1. Introduction
Neurobehavioral development during early childhood lays the foundation for later cognitive functioning and social adaptation. This developmental process depends heavily on tightly regulated neurobiological events such as neuronal differentiation, migration, myelination, and synaptic plasticity [
1,
2]. The preschool period (ages 3–6) is a particularly sensitive window, characterized by rapid structural and functional maturation of the central nervous system [
3]. While neurobehavioral disorders have traditionally been attributed to genetic, psychosocial, and nutritional factors, growing evidence indicates that environmental pollutants represent an important and often underrecognized contributor, particularly when exposure occurs during early life [
4,
5]. During this stage, the brain is highly susceptible to external insults, and adverse environmental exposures may result in long-term or even irreversible disruptions to neurodevelopment [
6]. Such exposures may disrupt neurodevelopment through mechanisms including interference with neurotransmitter systems, induction of oxidative stress, and modulation of synaptic function [
7,
8].
At present, several academic studies have reported associations between environmental factors such as heavy metals, organic pollutants, and air pollution with neurobehavioral abnormalities in children [
9,
10]. However, neonicotinoid insecticides (NEOs), as a class of emerging environmental contaminants, although their widespread presence in the environment has been well documented and animal as well as in vitro studies have demonstrated various adverse health effects including neurotoxicity, their neurotoxic impacts on human populations remain insufficiently understood and require further systematic investigation [
11,
12]. NEOs are synthetic insecticides structurally derived from nicotine and have been widely applied in agriculture, horticulture, and animal husbandry since the 1990s [
13]. Due to their high water solubility, strong systemic activity, and environmental persistence, NEOs are widely present in surface water, groundwater, soil, agricultural products, and even the atmosphere, thereby posing potential exposure risks to humans [
14,
15].
Existing studies have shown that at least one neonicotinoid compound was detected in over 90% of surface water samples collected from major water bodies in China [
16,
17]. Moreover, neonicotinoids can be bioaccumulated in aquatic vertebrates (such as fish and amphibians) as well as invertebrates (including mayflies and water fleas), suggesting their potential transmission through the food chain and ecological threat [
18]. More importantly, urine biomonitoring in some regions has shown detection rates of neonicotinoids and their metabolites approaching 100%. This indicates long-term, low-level exposure in the general population of China, with particularly elevated risks in agriculturally intensive and downstream urban areas [
19]. Previous studies have demonstrated that NEOs can activate mammalian nicotinic acetylcholine receptors (nAChRs), leading to abnormal neuronal excitability and impaired synaptic formation, potentially leading to deficits in learning, memory, and behavioral regulation in animal models [
20,
21]. Regarding the neurotoxic mechanism of NEOs, some studies reported that exposure to NEOs may induce oxidative stress, mitochondrial dysfunction, and apoptosis, all of which are key mechanisms implicated in neurodevelopmental impairment [
22,
23]. Although an increasing body of evidence suggests that NEOs may exert neurotoxic effects on non-target organisms, including mammals, most of this evidence comes from laboratory studies using cell cultures or animal models. Furthermore, the NEO concentrations applied in such experiments are often several orders of magnitude higher than those encountered under real-world environmental exposure conditions [
24]. Therefore, the impact of NEO exposure on human neurobehavioral development, particularly in children, requires further investigation under environmentally relevant conditions through population-based epidemiological study.
The Pearl River Delta (PRD) region in southern China provides a representative setting for such research. Due to highly intensive forestry and protected agriculture in the upper and middle reaches of the Pearl River, coupled with substantial pesticide application, large quantities of NEOs are introduced into water bodies through atmospheric deposition and surface runoff, resulting in downstream migration of contamination to urban areas [
25]. Consequently, residents of PRD cities with high water utilization—such as Guangzhou, Shenzhen, and Foshan—are at increased risk of NEO pollution. Environmental monitoring has also revealed that residual NEO concentrations in the Pearl River rank among the highest in China, corroborating that urban residents in this region—particularly children and other vulnerable populations—are at considerable exposure risk [
26]. These regional characteristics provide an ideal context for assessing the health impacts of chronic, low-level, mixed NEO exposure under real-world environmental conditions.
Previous research indicates that urinary neonicotinoids and their metabolites can serve as biomarkers of total exposure from multiple sources based on human metabolic characteristics [
27]. Therefore, this study aimed to assess the associations between real-life mixed exposures to NEOs and their urinary metabolites and neurobehavioral development in preschool children living in Shenzhen in the Pearl River Delta of China. Our findings are expected to provide epidemiological evidence and scientific insights to support the identification of environmental health risks during critical windows of childhood neurodevelopment and inform future policy interventions at the national and global levels.
4. Discussion
This study is an epidemiological investigation to systematically assess urinary concentrations of NEOs and their associations with neurobehavioral development in a preschool-age population. Among the 11 neonicotinoid substances and their metabolites measured, five neonicotinoid prototypes, including IMI, CLO, THM, DNT, and FLO, and two of their metabolites (NACE and NTHM) were detected in over 90% of samples. The mean total urinary concentration of NEOs reached 234.145 μg/g Cr, which exceeds the exposure levels reported in previous studies, suggesting that preschool children in China may be facing substantial exposure risks to neonicotinoids [
44]. The detection rates observed in this study are consistent with the results reported from recent population studies in China, in which most of the neonicotinoids tested showed high detection frequencies [
26]. However, in earlier population studies, the reported detection rates were generally lower [
45,
46]. In addition, the overall human exposure to neonicotinoids and the detection rates of individual compounds reported in the literature have shown an upward trend over time. For instance, a 2020 epidemiological survey among children in Shanghai reported detection rates ranging from 0.6% to 62.6% [
46], whereas a 2016 survey conducted in Japan reported detection rates of ≤57.8%. This upward trend in detection metrics suggests that preschool children and the general population may now be facing higher exposure risks [
45].
Notably, this study observed considerable inter-individual differences in urinary neonicotinoid concentrations. Such variations may reflect disparities in the recent living environments of the preschool participants, including the use of household insecticides and the presence of pesticide residues in fruits, vegetables, and drinking water. Only a very small proportion of participants exhibited extremely high concentrations, which are likely to represent short-term, episodic exposure events rather than chronic accumulation. Nevertheless, these abnormally elevated levels merit future attention, as such episodic exposures may pose potential health risks to sensitive populations, particularly preschool children.
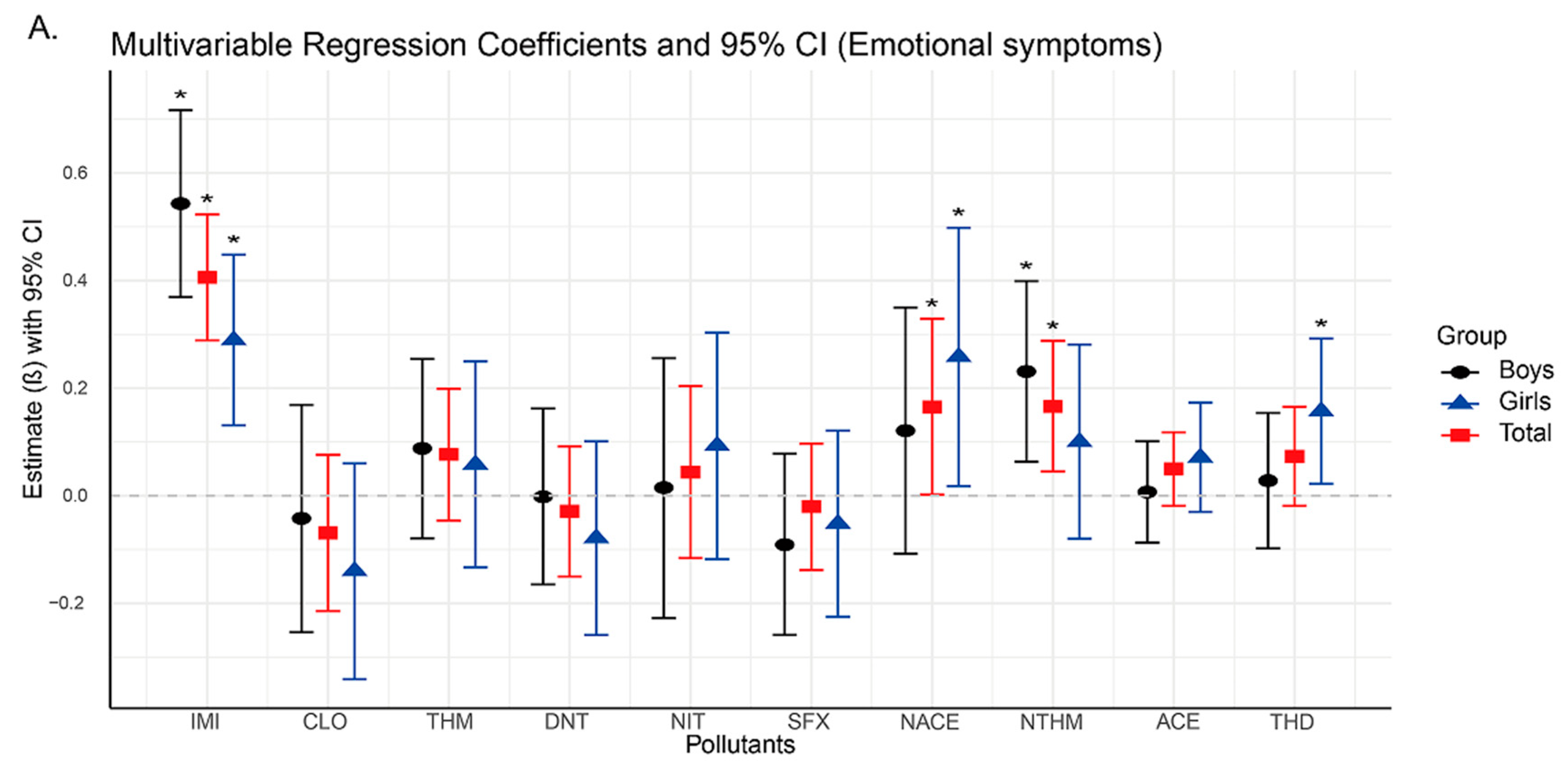
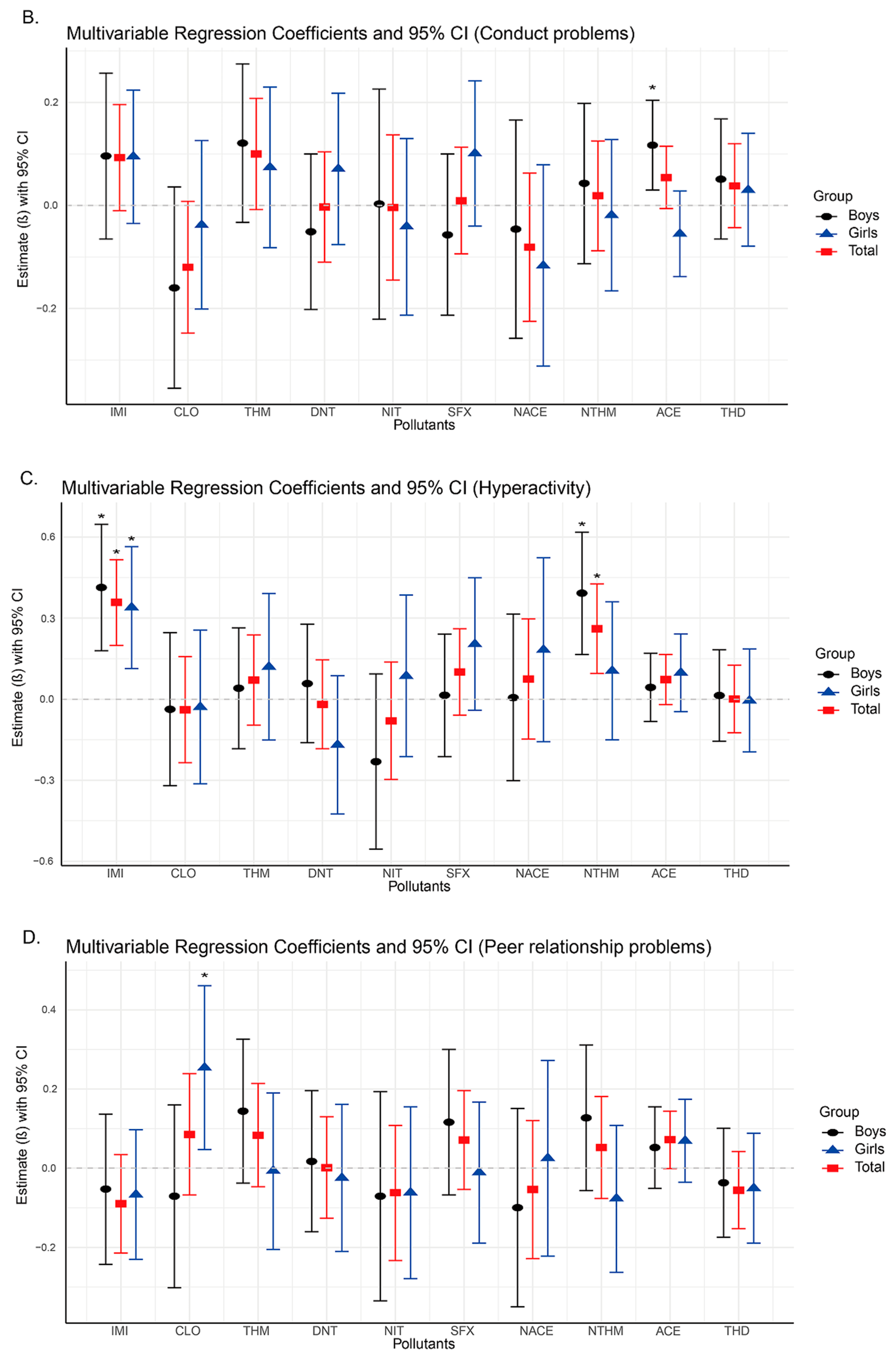
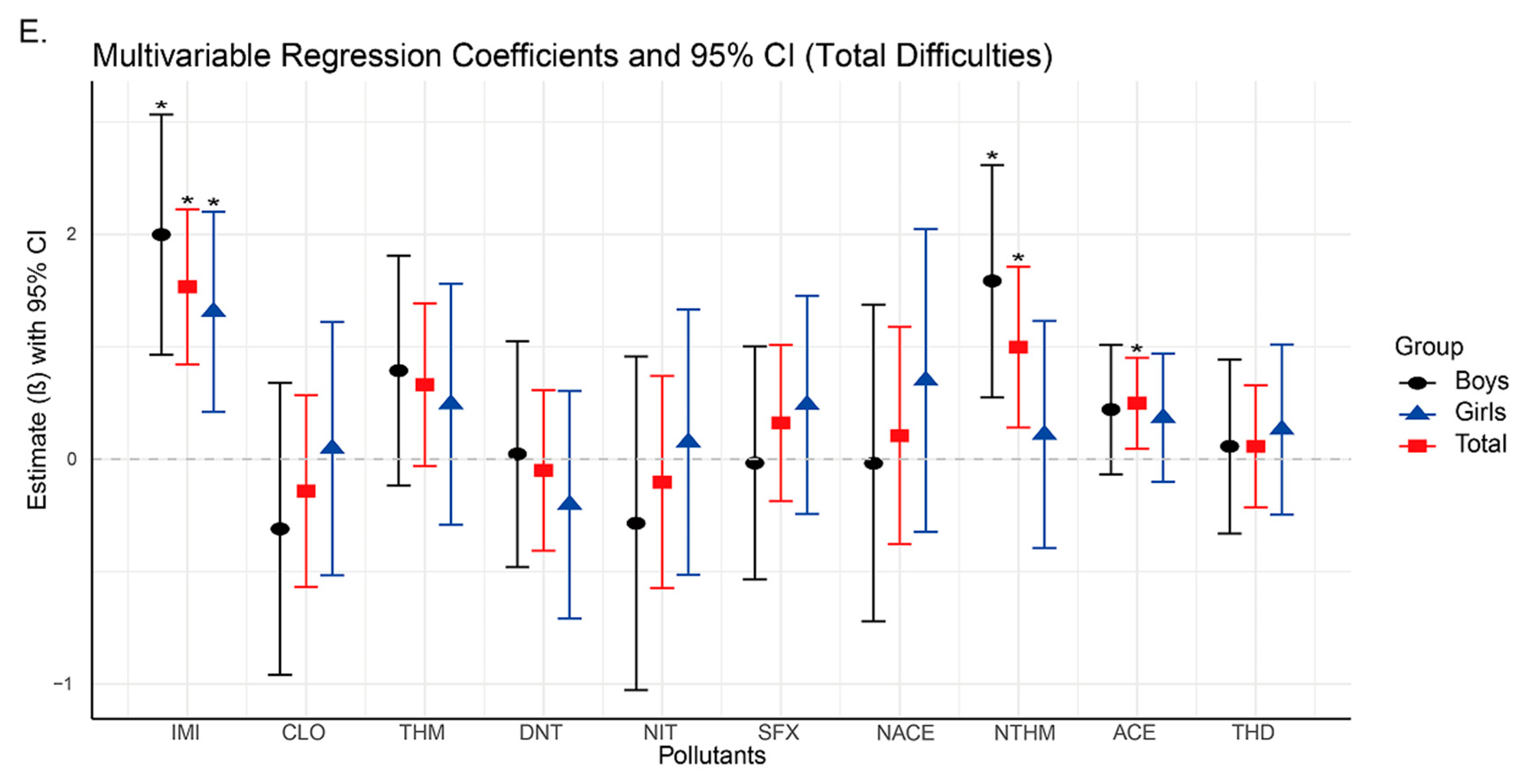
Subsequent regression analyses revealed significant positive associations between urinary concentrations of IMI, NTHM, and NACE and neurobehavioral problems in preschoolers, particularly in the domains of emotional symptoms, hyperactivity, and total difficulties. As the concentrations of IMI, NTHM, and NACE increased, the risk of neurobehavioral abnormalities also appeared to rise. These compounds may exert adverse neurodevelopmental effects by activating nicotinic acetylcholine receptors (nAChRs) in the nervous system [
21]. Early studies suggested that the neurotoxicity of neonicotinoids was low because mammals, especially humans, have relatively few nAChRs in the brain with low binding affinity [
47]. However, more recent animal and mechanistic studies have challenged this view [
24]. Several commonly used insecticides, including neonicotinoids, have demonstrated neurotoxic effects on the developing nervous system to varying degrees. IMI has been shown in brainstem slices from neonatal mice to induce depolarizing inward currents, increase neuronal excitability, and provide direct physiological evidence of neurotoxicity [
21]. In a mouse study, low-dose exposure to neonicotinoids such as sulfoxaflor impaired performance in elevated plus maze tests and motor coordination in juvenile mice [
48]. Mechanistic data suggested that nAChRs and other mediators of neurodevelopment play crucial roles during fetal and early postnatal brain development, with particularly high expression during infancy [
49]. Exposure to certain neonicotinoids during this developmental window may disrupt nAChR expression and regulation, interfere with neuronal migration and synaptogenesis, and affect other developmental processes, potentially yielding adverse neurobehavioral outcomes [
49]. Collectively, the above evidence indicates that neonicotinoid insecticides or their metabolites can act on the nervous system of mammals, including humans, altering neuronal signaling and inducing toxic or oxidative stress–related effects in developing neurons. Therefore, the neurotoxic potential of neonicotinoids in mammals may have been underestimated, and continued scientific and regulatory attention to their safety is warranted.
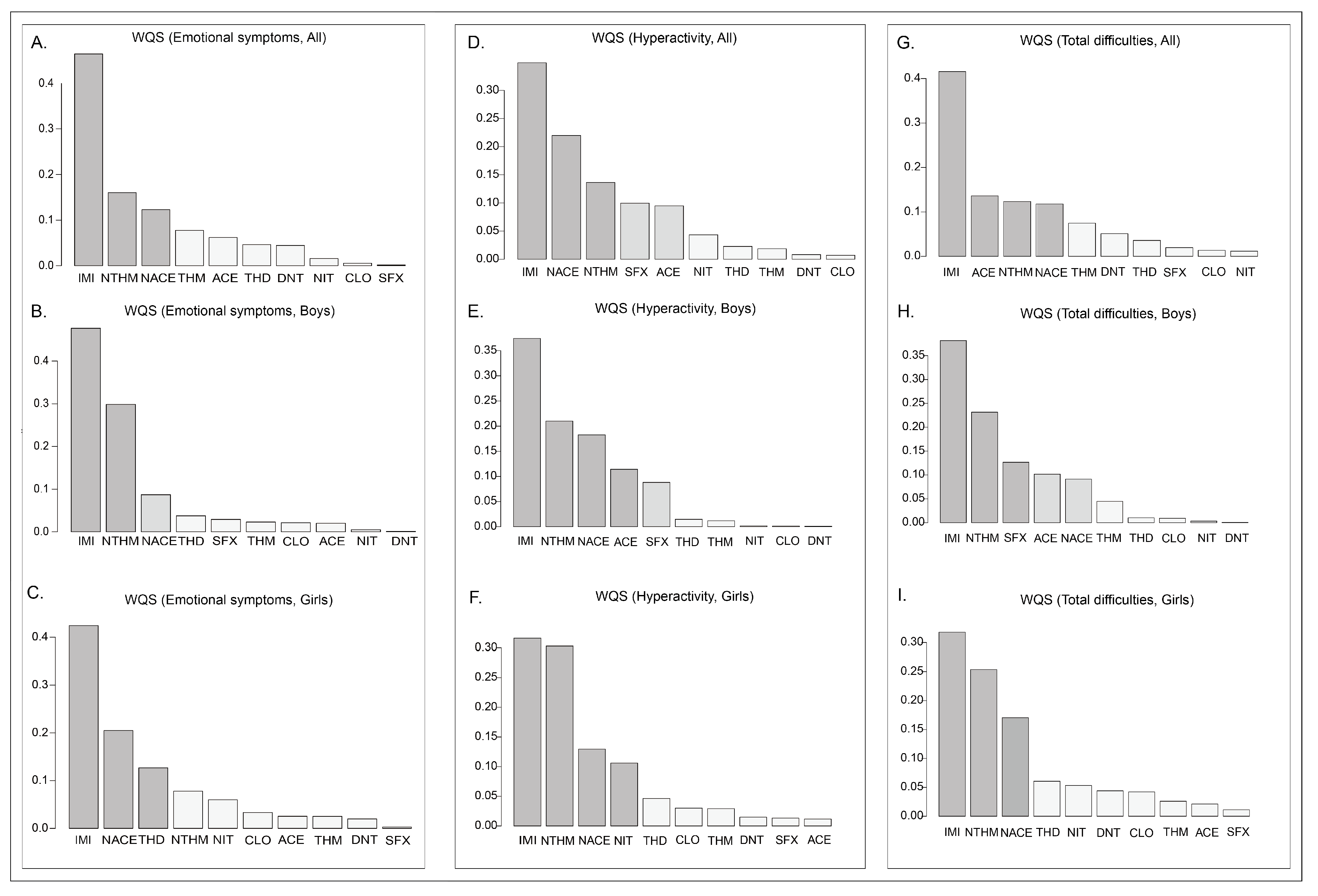
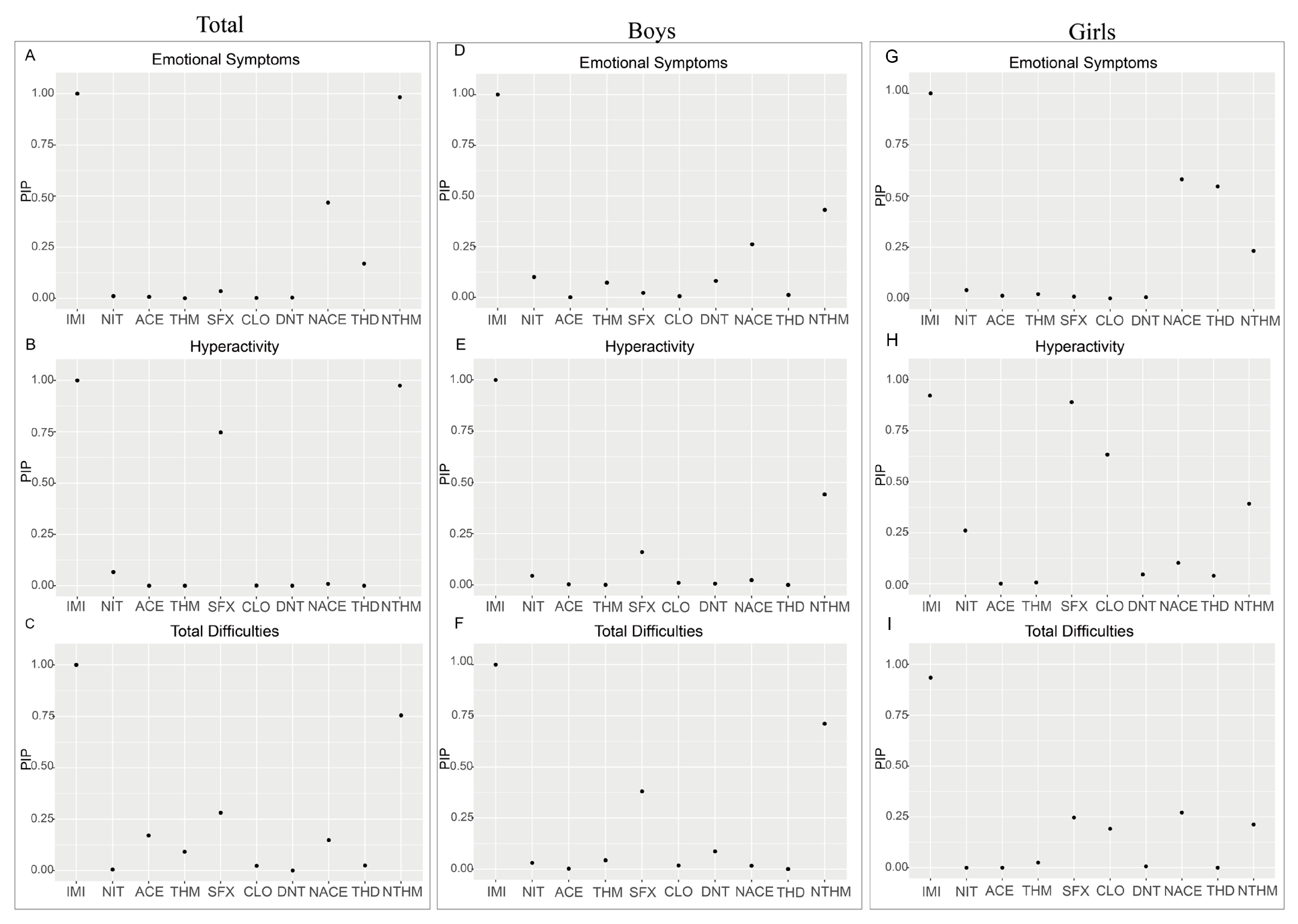
The findings of this study suggest that among the detected neonicotinoid insecticides, IMI, NTHM, and NACE are the most influential compounds associated with neurobehavioral outcomes in preschoolers. Notably, IMI showed consistent and statistically significant positive associations across all strata (overall population, boys, and girls). Together with the WQS regression results, IMI emerged as a key contributor within the mixture. To date, no epidemiological studies have directly examined IMI exposure and neurobehavioral outcomes in humans; however, experimental evidence suggests that IMI’s potent effects may arise from interference with neurotransmitter systems and the induction of oxidative stress [
50]. For instance, in a prior study using cultured cerebellar neurons from neonatal rats, IMI significantly enhanced the activity of nAChR subunits α3, α4, and α7, an effect not observed with other neonicotinoids [
21]. In a mouse low-dose IMI exposure reduced SOX2-positive neural stem cells and GFAP-positive astrocytes, indicating suppressed neurogenesis and glial development in the dentate gyrus (DG) [
51]. Behavioral impairments were observed in the elevated plus maze (EPM) and fear conditioning (FC) tests, suggesting anxiety-like behavior and deficits in learning and memory [
48]. Collectively, these mechanistic studies suggest that IMI may exert a more pronounced neurotoxic impact than other neonicotinoids and threaten neurobehavioral development in preschool-aged children during early life. Therefore, given the consistently observed adverse effects of IMI across different subgroups, targeted mitigation strategies addressing IMI exposure may reduce health risks from neonicotinoids in young children and the general population.
In this study, both NTHM (a metabolite of THM) and NACE (a metabolite of ACE) showed significant positive associations with neurobehavioral outcomes, whereas the parent compounds (THM and ACE) showed no such associations. Prior toxicokinetic studies have shown that demethylated metabolites such as NTHM and NACE are more hydrophilic and possess slower metabolic clearance and longer biological half-lives, resulting in more persistent internal exposure and potentially stronger biological effects than their parent compounds [
52]. For example, a 2022 study reported that NACE could be consistently detected in the cerebrospinal fluid (CSF) of children, whereas its parent compound was less frequently detected, and that CSF NACE concentrations correlated linearly with those in blood and urine. These findings suggest that these NEO metabolites can remain in the human body for a longer time and penetrate the central nervous system [
53]. Mechanistic studies further revealed that demethylated THM has 28–3600 times higher receptor affinity than THM, and THM itself resembles a pro-pesticide, with its neuroactivity primarily mediated by its transformation products [
52].
In addition, NTHM and NACE show notable sex-specific differences in stratified analyses. Among boys, NTHM is positively associated with hyperactivity and total difficulty scores, whereas no such associations are observed among girls. These findings may partly reflect sex-related differences in the metabolism of neonicotinoids [
54,
55]. However, the mechanisms underlying the observed sex differences in NACE and NTHM effects remain poorly understood and require further investigation to elucidate how these compounds may differentially influence neurobehavioral development in boys and girls through distinct physiological pathways.
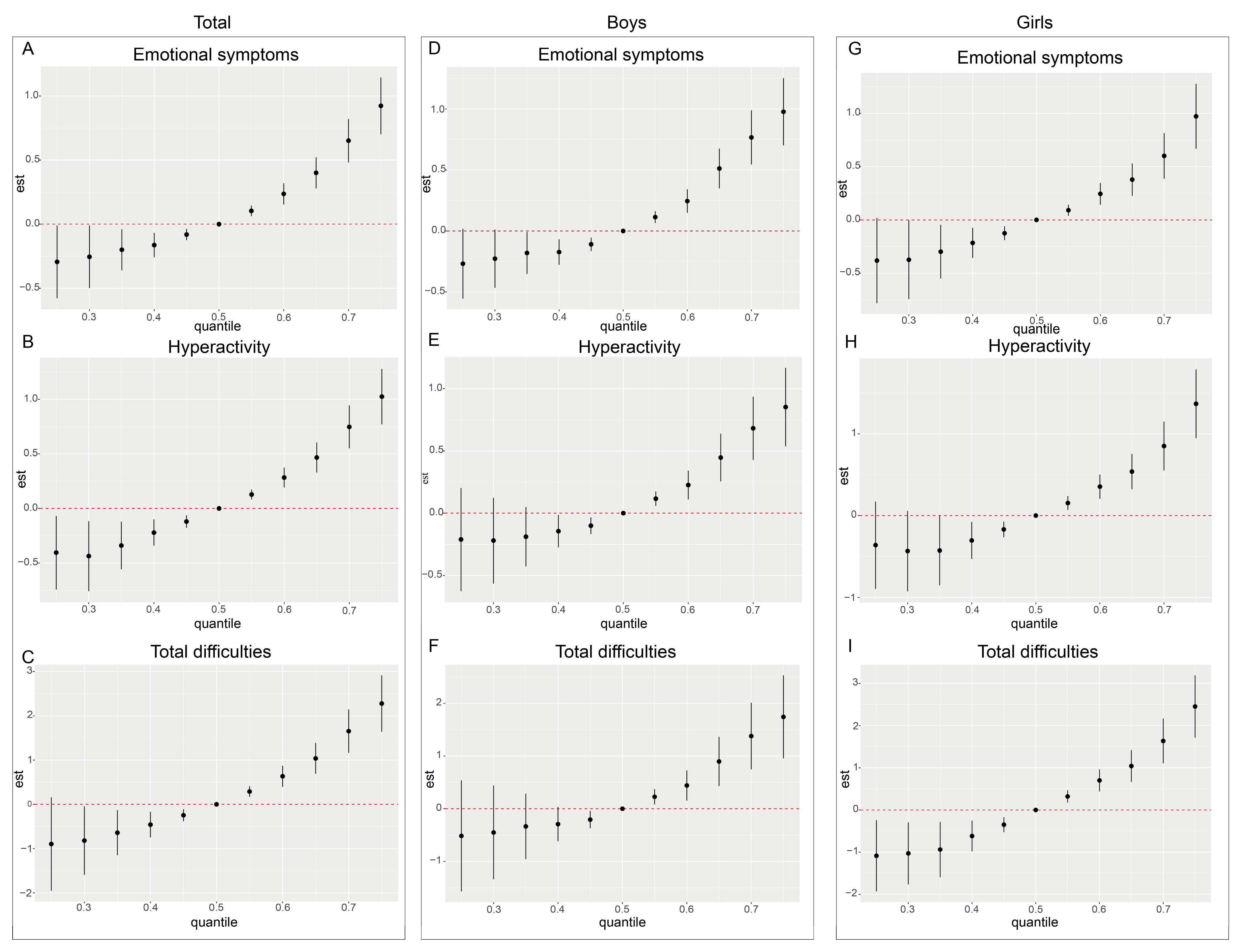
Subsequent BKMR analysis shows that urinary neonicotinoid concentrations are positively associated with neurobehavioral problem scores across all population subgroups, with prominent effects on emotional symptoms, hyperactivity, and overall difficulties. Notably, effect estimates rose with exposure and remained statistically significant across all percentile levels. These results suggest that even low levels of neonicotinoid exposure may have adverse effects on the neurobehavioral development of preschool children. This finding aligns with prior in vivo and in vitro studies showing that low-dose neonicotinoid exposure can induce nicotine-like neurotoxicity [
56]. Additional experimental work indicates that neonicotinoids, even at environmentally relevant concentrations, can activate nAChRs, induce oxidative stress, and damage synaptic structure, thereby disrupting neuroplasticity and regulatory function in the developing nervous system [
57]. Taken together, IMI, NTHM, and NACE showed significant positive associations in the regression models, whereas the contributions of other neonicotinoids were relatively limited. This suggests that particular attention should be paid to the potential health risks of IMI, THM (the parent compound of NTHM), and ACE (the parent compound of NACE). From a public health perspective, reducing the use of these compounds and exploring safer alternatives may help mitigate their potential adverse effects on neurobehavioral development in preschool children.
Nevertheless, this study has several limitations that warrant acknowledgment. First, because this is a cross-sectional study, it cannot establish causal relationships between neonicotinoid exposure and neurobehavioral outcomes. Second, most neonicotinoids have relatively short biological half-lives in humans, and the spot urine samples collected in this study may not accurately reflect long-term cumulative exposure. In addition, because urine collection did not occur immediately after potential exposure events, transient peak concentrations may have been missed, meaning that the actual short-term exposure levels could have been underestimated. Meanwhile, the total IMIeq concentrations calculated using RPFs based on EPA reference doses primarily serve to reflect overall exposure levels for comparability across studies, rather than to precisely represent differences in neurotoxic potency, as the underlying RfDs were derived from heterogeneous toxicological endpoints. Third, neurodevelopmental outcomes were assessed with the SDQ, which may be affected by recall bias and other reporting inaccuracies. Finally, the sample size of this study was relatively modest (n = 506), and all participants were recruited from a single city (Shenzhen), which may limit generalizability and robustness of the findings. Future prospective cohort studies and experimental investigations are warranted to provide more comprehensive evidence on the impact of neonicotinoid exposure on human neurobehavioral development.