height (12 cm) of Chinese cabbage, with Cu and Cd removal rates reaching 73.2% and 80%, respectively. Excessive biochar (5% and 7%) led to reduced plant growth, confirming that crop growth responses to biochar addition follow a “promotion then inhibition” pattern.
- Synergistic remediation mechanism clarification: The combined amendment regulates soil pH from acidic (initial pH 5.4) to slightly alkaline (pH 7.2–7.5), reducing the exchangeable fraction of heavy metals (Cd exchangeable fraction decreased by ~10% compared to non-biochar groups) and increasing the residual fraction (Cu residual fraction accounting for 60–70%, Cd for 45–60%), thereby lowering heavy metal bioavailability and ecological toxicity. Biochar’s porous structure (observed via SEM) reduces soil compaction and provides habitats for microorganisms, while microbial organic fertilizer enriches exogenous beneficial flora. Together, they optimize soil microbial abundance: Acidobacteria (associated with plant residue degradation) increased to 15–32%, Chloroflexi (adapting to nutrient-rich environments) and Verrucomicrobia (involved in carbon cycling) showed enhanced abundance, and Proteobacteria (key for nitrogen fixation) was significantly higher than the control group, improving soil self-remediation capacity. The amendment enhances soil water-holding capacity and reduces bulk density, minimizing heavy metal leaching—particularly evident after rainfall, where biochar-amended groups showed 30–50% lower heavy metal concentrations in leachate compared to non-amended groups.
- Applicability verification: The combined application of biochar and microbial organic fertilizer effectively addresses concurrent issues of heavy metal pollution and soil compaction in farmland. It creates a virtuous cycle: biochar adsorbs heavy metals and improves soil structure, microbial organic fertilizer regulates flora and enhances nutrient supply, collectively promoting crop growth and reducing heavy metal accumulation in plants (no detectable Cu/Cd in Chinese cabbage). This approach provides a practical and sustainable technical solution for the remediation of Cu/Cd-contaminated agricultural soils.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Ting, L.; Zhen, H.; Xiao, Y. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, C.; Zhang, Y.; Hou, Y.; Chang, R. Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 2020, 252, 126591. [Google Scholar] [CrossRef]
- Liu, L.; Wei, L.; Wei, S.; Ming, G. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Yuchen, W.; Jiayu, G.; Junjun, N. Influence of biochar on soil air permeability and greenhouse gas emissions in vegetated soil: A review. Biogeotechnics 2023, 1, 100040. [Google Scholar] [CrossRef]
- Wang, Y. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2022, 9, 135–148. [Google Scholar] [CrossRef]
- Snigdhendubala, P.; Prakash, P.; Mackey, R.; Ansari, T.; McKay, G. Food waste biochar: A sustainable solution for agriculture application and soil–water remediation. Carbon Res. 2024, 3, 41. [Google Scholar] [CrossRef]
- Salam, A.; Bashir, S.; Khan, I.; Shahid, R.; Muhammad, A. Biochars Immobilize Lead and Copper in Naturally Contaminated Soil. Environ. Eng. Sci. 2018, 35, 1349–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Muhammad, A.; Dalia, F.; Vita, T.; Kashif, A.; Urte, S.; Rashid, I. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Jian, L.; Feng, Z.; Zhang, G.; Yang, H.; Gao, C.; Jin, L.; Yu, J. Chemical fertilizer reduction combined with bio-organic fertilizers increases cauliflower yield via regulation of soil biochemical properties and bacterial communities in Northwest China. Front. Microbiol. 2022, 13, 922149. [Google Scholar] [CrossRef]
- Medynska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Liu, X.; Bi, Q.; Qiu, L.; Li, K.; Yang, X.; Lin, X. Increased risk of phosphorus and metal leaching from paddy soils after excessive manure application: Insights from a mesocosm study. Sci. Total Environ. 2019, 666, 778–785. [Google Scholar] [CrossRef]
- Glab, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Chen, C.; Lv, Q.; Tang, Q. Impact of bio-organic fertilizer and reduced chemical fertilizer application on physical and hydraulic properties of cucumber continuous cropping soil. Biomass Convers. Biorefinery 2024, 14, 921–930. [Google Scholar] [CrossRef]
- Yan, S.; Wei, Y.; Ting, Y.; Qi, L.; Guo, J. The Adsorption of Corn Stalk Biochar for Pb and Cd: Preparation, Characterization, and Batch Adsorption Study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Ma, S.; Wang, X.; Wang, S.; Feng, K. Effects of temperature on physicochemical properties of rice straw biochar and its passivation ability to Cu2+ in soil. J. Soils Sediments 2022, 22, 1418–1430. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.; Taušnerová, H.; Hanč, A.; Tlustoš, P. Stabilization of different starting materials through vermicomposting in a continuous-feeding system: Changes in chemical and biological parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, F.T.J.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Scott, X. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–20. [Google Scholar] [CrossRef]
- Mukherjee, S.; Binoy, S.; Kumar, V.A.; Raj, M.; Prashant, S. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef]
- Buss, W.; Masek, O. Mobile organic compounds in biochar—A potential source of contamination—Phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, C.; Wang, H.; Zhao, C.; Yin, D.; Yuan, Y.; Yang, K.; Li, Z. Effect of Different Amounts of Biochar on Meadow Soil Characteristics and Maize Yields Over Three Years. Bioresources 2019, 14, 4194–4209. [Google Scholar] [CrossRef]
- Baek, K.H.; Kim, H.S.; Oh, H.M.; Yoon, B.D.; Kim, J.; Lee, I.S. Effects of crude oil, oil components, and bioremediation on plant growth. J. Environ. Sci. Health Part A 2004, 39, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, F.; Bian, Y.; Jin, X.; Song, Y.; Kengara, F.O.; Xu, R.; Jiang, X. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour. Technol. 2013, 136, 87–93. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Cornelissen, G.; Mulder, J.; Hale, E.S.; Breedveld, G.D.; Rutherford, D.W.; Sparrevik, M. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Jiang, W.; He, J.; Peng, Y.; Wu, Q.; Yang, Q.; Heděnec, P.; Huang, Y.; Wu, Y.; Yue, K.; Surampalli, R.Y. Fluxes of Cadmium, Chromium, and Lead Along with Throughfall and Stemflow Vary Among Different Types of Subtropical Forests. Forests 2025, 16, 152. [Google Scholar] [CrossRef]
- Rao, Z.; Huang, D.; Zhu, H.; Zhu, Q.; Wang, J.; Luo, Z.; Xu, C.; Shen, X.; He, Y. Effect of rice straw mulching on migration and transportation of Cd, Cu, Zn, and Ni in surface runoff under simulated rainfall. J. Soils Sediments 2016, 16, 2021–2029. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Brar, S.K.; Rao, Y.; Surampalli, R.Y. Development of biochar-based green functional materials using organic acids for environmental applications. J. Clean. Prod. 2020, 244, 118841. [Google Scholar] [CrossRef]
- Nóvoa-Muñoz, J.C.; Queijeiro, J.M.; Blanco-Ward, D.; Álvarez-Olleros, C.; Martínez-Cortizas, A.; García-Rodeja, E. Total copper content and its distribution in acid vineyards soils developed from granitic rocks. Sci. Total Environ. 2007, 378, 23–27. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, X.; Zhao, Z.; He, Q.; Wang, S.; Zhu, Y.; Yan, Y.; Liu, X.; Sun, K.; Zhao, Y.; et al. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ. Pollut. 2016, 218, 513–522. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, C.; Farmer, J.; Sun, J. Effects of bio-organic fertilizer on pepper growth and Fusarium wilt biocontrol. Sci. Hortic. 2015, 193, 114–120. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, J.; Chen, C.; Xiang, Y.; Mehran, R.R.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. Glob. Change Biol. Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 182. [Google Scholar] [CrossRef]
- Xza, B.; Lm, C.; Bca, B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y.; Jin, D.; Zhou, Y.; Mukhamed, B.; Liu, D.; Feng, B. The application of biochar and organic fertilizer substitution regulates the diversities of habitat specialist bacterial communities within soil aggregates in proso millet farmland. Biochar 2025, 7, 6. [Google Scholar] [CrossRef]
- Liu, Y.L.; Gu, Y.; Wu, C.; Zhao, H.; Hu, W.; Xu, C.; Chen, X. Short-Term Straw Returning Improves Quality and Bacteria Community of Black Soil in Northeast China. Pol. J. Environ. Stud. 2022, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Klatt, C.G.; Liu, Z.; Ludwig, M.; Kühl, M.; Jensen, S.; Bryant, D.A.; Ward, D.M. Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring. ISME J. 2013, 7, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Bünger, W.; Jiang, X.; Müller, J.; Hurek, T.; Reinhold-Hurek, B. Novel cultivated endophytic Verrucomicrobia reveal deep-rooting traits of bacteria to associate with plants. Sci. Rep. 2020, 10, 8692. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.S.; Sharma, R.; Dahariya, N.S.; Patel, R.K.; Blazhev, B.; Matini, L. Black Carbon and Heavy Metal Contamination of Soil. Pol. J. Environ. Stud. 2016, 25, 717–724. [Google Scholar] [CrossRef]
| Serial Number | Inspection Test Items | Unit | Inspection Test Results |
|---|---|---|---|
| 1 | Appearance | The color is brown, powdery. Uniform, odorless, inorganic Mechanical impurities. | |
| 2 | Mass fraction of organic matter (dry basis), % | 46 | |
| 3 | Total nutrient (nitrogen + phosphorus pentoxide + potassium oxide) mass fraction (based on drying), % | 5.6 | |
| 4 | Moisture (fresh sample) mass fraction, % | 27 | |
| 5 | pH | 7.2 | |
| 6 | As (Measured by drying base) | mg/kg | 0.1 |
| 7 | Cu (Measured by drying base) | mg/kg | Not detected (detection limit: 0.01 mg/kg). |
| 8 | Pb (Measured by drying base) | mg/kg | 2 |
| 9 | Cd (Measured by drying base) | mg/kg | Not detected (detection limit: 0.01 mg/kg). |
| 10 | Cr (Measured by drying base) | mg/kg | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wang, W.; Kang, B.; Yin, Y.; Yang, J. Effects of Biochar and Microbial Organic Fertilizers on Agricultural Productivity and Their Microbial Mechanisms Under Heavy Metal Stress. Toxics 2025, 13, 997. https://doi.org/10.3390/toxics13110997
He Z, Wang W, Kang B, Yin Y, Yang J. Effects of Biochar and Microbial Organic Fertilizers on Agricultural Productivity and Their Microbial Mechanisms Under Heavy Metal Stress. Toxics. 2025; 13(11):997. https://doi.org/10.3390/toxics13110997
Chicago/Turabian StyleHe, Zhenyu, Wenming Wang, Bo Kang, Yonggao Yin, and Jie Yang. 2025. "Effects of Biochar and Microbial Organic Fertilizers on Agricultural Productivity and Their Microbial Mechanisms Under Heavy Metal Stress" Toxics 13, no. 11: 997. https://doi.org/10.3390/toxics13110997
APA StyleHe, Z., Wang, W., Kang, B., Yin, Y., & Yang, J. (2025). Effects of Biochar and Microbial Organic Fertilizers on Agricultural Productivity and Their Microbial Mechanisms Under Heavy Metal Stress. Toxics, 13(11), 997. https://doi.org/10.3390/toxics13110997






