The Silent Conquest: The Journey of Micro- and Nanoplastics Through Children’s Organs
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection and Data Extraction
2.5. Critical Appraisal and Synthesis
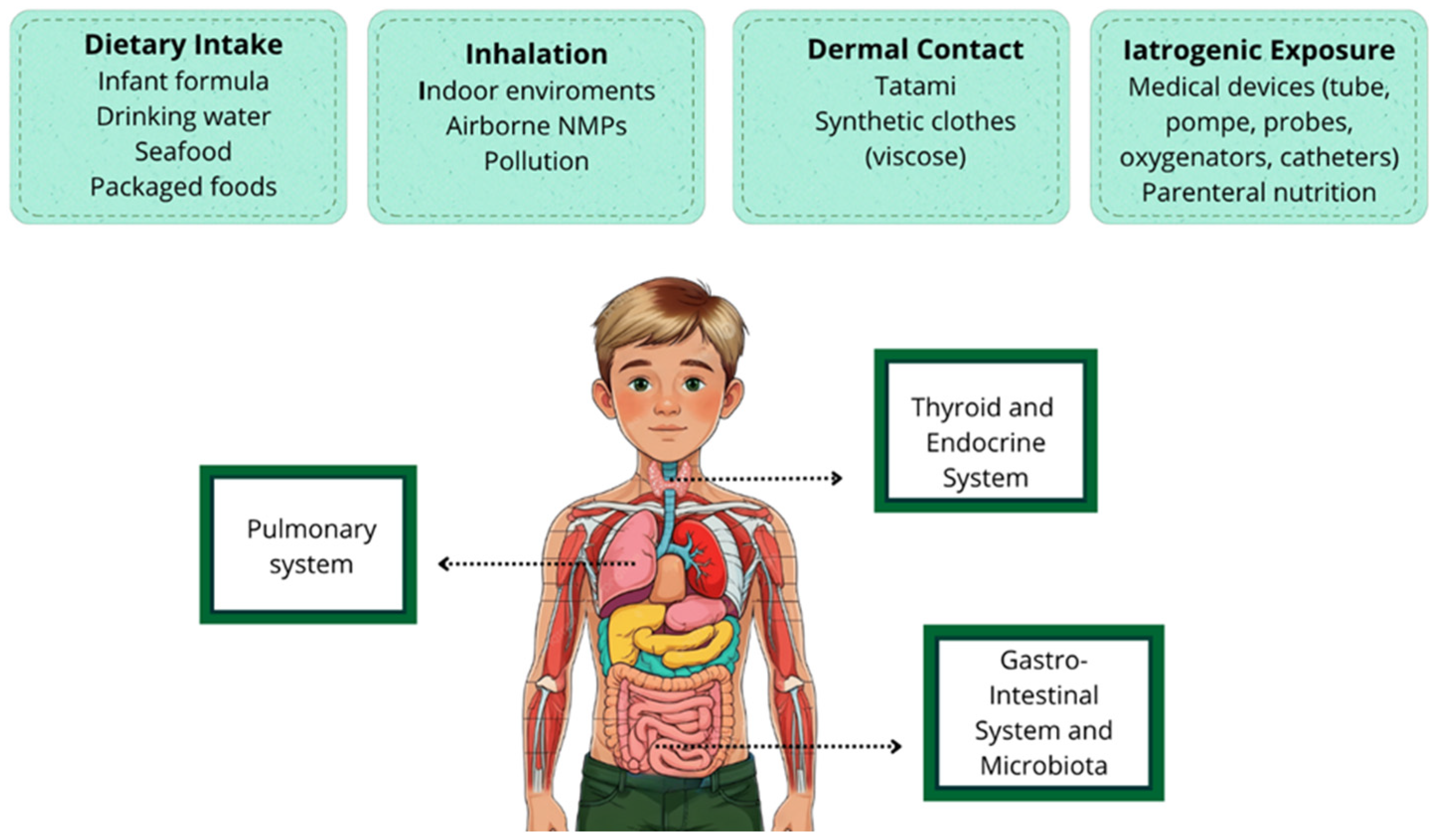
3. Exposure Pathways and Sources of MNPs in Children
3.1. Dietary Intake
3.2. Inhalation
3.3. Dermal Contact
3.4. Iatrogenic Exposure
4. Organ-Specific Effects in Children
4.1. Pulmonary System
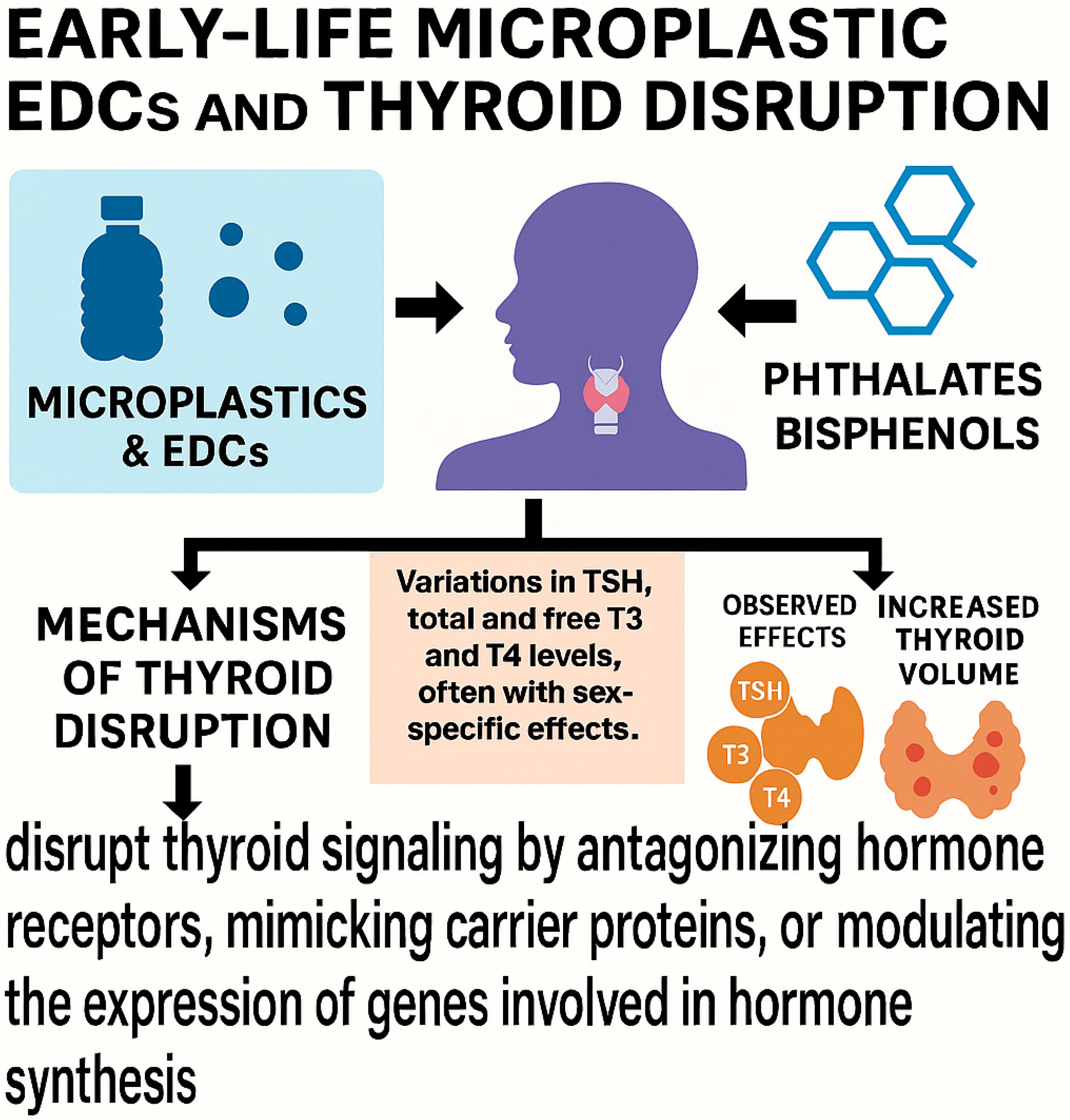
4.2. Thyroid and Endocrine System
4.3. Gastrointestinal System and Microbiota
5. Pathogenic Mechanisms
6. Exposure Biomarkers
7. Research Gaps and Limitations
7.1. Detection Limitations Due to Instrumental Constraints
7.2. Lack of Standardized Sampling Protocols
7.3. Lack of Information on All Organs in the Pediatric Age Group
7.4. Ethical and Practical Barriers to Tissue-Based Studies in Children
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, F.; Du, J.; Gao, J.; Liu, G.; Song, Y.; Yang, A.; Wang, H.; Ding, Y.; Wang, Q. Bibliometric Profile of Global Microplastics Research from 2004 to 2019. Int. J. Environ. Res. Public Health 2020, 17, 5639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.W. Further research on impact of microplastics on children’s health is essential to protecting future generations. Clin. Exp. Pediatr. 2025, 68, 359–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics exposure: Implications for human fertility, pregnancy and child health. Front. Endocrinol. 2024, 14, 1330396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, Q.; Gatheru Waigi, M.; Ling, W.; Qin, C.; Wang, J.; Gao, Y. Microplastics-sorbed phenanthrene and its derivatives are highly bioaccessible and may induce human cancer risks. Environ. Int. 2022, 168, 107459. [Google Scholar] [CrossRef] [PubMed]
- EEA. Communication from the Commission to the European Parliament, the Council, the European Economic and Socialcommittee and the Committee of the Regions A European Strategy for Plastics in a Circular Economy Brussels, 16.1.2018 COM. 2018; 28 Final; European Environment Agency: Copenhagen, Denmark, 2014. [Google Scholar]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ Sci Technol. 2019, 53, 7068–7074, Erratum in Environ. Sci. Technol. 2020, 54, 10974. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragusa, A.; Fanos, V. Microplastics and nanoplastics in the brain: A review of the neurodevelopmental risks. J. Pediatr. Neonatal Individ. Med. 2025, 14, e140206. [Google Scholar]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Etzel, R.A. The special vulnerability of children. Int. J. Hyg. Environ. Health 2020, 227, 113516, Erratum in Int. J. Hyg. Environ. Health 2021, 234, 113714. [Google Scholar] [CrossRef] [PubMed]
- Smerieri, A.; Testa, C.; Lazzeroni, P.; Nuti, F.; Grossi, E.; Cesari, S.; Montanini, L.; Latini, G.; Bernasconi, S.; Papini, A.M.; et al. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS ONE 2015, 10, e0117831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Street, M.E.; Bernasconi, S. Microplastics, environment and child health. Ital. J. Pediatr. 2021, 47, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behrendt, M.; Mahlik, S.; Zaleska-Medynska, A.; Grembecka, M. Isolation and identification of microplastics in infant formulas—A potential health risk for children. Food Chem. 2024, 440, 138246. [Google Scholar]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food. 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.; Mondal, P.; Haldar, D.; Halder, G. Beyond the cradle—Amidst microplastics and the ongoing peril during pregnancy and neonatal stages: A holistic review. J. Hazard. Mater. 2024, 469, 133963. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Lujic, T.; Cirkovic Velickovic, T. Nano- and Microplastics Migration from Plastic Food Packaging into Dairy Products: Impact on Nutrient Digestion, Absorption, and Metabolism. Foods 2023, 12, 3043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trivedi, R.; Spill, M.K.; Saha, S.; Thoerig, R.C.; Davis, J.S.; MacFarlane, A.J. Seafood Toxicant Exposure During Pregnancy, Lactation, and Childhood and Child Outcomes: A Scoping Review. Adv. Nutr. 2025, 16, 100353. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, F.; Quan, S.; Chen, L.; Shen, A.; Jiao, A.; Qi, H.; Yu, G. Microplastics in the Bronchoalveolar Lavage Fluid of Chinese Children: Associations with Age, City Development, and Disease Features. Environ. Sci. Technol. 2023, 57, 12594–12601. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Moradalizadeh, S.; Jahani, Y.; Nasiri, A. Biomonitoring of microplastics in saliva and hands of young children in kindergartens: Identification, quantification, and exposure assessment. Environ. Monit. Assess. 2025, 197, 859. [Google Scholar] [CrossRef]
- Scopetani, C.; Esterhuizen-Londt, M.; Chelazzi, D.; Cincinelli, A.; Setälä, H.; Pflugmacher, S. Self-contamination from clothing in microplastics research. Ecotoxicol. Environ. Saf. 2020, 189, 110036. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Ding, R.; Sun, C.; Yao, L.; Zhang, W. Microparticles and microplastics released from daily use of plastic feeding and water bottles and plastic injectors: Potential risks to infants and children in China. Environ. Sci. Pollut. Res. 2021, 28, 59813–59820. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.; He, R.; Zhao, T.; Chen, Z. Micronanoplastic exposure due to cardiopulmonary bypass in children: A prospective observational study. J. Hazard. Mater. 2025, 489, 137732. [Google Scholar] [CrossRef]
- Krzciuk, K.; Dołęgowska, S.; Gałuszka, A.; Michalik, A. Factors affecting microplastic pollution of sandboxes in urban residential areas: Simple methodology for quality control in the context of potential exposure assessment for children. Environ. Toxicol. Chem. 2025, 44, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, V.S.; Leonard, J.; El Rassi, L.A.; Choy, M.M.; Brar, J.; Glasman, J.B.; Cowger, W.; Mohanty, S.K. Children’s playgrounds contain more microplastics than other areas in urban parks. Sci. Total Environ. 2023, 854, 158866. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Panneel, L.; Jorens, P.G.; Covaci, A.; Cleys, P.; Mulder, A.; Janssen, C.R.; Asselman, J. An Ex Vivo Study Examining Migration of Microplastics from an Infused Neonatal Parenteral Nutrition Circuit. Environ. Health Perspect. 2024, 132, 37703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirel, A.; Çoban, A.; Yıldırım, Ş.; Doğan, C.; Sancı, R.; İnce, Z. Hidden Toxicity in Neonatal Intensive Care Units: Phthalate Exposure in Very Low Birth Weight Infants. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 298–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856 (Pt 2), 159164. [Google Scholar] [CrossRef] [PubMed]
- Dzierżyński, E.; Blicharz-Grabias, E.; Komaniecka, I.; Panek, R.; Forma, A.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Choma, A.; Suśniak, K.; et al. Post-mortem evidence of microplastic bioaccumulation in human organs: Insights from advanced imaging and spectroscopic analysis. Arch Toxicol. 2025, 99, 4051–4066. [Google Scholar] [CrossRef] [PubMed]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in human urine: Characterisation using μFTIR and sampling challenges using healthy donors and endometriosis participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef] [PubMed]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med. 2025, 31, 1114–1119, Erratum in Nat. Med. 2025, 31, 1367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solleiro-Villavicencio, H.; Gomez-De León, C.T.; Del Río-Araiza, V.H.; Morales-Montor, J. The detrimental effect of microplastics on critical periods of development in the neuroendocrine system. Birth Defects Res. 2020, 112, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Shulhai, A.-M.; Petraroli, M.; Patianna, V.; Donini, V.; Giudice, A.; Gnocchi, M.; Masetti, M.; Montani, A.G.; Rotondo, R.; et al. The impact of environmental factors and contaminants on thyroid function and disease from fetal to adult life: Current evidence and future directions. Front. Endocrinol. 2024, 15, 1429884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maia, A.; Vieira-Coelho, M.A. The impact of exposure to phthalates in thyroid function of children and adolescents: A systematic review. Eur. J. Pediatr. 2024, 184, 111. [Google Scholar] [CrossRef]
- Ke, D.; Zheng, J.; Liu, X.; Xu, X.; Zhao, L.; Gu, Y.; Yang, R.; Liu, S.; Yang, S.; Du, J.; et al. Occurrence of microplastics and disturbance of gut microbiota: A pilot study of preschool children in Xiamen, China. EBioMedicine 2023, 97, 104828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Röllin, H.B.; Weihe, P.; Díaz, G.J.; Singh, R.R.; Visnes, T.; et al. A Children’s Health Perspective on Nano- and Microplastics. Environ. Health Perspect. 2022, 130, 15001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2022, 302 Pt A, 113995. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, X.; Zhang, Y.; Liu, B.; Zhang, X.; Yang, L. Urinary microplastic contaminants in primary school children: Associations with behavioral development. Ecotoxicol. Environ. Saf. 2025, 295, 118097. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jin, C.; Bai, Y.; Ma, R.; Deng, Y.; Gao, Y.; Pan, G.; Yang, Z.; Yan, L. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci. Total Environ. 2022, 848, 157639. [Google Scholar] [CrossRef] [PubMed]
- VLLeonard, S.; Liddle, C.R.; Atherall, C.A.; Chapman, E.; Watkins, M.; DJCalaminus, S.; Rotchell, J.M. Microplastics in human blood: Polymer types, concentrations and characterisation using μFTIR. Environ. Int. 2024, 188, 108751. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, K.; Zhao, Y. The development and application of advanced analytical methods in microplastics contamination detection: A critical review. Sci. Total Environ. 2022, 818, 151851. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’oNofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ethics in pediatric research. Med 2023, 4, 5–7. [CrossRef] [PubMed]
- Zheng, D.; Wang, D.; Zhang, S.; Liu, Y.; Xi, Q.; Weng, Y. Impact of urinary microplastic exposure on cognitive function in primary school children. Ecotoxicol. Environ. Saf. 2025, 302, 118532. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue accumulation of microplastics and potential health risks in human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef] [PubMed]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23, Erratum in Ann. Glob. Health 2023, 89, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| What Was Already Known | What This Work Adds |
|---|---|
| Micro- and nanoplastics are ubiquitous environmental contaminants capable of bioaccumulating in human tissues, with potential toxicological effects. Most available knowledge derives from studies on adults or animal models, while data concerning the pediatric population are sporadic and fragmented. | This work represents the first narrative review entirely focused on the pediatric population. It provides a structured analysis of exposure routes specific to children (dietary intake, inhalation, dermal, and iatrogenic), describes the presence of MNPs in organs such as lungs, thyroid, and gut microbiota, and proposes possible non-invasive biomarkers. It offers an organized basis for the development of future clinical and experimental studies. |
| Tissue/Fluid | MPs Concentration |
|---|---|
| Placenta | 2.7–18 MPs/g |
| Endometrium | 21 MPs/100 mg |
| Heart | 0–7.75 MPs/g |
| Lungs | 0.69–14.1 MPs/g |
| Kidneys | 40.4 MPs/g |
| Liver | 4.6 MPs/g |
| Spleen | 1.1 MPs/g |
| Brain | 4 800 µg/g |
| Colon | 7.91 and 9.45 MPs/g |
| Endometrium | 21 MPs/100 mg |
| Skin (hair, hands, and facial skin) | 3.5; 2.1; 2.02 MPs per individual per day |
| Testis | 11.6 MPs/g |
| Semen | 0.23 MPs/g |
| Bronchoalveolar Lavage Fluid | 9.18 ± 2.45 MPs/100 ml |
| Sputum | 39.5 MPs/10 mL |
| Saliva | 0.33 MPs per individual per day |
| Feces | 1 to 50 MPs/g |
| Urine | 0–9600 MPs/L |
| Blood | 1.6 µg/ml |
| Breast Milk | 0–2.72 Mps/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, E.; Comisi, F.F.; Fanos, V.; Ragusa, A. The Silent Conquest: The Journey of Micro- and Nanoplastics Through Children’s Organs. Toxics 2025, 13, 812. https://doi.org/10.3390/toxics13100812
Esposito E, Comisi FF, Fanos V, Ragusa A. The Silent Conquest: The Journey of Micro- and Nanoplastics Through Children’s Organs. Toxics. 2025; 13(10):812. https://doi.org/10.3390/toxics13100812
Chicago/Turabian StyleEsposito, Elena, Francesco Fabrizio Comisi, Vassilios Fanos, and Antonio Ragusa. 2025. "The Silent Conquest: The Journey of Micro- and Nanoplastics Through Children’s Organs" Toxics 13, no. 10: 812. https://doi.org/10.3390/toxics13100812
APA StyleEsposito, E., Comisi, F. F., Fanos, V., & Ragusa, A. (2025). The Silent Conquest: The Journey of Micro- and Nanoplastics Through Children’s Organs. Toxics, 13(10), 812. https://doi.org/10.3390/toxics13100812








