Abstract
Phenol-derived compounds are among the most hazardous organic pollutants for aquatic environments due to their relatively high aqueous solubility. Microalgae harbor metabolic pathways that enable the degradation of phenolic compounds into less toxic derivatives, highlighting their potential for the bioremediation of these contaminants. In this study, four microalgal species were evaluated for their tolerance and biodegradation capacity of a mixture of phenolic compounds which include phenol, p-cresol, and acetophenone. The results revealed that Chlorella sorokiniana and Nannochloropsis gaditana could tolerate higher concentrations of the mixture (60, 50, and 25 mg L−1 of phenol, p-cresol, and acetophenone) than Chlamydomonas reinhardtii and Tetraselmis chuii (60, 30, and 20 mg L−1 of phenol, p-cresol, and acetophenone). Notably, Tetraselmis chuii could biodegrade these compounds with the highest efficiency (32, 45, and 85% of initial phenol, p-cresol, and acetophenone, respectively) after 72 h of cultivation. In the absence of alternative carbon sources in the medium, Tetraselmis chuii also biodegraded 45, 60, and 51% of initial phenol, p-cresol, and acetophenone, at 72 h, highlighting its potential for bioremediation processes. Finally, the ascorbate peroxidase, catalase, and phenol hydroxylase enzymatic activities of Tetraselmis chuii were studied in presence of the pollutants, showing increasing activity levels of these enzymes (123, 135, and 173% of control cultures for APX, CAT, and PH, respectively) involved in the antioxidant system and the degradation of phenolic compounds.
1. Introduction
The pollution of aquatic environments produced by industrial wastewaters has been one of the main worldwide problems in these years. The growing demand for energy consumption and industrial products has contributed to the degradation of water quality in diverse ecosystems [1]. The presence of several pollutants, such as ammonium, phosphate, heavy metals, and organic compounds (pesticides, phenol derivatives, BTEXs), in industrial wastewaters is one of the main causes of this water quality degradation [2]. The increasing demand for diesel, petroleum, and plastic-derived products over the past century has promoted the petrochemical industry as one of the most important industries in the world. Industrial effluents originating from petrochemical industries contain high levels of phenolic compounds, aliphatic hydrocarbons, halogen-derived compounds, and mono- and polycyclic aromatic hydrocarbons (PAHs) [3]. Short-term exposure to these pollutants may cause different adverse effects, including pulmonary toxicity, immune responses, and thyroid destruction in humans and animals, as well as metabolic and DNA damage in microorganisms, even at low concentrations [4,5].
Phenol derivatives are one of the most abundant organic compounds in industrial and petrochemical effluents. They are spelled to the environment not only from petrochemical industries but also from leather, textile, and pharmaceuticals. Their ubiquity and acceptable solubility in water, compared to PAHs or aliphatic hydrocarbons, make them one of the most problematic organic pollutants [6]. The removal of phenolic compounds has been performed with physical and chemical methods, such as adsorption, flocculation, and oxidation [7,8]. However, these approaches have serious problems with their operational costs at low concentrations of these compounds [9].
The microbial degradation of these pollutants has emerged as a promising approach to addressing this issue in aquatic environments. Microalgae are a group of photosynthetic organisms able to grow under different stress conditions, including heavy metals and both organic and inorganic contaminants [10,11,12]. Some freshwater microalgae, such as Chlamydomonas reinhardtii and Chlorella sorokiniana, have been described to use phenol derivatives compounds as alternative carbon sources [13,14]. Moreover, the increasing interest in marine microalgal strains is related to the high salinity typically found in petrochemical and phenol-derived wastewaters [15]. In this context, marine microalgae with rigid cell walls, such as Nannochloropsis gaditana and Tetraselmis chuii, represent promising candidates, as they have previously demonstrated their capacity to remove phenol derivative compounds from wastewaters [16,17]. Different microalgae have been tested for the biodegradation of phenolic compounds, with a wide range of results. While most microalgae can remove phenol or its derivatives when present as single pollutant in the culture medium, their efficiency decreases when there is a mix of different compounds [17,18]. These limitations in the simultaneous biodegradation of more than one pollutant in the culture medium may be caused by additive or synergetic toxicity effect, which reduces the microalgal biodegradation capacity [19]. However, due to the lack of studies involving mixtures of phenol derivative compounds, the underlying mechanisms still remain unclear. Thus, further research is needed to evaluate microalgal responses to pollutant mixtures.
The presence of phenolic compounds in the culture medium of microalgae can also produce alterations in microalgae metabolism. Previous studies have reported an increase in reactive oxygen species (ROS) production and, consequently, an induction of the antioxidant system, including catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD) activity, as well as an increase in lipid content [18,20,21]. Additionally, enzymes involved in the biodegradation of these compounds, such as phenol hydroxylase (PH) and catechol 2,3-dioxygenase, are also induced under these stress conditions [13,22,23]. Thus, obtaining a better comprehension of the effect that phenol derivative compounds produce on microalgae metabolism could be a first step for the improvement of pollutant biodegradation. However, to date, there are just a few reports on the removal of phenolic compounds in a mixture of three compounds. In this work, four species of microalgae were tested to elucidate their tolerance and biodegradation capacity for a mixture of pollutants that includes phenol, p-cresol, and acetophenone. The microalgal strains were Chlamydomonas reinhardtii, Chlorella sorokiniana, Tetraselmis chuii, and Nannochloropsis gaditana, which were cultured with concentrations of 60 mg L−1 of phenol, 30 or 50 mg L−1 of p-cresol, and 20 or 25 mg L−1 of acetophenone, which are typical concentrations of these compounds after secondary wastewater treatments in phenol production plants [6,24]. Additionally, the enzymatic activity of the enzymes PH (EC:1.14.13.7), APX (EC:1.11.1.11), and CAT (EC:1.11.1.6) were studied after 72 h of culture under phenolic compound stress in the microalga T. chuii to check how these compounds alter the metabolism of this strain.
2. Materials and Methods
2.1. Microalgae Strain and Culture Conditions
Microalgae used in this work were the freshwater species Chlamydomonas reinhardtii cc-1690 WTmt-21gr and Chlorella sorokiniana 211-32, kindly provided by the University of Córdoba and the Institute of Plant Biochemistry and Photosynthesis (IVBF), respectively, and the seawater species Tetraselmis chuii CCMM 03/0201 and Nannochloropsis gaditana CCMM 04/0201 from the culture collection of the Institute of Marine Sciences of Andalusia (ICMAN). Freshwater strains were cultured in Tris-Acetate-Phosphate (TAP) medium [25], while marine strains were cultured in Guillard F/2 medium [26] using CO2 as carbon source with continuous aeration (3% CO2). Microalgal pre-inocula were added to the medium at an initial optical density OD660 of 1.2. Phenolic compounds were added to the culture medium after autoclaving at the concentrations described in Table 1, and pH was adjusted to 6.7. The microalgae were cultured at 25 ˚C under continuous agitation (120 rpm) and light irradiation (120 µmol m−2 s−1).

Table 1.
Initial concentration of pollutants tested.
2.2. Biodegradation Experiments
The four microalgal strains were cultured as indicated in 2.1. For cultures without a carbon source, pre-inocula were harvested and resuspended in fresh medium without carbon source at an initial optical density OD660 = 1.2. TAP medium was prepared without acetate, the pH was adjusted to 6.7 before autoclaving, and F/2 strains were cultured without CO2 aeration. The high cell density prevented the occurrence of a lag phase in the cultures and provided a protective effect against the toxicity of the tested pollutants, and samples were taken at 24 and 72 h of culture to determine the pollutant concentration. These 2 mL aliquots were centrifuged at 13,400× g, the supernatants were filtered using 0.22 nylon filters, and the samples were stored at −20 °C for subsequent phenolic compound determination.
2.3. Determination of Phenolic Compounds
Phenolic compounds were determined by HPLC using an Agilent 1100 series system (Agilent, Santa Clara, CA, USA) equipped with a binary pump system, vacuum degasser, a thermos stated column, and a diode array detector (DAD). Separation of phenolic compounds was performed in a Nucleosil 100C18 (4.6 mm × 250 mm, 5 μm) analytical column. For the successful resolution of the compounds, the elution program was performed using an isocratic flow, with two mobile phases at a flow rate of 1.2 mL min−1 and a temperature of 40 °C. Solvents were acetonitrile (A) and water (B) in a proportion of 55:45. The injection volume was 20 μL, and the program was recording the absorbance for 12 min until the three peaks were detected. Chromatograms were recorded at 271 and 290 nm. Retention times for the compounds were 2.92 min for phenol, 4.89 min for p-cresol, and 6.34 min for acetophenone. Quantification was carried out by comparing the areas with the calibration standards (from 1 to 100 mg L−1 of each compound).
2.4. Enzymatic Assay Experiments
Enzymatic assays were performed using crude extract of the microalga T. chuii. For the extraction of soluble proteins, samples were taken from the control and phenol derivatives-treated cultures at 72 h. These aliquots were centrifuged at 4400× g, and the supernatants were discarded. The wet biomass was resuspended in 50 mM phosphate buffer (pH 7.5) and disrupted using glass beads in a Digital Disruptor Genie® (Scientific Industries, Bohemia, NY, USA) for 3 cycles of 30 s. After that, the homogenate was centrifuged at 14,000× g for 15 min, and the supernatants were used as crude extract source. The Bio-Rad Bradford assay was used to determine protein in a crude extract according to the manufacture’s protocol, using BSA as the standard.
APX and CAT enzymatic activity were determined kinetically as previously described by Romero-Cruz et al. [10]. One unit of APX was defined as the amount of enzyme that oxidizes 1 µmol of ascorbate min−1. One unit of CAT was defined as the amount of enzyme required to decompose 1 µmol of H2O2 min−1. PH activity assay was determined spectrophotometrically by measuring the NADPH content at 340 nm. The kinetics of NADPH disappearance was analyzed as described by Wang et al., [27] with minor modifications. The measured mixture contained, in a final volume of 1 mL, 50 mM Tris-HCl (pH 7.5), 5 μM NADPH, 5 nM FAD, 60 μM phenol, and 100 μL of crude extract. One unit of PH was defined as the amount of enzyme required to oxidize 1 μmol of NADPH min−1.
2.5. Statistical Analysis
All the experiments were carried out using biological triplicates and represented as mean value ± SD. One-way analysis of variance (ANOVA) was applied to identify significant differences between conditions. Significant differences were considered for values with p < 0.05 and p < 0.01. Statistical analyses were performed using IBM SPSS Statistics v29.0 software (Armonk, New York, NY, USA).
3. Results and Discussion
3.1. Tolerance of Microalgae to a Mixture of Phenol-Derived Compounds
The mix of phenolic compounds was tested using two different concentrations, depending on the tolerance of each species. While Chlorella sorokiniana (C. sorokiniana) and Nannochloropsis gaditana (N. gaditana) tolerated higher concentrations of p-cresol and acetophenone (50 and 25 mg L−1, respectively), Chlamydomonas reinhardtii (C. reinhardtii) and Tetraselmis chuii (T. chuii) could not grow under these conditions. Thus, the studies with these two strains were performed using a mixture of phenol derivate compounds with lower concentrations of p-cresol and acetophenone (30 and 20 mg L−1, respectively), as described in Table 1. Exposure to the mixture led to a slight decrease in C. reinhardtii and T. chuii growth, whose biomass decreased only 5% compared to control cultures (Figure 1A,C). In contrast, there was a significant (p < 0.01) reduction in C. sorokiniana and N. gaditana biomass, with 15 and 27% less biomass than in control cultures, respectively (Figure 1B,D). It is noteworthy that C. sorokiniana and N. gaditana mix cultures had similar growth curves to the control at the beginning of the experiment, with the differences emerging at the mid-exponential phase (2 and 5 days, respectively). This change in C. sorokiniana can be related to metabolic modifications. Previous studies demonstrated that C. sorokiniana can assimilate the initial concentration of acetate in TAP medium after 24–48 h [28]; consequently, the microalga could use the tested pollutants as a carbon source once acetate was depleted. There are also numerous studies which have reported that different microalgae can grow at phenol concentrations up to 200 mg L−1, demonstrating the high tolerance of certain species to this organic compound [22,29,30]. However, only a few studies have tackled the tolerance and cometabolic biodegradation of phenol and o/p-cresol, and these have consistently shown reduced tolerance when microalgae were exposed to a mixture of pollutants rather than individual compounds [17,18]. These results may explain the low tolerance of the microalgae tested in this work compared with previous studies using a single pollutant [13,17].
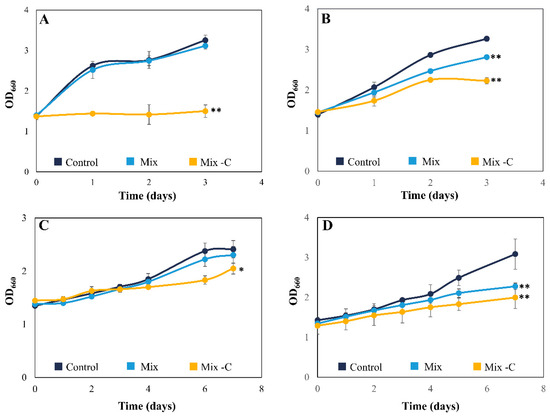
Figure 1.
Growth curves expressed as mean ± SD of C. reinhardtii (A), C. sorokiniana (B), T. chuii (C), and N. gaditana (D) under a mixture of phenol, p-cresol, and acetophenone cultured with the standard medium (blue line) and without the carbon source of the medium (yellow line). Initial concentrations of the phenol derivate compounds were 60 mg L−1 of phenol, 30 mg L−1 of p-cresol, and 20 mg L−1 of acetophenone for C. reinhardtii and T. chuii; 60 mg L−1 of phenol, 50 mg L−1 of p-cresol, and 25 mg L−1 of acetophenone for C. sorokiniana and N. gaditana. * Significant differences in biomass between control and mixture of pollutants treatment at p < 0.05 and ** at p < 0.01.
The four microalgal strains were also exposed to the mix of phenol derivate compounds in the absence of another carbon source in the medium (acetate or CO2 aeration) (-C). Under these stress conditions, the strains could adapt their metabolism to use these compounds as alternative carbon source, as previously described [13]. The results shown in Figure 1 for -C conditions were diverse. On the one hand, C. reinhardtii was not able to grow, obtaining similar biomass values throughout the experiment (Figure 1A). On the other hand, C. sorokiniana and N. gaditana showed a significant decrease (p < 0.01) compared to the control, with 32 and 38% less biomass, respectively (Figure 1B,D). T. chuii was the only species whose growth curve was similar to the control, although there were minor differences at the end of the exponential phase (Figure 1C). These results confirm that C. sorokiniana, T. chuii, and N. gaditana can use phenol derivative compounds as a carbon source for their development. Microalgae usually need an adaptation period to modify their metabolism in order to change their preferred carbon source [22]; however, T. chuii was able to perform this metabolic adjustment without this period, as Figure 1C shows. These results are consistent with the rapid adaptation to oxidative microenvironments that T. chuii showed through alterations in its transcriptome and metabolome [31]. Although most of the studies were carried out with freshwater species, the interest of the genera Tetraselmis for removing phenolic compounds is increasing in recent years [17,32,33]. The use of seawater species, such as T. chuii, has also the advantage that these species are more adapted to the high salinity typically found in industrial wastewaters (up to 100 g L−1 of salts) [15,34].
3.2. Biodegradation of Phenolic Compounds
The capacity of the four microalgal strains to degrade phenol, p-cresol, and acetophenone from the mixture was also reported at 24 and 72 h of culture. Additionally, a control without microalgae was included, demonstrating that the concentrations of the pollutants remained stable after 72 h (losses of less than 2.5%). The results demonstrate that these four microalgae could biodegrade these pollutants from the culture medium (Figure 2 and Figure 3). The biodegradation levels of phenol were slightly higher in seawater than in freshwater microalgae in the standard experiment (Figure 2). While T. chuii and N. gaditana degraded 32 and 45% of the initial phenol, respectively, C. reinhardtii and C. sorokiniana biodegraded 38 and 39%, after 72 h (Figure 2A). These results changed in the -C cultures (Figure 3). In this experiment, the removal values in freshwater species were lower than with a carbon source (31 and 25% for C. reinhardtii and C. sorokiniana, respectively), after 72 h. However, T. chuii was able to biodegrade 45% of the total phenol from the culture medium in the same period (Figure 3A), demonstrating its rapid metabolic shift from phototrophic to mixotrophic growth using phenol as a carbon source in a short period of time. Different studies have reported the capacity of the genus Tetraselmis to rapidly adapt to stress conditions [31,35], which may underline its capacity to efficiently assimilate these pollutants. N. gaditana suffered a significant decrease in phenol biodegradation levels in these conditions compared with standard cultivation (Figure 2A), keeping similar concentrations of this pollutant than the initial ones after 72 h (Figure 3A). Although there was no cell growth in C. reinhardtii -C cultures, as Figure 1A shows, some phenol removal was observed (Figure 1A), which means that phenol decrease can be mainly produced by cell surface adsorption instead of biodegradation. Though the mixture of pollutants seems to be toxic for this microalga, Nazos et al. [13] reported that C. reinhardtii could biodegrade phenol as a single carbon source, showing also a production of catechol in the medium. Biodegradation results of phenol in the four microalgae tested were lower than previous studies using this pollutant as a single one (Table 2) [11,18,30], which confirms that mixtures of pollutants, such as those used in our work, are more difficult to remove. These results are consistent with findings by Meza-Escalante et al. [17], which demonstrated that in a mixture of phenol derivate compounds (phenol, o- and p-cresol), biodegradation of phenol decrease significantly compared with its degradation as a single pollutant, being the biodegradation capacity of o/p-cresol higher than phenol (Table 2), in concordance with the results reported in Figure 2 of our work.
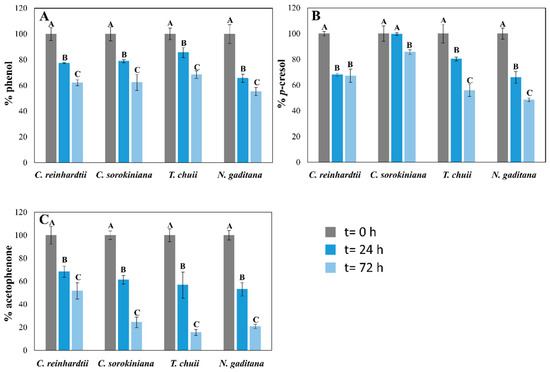
Figure 2.
Biodegradation of phenol (A), p-cresol (B), and acetophenone (C), expressed as mean ± SD, after 24 and 72 h of cultured in the mixture with the standard medium (filled) cultures of the four microalgae tested. Initial concentrations of the phenol derivate compounds were 60 mg L−1 of phenol, 30 mg L−1 of p-cresol, and 20 mg L−1 of acetophenone for C. reinhardtii and T. chuii; 60 mg L−1 of phenol, 50 mg L−1 of p-cresol, and 25 mg L−1 of acetophenone for C. sorokiniana and N. gaditana. For the same microalgae, different capital letters indicate statistically significant differences (p < 0.05) between timepoints.
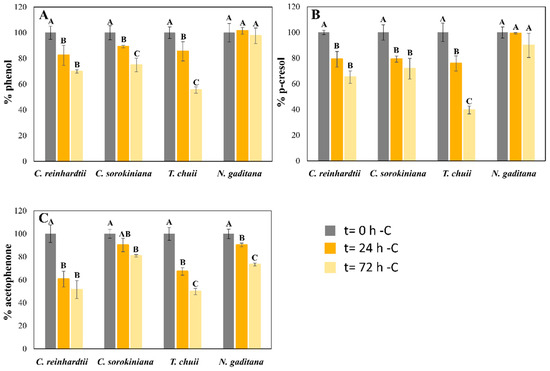
Figure 3.
Biodegradation of phenol (A), p-cresol (B), and acetophenone (C), expressed as mean ± SD after 24 and 72 h of cultured in the mixture, with and without a carbon source, in cultures of the four microalgae tested. Initial concentrations of the phenol derivate compounds were 60 mg L−1 of phenol, 30 mg L−1 of p-cresol, and 20 mg L−1 of acetophenone for C. reinhardtii and T. chuii; 60 mg L−1 of phenol, 50 mg L−1 of p-cresol, and 25 mg L−1 of acetophenone for C. sorokiniana and N. gaditana. For the same microalgae, different capital letters indicate statistically significant differences (p < 0.05) between timepoints.

Table 2.
Comparison of biodegradation of phenolic compounds in different microalgae.
The biodegradation of p-cresol reported similar behaviors than phenol. The removal values were from 15% in C. sorokiniana cultures to 52% in N. gaditana cultures in the carbon source supplied (+C) experiment after three days (Figure 2B). Although C. sorokiniana tolerated high concentrations of this pollutant, it exhibited limited biodegradation in the mix with other pollutants, being the affinity for phenol and acetophenone higher than p-cresol under +C conditions (Figure 2). However, under -C conditions, different behaviors were reported for the four microalgae (Figure 3B). While N. gaditana could not biodegrade p-cresol in -C conditions, T. chuii and C. sorokiniana had higher biodegradation levels than in +C conditions (60 vs. 45% and 29 vs. 15%, respectively) after 72 h (Figure 2B and Figure 3B). Finally, C. reinhardtii could adsorb 35% of initial p-cresol in -C conditions, as happened with phenol (Figure 2A and Figure 3A). The removal of carbon source (-C) also produced an increase in T. chuii degradation rates from 45% to 60% of the initial p-cresol concentration (Figure 2B and Figure 3B). These results are in agreement with previous works showing that Tetraselmis suecica degrades o- and p-cresol more efficiently than phenol in a mix of pollutants [17]. Xiao et al. [18] also tested the microalga Chlorella vulgaris in the presence of different concentrations of phenol and p-cresol, demonstrating that low concentrations of phenol in the culture medium promoted the biodegradation of p-cresol in microalgae (Table 2).
Acetophenone biodegradation exhibited similar patterns in three of the studied microalgae, where biodegradation levels in +C conditions were higher than in -C. C. reinhardtii was the only strain which removed similar concentrations in both conditions (Figure 2C and Figure 3C), as for the other pollutants (Figure 2 and Figure 3), probably as an adsorption process in the -C experiment due to the lack of cellular growth (Figure 1A). Nevertheless, C. sorokiniana, T. chuii, and N.gaditana could remove 75, 85, and 80%, respectively, of initial acetophenone in the +C cultures after 72 h. These removal rates decreased in -C experiments until 20, 50, and 25% of initial concentration (Figure 2C), probably due to its higher metabolic complexity in degradation pathway compared with phenol and p-cresol [36]. Although it is more toxic to microalgae than the other pollutants tested, acetophenone was the least studied of the proposed mix in microalgae. The lack of studies in literature limits direct comparisons with previous research. The microalga Tribonema sp. could remove high amounts of this pollutant using catalytic intense pulse light as a activating (Table 2) [20]. Nevertheless, the contribution of the microalga cannot be clearly established. Thus, acetophenone could produce more alterations in microalgae metabolism, being its biodegradation a priority for microalgae development, which could explain the results shown in Figure 2. However, their preference could change when the carbon source is removed, and phenol can be the most adequate nutrient for them because of its biodegradation pathway, which makes this pollutant easier than the others to be assimilated by microalgae [36].
3.3. Effect of Phenolic Compounds on the Activity Level of Antioxidant Enzymes in Different Microalgae
The presence of these pollutants in the culture medium could produce alterations in microalgal metabolism, increasing the concentration of ROS species and activating the antioxidant system of the microorganisms [3]. Furthermore, some enzymes that can biodegrade these compounds are also expressed under these stress conditions [22]. Thus, the enzymatic activity of ascorbate peroxidase (APX), catalase (CAT), and phenol hydroxylase (PH) was studied in the marine microalga T. chuii, as selected model due to its high capacity to biodegrade the selected pollutants under both standard and -C conditions, after 72 of cultivation under phenol derivative stress. The results, represented in Figure 4, showed a significant (p < 0.01) increase in antioxidant enzymatic activities with APX and CAT activities of 1.24 and 1.35 times higher than control cultures, respectively. These results after that period of exposure are expected due to the harmful effects of these pollutants in microorganisms. It has been demonstrated that CAT plays a crucial role in the elimination of ROS species under phenol stress in microalgae. In this context, a transcriptomic study performed by Zhou et al. [37] demonstrated that three different genes related with CAT synthesis were upregulated from four to seven times in the microalga Chlorella sp. L5 after 96 h of exposure to 500 mg L−1 of phenol. Moreover, other studies reported increases from 1.3 to 4 times in CAT enzymatic activity due to the presence of phenol (from 50 to 500 mg L−1) in the microalgal species Chlorella sp., Lingulodinium polyedrum, and Scenedesmus abundans [23,38,39], which are in agreement with the results obtained for T. chuii in this work. However, the role of APX in microalgae under phenol stress is more variable. While Zhou et al. [37] reported an upregulation of different genes related to this enzyme at high concentrations of phenol (500 mg L−1) in Chlorella sp. L5, other studies showed no significant differences in the microalga Lingulodinium polyedrum with a phenol concentration of 9.5 mg L−1, or a significant decrease in APX activity at concentrations from 50 to 300 mg L−1 in the microalga Scenedesmus abundans [23,39]. In contrast, a recent study performed by Lu et al. [40] reported an increase in monodehydroascorbate (MDA) production due to a p-cresol derivative in the microalga Phaeodactylum tricornutm, being MDA a direct product of APX activity. These results suggest that the increase in APX reported by T. chuii in our work may be related to p-cresol exposure. Moreover, APX activity tends to decrease at high pollutant concentrations due to enzyme inactivation or depletion of ascorbate, as was described by Romero-Cruz et al. under Cu2+, Cd2+, Hg2+, and As (III) stress [10].
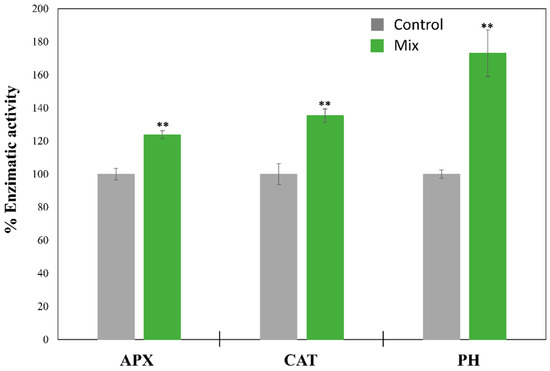
Figure 4.
Effect of a mixture of phenol, p-cresol, and acetophenone on the enzymatic activities of APX, CAT, and PH, expressed as mean ± SD in Tetraselmis chuii after 72 h of cultivation. Initial concentrations of the phenol derivate compounds were 60 mg L−1 of phenol, 30 mg L−1 of p-cresol, and 20 mg L−1 of acetophenone. 100% APX, CAT, and PH activity corresponded to 4.428 ± 0.153 U mg−1, 10.073 ± 0.648 U mg−1, and 296.611 ± 7.535 U mg−1, respectively. ** Significant differences in biomass between control and mixture of pollutant treatment at p < 0.01.
The enzymatic activity of T. chuii PH was also significantly (p < 0.01) enhanced in the presence of a mixture of phenol derivate compounds, with an increase of 1.73 times compared with the control cultures activity (Figure 4). This enzyme, which is responsible of phenol oxidation to 2,3-chatecol, constitutes the first step of the phenol biodegradation pathway, which ends in the production of acetyl CoA or pyruvate [13]. Moreover, phenol is a secondary metabolite in p-cresol and acetophenone biodegradation, which is produced through oxidation [20,41]. Thus, these results in PH enzymatic activity confirm that T. chuii is able to biodegrade phenol into catechol, introducing this compound into the carbon assimilation pathway, as was previously described for other microalgal strains such as Chlorella vulgaris and Chlamydomonas reinhardtii [11,13]. Similar increases in PH activity (1.63 times) was previously reported in the marine microalga Isochrysis galbana at 50 mg L−1 of phenol [27]. However, the enzymatic activity of PH was inhibited at higher concentrations of phenol in this study, just as their biodegradation rates. Furthermore, it has been reported that the cometabolic degradation of phenol and p-cresol increased the PH activity in the microalga C. vulgaris [18], which is in concordance with the results obtained for T. chuii in this work (Figure 4). Thus, although PH activity is an outstanding first approach to understanding the mechanisms that T. chuii uses to biodegrade these pollutants, further research focusing on molecular or omics approaches is needed for a full comprehension of T. chuii metabolism under phenol derivative stress.
4. Conclusions
In this study, the high tolerance of different microalgae to a mixture of phenolic compounds has been demonstrated, Chlorella sorokiniana and Nannochloropsis gaditana being the most tolerant strains. Moreover, the capacity of these microalgae for the biodegradation of phenolic compounds was also tested in the four strains. In this case, Tetraselmis chuii showed higher biodegradation capacity, both with and without a carbon source in the medium. The metabolic alterations produced by these compounds in Tetraselmis chuii were also studied, revealing an enhancement of the antioxidant system through APX and CAT activities as well as the capacity to biodegrade these compounds with an increase in PH enzymatic activity. These results confirmed that Tetraselmis chuii could be an outstanding tool for the biodegradation of phenol derivative pollutants spilled in different industry wastewaters, due to its robustness, biodegradation capacity in the absence of a carbon source, and tolerance to high salinity conditions, thereby mitigating the impact of these effluents on aquatic and marine ecosystems.
Author Contributions
Conceptualization, J.V. and A.L.-V.; Funding Acquisition, J.V. and A.L.-V.; Methodology, J.M.R.-V., A.S., and A.L.-V.; Project Administration, J.V.; Supervision, J.V.; Validation, R.L. and A.S.; Writing—Original Draft, J.M.R.-V. and A.L.-V.; Writing—Review and Editing, R.L. and A.F.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Huelva and Cepsa Foundation (Cátedra Fundación Cepsa Universidad de Huelva) and by Spanish MICIU/AEI/10.13039/501100011033 (research grant n°: JDC2022-049636-I) and the European Union NextGenerationEU/PRTR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| PH | Phenol hydroxylase |
References
- Murtaza, B.; Rahman, S.; Charles, C.; Tingheng, X.; Wensheng, Z. Environmental Impact Associated with Oil and Grease and Their Emerging Mitigation Strategies. Waste Biomass Valorization 2024, 15, 3913–3928. [Google Scholar] [CrossRef]
- Jan, S.; Mishra, A.K.; Bhat, M.A.; Bhat, M.A.; Jan, A.T. Pollutants in Aquatic System: A Frontier Perspective of Emerging Threat and Strategies to Solve the Crisis for Safe Drinking Water. Environ. Sci. Pollut. Res. 2023, 30, 113242–113279. [Google Scholar] [CrossRef]
- Chen, M.; Yin, G.; Zhao, N.; Gan, T.; Feng, C.; Gu, M.; Qi, P.; Ding, Z. Rapid and Sensitive Detection of Water Toxicity Based on Photosynthetic Inhibition Effect. Toxics 2021, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liao, X.; Ma, R.; Deng, N.; Wu, H.; Zhang, Z.; Chen, L.; Wang, Q.; Liao, Q.; Li, Q.; et al. Effects of Co-Exposure to Benzene, Toluene, and Xylene, Polymorphisms of MicroRNA Genes, and Their Interactions on Genetic Damage in Chinese Petrochemical Workers. Toxics 2024, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, Y.; Wei, Y.; Li, X.; Liu, Y.; Su, G.; Wang, X.; Jia, J.; Yan, B. Threats of Per- and Poly-Fluoroalkyl Pollutants to Susceptible Populations. Sci. Total Environ. 2024, 921, 171188. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Nassar, H.N. Phycoremediation of Phenol-Polluted Petro-Industrial Effluents and Its Techno-Economic Values as a Win-Win Process for a Green Environment, Sustainable Energy and Bioproducts. J. Appl. Microbiol. 2021, 131, 1621–1638. [Google Scholar] [CrossRef]
- Narimani, M.; Gonbadi, M.; Navabi, M.; Khezri-shooshtari, F.; Ebrahim, A.A.; Zerafat, M.M. Photocatalytic Performance of N-Doped Ti 3 O 5 Nano-Catalyst for Phenolic Compounds Removal from Industrial Wastewaters. Sci. Rep. 2025, 15, 10511. [Google Scholar] [CrossRef]
- Chang, B.; Li, G.; Guo, F.; Lu, S.; Peng, Y.; Hou, J. Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water. Water 2024, 16, 1715. [Google Scholar] [CrossRef]
- Li, H.; Meng, F. Efficiency, Mechanism, Influencing Factors, and Integrated Technology of Biodegradation for Aromatic Compounds by Microalgae: A Review. Environ. Pollut. 2023, 335, 122248. [Google Scholar] [CrossRef]
- Romero-Cruz, M.d.C.; Leon-Vaz, A.; Giráldez, I.; Vega, J.M.; Vigara, J. Effect of Heavy Metals on the Antioxidant System of the Acid-Tolerant Microalga Coccomyxa Onubensis. Algal Res. 2024, 77, 103337. [Google Scholar] [CrossRef]
- León-Vaz, A.; Torres-Franco, A.F.; García-Encina, P.A.; Muñoz, R. Developing a Microalgal-Bacterial Consortium for the Removal of Organic Pollutants from Petrochemical Industry. J. Water Process Eng. 2025, 73, 107663. [Google Scholar] [CrossRef]
- Cavalcanti Pessoa, L.; Pinheiro Cruz, E.; Mosquera Deamici, K.; Bomfim Andrade, B.; Santana Carvalho, N.; Rocha Vieira, S.; Alves da Silva, J.B.; Magalhaes Pontes, L.A.; Oliveira de Souza, C.; Druzian, J.I.; et al. A Review of Microalgae-Based Biorefineries Approach for Produced Water Treatment: Barriers, Pretreatments, Supplementation, and Perspectives. J. Environ. Chem. Eng. 2022, 10, 108096. [Google Scholar] [CrossRef]
- Nazos, T.T.; Mavroudakis, L.; Pergantis, S.A.; Ghanotakis, D.F. Biodegradation of Phenol by Chlamydomonas Reinhardtii. Photosynth. Res. 2020, 144, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Kumar Jaiswal, K.; Kumar, V.; Arora, N.; Vlaskin, M.S. Evaluation of the Mechanisms Underlying Altered Fatty Acid Biosynthesis in Heterotrophic Microalgal Strain Chlorella Sorokiniana during Biodegradation of Phenol and p - Nitrophenol. Environ. Sci. Pollut. Res. 2023, 30, 87866–87879. [Google Scholar] [CrossRef] [PubMed]
- Parsy, A.; Ficara, E.; Mezzanotte, V.; Mantovani, M.; Guyoneaud, R.; Monlau, F.; Sambusiti, C. Culture of Photosynthetic Microalgae Consortium in Artificial Produced Water Supplemented with Liquid Digestate in Closed Column Photobioreactors and Open-Pond Raceway. Biomass Bioenergy 2024, 184, 107165. [Google Scholar] [CrossRef]
- Serrano, A.; Contreras, C.; Ruiz-Filippi, G.; Borja, R.; Fermoso, F.G. Sequential Adaptation of Nannochloropsis Gaditana to Table Olive Processing Water. J. Environ. Sci. Health Part A 2017, 52, 986–991. [Google Scholar] [CrossRef]
- Meza-Escalante, E.R.; Lepe-Martinié, L.; Díaz-Quiroz, C.; Serrano-Palacios, D.; Álvarez-Valencia, L.H.; Rentería-Mexía, A.; Gortáres-Moroyoqui, P.; Ulloa-Mercado, G. Capacity of Marine Microalga Tetraselmis Suecica to Biodegrade Phenols in Aqueous Media. Sustainability 2022, 14, 6674. [Google Scholar] [CrossRef]
- Xiao, M.; Ma, H.; Sun, M.; Yin, X.; Feng, Q.; Song, H.; Gai, H. Characterization of Cometabolic Degradation of P-Cresol with Phenol as Growth Substrate by Chlorella Vulgaris. Bioresour. Technol. 2019, 281, 296–302. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.; Huang, K.; Chen, D.; Gao, F. Mechanisms and Application of Microalgae on Removing Emerging Contaminants from Wastewater: A Review. Bioresour. Technol. 2022, 364, 128049. [Google Scholar] [CrossRef]
- Chen, D.; Huo, S.; Cheng, P.; Cheng, Y.; Zhou, N. Treatment and Nutrient Recovery from Acetophenone Based Wastewater by an Integrated Catalytic Intense Pulsed Light and Tribonema Sp. Cultivation. Chem. Eng. Process. Process Intensif. 2021, 160, 108276. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Lin, Y.; Wang, G. Toxicological Effects of Phenol on Four Marine Microalgae. Environ. Toxicol. Pharmacol. 2017, 52, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, J.; Sun, T.; Wang, Y.; Meng, F. Safety Acclimation of Isochrysis Galbana Parke (Isochrysidaceae) for Enhancing Its Tolerance and Biodegradation to High-Level Phenol in Seawater. Ecotoxicol. Environ. Saf. 2021, 207, 111571. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.L.G.; Marques, L.G.; Colepicolo, P. Antioxidant Enzymes Are Induced by Phenol in the Marine Microalga Lingulodinium Polyedrum. Ecotoxicol. Environ. Saf. 2015, 116, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Bastos, P.D.A.; Galinha, C.F.; Santos, M.A.; Carvalho, P.J.; Crespo, J.G. Predicting the Concentration of Hazardous Phenolic Compounds in Refinery Wastewater—A Multivariate Data Analysis Approach. Environ. Sci. Pollut. Res. 2022, 29, 1482–1490. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: Laboratory Use. In Chlamydomonas Sourceb; Elsevier: San Diego, CA, USA, 2009; pp. 241–302. [Google Scholar]
- Guillard, R. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrates Animals; Smith, M.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Wang, Y.; Meng, F.; Li, H.; Zhao, S.; Liu, Q.; Lin, Y.; Wang, G.; Wu, J. Biodegradation of Phenol by Isochrysis Galbana Screened from Eight Species of Marine Microalgae: Growth Kinetic Models, Enzyme Analysis and Biodegradation Pathway. J. Appl. Phycol. 2019, 31, 445–455. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using Agro-Industrial Wastes for Mixotrophic Growth and Lipids Production by the Green Microalga Chlorella Sorokiniana. N. Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Zhang, F.; Wang, Y.; Wu, Y.; Jiang, Z.; Hao, S.; Ye, S.; Zhang, H.; Zhang, X. Use Microalgae to Treat Coke Wastewater for Producing Biofuel: Influence of Phenol on Photosynthetic Properties and Intracellular Components of Microalgae. Chemosphere 2024, 349, 140805. [Google Scholar] [CrossRef]
- Das, B.; Mandal, T.K.; Patra, S. A Comprehensive Study on Chlorella Pyrenoidosa for Phenol Degradation and Its Potential Applicability as Biodiesel Feedstock and Animal Feed. Appl. Biochem. Biotechnol. 2015, 176, 1382–1401. [Google Scholar] [CrossRef]
- Koletti, A.; Skliros, D.; Kalloniati, C.; Marka, S.; Eleftheria, M.-E.; Infante, C.; Mantecón, L.; Flemetakis, E. Global Omics Study of Tetraselmis Chuii Reveals Time - Related Metabolic Adaptations upon Oxidative Stress. Appl. Microbiol. Biotechnol. 2024, 108, 138. [Google Scholar] [CrossRef]
- Abu Jayyab, M.; Al-Zuhair, S. Use of Microalgae for Simultaneous Industrial Wastewater Treatment and Biodiesel Production. Int. J. Environ. Res. 2020, 14, 311–322. [Google Scholar] [CrossRef]
- Radziff, S.B.M.; Ahmad, S.A.; Shaharuddin, N.A.; Merican, F.; Kok, Y.Y.; Zulkharnain, A.; Gomez-Fuentes, C.; Wong, C.Y. Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study. Plants 2021, 10, 2677. [Google Scholar] [CrossRef]
- Ali, N.; Hellen, B.J.; Duanmu, C.; Yang, Y.; Nawaz, S.; Khan, A.; Ali, F.; Gao, X.; Bilal, M.; Iqbal, H.M.N. Effective Remediation of Petrochemical Originated Pollutants Using Engineered Materials with Multifunctional Entities. Chemosphere 2021, 278, 130405. [Google Scholar] [CrossRef]
- Patelou, M.; Koletti, A.; Infante, C.; Skliros, D.; Komaitis, F.; Kalloniati, C.; Tsiplakou, E.; Mavrommatis, A.; Mantecón, L. Fleme Omics Exploration of Tetraselmis Chuii Adaptations to Diverse Light Regimes. Antonie Van Leeuwenhoek 2025, 118, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hook, I.L.; Ryan, S.; Sheridan, H. Biotransformation of Aliphatic and Aromatic Ketones, Including Several Monoterpenoid Ketones and Their Derivatives by Five Species of Marine Microalgae. Phytochemistry 2003, 63, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cheng, D.; Wang, L.; Gao, J.; Zhao, Q.; Wei, W.; Sun, Y. Comparative Transcriptomic Analysis Reveals Phenol Tolerance Mechanism of Evolved Chlorella Strain. Bioresour. Technol. 2017, 227, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, Y.; Li, X.; Mei, S.; Gao, J.; Zhao, Q.; Wei, W.; Sun, Y. Exploration of Phenol Tolerance Mechanism through Antioxidative Responses of an Evolved Strain, Chlorella Sp. L5. J. Appl. Phycol. 2018, 30, 2379–2385. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Alharthi, S. Cellular Responses and Phenol Bioremoval by Green Alga Scenedesmus Abundans: Equilibrium, Kinetic and Thermodynamic Studies. Environ. Technol. Innov. 2021, 22, 101463. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Xiao, X.; Xue, J.; Cheng, D.; Zhang, L. The Influence of 2, 6-Di-Tert-Butyl-p-Cresol Stress on the Microalga Phaeodactylum Tricornutum and Phycosphere Bacteria Community. World J. Microbiol. Biotechnol. 2025, 41, 150. [Google Scholar] [CrossRef]
- Papazi, A.; Kotzabasis, K. Bioenergetic Strategy of Microalgae for the Biodegradation of Phenolic Compounds-Exogenously Supplied Energy and Carbon Sources Adjust the Level of Biodegradation. J. Biotechnol. 2007, 129, 706–716. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).