Mytilus galloprovincialis as a Biomarker for Personal Care Product (PCP) Ingredients and UV Filters (UVFs) in Tunisian Coastal Waters: Correlation with the Chemical Composition of Polluted Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
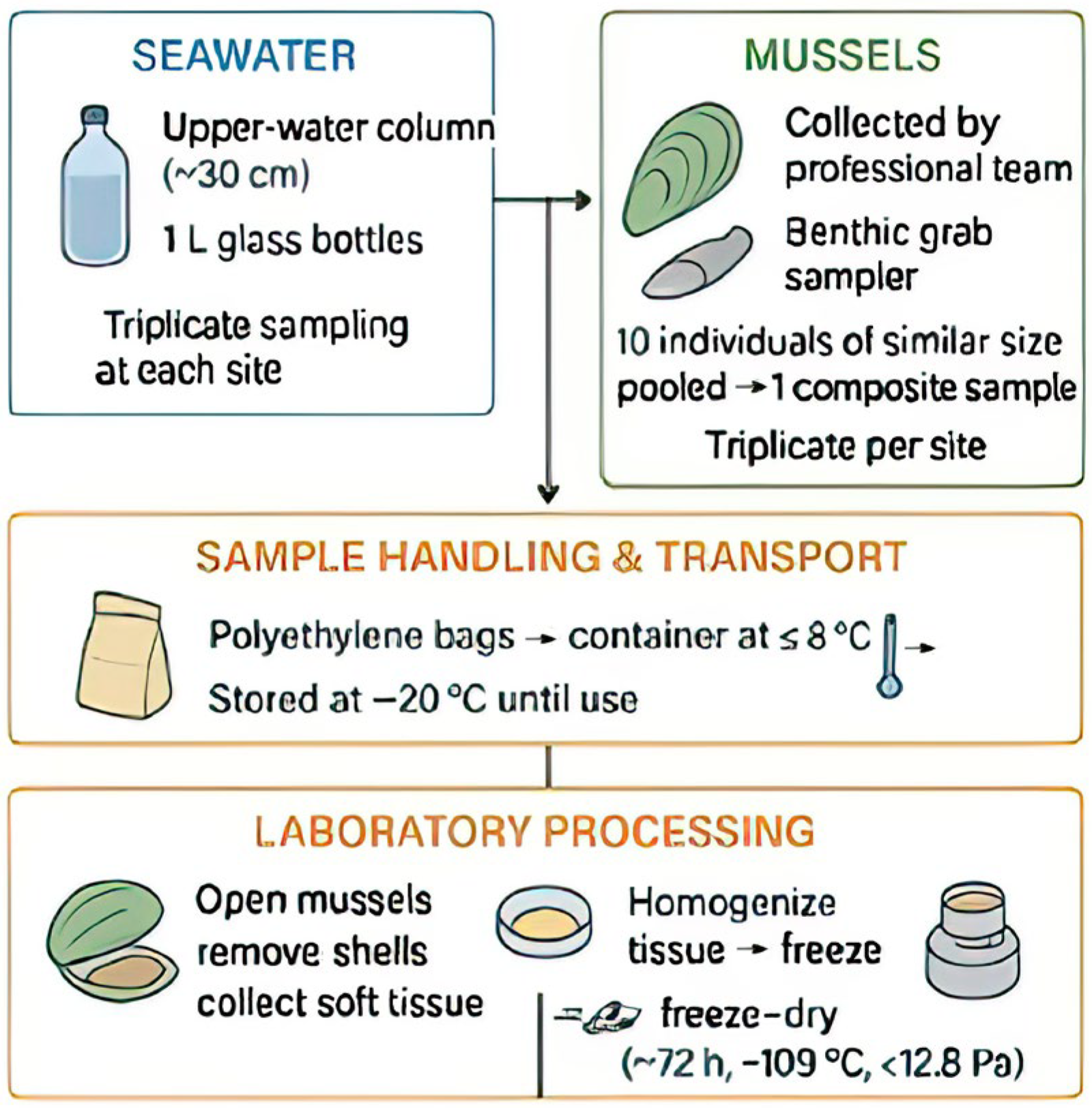
2.2. Sampling
- Seawater: Collected from the upper water column (≈30 cm) in 1 L glass bottles.
- Mussels: Collected using a benthic grab sampler. To reduce variability (size, age, environmental conditions, and health status), 10 mussels of a similar size were pooled per sample, resulting in 30 composite samples per month.
2.3. Samples Pretreatment
2.3.1. Seawater Pretreatment
2.3.2. Mussels Pretreatment
2.4. Analysis of Seawater Samples
2.5. Analysis by LC-MS/MS
2.5.1. Seawater Analysis
2.5.2. Mussels Analysis
2.6. QA/QC in Chemical Analysis
2.7. Estimation of Human Mussel’s Intake
2.8. Statistical Analysis
3. Results
3.1. Characterization of Seawater Samples
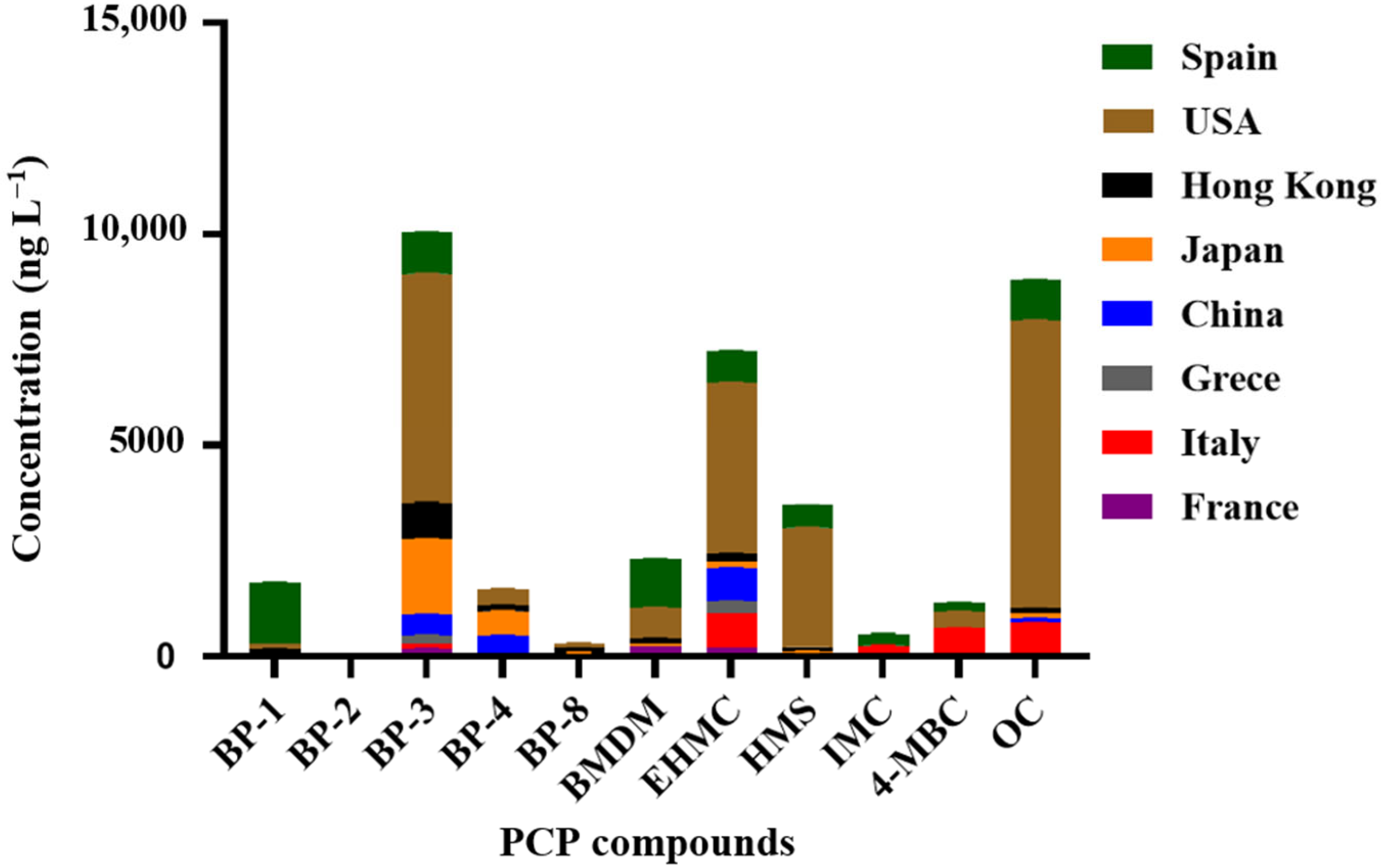
3.2. Occurrence of Personal Care Product Ingredients
3.2.1. Seawater
3.2.2. Mussels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Cruz, M.S.; Molins-Delgado, D.; Serra-Roig, M.P.; Kalogianni, E.; Skoulikidis, N.T.; Barceló, D. Personal Care Products Reconnaissance in EVROTAS River (Greece): Water-Sediment Partition and Bioaccumulation in Fish. Sci. Total Environ. 2019, 651, 3079–3089. [Google Scholar] [CrossRef]
- Carve, M.; Nugegoda, D.; Allinson, G.; Shimeta, J. A Systematic Review and Ecological Risk Assessment for Organic Ultraviolet Filters in Aquatic Environments. Environ. Pollut. 2021, 268, 115894. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and Removal of Chemicals of Emerging Concern in Wastewater Treatment Plants and Their Impact on Receiving Water Systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Volpe, A.; Pagano, M.; Mascolo, G.; Grenni, P.; Rossetti, S. Biodegradation of UV-Filters in Marine Sediments. Sci. Total Environ. 2017, 575, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Liñán, L.; Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Beiras, R. Bioaccumulation of UV Filters in Mytilus Galloprovincialis Mussel. Chemosphere 2018, 190, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Lanzinger, A.C.; Dolich, T.; Füßl, S.; Salinas, E.R.; Zok, S.; Weiss, B.; Hefner, N.; Van Sloun, P.; Hombeck, H.; et al. Evaluation of the Bioaccumulation of Octocrylene after Dietary and Aqueous Exposure. Sci. Total Environ. 2019, 672, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.; Sakamaki, T.; Dong Tian, W.; Moyal, D.; Hamzavi, I.H.; Kollias, N. The Dynamics of Pigment Reactions of Human Skin to Ultraviolet a Radiation. Photodermatol. Photoimmunol. Photomed. 2019, 35, 387–392. [Google Scholar] [CrossRef]
- Soler De La Vega, A.C.; Cruz-Alcalde, A.; Sans Mazón, C.; Barata Martí, C.; Diaz-Cruz, M.S. Nano-TiO2 Phototoxicity in Fresh and Seawater: Daphnia Magna and Artemia Sp. as Proxies. Water 2020, 13, 55. [Google Scholar] [CrossRef]
- Vione, D.; Calza, P.; Galli, F.; Fabbri, D.; Santoro, V.; Medana, C. The Role of Direct Photolysis and Indirect Photochemistry in the Environmental Fate of Ethylhexyl Methoxy Cinnamate (EHMC) in Surface Waters. Sci. Total Environ. 2015, 537, 58–68. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An Overview of UV-Absorbing Compounds (Organic UV Filters) in Aquatic Biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhu, L.; Zhu, L.; Kun, Y.; Zhu, X. Individual and Joint Toxic Effects of Pentachlorophenol and Bisphenol A on the Development of Zebrafish (Danio rerio) Embryo. Ecotoxicol. Environ. Saf. 2008, 71, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A Review of Organic UV-Filters in Wastewater Treatment Plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Silori, R.; Shrivastava, V.; Singh, A.; Sharma, P.; Aouad, M.; Mahlknecht, J.; Kumar, M. Global Groundwater Vulnerability for Pharmaceutical and Personal Care Products (PPCPs): The Scenario of Second Decade of 21st Century. J. Environ. Manag. 2022, 320, 115703. [Google Scholar] [CrossRef]
- Afsa, S.; Hamden, K.; Lara Martin, P.A.; Mansour, H.B. Occurrence of 40 Pharmaceutically Active Compounds in Hospital and Urban Wastewaters and Their Contribution to Mahdia Coastal Seawater Contamination. Environ. Sci. Pollut. Res. 2020, 27, 1941–1955. [Google Scholar] [CrossRef]
- Ngo, T.H.; Van, D.-A.; Tran, H.L.; Nakada, N.; Tanaka, H.; Huynh, T.H. Occurrence of Pharmaceutical and Personal Care Products in Cau River, Vietnam. Environ. Sci. Pollut. Res. 2021, 28, 12082–12091. [Google Scholar] [CrossRef]
- Huang, J.; Ding, J.; Jiang, H.; Wang, Z.; Zheng, L.; Song, X.; Zou, H. Pharmaceuticals and Personal Care Products across Different Water Bodies in Taihu Lake Basin, China: Occurrence, Source, and Flux. Int. J. Environ. Res. Public Health 2022, 19, 11135. [Google Scholar] [CrossRef]
- Fenni, F.; Sunyer-Caldú, A.; Ben Mansour, H.; Diaz-Cruz, M.S. Contaminants of Emerging Concern in Marine Areas: First Evidence of UV Filters and Paraben Preservatives in Seawater and Sediment on the Eastern Coast of Tunisia. Environ. Pollut. 2022, 309, 119749. [Google Scholar] [CrossRef]
- Nasri, E.; De La Vega, A.C.S.; Martí, C.B.; Ben Mansour, H.; Diaz-Cruz, M.S. Pharmaceuticals and Personal Care Products in Tunisian Hospital Wastewater: Occurrence and Environmental Risk. Environ. Sci. Pollut. Res. 2023, 31, 2716–2731. [Google Scholar] [CrossRef]
- Glanz, K.; Kwong, P.L.; Avelis, J.; Cassel, K. Development of a Survey of Sunscreen Use and Attitudes among Adults in Two Coastal States, 2019. Int. J. Environ. Res. Public Health 2022, 19, 2677. [Google Scholar] [CrossRef]
- Wang, W.; Lee, I.-S.; Oh, J.-E. Specific-Accumulation and Trophic Transfer of UV Filters and Stabilizers in Marine Food Web. Sci. Total Environ. 2022, 825, 154079. [Google Scholar] [CrossRef]
- Nasri, E.; Machreki, M.; Beltifa, A.; Aroui, S.; Ghorbel, A.; Saad, A.; Feriani, A.; Borgi, M.A.; Ghazouani, L.; Sire, O.; et al. Cytotoxic Effects of Seven Tunisian Hospital Wastewaters on the Proliferation of Human Breast Cancer Cell Line MDA-231: Correlation with Their Chemical Characterization. Environ. Sci. Pollut. Res. 2017, 24, 20422–20428. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV Filters Bioaccumulation in Fish from Iberian River Basins. Sci. Total Environ. 2015, 518–519, 518–525. [Google Scholar] [CrossRef]
- García-Gil, A.; Garrido Schneider, E.; Mejías, M.; Barceló, D.; Vázquez-Suñé, E.; Díaz-Cruz, S. Occurrence of Pharmaceuticals and Personal Care Products in the Urban Aquifer of Zaragoza (Spain) and Its Relationship with Intensive Shallow Geothermal Energy Exploitation. J. Hydrol. 2018, 566, 629–642. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Silvia Díaz-Cruz, M.; Barceló, D. Removal of Polar UV Stabilizers in Biological Wastewater Treatments and Ecotoxicological Implications. Chemosphere 2015, 119, S51–S57. [Google Scholar] [CrossRef]
- European Commission 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified Under Document Number C(2002) 3044). 2002. Available online: https://www.eurl-pesticides.eu/library/docs/fv/2ndws2007_ValidConceptsLippold.pdf (accessed on 25 August 2025).
- Onsanit, S.; Ke, C.; Wang, X.; Wang, K.-J.; Wang, W.-X. Trace Elements in Two Marine Fish Cultured in Fish Cages in Fujian Province, China. Environ. Pollut. 2010, 158, 1334–1342. [Google Scholar] [CrossRef]
- Ben Salem, Z.; Ayadi, H. Assessment of Trace Metals Contamination in Diplodus Annularis (Linnaeus, 1758) from the South Coast of Sfax, Tunisia. Euro-Mediterr. J. Environ. Integr. 2017, 2, 13. [Google Scholar] [CrossRef][Green Version]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 1999; p. 944. [Google Scholar][Green Version]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; Sage: Singapore, 2012. [Google Scholar][Green Version]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and Spatial Distribution of Pharmaceuticals and Personal Care Products (PPCPs) in the Aquatic Environment, Their Characteristics, and Adopted Legislations. J. Water Process. Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- N.T. 106.002; Relative Aux Rejets d’Effluents Dans le Milieu Hydrique (Protection de L’Environnement). Normes Tunisiennes. Ministre de l’Economie Nationale: Paris, France, 1989.
- Giokas, D.L.; Sakkas, V.A.; Albanis, T.A. Determination of Residues of UV Filters in Natural Waters by Solid-Phase Extraction Coupled to Liquid Chromatography–Photodiode Array Detection and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2004, 1026, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bratkovics, S.; Sapozhnikova, Y. Determination of Seven Commonly Used Organic UV Filters in Fresh and Saline Waters by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Methods 2011, 3, 2943. [Google Scholar] [CrossRef]
- Román, I.P.; Chisvert, A.; Canals, A. Dispersive Solid-Phase Extraction Based on Oleic Acid-Coated Magnetic Nanoparticles Followed by Gas Chromatography–Mass Spectrometry for UV-Filter Determination in Water Samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef]
- Benedé, J.L.; Chisvert, A.; Salvador, A.; Sánchez-Quiles, D.; Tovar-Sánchez, A. Determination of UV Filters in Both Soluble and Particulate Fractions of Seawaters by Dispersive Liquid–Liquid Microextraction Followed by Gas Chromatography–Mass Spectrometry. Anal. Chim. Acta 2014, 812, 50–58. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, Distribution and Ecological Risk Assessment of Multiple Classes of UV Filters in Surface Waters from Different Countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Slomberg, D.; Catalano, R.; Robert, S.; Apers-Tremelo, M.-L.; Boudenne, J.-L.; Manasfi, T.; Radakovitch, O. Assessing UV Filter Inputs into Beach Waters during Recreational Activity: A Field Study of Three French Mediterranean Beaches from Consumer Survey to Water Analysis. Sci. Total Environ. 2020, 706, 136010. [Google Scholar] [CrossRef] [PubMed]
- Thallinger, D.; Labille, J.; Milinkovitch, T.; Boudenne, J.; Loosli, F.; Slomberg, D.; Angeletti, B.; Lefrançois, C. UV Filter Occurrence in Beach Water of the Mediterranean Coast—A Field Survey over 2 Years in Palavas-les-Flots, France. Intern. J. Cosmet. Sci. 2023, 45, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Klimová, Z.; Hojerová, J.; Beránková, M. Skin Absorption and Human Exposure Estimation of Three Widely Discussed UV Filters in Sunscreens—In Vitro Study Mimicking Real-Life Consumer Habits. Food Chem. Toxicol. 2015, 83, 237–250. [Google Scholar] [CrossRef]
- Jeon, H.-K. Comparative Toxicity Related to Metabolisms of Benzophenone-Type UV Filters, Potentially Harmful to the Environment and Humans. Mol. Cell. Toxicol. 2017, 13, 337–343. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Occurrence and Removal of Benzotriazoles and Ultraviolet Filters in a Municipal Wastewater Treatment Plant. Environ. Pollut. 2012, 165, 225–232. [Google Scholar] [CrossRef]
- Magi, E.; Di Carro, M.; Scapolla, C.; Nguyen, K.T.N. Stir Bar Sorptive Extraction and LC–MS/MS for Trace Analysis of UV Filters in Different Water Matrices. Chromatographia 2012, 75, 973–982. [Google Scholar] [CrossRef]
- Kaiser, D.; Wappelhorst, O.; Oetken, M.; Oehlmann, J. Occurrence of Widely Used Organic UV Filters in Lake and River Sediments. Environ. Chem. 2012, 9, 139. [Google Scholar] [CrossRef]
- Kameda, Y.; Kimura, K.; Miyazaki, M. Occurrence and Profiles of Organic Sun-Blocking Agents in Surface Waters and Sediments in Japanese Rivers and Lakes. Environ. Pollut. 2011, 159, 1570–1576. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen Products as Emerging Pollutants to Coastal Waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Moyano, C.; Giokas, D.L.; Salvador, A. Expanding the Application of Stir Bar Sorptive-Dispersive Microextraction Approach to Solid Matrices: Determination of Ultraviolet Filters in Coastal Sand Samples. J. Chromatogr. A 2018, 1564, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Rodríguez, A.; Rodrigo Sanz, M.; Betancort Rodríguez, J.R. Occurrence of Eight UV Filters in Beaches of Gran Canaria (Canary Islands). An Approach to Environmental Risk Assessment. Chemosphere 2015, 131, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Picot-Groz, M.; Fenet, H.; Martinez Bueno, M.J.; Rosain, D.; Gomez, E. Diurnal Variations in Personal Care Products in Seawater and Mussels at Three Mediterranean Coastal Sites. Environ. Sci. Pollut. Res. 2018, 25, 9051–9059. [Google Scholar] [CrossRef]
- Mozas-Blanco, S.; Rodríguez-Gil, J.L.; Kalman, J.; Quintana, G.; Díaz-Cruz, M.S.; Rico, A.; López-Heras, I.; Martínez-Morcillo, S.; Motas, M.; Lertxundi, U.; et al. Occurrence and Ecological Risk Assessment of Organic UV Filters in Coastal Waters of the Iberian Peninsula. Mar. Pollut. Bull. 2023, 196, 115644. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; Allan, I.J.; González-Mazo, E.; Lara-Martín, P.A. Passive Samplers vs Sentinel Organisms: One-Year Monitoring of Priority and Emerging Contaminants in Coastal Waters. Environ. Sci. Technol. 2020, 54, 6693–6702. [Google Scholar] [CrossRef]
- Sankoda, K.; Murata, K.; Tanihata, M.; Suzuki, K.; Nomiyama, K.; Shinohara, R. Seasonal and Diurnal Variation of Organic Ultraviolet Filters from Personal Care Products Used Along the Japanese Coast. Arch. Environ. Contam. Toxicol. 2015, 68, 217–224. [Google Scholar] [CrossRef]
- Widz, M.; Brzezińska-Wójcik, T. Assessment of the Overtourism Phenomenon Risk in Tunisia in Relation to the Tourism Area Life Cycle Concept. Sustainability 2020, 12, 2004. [Google Scholar] [CrossRef]
- Papageorgiou, M. Coastal and Marine Tourism: A Challenging Factor in Marine Spatial Planning. Ocean. Coast. Manag. 2016, 129, 44–48. [Google Scholar] [CrossRef]
- Rodil, R.; Villaverde-de-Sáa, E.; Cobas, J.; Quintana, J.B.; Cela, R.; Carro, N. Legacy and Emerging Pollutants in Marine Bivalves from the Galician Coast (NW Spain). Environ. Int. 2019, 129, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Matthies, M.; Solomon, K.; Vighi, M.; Gilman, A.; Tarazona, J.V. The Origin and Evolution of Assessment Criteria for Persistent, Bioaccumulative and Toxic (PBT) Chemicals and Persistent Organic Pollutants (POPs). Environ. Sci. Process. Impacts 2016, 18, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- P.C. 2000-348; Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations. SOR/2000-107. Canada Gazette: Ottawa, ON, Canada, 2000; Part II. Volume 134, pp. 607–612.
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental Occurrence and Hazard of Organic UV Stabilizers and UV Filters in the Sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Fagervold, S.K.; Rodrigues, A.S.; Rohée, C.; Roe, R.; Bourrain, M.; Stien, D.; Lebaron, P. Occurrence and Environmental Distribution of 5 UV Filters During the Summer Season in Different Water Bodies. Water Air Soil. Pollut. 2019, 230, 172. [Google Scholar] [CrossRef]
- Peng, X.; Fan, Y.; Jin, J.; Xiong, S.; Liu, J.; Tang, C. Bioaccumulation and Biomagnification of Ultraviolet Absorbents in Marine Wildlife of the Pearl River Estuarine, South China Sea. Environ. Pollut. 2017, 225, 55–65. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Biodegradation of Three Selected Benzotriazoles under Aerobic and Anaerobic Conditions. Water Res. 2011, 45, 5005–5014. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Lee, W.-N.; Wang, X.-H. Ketamine and the Metabolite Norketamine: Persistence and Phototransformation Toxicity in Hospital Wastewater and Surface Water. Water Res. 2014, 53, 351–360. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A.; et al. Toxicological Effects of the Sunscreen UV Filter, Benzophenone-2, on Planulae and in Vitro Cells of the Coral, Stylophora Pistillata. Ecotoxicology 2014, 23, 175–191. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative Toxicities of Four Benzophenone Ultraviolet Filters to Two Life Stages of Two Coral Species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- Jovanović, B.; Guzmán, H.M. Effects of Titanium Dioxide (TiO2) Nanoparticles on Caribbean Reef-building Coral (Montastraea Faveolata). Environ. Toxic. Chem. 2014, 33, 1346–1353. [Google Scholar] [CrossRef]
- Araújo, M.J.; Rocha, R.J.M.; Soares, A.M.V.M.; Benedé, J.L.; Chisvert, A.; Monteiro, M.S. Effects of UV Filter 4-Methylbenzylidene Camphor during Early Development of Solea Senegalensis Kaup, 1858. Sci. Total Environ. 2018, 628–629, 1395–1404. [Google Scholar] [CrossRef]
- Barone, A.N.; Hayes, C.E.; Kerr, J.J.; Lee, R.C.; Flaherty, D.B. Acute Toxicity Testing of TiO2-Based vs. Oxybenzone-Based Sunscreens on Clownfish (Amphiprion ocellaris). Environ. Sci. Pollut. Res. 2019, 26, 14513–14520. [Google Scholar] [CrossRef]
- European Commission. CosIng—Cosmetic Ingredients Database. Available online: https://single-market-economy.ec.europa.eu/sectors/cosmetics/cosmetic-ingredient-database_en (accessed on 20 September 2025).
- PubChem. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 20 September 2025).
- SCCS/1625/20; Opinion on Benzophenone-3 (CAS No 131-57-7, EC No 205-031-5)—Final Opinion. Scientific Committee on Consumer Safety: Luxembourg, April 2021.
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are Sunscreens a New Environmental Risk Associated with Coastal Tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Fent, K.; Zenker, A.; Rapp, M. Widespread Occurrence of Estrogenic UV-Filters in Aquatic Ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of Multiclass UV Filters in Treated Sewage Sludge from Wastewater Treatment Plants. Chemosphere 2011, 84, 1158–1165. [Google Scholar] [CrossRef]
- SCCS/1771/20; Opinion on Butyl Methoxydibenzoylmethane (Avobenzone). Scientific Committee on Consumer Safety: Luxembourg, 2020.
- SCCS/1517/13; Opinion on 4-Methylbenzylidene Camphor (4-MBC). Scientific Committee on Consumer Safety: Luxembourg, 2013.
- SCCS//1671/24; Opinion on Ethylhexyl Methoxycinnamate (EHMC) (CAS No. 5466-77-3/83834-59-7, EC No. 226-775-7/629-661-9). Scientific Committee on Consumer Safety: Luxembourg, June 2025.
- SCCS/1501/13; Opinion on Ensulizole (2-Phenylbenzimidazole-5-Sulfonic Acid). Scientific Committee on Consumer Safety: Luxembourg, 2013.
- SCCS/1348/10; Opinion on Parabens (Methyl-, Ethyl-, Propyl-, Butyl-, Benzylparaben). Scientific Committee on Consumer Safety: Luxembourg, 2011.


| Governorates Parameters | Monastir | Sousse | Mahdia |
|---|---|---|---|
| BOD5 (mgO2 L−1) | 9.3 ± 0.3 | 8.28 ± 0.7 | 8.5 ± 0.6 |
| COD (mgO2 L−1) | 24.9 ± 1.8 | 23.24 ± 1.2 | 19.60 ± 0.2 |
| TSS (mg L−1) | 48.6 ± 0.4 | 37.5 ± 0.5 | 25.55 ± 0.3 |
| TOC (mg L−1) | 2.62 ± 0.5 | 3.05 ± 0.2 | 2.56 ± 5.6 |
| NO3 (mg L−1) | <0.5 | <0.5 | <0.5 |
| AOX (mg L−1) | <0.5 | <0.5 | <0.5 |
| Turbidity (NTU) | 0.81 ± 0.1 | 0.73 ± 0.5 | 0.3.6 ± 0.2 |
| Conductivity (ms cm−1) | 58.51 ± 0.5 | 55.15 ± 1 | 35.69 ± 1 |
| pH | 7.09 ± 0.2 | 7.67 ± 0.3 | 7.01 ± 0.1 |
| COD/BOD5 | 2.69 ± 0.6 | 2.8 ± 0.2 | 2.24 ± 0.2 |
| OM (mg L−1) | 2.4 ± 0.8 | 3.33 ± 0.8 | 1.66 ± 0.7 |
| PCP Ingredients | Seawater | LOD (ng L−1) | LOQ (ng L−1) | ||
|---|---|---|---|---|---|
| City | Monastir | Sousse | Mahdia | ||
| BP1 | 82.23 ± 0.8 b | 91.98 ± 2.4 a | 89.46 ± 2.3 a | 1.09 | 3.56 |
| BP2 | 62.86 ± 2.7 a | 74.51 ± 3.9 b | 71.03 ± 2.7 b | 1.17 | 3.90 |
| BP3 | 25.27 ± 1 b | 29.01 ± 1 a | 18.76 ± 1.3 b | 0.74 | 2.47 |
| 4HB | 132, 45 ± 1.5 a | 139.75 ± 1.9 a | 125. 61 ± 2.2 ab | 1.04 | 3.47 |
| 4DHB | 67. 57 ± 2.8 a | 71.05 ± 1.8 a | 61. 85 ± 2.7 b | 0.99 | 3.30 |
| AVO | 524.94 ± 2 a | 603.70 ± 4.4 a | 621.02 ±3.9 b | 0.93 | 3.75 |
| EtPABA | 51.72 ± 2.9 b | 41.89 ± 1.4 ab | 40.94 ± 1.2 a | 0.3 | 1.1 |
| ODPABA | 36.72 ± 0.8 a | 45.27 ± 1.4 a | 35.83 ± 0.4 a | 0.73 | 2.44 |
| EHMC | 32.64 ± 0.9 a | 19 ± 0.7 c | 43.87 ± 0.7 b | 1.71 | 5.69 |
| TBHPBT | 83.42 ± 1.2 a | 74.27 ± 1.3 a | 62.77 ± 0.8 b | 0.1 | 0.3 |
| UVP | 59.72 ± 0.9 a | 66.97 ± 3.4 b | 19.43 ± 0.2 c | 1.03 | 3.42 |
| UV328 | 60.47 ± 1 b | 58.27 ± 2.4 a | 58.01 ± 2.7 a | 1.03 | 3.42 |
| DMBZT | 35.073 ± 0.7 b | 19.29 ± 0.9 a | 22.46 ± 0.8 b | 0.90 | 2.99 |
| PCP Ingredients | Mussels | LOD (ng g−1) | LOQ (ng g−1) | ||
|---|---|---|---|---|---|
| City | Monastir | Sousse | Mahdia | ||
| BP1 | 12.53 ± 0.5 b | 7.216 ± 0.4 a | 8.246 ± 0.2 a | 1.09 | 3.56 |
| BP2 | 4.360 ± 0.1 a | 4.503 ± 0.1 a | 7.103 ± 0.2 ab | 1.17 | 3.90 |
| BP3 | n.d. | 0.391 ± 0.1 a | n.d. | 0.74 | 2.47 |
| 4HB | 24.645 ± 0.5 a | 26.745 ± 0.4 a | 25.465 ± 0.3 ab | 1.04 | 3.47 |
| 4DHB | 6.652 ± 1.5 a | 7.85 ± 2.7 a | 1.025 ± 0.5 b | 0.99 | 3.30 |
| AVO | 164.244 ± 2.8 a | 193.481 ± 5.5 a | 121.076 ± 1.6 b | 0.93 | 3.75 |
| EtPABA | 5.553 ± 1 b | 4.030 ± 0.9 ab | 3.091 ± 0.7 a | 0.3 | 1.1 |
| ODPABA | 6.612 ± 0.26 a | 5.96 ± 1.79 a | 5.553 ± 0.7 a | 0.73 | 2.44 |
| EHMC | 2.316 ± 0.8 a | 9 ± 1.43 c | 4.360 ± 0.8 b | 1.71 | 5.69 |
| TBHPBT | 23.886 ± 2.2 a | 26.704 ± 1.7 a | 20.987 ± 0.7 b | 0.1 | 0.3 |
| UVP | 9.652 ± 0.7 a | 16.566 ± 1.9 ab | 8.043 ± 0.8 b | 1.03 | 3.42 |
| UV328 | 5.224 ± 1.46 b | 14.747 ± 3.01 a | 13.045 ± 1.9 a | 1.03 | 3.42 |
| DMBZT | 4.503 ± 0.5 b | 1.224 ± 0.5 a | 2.96 ± 0.6 b | 0.90 | 2.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, E.; Bouchiba, E.; Brahmi, B.; Bouyahi, S.; López-Maldonado, E.A.; Borgi, M.A. Mytilus galloprovincialis as a Biomarker for Personal Care Product (PCP) Ingredients and UV Filters (UVFs) in Tunisian Coastal Waters: Correlation with the Chemical Composition of Polluted Seawater. Toxics 2025, 13, 847. https://doi.org/10.3390/toxics13100847
Nasri E, Bouchiba E, Brahmi B, Bouyahi S, López-Maldonado EA, Borgi MA. Mytilus galloprovincialis as a Biomarker for Personal Care Product (PCP) Ingredients and UV Filters (UVFs) in Tunisian Coastal Waters: Correlation with the Chemical Composition of Polluted Seawater. Toxics. 2025; 13(10):847. https://doi.org/10.3390/toxics13100847
Chicago/Turabian StyleNasri, Emna, Elhem Bouchiba, Bouthaina Brahmi, Siwar Bouyahi, Eduardo Alberto López-Maldonado, and Mohamed Ali Borgi. 2025. "Mytilus galloprovincialis as a Biomarker for Personal Care Product (PCP) Ingredients and UV Filters (UVFs) in Tunisian Coastal Waters: Correlation with the Chemical Composition of Polluted Seawater" Toxics 13, no. 10: 847. https://doi.org/10.3390/toxics13100847
APA StyleNasri, E., Bouchiba, E., Brahmi, B., Bouyahi, S., López-Maldonado, E. A., & Borgi, M. A. (2025). Mytilus galloprovincialis as a Biomarker for Personal Care Product (PCP) Ingredients and UV Filters (UVFs) in Tunisian Coastal Waters: Correlation with the Chemical Composition of Polluted Seawater. Toxics, 13(10), 847. https://doi.org/10.3390/toxics13100847









