Sublethal Effects of Hexaflumuron on Adults of Apolygus lucorum (Hemiptera: Miridae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Insecticide Preparation
2.2. Acute Toxicity of Hexaflumuron to A. lucorum
2.3. Sublethal Effects of Hexaflumuron on Adults That Developed from 3rd-Instar Nymphs of A. lucorum
2.4. Sublethal Effects of Hexaflumuron on One-Day-Old Adults of A. lucorum
2.5. Statistical Analysis
3. Results
3.1. Acute Toxicity
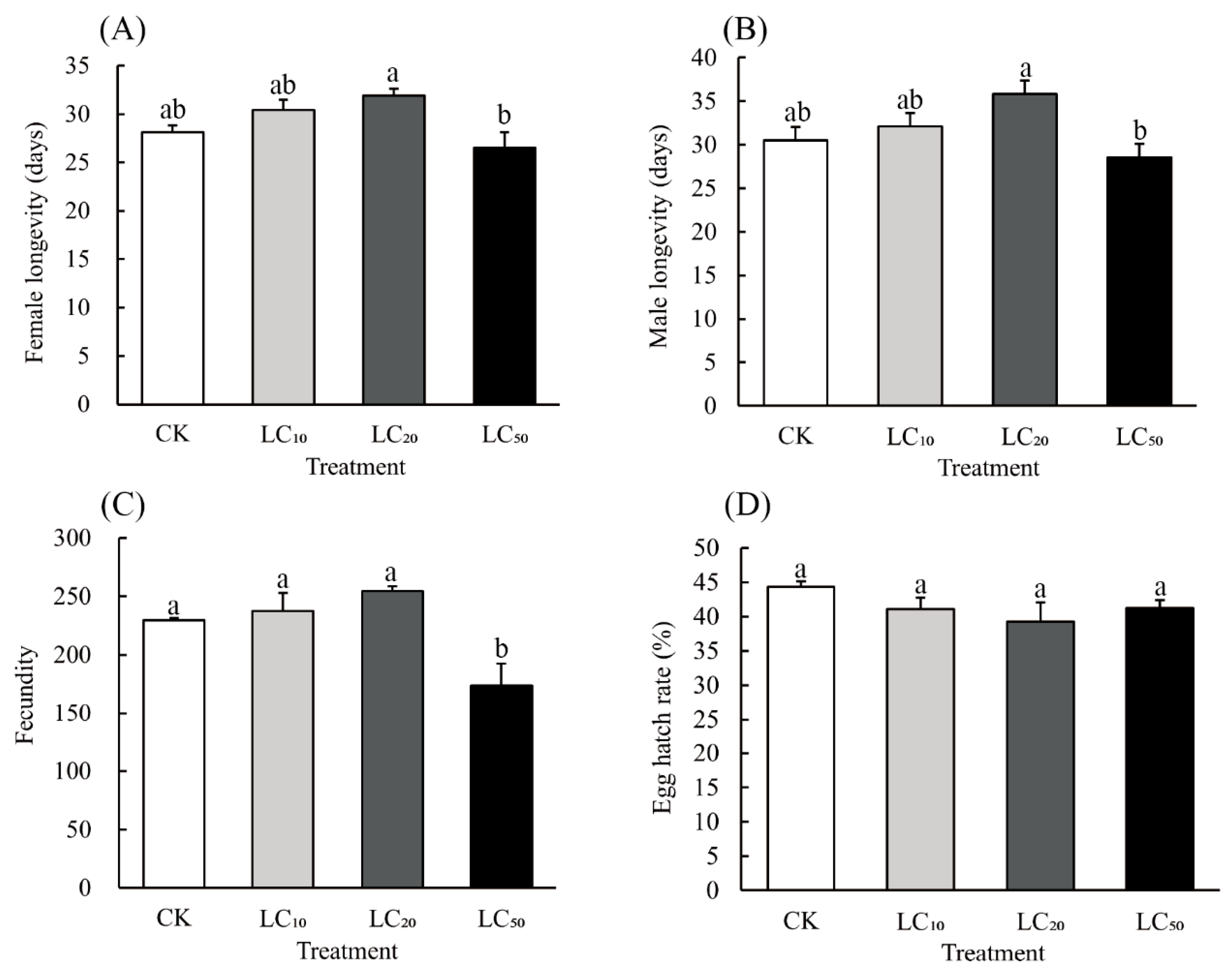
3.2. Sublethal Effects of Hexaflumuron on Adults That Developed from Nymphs That Survived Pesticide Exposure as 3rd Instars
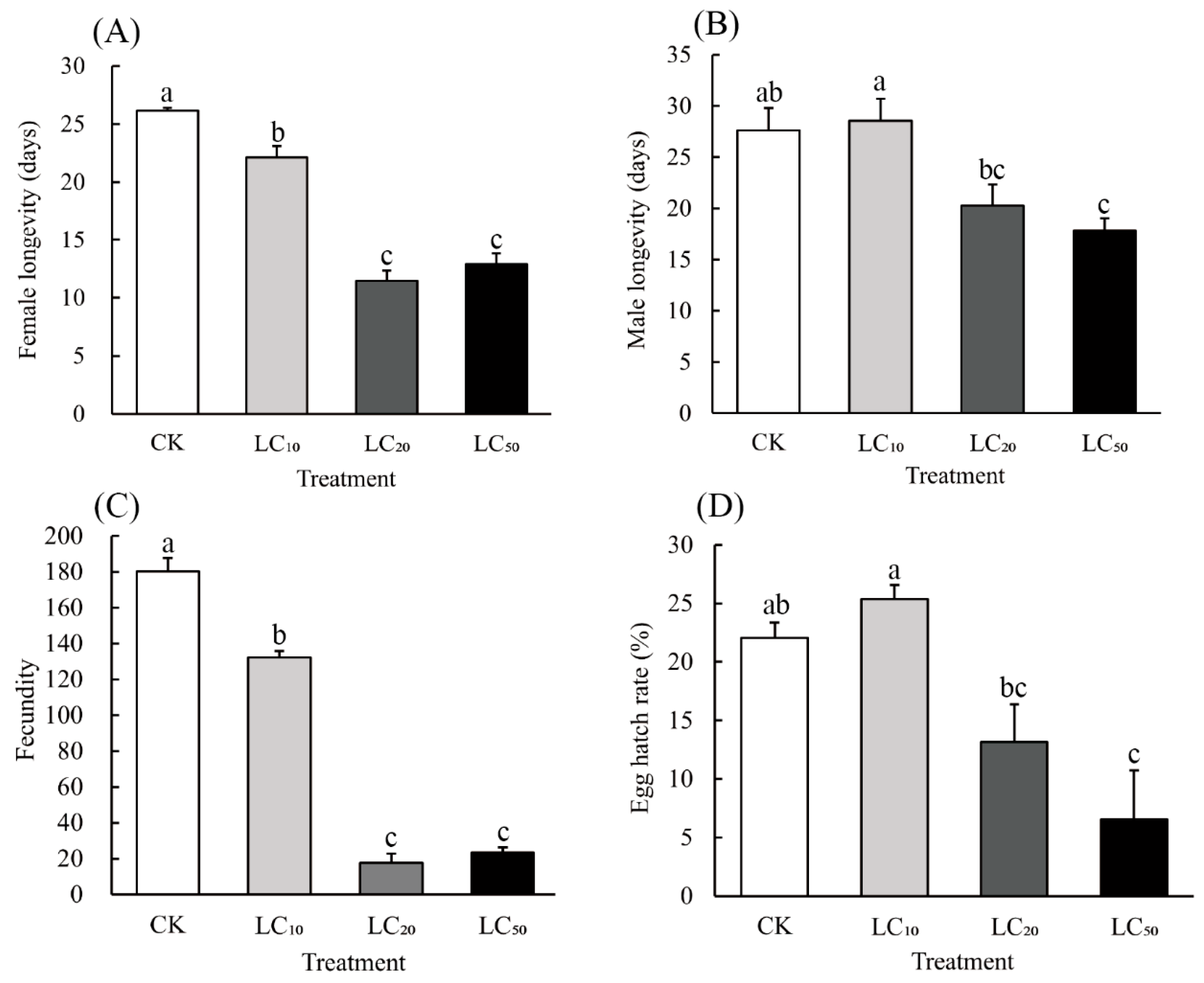
3.3. Sublethal Effects of Hexaflumuron on Adults That Survived Pesticide Exposure When Treated as One-Day-Old Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, J.; Glover, J.; Gore, J.; Crow, W.; Reddy, G. Biology, ecology, and pest management of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) in southern row crops. Insects 2021, 12, 807. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Zhao, Y.; Li, B.; Lin, J.; Zhang, X.; Liu, F.; Mu, W. Nitenpyram seed treatment effectively controls against the mirid bug Apolygus lucorum in cotton seedlings. Sci. Rep. 2017, 7, 8573. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Zhang, J.; Li, P.; Tang, Y.; Mu, Y.; Wang, M.; Wang, W.; Mao, Y. Gut microbiota facilitates the adaptation of Apolygus lucorum (Hemiptera: Miridae) to its host plant. J. Econ. Entomol. 2025, 118, 1553–1564. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.; Guo, Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef]
- Tan, Y.; Xiao, L.; Sun, Y.; Zhao, J.; Bai, L. Sublethal effects of the chitin synthesis inhibitor, hexaflumuron, in the cotton mirid bug, Apolygus lucorum (Meyer-Dür). Pestic. Biochem. Phys. 2014, 111, 43–50. [Google Scholar] [CrossRef]
- Dhadialla, T.; Carlson, G.; Dat, L. New insecticides with ecdysteroidal and juvenile hormone activity. Annu. Rev. Entomol. 1998, 43, 545–569. [Google Scholar] [CrossRef]
- Nassima, R.; Soltani, N. Laboratory evaluation of Alsystin, a chitin synthesis inhibitor, against Culex pipiens pipiens L. (Dip., Culicidae): Effects on development and cuticle secretion. J. Appl. Entomol. 2001, 123, 437–441. [Google Scholar] [CrossRef]
- Sheets, J.; Karr, L.; Dripps, J. Kinetics of uptake, clearance, transfer, and metabolism of hexaflumuron by eastern subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2000, 93, 871–877. [Google Scholar] [CrossRef]
- Khajepour, S.; Izadi, H.; Assari, M.J. Evaluation of two formulated chitin synthesis inhibitors, hexaflumuron and lufenuron against the raisin moth, Ephestia figulilella. J. Insect Sci. 2012, 12, 102. [Google Scholar] [CrossRef]
- Pineda, S.; Schneider, M.; Smagghe, G.; Martínez, C.A.; Mabel; Estal, P.; Viñuela, E.; Valle, M.J.; Budia, F. Lethal and sublethal effects of methoxyfenozide and spinosad on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2007, 100, 773–780. [Google Scholar] [CrossRef]
- Yu, C.; Fu, M.; Lin, R.; Zhang, Y.; Liu, Y.; Jiang, H.; Brock, T.C.M. Toxic effects of hexaflumuron on the development of Coccinella septempunctata. Environ. Sci. Pollut. R. 2014, 21, 1418–1424. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Abbasipour, H.; Sheikhigarjan, A.; Bandani, A.R. Sublethal effects of hexaflumuron on development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Insect Sci. 2011, 18, 689–696. [Google Scholar] [CrossRef]
- Bakr, R.; Mohammed, M.I.; Abd, E.M.; Mahdy, M. Biological effects of chitin-synthesis inhibitor, hexaflumuron compound on the desert locust, Schistocerca gregaria (Forskal). Egypt. Acad. J. biolog. Sci. 2009, 1, 49–57. [Google Scholar] [CrossRef]
- Hsiao, T.; Meng, L. The plant bugs collected from cotton fields in China (Hemiptera—Heteroptera, Miridae). Acta Zool. Sin. 1963, 15, 439–449. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Dang, Z.; Guo, Z.; Gao, Z.; Li, Y.; Ma, Y.; Pan, W. Bioactivity and sublethal effects of benzoylphenylurea insecticides on Apolygus lucorum. Chin. J. Appl. Entomol. 2012, 49, 660–665. [Google Scholar]
- Catchot, B.D.; Musser, F.R.; Gore, J.; Krishnan, N.; Knighten, K.S. Sublethal impacts of novaluron on tarnished plant bug (Hemiptera: Miridae) Adults. J. Econ. Entomol. 2021, 114, 739–746. [Google Scholar] [CrossRef]
- Gandara, L.; Jacoby, R.; Laurent, F.; Spatuzzi, M.; Vlachopoulos, N.; Borst, N.O.; Gülina, E.; Potel, C.M.; Garrido-Rodriguez, M.; Martin; et al. Pervasive sublethal effects of agrochemicals on insects at environmentally relevant concentrations. Science 2024, 386, 446–453. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Su, N. Field evaluation of a hexaflumuron bait for population suppression of subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 1994, 87, 389–397. [Google Scholar] [CrossRef]
- Su, N.Y.; La Fage, J.P. Effects of soldier proportion on the wood-consumption rate of the formosan subterranean termite (Isoptera: Rhinotermitidae). Sociobiology 1987, 13, 145–151. [Google Scholar] [CrossRef]
- Catchot, B.; Anderson, C.J.H.; Gore, J.; Jackson, R.; Krishnan, N. Novaluron prevents oogenesis and oviposition by inducing ultrastructural changes in ovarian tissue of young adult Lygus lineolaris. Pest Manag. Sci. 2020, 76, 4057–4063. [Google Scholar] [CrossRef]
- Tan, Y.; Biondi, A.; Desneux, N.; Gao, X. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology 2012, 21, 1989–1997. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Toxicology rethinks its central belief. Nature 2003, 421, 691–692. [Google Scholar] [CrossRef]
- Chu, H.F.; Meng, H.L. Studies on three species of cotton plant bugs, Adelphocoris taeniophorus Reuter, A. lineolatus (Goeze), and Lygus lucorum Meyer-Dür (Hemiptera: Miridae). Acta Entomol. Sin. 1958, 8, 97–118. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; An, J.; Gao, Z.; Dang, Z.; Pan, W.; Yuan, W.; Li, Y. Bioactivity of Benzoylphenylurea Insecticides Apolygus lucorum (Meyer-Dür) by Different Bioassay Methods. Xinjiang Agric. Sci. 2019, 56, 61–66. [Google Scholar]
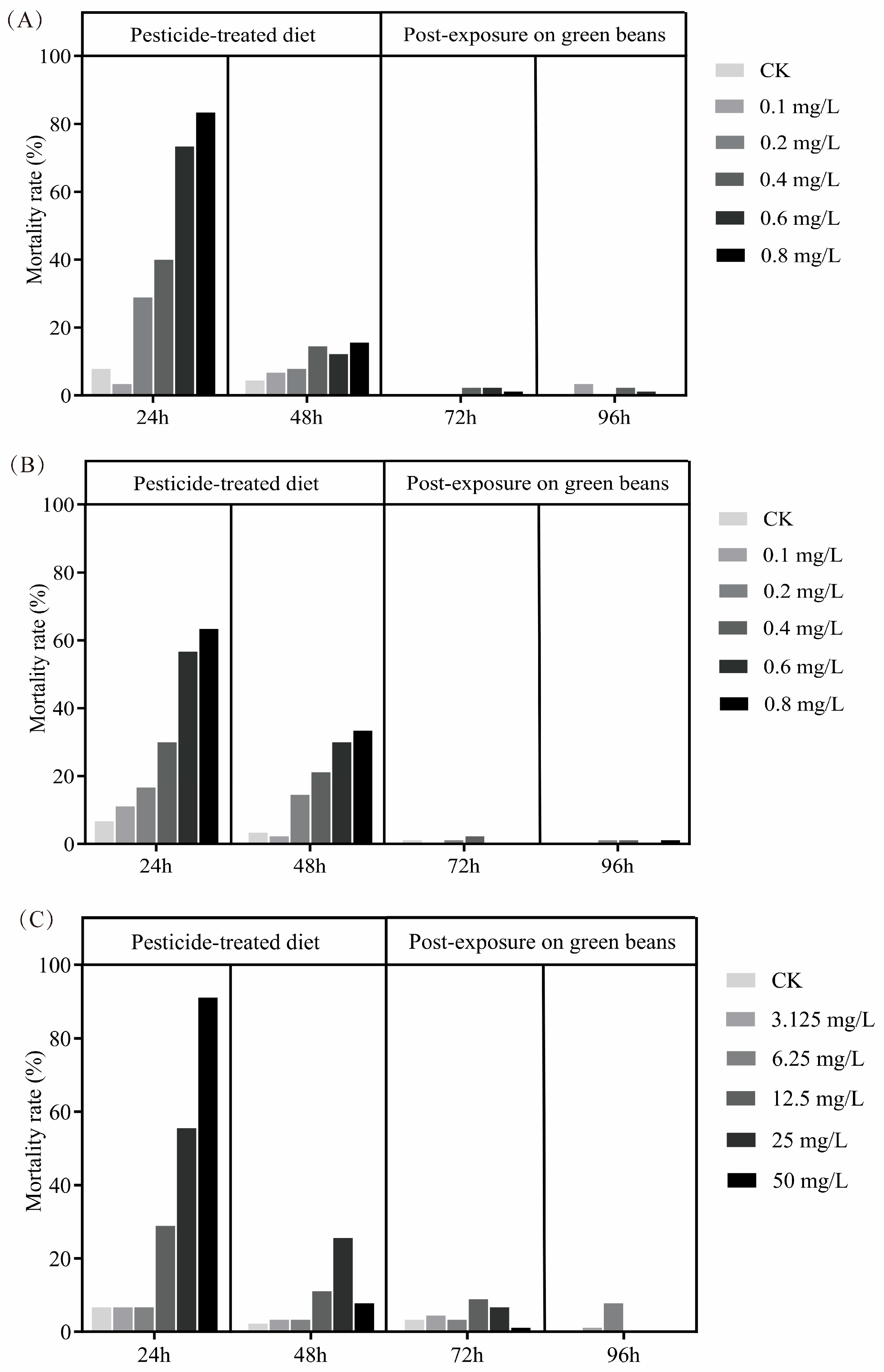
| LC Values (mg/L) and 95% CL | ||||||
|---|---|---|---|---|---|---|
| Life Stage | Slope ± SE | LC10 | LC20 | LC50 | LC90 | χ2 |
| 1st-instars | 4.303 ± 1.339 | 0.013 | 0.116 | 0.311 | 0.609 | 6.655 |
| (N/A~0.058) | (0.073~0.152) | (0.281~0.343) | (0.560~0.671) | |||
| 3rd-instars | 4.023 ± 1.354 | 0.018 | 0.127 | 0.337 | 0.655 | 3.777 |
| (N/A~0.065) | (0.082~0.165) | (0.304~0.370) | (0.603~0.721) | |||
| One-day-old adults | 0.098 ± 1.290 | 0.089 | 4.587 | 13.193 | 26.902 | 1.387 |
| (N/A~1.969) | (2.799~6.085) | (11.762~14.836) | (23.560~30.012) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, Y.; Liu, Y.; Wang, P.; Lu, Y. Sublethal Effects of Hexaflumuron on Adults of Apolygus lucorum (Hemiptera: Miridae). Toxics 2025, 13, 846. https://doi.org/10.3390/toxics13100846
Wang X, Gao Y, Liu Y, Wang P, Lu Y. Sublethal Effects of Hexaflumuron on Adults of Apolygus lucorum (Hemiptera: Miridae). Toxics. 2025; 13(10):846. https://doi.org/10.3390/toxics13100846
Chicago/Turabian StyleWang, Xie, Yu Gao, Yongqiang Liu, Peiling Wang, and Yanhui Lu. 2025. "Sublethal Effects of Hexaflumuron on Adults of Apolygus lucorum (Hemiptera: Miridae)" Toxics 13, no. 10: 846. https://doi.org/10.3390/toxics13100846
APA StyleWang, X., Gao, Y., Liu, Y., Wang, P., & Lu, Y. (2025). Sublethal Effects of Hexaflumuron on Adults of Apolygus lucorum (Hemiptera: Miridae). Toxics, 13(10), 846. https://doi.org/10.3390/toxics13100846







