Variability Between Datasets and Statistical Approaches—Rethinking Estimation of Default Dermal Absorption Values for Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Dermal Absorption Dataset (BfR2024)
2.2. Statistical Analyses
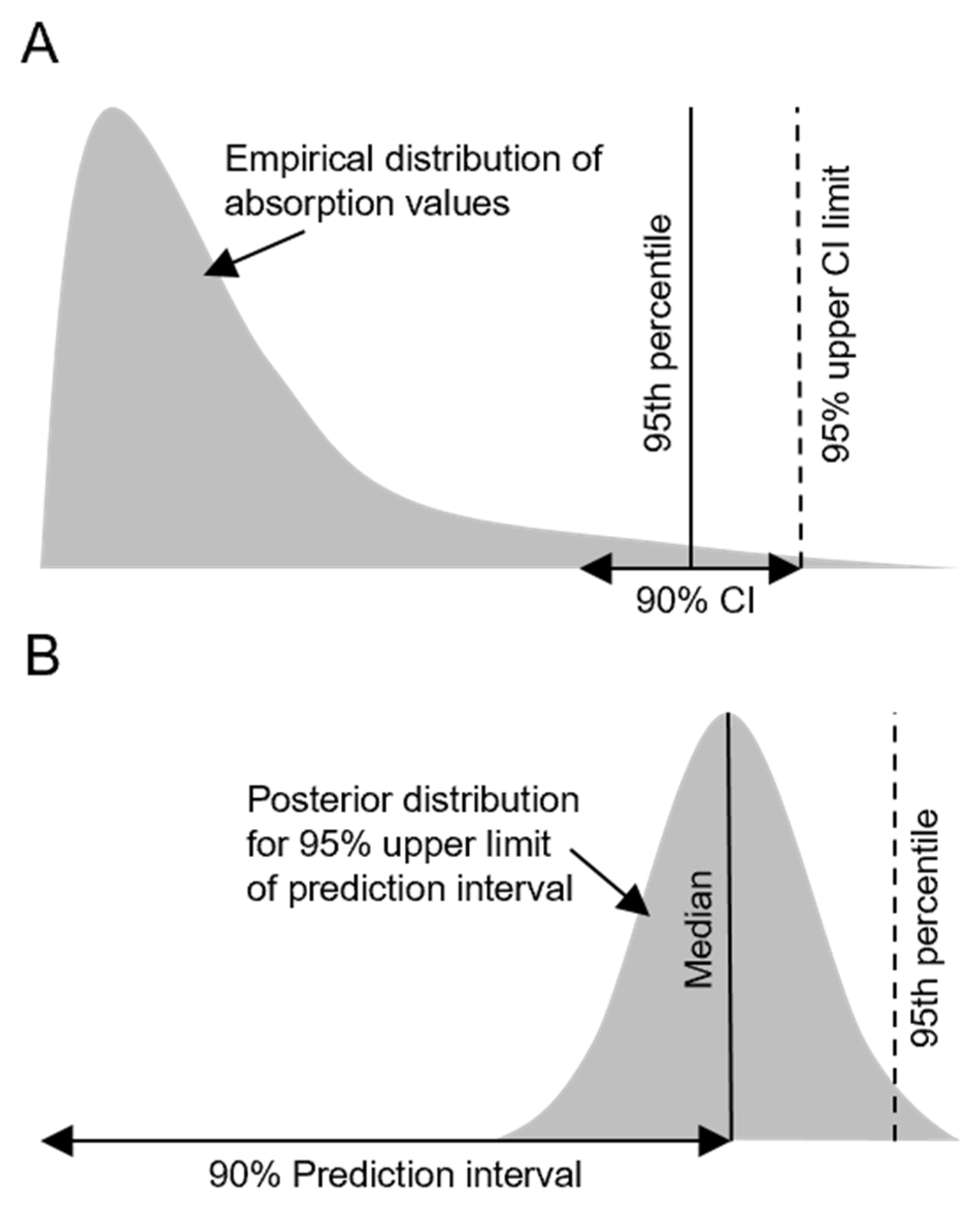
2.2.1. Default Values Based on Empirical Percentiles
2.2.2. Default Values Based on Bayesian Mixed Models
2.3. Comparative Datasets
2.3.1. EFSA2017
2.3.2. Sarti et al. 2025
3. Results
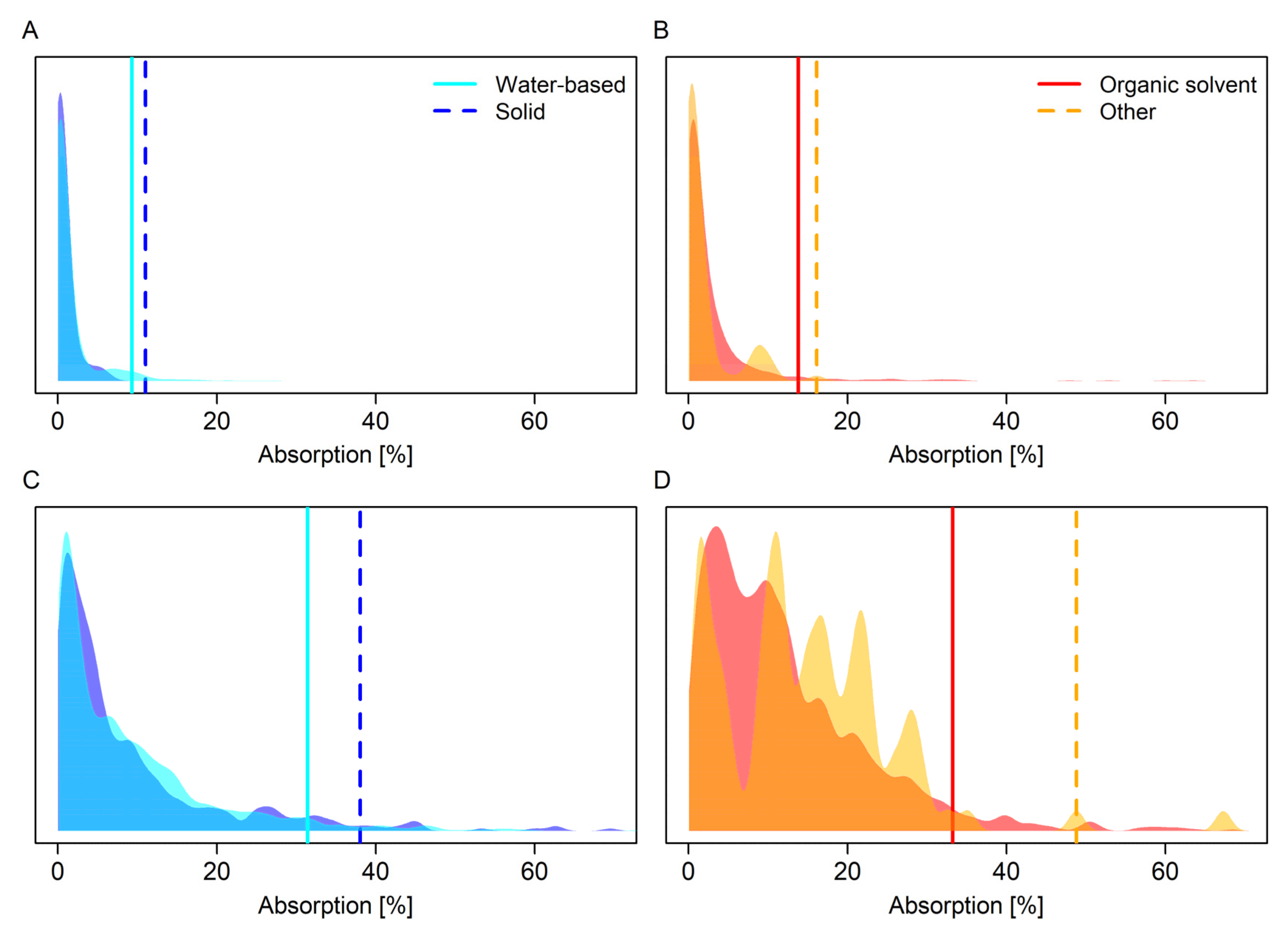
3.1. Comparison of Default Values Based on Empirical Percentiles
3.2. Model-Derived Average Effects of Formulation Type Category and Concentration Status on Absorption
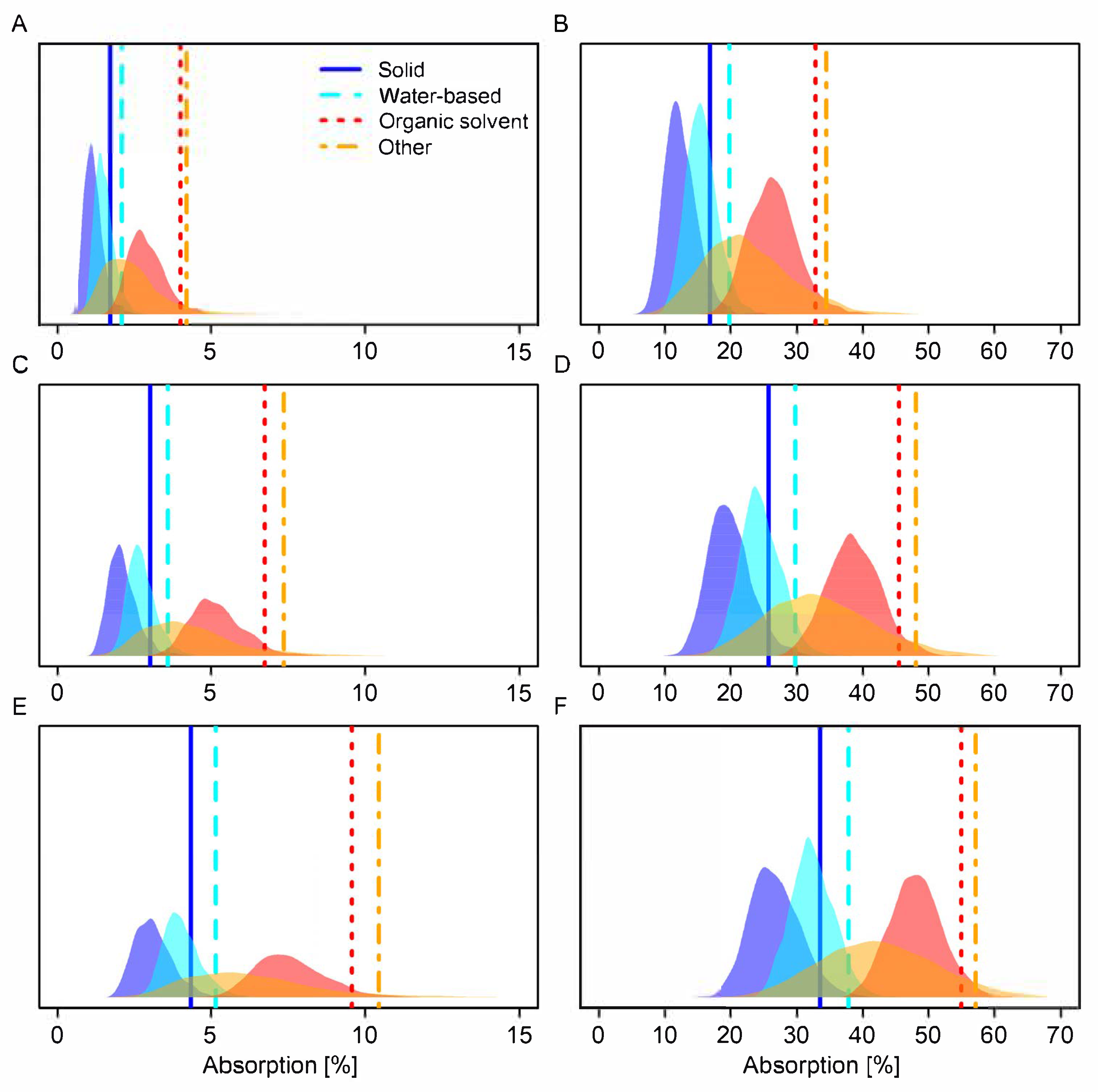
3.3. Comparison of Default Values Based on Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPP | Plant protection product |
| EFSA | European Food Safety Authority |
| GLP | Good Laboratory Practice |
| BfR | German Federal Institute for Risk Assessment |
| SCoPAFF | Standing Committee on Plants, Animals, Food and Feed |
| OECD | Organization for Economic Co-operation and Development |
| ECPA | European Crop Protection Association |
| HPDI | Highest posterior density interval |
Appendix A
| Formulation Type Category | Dilution/ Concentrate | 95th Perc. 1 | 95% CI Limit 2 | Replicates |
|---|---|---|---|---|
| Organic solvent | Concentrate | 11 (11) | 15 (14) | 595 |
| Other | Concentrate | 10 (9) | 16 (16) | 67 |
| Water-based | Concentrate | 8 (8) | 9 (9) | 1143 |
| Solid | Concentrate | 6 (7) | 11 (11) | 273 |
| Organic solvent | Dilution | 30 (31) | 32 (32) | 1742 |
| Other | Dilution | 29 (29) | 49 (49) | 119 |
| Water-based | Dilution | 29 (30) | 31 (31) | 1896 |
| Solid | Dilution | 31 (33) | 35 (38) | 505 |
| Parameter | Mean | 95% CI Lower Bound | 95% CI Upper Bound | Effective Samples |
|---|---|---|---|---|
| α Intercept | −2.61 | −2.88 | −2.36 | 2134 |
| β Concentrate | −2.33 | −2.53 | −2.12 | 2000 |
| β Other | −0.25 | −0.99 | 0.48 | 2000 |
| β Solid | −0.92 | −1.34 | −0.50 | 2000 |
| β Water-based | −0.53 | −0.84 | −0.27 | 2000 |
| σ2 Substance:Dilution 1 | 0.56 | 0.32 | 0.82 | 1687 |
| σ2 Substance:Concentrate 2 | 0.31 | 0.12 | 0.53 | 779 |
| σ2 Study ID 3 | 0.49 | 0.28 | 0.71 | 986 |
| σ2 Within-study 4 | 1.20 | 1.04 | 1.39 | 1310 |
| ρ (σ2 S:D, σ2 S:C) 5 | 0.31 | 0.12 | 0.53 | 898 |
References
- EC. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- EC. Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Off. J. Eur. Union 2013, 93, 85–152. [Google Scholar]
- EC. Commision Regulation (EU) 2022/1440 of 31 August 2022 amending Regulation (EU) No 284/2013 as regards the information to be submitted for plant protection products and the specific data requirements for plant protection products containing micro-organisms. Off. J. Eur. Union 2022, 227, 38–69. [Google Scholar]
- OECD. Environment Directorate. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Guidance Notes on Dermal Absorption Studies. ENV/JM/MONO(2011)36/REV1. In Series on Testing and Assessment No. 156, 2nd ed.; OECD Environment, Health and Safety Publications: Paris, France, 2022. [Google Scholar]
- OECD. Test No. 427: Skin Absorption: In Vivo Method. In OECD Guideline for the Testing of Chemicals; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 428: Skin Absorption: In Vitro Method. In OECD Guideline for the Testing of Chemicals; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Environment Directorate. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Guidance Document for the Conduct of Skin Absorption Studies. ENV/JM/MONO(2004)2. In Series on Testing and Assessment Number 28; Environmental Health and Safety Publications: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Environment Directorate. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Guidance notes on dermal absorption. ENV/JM/MONO(2011)36. In Series on Testing and Assessment No. 156; OECD Environment, Health and Safety Publications: Paris, France, 2011. [Google Scholar]
- EFSA; Buist, H.; Craig, P.; Dewhurst, I.; Hougaard Bennekou, S.; Kneuer, C.; Machera, K.; Pieper, C.; Marques, D.C.; Guillot, G.; et al. Guidance on dermal absorption. EFSA J. 2017, 15, e04873. [Google Scholar] [CrossRef] [PubMed]
- Sarti, D.; Wagner, J.; Palma, F.; Kalvan, H.; Giachini, M.; Lautenschalaeger, D.; Lupianhez, V.; Pires, J.; Sales, M.; Faria, P.; et al. Interpretable machine learning unveils key predictors and default values in an expanded database of human in vitro dermal absorption studies with pesticides. Regul. Toxicol. Pharmacol. 2025, 159, 105801. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Fisher, P.; Kluxen, F.M.; Maas, W.; Morgan, N.; Parr-Dobrzanski, R.; Strupp, C.; Wiemann, C. Assessing in vitro dermal absorption of dry residues of agrochemical sprays using human skin within OECD TG 428. Regul. Toxicol. Pharmacol. 2019, 106, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kluxen, F.M.; Felkers, E.; McEuen, S.; Fisher, P.; Strupp, C.; Lorez, C.; Domoradzki, J.Y.; Wiemann, C. A new conceptional model for deriving average dermal absorption estimates from studies with multiple tested concentrations for non-dietary risk assessment of pesticides. Arch. Toxicol. 2022, 96, 2429–2445. [Google Scholar] [CrossRef] [PubMed]
- Pieper, C.; Engel, N.; Wend, K.; Kneuer, C.; Martin, S. In Vitro Human Dermal Absorption Studies on Pesticides in Complex Mixtures: Investigation of Guidance Criteria and Possible Impact Parameters. Toxics 2024, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Kuster, C.J.; Baumann, J.; Braun, S.M.; Fisher, P.; Hewitt, N.J.; Beck, M.; Weysser, F.; Goerlitz, L.; Salminen, P.; Dietrich, C.R.; et al. In silico prediction of dermal absorption from non-dietary exposure to plant protection products. Comput. Toxicol. 2022, 24, 100242. [Google Scholar] [CrossRef]
- SCoPAFF. Note for Agreement by Member States’s Competent Authorities in the SCoPAFF: Phytopharmaceutical Legislation Section Guidance on Dermal Absorption. SANTE/2018/10591 rev.1. 2018. Available online: https://food.ec.europa.eu/system/files/2018-11/pesticides_ppp_app-proc_guide_tox_dermal-absorp-2018-paff.pdf (accessed on 16 October 2025).
- EFSA. Dermal Absorption: Refined BfR Template for in Vitro Calculations. Available online: https://www.efsa.europa.eu/en/press/news/171207-0 (accessed on 26 August 2025).
- OECD. Environment Directorate. Chemicals Group and Management Commitee. OECD Principles on Good Laboratory Practice. ENV/MC/CHEM(98)17. In OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring Number 1; OECD: Paris, France, 1998. [Google Scholar] [CrossRef]
- FAO/WHO. Joint Meeting on Pesticide Specifications (JMPS). Manual on Development and Use of FAO and WHO Specifications for Pesticides. Pesticide Specifications, 1st ed.; Yadav, R., Ed.; FAO: Rome, Italy, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; 4.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Montesinos López, O.A.; Montesinos López, A.; Crossa, J. Linear Mixed Models. In Multivariate Statistical Machine Learning Methods for Genomic Prediction; Springer International Publishing: Cham, Switzerland, 2022; pp. 141–170. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Guidance on Dermal Absorption. EFSA J. 2012, 10, 2665. [Google Scholar] [CrossRef]
- EFSA. Assessment of new scientific studies on human in vitro dermal absorption. EFSA J. 2015, 13, 4304. [Google Scholar] [CrossRef]
- Buist, H.E.; Schaafsma, G.; van de Sandt, J.J. Relative absorption and dermal loading of chemical substances: Consequences for risk assessment. Regul. Toxicol. Pharmacol. 2009, 54, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Frasch, H.F.; Dotson, G.S.; Bunge, A.L.; Chen, C.P.; Cherrie, J.W.; Kasting, G.B.; Kissel, J.C.; Sahmel, J.; Semple, S.; Wilkinson, S. Analysis of finite dose dermal absorption data: Implications for dermal exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Battalora, M.; Fisher, P.; Huser, A.; Parr-Dobrzanski, R.; Soufi, M.; Mostert, V.; Strupp, C.; Whalley, P.; Wiemann, C.; et al. Assessment of in vitro human dermal absorption studies on pesticides to determine default values, opportunities for read-across and influence of dilution on absorption. Regul. Toxicol. Pharmacol. 2014, 68, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Hazra, D.K.; Purkait, A. Role of pesticide formulations for sustainable crop protection and environment management: A review. J. Pharmacogn. Phytochem. 2019, 8, 686–693. [Google Scholar]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the Science behind the Revision of the Guidance Document on Dermal Absorption. EFSA J. 2011, 9, 2294. [Google Scholar] [CrossRef]
- Rabha, B.; Saha, D.; Bhuyan, C. A Brief Review on Topical Gels as Drug Delivery System. J. Pharm. Res. Int. 2021, 33, 344–357. [Google Scholar] [CrossRef]
- Wend, K.; Lemoine, L.; Pieper, C. Dermal Absorption: Considerations on Risk Assessment, Drug Administration, and the Human Skin Microbiome. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Hock, F.J., Pugsley, M.K., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Lemoine, L.; Bayrambey, D.; Roloff, A.; Hutzler, C.; Luch, A.; Tralau, T. Commensal-Related Changes in the Epidermal Barrier Function Lead to Alterations in the Benzo[a]Pyrene Metabolite Profile and Its Distribution in 3D Skin. Mbio 2021, 12, e0122321. [Google Scholar] [CrossRef] [PubMed]
- Vehtari, A.; Gelman, A.; Simpson, D.; Carpenter, B.; Bürkner, P.-C. Rank-normalization, folding, and localization: An improved R-hat for assessing convergence of MCMC (with Discussion). Bayesian Anal. 2021, 16, 667–718. [Google Scholar] [CrossRef]
- Bürkner, P.; Gabry, J.; Kay, M.; Vehtari, A. posterior: Tools for Working with Posterior Distributions, R Package Version 1.6.1.; 2025. Available online: https://cran.r-project.org/web/packages/posterior/index.html (accessed on 16 October 2025).


| Formulation Type Category | Dilution/ Concentrate | Replicates | Studies | Substances | Formulation Types |
|---|---|---|---|---|---|
| Organic solvent | Concentrate | 847 | 106 | 68 | 7 |
| Other | Concentrate | 83 | 11 | 8 | 3 |
| Water-based | Concentrate | 1268 | 153 | 86 | 4 |
| Solid | Concentrate | 351 | 47 | 37 | 6 |
| Organic solvent | Dilution | 1490 | 119 | 66 | 6 |
| Other | Dilution | 103 | 9 | 7 | 2 |
| Water-based | Dilution | 1771 | 153 | 83 | 3 |
| Solid | Dilution | 427 | 47 | 33 | 4 |
| Overall | 6340 | 356 | 155 | 20 |
| Formulation Type Category | Formulation Types |
|---|---|
| Organic solvent | EC, EW, DC, ME, OD, OL, SE |
| Other | GD, CS, ZC |
| Water-based | FS, SC, SD, SL |
| Solid | AP, DP, GR, SG, WG, WP |
| This Study | EFSA2017 | Sarti et al. 2025 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Formulation Type Category | Dilution/ Concentrate | 95th Perc. 1 | 95% CI Limit 2 | Replicates | 95th Perc. 1 | 95% CI Limit 2 | Replicates | 95% CI Limit 2 | Replicates |
| Organic solvent | Concentrate | 11 | 14 | 847 | 18 | 20 | 1153 | 10 | 2150 |
| Other | Concentrate | 9 | 16 | 83 | 20 | - | 131 | 8 | 73 |
| Water-based | Concentrate | 8 | 9 | 1268 | 8 | 10 | 1073 | 4 | 2509 |
| Solid | Concentrate | 7 | 11 | 351 | 8 | 11 | 471 | 3 | 949 |
| Organic solvent | Dilution | 31 | 33 | 1490 | 49 | 55 | 1553 | 42 | 3318 |
| Other | Dilution | 29 | 49 | 119 | 56 | 61 | 105 | - | 8 |
| Water-based | Dilution | 30 | 31 | 1771 | 40 | 44 | 1567 | 37 | 3463 |
| Solid | Dilution | 33 | 38 | 427 | 51 | 57 | 710 | 39 | 1510 |
| Parameter | Mean | 95% CI Lower Bound | 95% CI Upper Bound | Effective Samples |
|---|---|---|---|---|
| α Intercept | −2.34 | −2.63 | −2.11 | 2000 |
| β Concentrate | −2.37 | −2.56 | −2.17 | 2000 |
| β Other | −0.24 | −0.89 | 0.50 | 2000 |
| β Solid | −0.94 | −1.32 | −0.56 | 2000 |
| β Water-based | −0.67 | −0.95 | −0.39 | 2000 |
| σ2 Substance:Dilution 1 | 0.64 | 0.38 | 0.90 | 1753 |
| σ2 Substance:Concentrate 2 | 0.52 | 0.27 | 0.81 | 1472 |
| σ2 Study ID 3 | 0.67 | 0.48 | 0.85 | 1650 |
| σ2 Within-study 4 | 0.59 | 0.49 | 0.70 | 1684 |
| ρ (σ2 S:D, σ2 S:C) 5 | 0.20 | 0.01 | 0.40 | 1780 |
| Formulation Type Category | Dilution/ Concentrate | Posterior Median 1 | 95th Percentile 1 | Posterior Median 2 | 95th Percentile 2 | Posterior Median 3 | 95th Percentile 3 |
|---|---|---|---|---|---|---|---|
| Organic solvent | Concentrate | 3 (5) | 4 (7) | 5 (8) | 7 (10) | 7 (11) | 10 (14) |
| Other | Concentrate | 2 (3) | 4 (6) | 4 (5) | 7 (8) | 6 (7) | 10 (11) |
| Water-based | Concentrate | 1 (3) | 2 (4) | 3 (4) | 4 (6) | 4 (6) | 5 (8) |
| Solid | Concentrate | 1 (3) | 2 (4) | 2 (4) | 3 (6) | 3 (6) | 4 (8) |
| Organic solvent | Dilution | 26 (37) | 33 (45) | 38 (48) | 45 (56) | 48 (57) | 55 (64) |
| Other | Dilution | 22 (27) | 34 (39) | 33 (37) | 48 (49) | 42 (46) | 57 (58) |
| Water-based | Dilution | 15 (24) | 20 (30) | 24 (33) | 30 (39) | 32 (41) | 38 (47) |
| Solid | Dilution | 12 (24) | 17 (30) | 20 (33) | 26 (39) | 26 (41) | 33 (48) |
| Formulation Type Category | Dilution/ Concentrate | Posterior Median 1 | 95th Percentile 1 | Posterior Median 2 | 95th Percentile 2 | Posterior Median 3 | 95th Percentile 3 |
|---|---|---|---|---|---|---|---|
| Organic solvent | Concentrate | 2 (3) | 2 (4) | 3 (5) | 4 (7) | 7 (7) | 9 (10) |
| Other | Concentrate | 1 (2) | 2 (4) | 2 (4) | 4 (7) | 5 (7) | 9 (10) |
| Water-based | Concentrate | 1 (1) | 1 (2) | 2 (3) | 2 (4) | 4 (4) | 5 (5) |
| Solid | Concentrate | 1 (1) | 1 (2) | 1 (2) | 2 (3) | 3 (3) | 4 (4) |
| Organic solvent | Dilution | 20 (26) | 26 (33) | 28 (38) | 35 (45) | 46 (48) | 53 (55) |
| Other | Dilution | 16 (22) | 27 (34) | 23 (33) | 37 (48) | 40 (42) | 55 (57) |
| Water-based | Dilution | 13 (25) | 17 (20) | 19 (24) | 24 (30) | 34 (32) | 40 (38) |
| Solid | Dilution | 9 (12) | 13 (17) | 14 (20) | 19 (26) | 26 (26) | 33 (33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Städele, V.; Martin, S.; Wend, K. Variability Between Datasets and Statistical Approaches—Rethinking Estimation of Default Dermal Absorption Values for Risk Assessment. Toxics 2025, 13, 925. https://doi.org/10.3390/toxics13110925
Städele V, Martin S, Wend K. Variability Between Datasets and Statistical Approaches—Rethinking Estimation of Default Dermal Absorption Values for Risk Assessment. Toxics. 2025; 13(11):925. https://doi.org/10.3390/toxics13110925
Chicago/Turabian StyleStädele, Veronika, Sabine Martin, and Korinna Wend. 2025. "Variability Between Datasets and Statistical Approaches—Rethinking Estimation of Default Dermal Absorption Values for Risk Assessment" Toxics 13, no. 11: 925. https://doi.org/10.3390/toxics13110925
APA StyleStädele, V., Martin, S., & Wend, K. (2025). Variability Between Datasets and Statistical Approaches—Rethinking Estimation of Default Dermal Absorption Values for Risk Assessment. Toxics, 13(11), 925. https://doi.org/10.3390/toxics13110925






